Abstract
Background
Cutibacterium acnes is the primary cause of shoulder surgery infections, but the predisposition to larger skin counts and potentially higher risk for postoperative infection remains unclear. This study aimed to quantify risk factors influencing endogenous C. acnes burden and to compare counts among 4 shoulder sites.
Methods
C. acnes counts were quantified via a detergent scrub technique for 173 participants. Bivariate and multivariable stepwise linear regression statistical analyses were used to investigate the association of sex, age, ethnicity, degree of hirsutism, diabetes, smoking status, body mass index, and location with counts. A separate Wilcoxon rank-sum test was performed analyzing counts of East/Southeast Asians vs. all other ethnicities.
Results
Sex, age, degree of hirsutism, diabetes, smoking status, and body mass index were included in the multivariable stepwise linear regression analysis. The multiple regression analysis isolated individuals <40 years with the highest burden (P = .001). Males had a 191% increase in C. acnes counts compared with females (P = .001). Increased hirsutism was further indicated to be a risk factor for the male sex although not in a dose-dependent manner (P = .027). Wilcoxon rank-sum test results found that East/Southeast Asians had the lowest load (P = .019), although not significant in the multivariate model.
Conclusion
Surgical site C. acnes infections occur more frequently in younger males, and males <40 years with shoulder-specific hirsutism have the highest preoperative burden. East/Southeast Asians have lower raw counts of C. acnes compared with other ethnicities that may be related to less hirsutism.
Keywords: Cutibacterium acnes, C. acnes, shoulder surgery infection, shoulder burden, skin infection, bacteria
Cutibacterium acnes is a bacterium associated with skin infections. It is a Gram-positive, non-spore forming, and anaerobic bacillus.5 C. acnes mainly colonizes hair follicles of the upper body, including the head, neck, shoulders, and axilla.5 It predominantly resides on the skin and in the sebaceous glands associated with hair follicles. Although the bacterium has been linked to conditions such as endocarditis, endophthalmitis, osteomyelitis, and acne, C. acnes has become a substantial culprit for shoulder surgery infections, accounting for almost 56% of all cases.5
During surgical transection of the skin, C. acnes can seep into the wound.16 Presurgical sites on patients undergoing shoulder, hip, knee, and spine surgery can have up to 105 organisms per follicle, sheltered within the dermal sebaceous glands.8 Virulence can then be achieved by adhering to cells and surfaces or forming a biofilm.5 This is accomplished through the use of antigenic proteins, which subsequently can lead to an inflammatory response.5 Infection rates range from approximately 3% to 15%.9,17 A C. acnes infection can ultimately lead to chronic pain, prosthetic instability, sepsis, or shoulder arthroplasty failure.1
This study aimed to quantify risk factors for increased endogenous C. acnes burden on the shoulder. Sex, age, ethnicity, degree of shoulder hair, diabetes, smoking status, body mass index (BMI), and location on the shoulder were analyzed through both independent statistical and multiple linear regression analyses. We hypothesized that younger men would have the highest C. acnes burden along with the posterior site given its proximity to the back. We also hypothesized that East/Southeast Asians would have the lowest C. acnes burden.
Methods
This is a secondary data analysis of 2 cohorts (173 participants). The first cohort included patients (N = 71) who underwent any primary or revision surgery at our institution and the second cohort (N = 102) included prospectively enrolled healthy volunteers. Exclusion criteria for patients were those younger than 18 years of age or those with a history of shoulder infection. Volunteers younger than 18 years of age or those with a history of shoulder surgery or infection were excluded. All participants provided informed consent after institutional review board approval. Endogenous C. acnes counts were collected before surgery or any skin treatment.
A Williamson and Kligman detergent scrub technique was used to measure C. acnes levels.27 The modified Williamson and Kligman technique used is a standardized dermatologic method for C. acnes quantification that correlates with deep sebaceous gland burden.6,8,10,11,27 Each cohort had both shoulders scrubbed for bacteria analysis. For risk factor analysis, the nonoperative shoulder of patients was used for data collection to ensure no potential impact of shoulder pathology on C. acnes counts. A random shoulder was used for data collection of volunteer participants. Age was well distributed throughout the combined patient and volunteer cohorts.
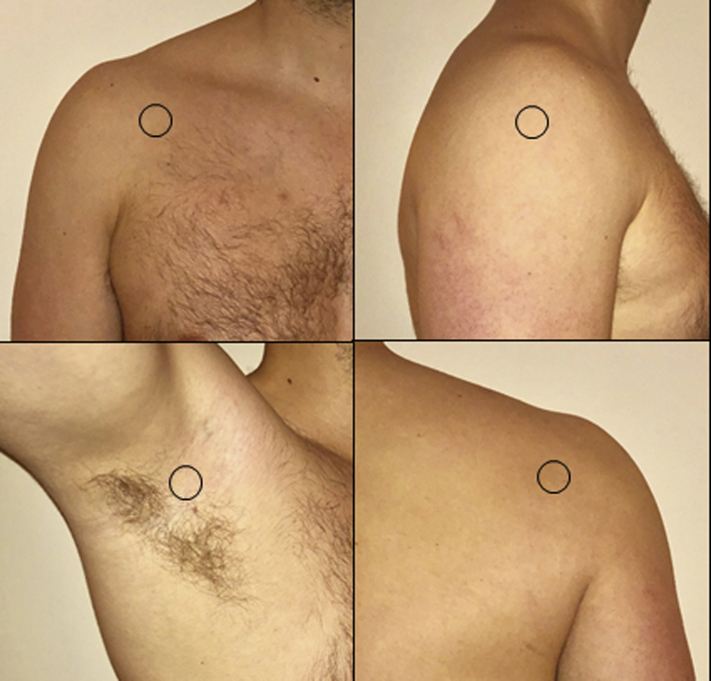
First, each site (anterior, lateral, posterior, and axilla; refer to Fig. 1) was cleansed of any possible surface bacteria by wiping the area for 30 seconds with sterile gauze soaked with 0.1% Triton X-100. Each site was delineated by a 3.8 cm2 diameter round hole in a business card–sized plastic template held firmly to the skin. Each sample area was then scrubbed for 30 seconds using moderate pressure with a sterile cotton swab that was dipped in Bacto-Letheen broth. The tip of each swab was then placed into a 2 mL tube of Bacto-Letheen broth for subsequent incubation.8
Figure 1.
Sample sites (starting top left and proceeding clockwise): anterior, lateral, posterior, and axilla.4
Patient samples were placed into individual transport bags, delivered on ice to an independent C. acnes laboratory, and subsequently serially diluted into 0.05% Tween-80. Samples from volunteers were obtained in the laboratory itself. Fifty microliters of each dilution was placed on Brucella agar plates, left to dry, and then incubated anaerobically at 35°C-37°C for 7 days. Quantification of C. acnes was measured in terms of colony-forming units and was counted at the lowest dilution that could be read.8
A priori power analysis, before volunteer enrollment, was performed based on the sex significance indicated in a previous paper that implied an 8000 colony-forming unit “effect size” when comparing the male and female sex.8 The results were then used to approximate how many participants would be necessary to generate statistical significance in a single risk factor. Power was set at 0.8, alpha at 0.05, and 39 subjects were determined as the minimum to detect a difference.
C. acnes counts were pooled between the patient and volunteer cohorts. Bacterial quantification was analyzed with respect to sex, age, ethnicity, degree of shoulder hair, diabetes, smoking status, BMI, and location on the shoulder. Average burden count among the 4 sites for each participant was calculated, and natural log transformed before statistical analysis as the data were not normally distributed.
Ethnicity was self-determined during admission into the study as participants were given the option to choose from East/Southeast Asian, Caucasian/Hispanic, South Asian/Middle Eastern, and African. Shoulder-specific hairiness was described on a 4-point scale ranging from 0 to 3 where 0 = no hair, 1 = minimal hair (more skin than hair), 2 = moderate hair (more hair than skin), and 3 = maximal hair. Because axillary hair removal was relatively common, but shoulder skin hair removal was not reported in our sample, a shoulder girdle/skin evaluation was conducted instead of an axillary evaluation when determining hairiness. A reliability analysis was conducted where 3 authors separately rated 10 sample shoulder images using our scale. An intraclass correlation score was computed using a 2-way random-effects model (R Core Team; R Foundation for Statistical Computing, Vienna, Austria). There was a high intraclass correlation coefficient indicating agreement among ratings (kappa = 0.88, P = 1.6 × 10−8). Age was specifically binned as <40, 40-60, and >60 years to provide clinical context to the findings, as ages 40-60 years are considered a young group for shoulder replacement and more than 60 years is considered the standard for arthroplasty.
Given the negative effects of increased blood sugar levels on skin healing, any participant with an A1C above 5.7% was placed in the “diabetes” group. Thus, diabetes was indicated as either present or absent, where type 1, type 2, and prediabetes were grouped together. Smoking status was indicated as “yes” or “no” where former smokers were included in the “yes” group. Former smokers were those who had quit within 1 year of enrollment, which is a common cutoff used in joint replacement infection analyses.
Sex, diabetes, and smoking were analyzed via the independent samples t-test assuming unequal variance (Microsoft Excel; Microsoft Corporation, Redmond, WA, USA). Age, ethnicity, degree of shoulder hair, BMI, and location on the shoulder were all evaluated for significance via a single-factor analysis of variation test (Microsoft Excel; Microsoft Corporation). Parameters with statistical significance (P < .05) in the bivariate analyses were subsequently included into a multivariable stepwise linear regression model using JMP data analysis software (JMP; Cary, NC, USA). Risk factors in this analysis with P < .05 were considered statistically significant. A separate Wilcoxon rank sum test was performed to analyze the nontransformed counts of East/Southeast Asians vs. all other ethnicities. An independent samples t-test assuming unequal variance was then conducted to analyze the degree of hirsutism difference between East/Southeast Asians and all other ethnicities to analyze for a potential covariate.
Results
Seventy-one patients and 102 healthy volunteers were enrolled in the study for a total of 173 participants. A total of 692 culture samples were obtained, and quantified and complete data were collected from 162 participants including shoulder hairiness score. Refer to Table I for a complete demographic breakdown of the compiled cohort.
Table I.
Study population demographic data
| Participants, n | Compiled cohort, 173 |
|---|---|
| Sex, n (%) | |
| Male | 98 (57) |
| Female | 75 (43) |
| Age∗ (yr), n (%) | |
| <40 | 111 (67) |
| 40-60 | 32 (19) |
| >60 | 23 (14) |
| BMI∗ (kg/m2), n (%) | |
| <25 | 91 (55) |
| 25-35 | 61 (37) |
| >35 | 14 (8) |
| Smoking∗, n (%) | |
| Yes/former† | 41 (25) |
| No | 125 (75) |
| Diabetes∗, n (%) | |
| Pre/type 1/type 2‡ | 14 (8) |
| No | 152 (92) |
| Ethnicity, n (%) | |
| South Asian/Middle Eastern | 18 (10) |
| Caucasian/Hispanic | 100 (68) |
| African | 32 (18) |
| East/Southeast Asian | 23 (13) |
| Degree of hirsutism§,‖, n (%) | |
| 0 | 108 (67) |
| 1 | 42 (26) |
| 2-3 merged | 12 (7) |
BMI, body mass index.
7 of 173 participants excluded for lack of demographic data.
Participants included 27 current smokers and 14 former smokers.
Participants included 1 prediabetic.
11 of 173 participants excluded for lack of shoulder hair data.
Shoulder hair key: 0 = minimal or no hair, 1 = some hair (more skin than hair), 2 = moderate hair (more hair than skin), 3 = significant hair (maximum).
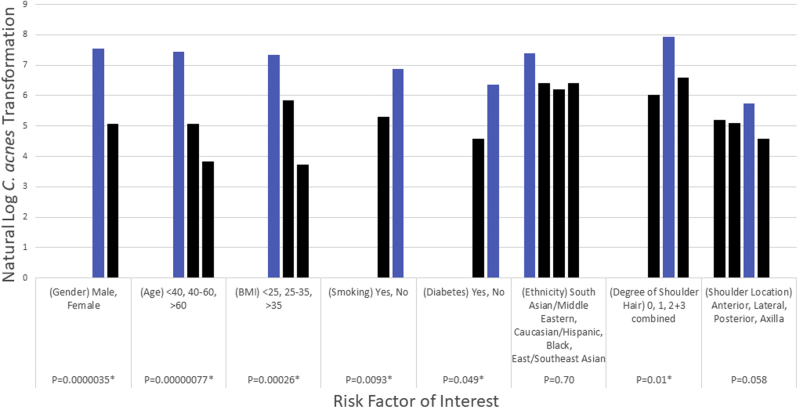
Bivariate analyses found sex, age, degree of shoulder hair, diabetes, smoking, and BMI were significant in relation to C. acnes burden (Fig. 2). The male sex was independently associated with increased counts (7.56 vs. 5.07; P < .001). Individuals with lower BMI (<25) were statistically associated with increased counts (P = .0003). Participants below the age of 40 years had the highest average counts with statistical significance (P < .001).
Figure 2.
Bivariate analyses show a statistically significant risk for increased burden in males, individuals with a BMI <25, individuals below the age of 40 years, those with increased amounts of shoulder hair, and positive smoking and diabetes status. BMI, body mass index.
Negative diabetes and smoking status were associated with increased counts (P = .049 and P = .009). Increased shoulder hair was associated with increased burden when subcategories 0, 1, and 2/3 combined were evaluated via analysis of variation analysis (P = .010).
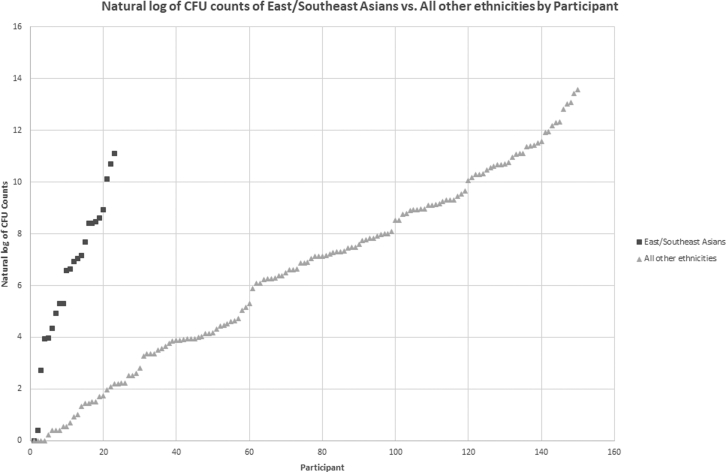
The posterior site was associated with increased burden without statistical significance after log transformation (P = .058). No significant difference was found among the different ethnicity groups (P = .70) when analyzed with logarithmically transformed counts (Table II). Wilcoxon rank-sum test results found East/Southeast Asians to have the lowest C. acnes load (P = .019; refer to Fig. 3). When an independent samples t-test assuming unequal variance was subsequently conducted to assess the degree of hirsutism difference between East/Southeast Asians and all other ethnicities, it was determined that East/Southeast Asians had a lower degree of shoulder hair on average than those of all other ethnicities with statistical significance (0.14 vs. 0.47, P = .002).
Table II.
Study population C. acnes average burden by risk factor univariate analysis with log transformation
| C. acnes burden (ln(CFU)) | P value | Type of test | |
|---|---|---|---|
| Sex | |||
| Male | 7.56 | <.001∗ | Unpaired t-test |
| Female | 5.07 | ||
| Age† (yr) | |||
| <40 | 7.44 | <.001∗ | One-factor ANOVA |
| 40-60 | 5.07 | ||
| >60 | 3.84 | ||
| BMI† (kg/m2) | |||
| <25 | 7.33 | <.001∗ | One-factor ANOVA |
| 25-35 | 5.85 | ||
| >35 | 3.72 | ||
| Smoking† | |||
| Yes/former | 5.31 | .009∗ | Unpaired t-test |
| No | 6.87 | ||
| Diabetes† | |||
| Pre/type 1/type 2 | 4.58 | .049∗ | Unpaired t-test |
| No | 6.36 | ||
| Ethnicity | |||
| South Asian/Middle Eastern | 7.40 | .70 | One-factor ANOVA |
| Caucasian/Hispanic | 6.42 | ||
| African | 6.21 | ||
| East/Southeast Asian | 6.43 | ||
| Degree of hirsutism‡ | |||
| 0 | 6.01 | .010∗ | One-factor ANOVA |
| 1 | 7.93 | ||
| 2-3 merged | 6.60 | ||
| Location§ | |||
| Anterior | 5.19 | .058 | One-factor ANOVA |
| Lateral | 5.09 | ||
| Posterior | 5.74 | ||
| Axilla | 4.59 | ||
BMI, body mass index; CFU, colony-forming unit; ANOVA, analysis of variation.
Indicated as statistically significant with P < .05.
7 of 173 participants excluded for lack of demographic data.
11 of 173 participants excluded for lack of shoulder hair data.
1 of 173 participants did not have anterior or axilla data and was excluded from these 2 bins.
Figure 3.
A scatter plot illustrating the transformed colony-forming unit (CFU) counts of East/Southeast Asians vs. all other ethnicities.
Sex, age, degree of shoulder hair, diabetes, smoking status, and BMI were all included into a multiple linear regression. After controlling for these risk factors, male sex (P = .0011) and individuals below the age of 40 years (P = .0095) have statistically significant risk for higher C. acnes burden. Furthermore, the analysis confirmed that BMI and negative diabetes and smoking statuses were not a statistically significant risk for increased burden suggesting that those with lower BMI were most likely young males. Insufficient sample variation in hairiness among the female sex prompted a separate multiple regression analysis run by sex. This separate analysis indicated that hairiness does have a non−dose-dependent statistically significant association with increased burden in the male sex (P = .0267) but not in the female sex (P = .6109). Refer to Tables III and IV for a detailed breakdown.
Table III.
Multiple linear regression of significant risk factors in the compiled cohort
| Risk factor included | Estimate (95% CI) | P value |
|---|---|---|
| Sex | .0011∗ | |
| Male | 1.91 (0.78, 3.04) | .0011∗ |
| Female | [REF] | N/A |
| Age | .0095∗ | |
| >60 | −2.61 (−4.42, −0.81) | .0049∗ |
| 40-60 | −1.88 (−3.44, −0.31) | .019∗ |
| <40 | [REF] | N/A |
| BMI | .35 | |
| >35 | −0.46 (−2.65, 1.73) | .68 |
| 25-35 | −0.87 (−2.05, 0.31) | .15 |
| <25 | [REF] | N/A |
| Degree of hirsutism | .07 | |
| 2-3 | −0.75 (−2.77, 1.26) | .46 |
| 1 | 1.22 (−0.01, 2.45) | .052 |
| 0 | [REF] | N/A |
| Diabetes | .81 | |
| Yes | −0.25 (−2.36, 185) | .81 |
| No | [REF] | N/A |
| Smoking | .85 | |
| Yes/former | 0.13 (−1.26, 1.52) | .85 |
| No | [REF] | N/A |
| Intercept | 6.23 (5.33, 7.13) | <.0001∗ |
| R2 Adj | 0.21 | N/A |
BMI, body mass index; CI, confidence interval.
Indicated as statistically significant with P < .05.
Table IV.
Multiple linear regression of significant risk factors by sex
| Risk factor | Estimate (95% CI) | P value (males) | P value (females) |
|---|---|---|---|
| Degree of hirsutism | .027∗ | .61 | |
| 2-3 | −0.88 (−2.98, 1.22) | .41 | N/A |
| 1 | 1.61 (0.22, 2.99) | .023∗ | N/A |
| 0 | [REF] | N/A | N/A |
| Age | N/A | .072 | .16 |
| BMI | N/A | .34 | .91 |
| Smoking | N/A | .39 | .34 |
| Diabetes | N/A | .58 | .68 |
BMI, body mass index; CI, confidence interval.
Indicated as statistically significant with P < .05.
Discussion
Our study indicates that younger hirsute males have an increased C. acnes burden. East/Southeast Asians harbor the least burden in comparison with all other ethnicities. Increased shoulder skin C. acnes burden might be a risk factor for postoperative infection.15
Age is a risk factor for infection after shoulder arthroplasty. Singh et al24,25 showed that older age is associated with lower rates of infection in total shoulder arthroplasty (hazard ratio, 0.97 [95% confidence interval, 0.95-1.00]). Richards et al22 found that with every 1-year increase in age, a 5% (95% confidence interval, 2%-8%) lower risk of infection was observed. This might be caused by the increasing sebaceous gland activity reported in postpuberty young adults that then declines over time; however, this has not been independently investigated in the context of endogenous C. acnes shoulder burden.2,12,13,18,21 This study suggests that the higher burden associated with younger age may explain the increased rates of infection seen in the younger population.
Male sex is also a risk factor for C. acnes postoperative infection.7,22,23 Although the presence of hair has been shown to be associated with increased C. acnes counts, no study has attempted to categorize levels of hair and correlate this with burden.5,7 To our knowledge, this is the first study to quantify shoulder hair in the context of C. acnes infection risk factors and describe the relationship between amount of hair and endogenous burden. Our study shows that the presence of shoulder hair in males is associated with higher C. acnes counts. We were unable to distinguish these findings in females because there were few hirsute females. However, our hirsutism model did not appear to be dose dependent. This might have been due to limited study participants enrolled in the hair categories 2 and 3 and further investigation is warranted.
Smoking and diabetes have been well documented to predispose patients to postoperative infection. This is most likely caused by poor wound healing and the effects of hyperglycemia on vascular changes.3,14
In the multiple regression analysis, positive diabetes status was not associated with increased burden. Although our sample variation for diabetes was low, this suggests that diabetes acts as a risk factor through weakening the ability to combat a postoperative infection rather than increasing the number of bacteria that can seep into a surgical wound to initiate infection. Similarly, positive smoking status has been extensively documented as a major risk factor of postoperative surgical site infections.3,19 In our participant cohort, positive smoking status was not associated with increased C. acnes counts. Smoking, like positive diabetes status, has no statistically significant impact on the C. acnes burden itself.
Our data specify that the posterior location has the highest average C. acnes counts although not statistically significant. This supported our hypothesis based on the premise that the upper back has been shown to have high bacterial loads.20
East/Southeast Asians had the lowest C. acnes burden compared with all other ethnicities in analysis of nontransformed data. This was not significant in the multivariate analysis. Richards et al22 found that race was not a statistically significant predictor of postoperative infection in primary shoulder arthroplasty. Sex, age, and hirsutism likely have a greater impact on C. acnes burden than ethnicity, and hirsutism may be a covariate for ethnicity as indicated from our univariate analysis of degree of shoulder hair in East/Southeast Asians vs. all other ethnicities. This study was not powered to evaluate C. acnes burden across a spectrum of hirsutism within ethnic groups. Nevertheless, an understanding of which ethnic groups may be at higher risk provides further clinical context to postoperative C. acnes infections.
Ultimately, this study provides a statistical model that suggests an explanation as to why younger males appear to have the highest predisposition for infection. Whether these populations would benefit from aggressive preoperative protocols could be investigated in future studies.
Limitations of our study include a lack of sample variation for age, ethnicity, and diabetes status, and relatively few participants with significant shoulder hair categories 2 and 3. The study design assumes that increased skin bacterial colonization implies an increased risk for postoperative infection as supported by past research. Turtiainen et al26 showed a statistically significant relationship between bacterial skin burden and risk of surgical site infection. Matsen et al15 similarly detailed that C. acnes infections have a high skin burden of C. acnes. Both studies validate this idea as a premise for study design. The standardized scrub technique used in this study was specifically chosen because it correlates with the C. acnes load in deeper sebaceous glands, therefore minimizing the possibility of improper quantification of endogenous counts. However, because a direct biopsy of the deep dermis was not performed, it remains a limitation of our study.
Conclusion
Males, participants below the age of 40 years, and individuals with shoulder hair all showed independent risk for increased C. acnes burden. Our study summarizes the populations at risk for higher C. acnes loads and highlights that increased burden in these populations correlates with increased postoperative infection rates from other studies. Future studies on shoulder C. acnes prevention and prophylaxis might target such high-risk populations.
Disclaimer
The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article.
Footnotes
Approval for this study was obtained from the University of Maryland, Baltimore Institutional Review Board (HP-00064296).
References
- 1.Dodson C.C., Craig E.V., Cordasco F.A., Dines D.M., Dines J.S., Dicarlo E. Propionibacterium acnes infection after shoulder arthroplasty: a diagnostic challenge. J Shoulder Elbow Surg. 2010;19:303–307. doi: 10.1016/j.jse.2009.07.065. [DOI] [PubMed] [Google Scholar]
- 2.Downing D.T., Stewart M.E., Strauss J.S. Changes in sebum secretion and the sebaceous gland. Dermatol Clin. 1986;4:419–423. [PubMed] [Google Scholar]
- 3.Durand F., Berthelot P., Cazorla C., Farizon F., Lucht F. Smoking is a risk factor of organ/space surgical site infection in orthopedic surgery with implant materials. Int Orthop. 2013;37:723–727. doi: 10.1007/s00264-013-1814-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duvall G., Kaveeshwar S., Sood A., Klein A., Williams K., Kolakowski L. Benzoyl peroxide use transiently decreases Cutibacterium acnes load on the shoulder. J Shoulder Elbow Surg. 2020;29:794–798. doi: 10.1016/j.jse.2019.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Kadler B.K., Mehta S.S., Funk L. Propionibacterium acnes infection after shoulder surgery. Int J Shoulder Surg. 2015;9:139–144. doi: 10.4103/0973-6042.167957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keyworth N., Millar M.R., Holland K.T. Swab-wash method for quantitation of cutaneous microflora. J Clin Microbiol. 1990;28:941–943. doi: 10.1128/jcm.28.5.941-943.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koh C.K., Marsh J.P., Drinkovic D., Walker C.G., Poon P.C. Propionibacterium acnes in primary shoulder arthroplasty: rates of colonization, patient risk factors, and efficacy of perioperative prophylaxis. J Shoulder Elbow Surg. 2016;25:846–852. doi: 10.1016/j.jse.2015.09.033. [DOI] [PubMed] [Google Scholar]
- 8.Kolakowski L., Lai J.K., Duvall G.T., Jauregui J.J., Dubina A.G., Jones D.L. Neer Award 2018: benzoyl peroxide effectively decreases preoperative Cutibacterium acnes shoulder burden: a prospective randomized controlled trial. J Shoulder Elbow Surg. 2018;27:1539–1544. doi: 10.1016/j.jse.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 9.Levy P.Y., Fenollar F., Stein A., Borrione F., Cohen E., Lebail B. Propionibacterium acnes postoperative shoulder arthritis: an emerging clinical entity. Clin Infect Dis. 2008;46:1884–1886. doi: 10.1086/588477. [DOI] [PubMed] [Google Scholar]
- 10.Leyden J.J. Efficacy of benzoyl peroxide (5.3%) emollient foam and benzoyl peroxide (8%) wash in reducing Propionibacterium acnes on the back. J Drugs Dermatol. 2010;9:622–625. [PubMed] [Google Scholar]
- 11.Leyden J.J., Del Rosso J.Q. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J Drugs Dermatol. 2012;11:830–833. [PubMed] [Google Scholar]
- 12.Leyden J.J., McGinley K.J., Mills O.H., Kligman A.M. Age-related changes in the resident bacterial flora of the human face. J Invest Dermatol. 1975;65:379–381. doi: 10.1111/1523-1747.ep12607630. [DOI] [PubMed] [Google Scholar]
- 13.Leyden J.J., McGinley K.J., Vowels B. Propionibacterium acnes colonization in acne and nonacne. Dermatology. 1998;196:55–58. doi: 10.1159/000017868. [DOI] [PubMed] [Google Scholar]
- 14.Martin E.T., Kaye K.S., Knott C., Nguyen H., Santarossa M., Evans R. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37:88–99. doi: 10.1017/ice.2015.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsen F.A., III, Butler-Wu S., Carofino B.C., Jette J.L., Bertelsen A., Bumgarner R. Origin of propionibacterium in surgical wounds and evidence-based approach for culturing propionibacterium from surgical sites. J Bone Joint Surg Am. 2013;95:e1811–e1817. doi: 10.2106/JBJS.L.01733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsen F.A., III, Russ S.M., Bertelsen A., Butler-Wu S., Pottinger P.S. Propionibacterium can be isolated from deep cultures obtained at primary arthroplasty despite intravenous antimicrobial prophylaxis. J Shoulder Elbow Surg. 2015;24:844–847. doi: 10.1016/j.jse.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 17.Mayne A.I.W., Bidwai A.S., Clifford R., Smith M.G., Guisasola I., Brownson P. The incidence and causative organisms of infection in elective shoulder surgery. Shoulder Elbow. 2018;10:179–185. doi: 10.1177/1758573217711888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourelatos K., Eady E.A., Cunliffe W.J., Clark S.M., Cove J.H. Temporal changes in sebum excretion and propionibacterial colonization in preadolescent children with and without acne. Br J Dermatol. 2007;156:22–31. doi: 10.1111/j.1365-2133.2006.07517.x. [DOI] [PubMed] [Google Scholar]
- 19.Nolan M.B., Martin D.P., Thompson R., Schroeder D.R., Hanson A.C., Warner D.O. Association between smoking status, preoperative exhaled carbon monoxide levels, and postoperative surgical site infection in patients undergoing elective surgery. JAMA Surg. 2017;152:476–483. doi: 10.1001/jamasurg.2016.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel A., Calfee R.P., Plante M., Fischer S.A., Green A. Propionibacterium acnes colonization of the human shoulder. J Shoulder Elbow Surg. 2009;18:897–902. doi: 10.1016/j.jse.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 21.Pochi P.E., Strauss J.S., Downing D.T. Age-related changes in sebaceous gland activity. J Invest Dermatol. 1979;73:108–111. doi: 10.1111/1523-1747.ep12532792. [DOI] [PubMed] [Google Scholar]
- 22.Richards J., Inacio M.C., Beckett M., Navarro R.A., Singh A., Dillon M.T. Patient and procedure-specific risk factors for deep infection after primary shoulder arthroplasty. Clin Orthop Relat Res. 2014;472:2809–2815. doi: 10.1007/s11999-014-3696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saper D., Capiro N., Ma R., Li X. Management of Propionibacterium acnes infection after shoulder surgery. Curr Rev Musculoskelet Med. 2015;8:67–74. doi: 10.1007/s12178-014-9256-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh J.A., Sperling J.W., Schleck C., Harmsen W., Cofield R.H. Periprosthetic infections after shoulder hemiarthroplasty. J Shoulder Elbow Surg. 2012;21:1304–1309. doi: 10.1016/j.jse.2011.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh J.A., Sperling J.W., Schleck C., Harmsen W.S., Cofield R.H. Periprosthetic infections after total shoulder arthroplasty: a 33-year perspective. J Shoulder Elbow Surg. 2012;21:1534–1541. doi: 10.1016/j.jse.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turtiainen J., Hakala T., Hakkarainen T., Karhukorpi J. The impact of surgical wound bacterial colonization on the incidence of surgical site infection after lower limb vascular surgery: a prospective observational study. Eur J Vasc Endovasc Surg. 2014;47:411–417. doi: 10.1016/j.ejvs.2013.12.025. [DOI] [PubMed] [Google Scholar]
- 27.Williamson P., Kligman A.M. A new method for the quantitative investigation of cutaneous bacteria. J Invest Dermatol. 1965;45:498–503. doi: 10.1038/jid.1965.164. [DOI] [PubMed] [Google Scholar]