Abstract
Objectives
Cholestasis refers to a reduction in bile flow from the liver into the biliary system. Ursodeoxycholic acid (UDCA) is commonly used for the treatment of hepatic cholestasis. This study aimed to explore the role of UDCA in the treatment and prevention of lipopolysaccharide (LPS)-induced cholestasis.
Methods
Sixty male albino rats were randomly classified into five groups of 12 rats each: the control group (received saline and water), UDCA group (received UDCA), LPS group (received LPS), treatment group (received LPS followed by UDCA), and prevention group (received UDCA followed by LPS). Changes in gamma-glutamyl transferase (GGT), plasma aspartate transferase (AST), plasma alkaline transferase (ALT), plasma alkaline phosphatase (ALP), total bilirubin (TBIL), hepatocyte apoptosis, immunomodulatory activity, plasma pro-inflammatory cytokines (TNF-α, IL-1α, and IL-4), and liver histology were assessed.
Results
UDCA improved serum liver chemical markers (GGT, ALP, and AST) in both the prevention and treatment groups (p < 0.05 and p < 0.05, respectively). CD3 count was higher in the UDCA treatment group compared to the LPS group (p < 0.001). UDCA caused a reduction in plasma TNF-α in the prevention group (P < 0.05); however, it had no effect on the treatment group, as compared to the LPS group. Similarly, UDCA had no effect on IL-1α or IL-4. UDCA treatment resulted in improved liver histological features and a significant reduction in liver tissue apoptosis in both the treatment and prevention groups, as compared to the LPS group (p = 0.013 and p = 0.002, respectively).
Conclusions
This study provides evidence of the effectiveness of UDCA for the treatment and prevention of sepsis-induced cholestasis.
Keywords: Apoptosis, Cytokine, Hepatocyte sepsis, Immunomodulatory activity, Sepsis-induced cholestasis, Ursodeoxycholic acid
الملخص
أهداف البحث
الركود الصفراوي هو انخفاض في تدفق المادة الصفراء من الكبد إلى الجهاز الصفراوي. يستخدم حمض أورسودويكسيكوليك بشكل شائع لعلاج الركود الصفراوي. الهدف من الدراسة هو استكشاف دور حمض أورسودويكسيكوليك في العلاج والوقاية من حدوث الركود الصفراوي الناتج عن استخدام عديد السكاريد الدهني.
طرق البحث
تم اختيار ٦٠ من ذكور الجرذان البيضاء بشكل عشوائي، وتقسيمها إلى خمس مجموعات؛ في كل مجموعة ١٢ جرذا: المجموعة الضابطة (أخذت محلول ملحي وماء)، مجموعة حمض أورسودويكسيكوليك (أخذت حمض أورسودويكسيكوليك،) ومجموعة عديد السكاريد الدهني (أخذت عديد السكاريد الدهني)، مجموعة العلاج (أخذت عديد السكاريد الدهني ثم حمض أورسودويكسيكوليك)، ومجموعة الوقاية أخذت حمض أورسودويكسيكوليك ثم عديد السكاريد الدهني). حيث تمت دراسة ناقلات جاما غلوتاميل، وناقلات الاسبارتات، وناقلات القلويات، والفوسفاتيز القاعدي، والبيلوروبين الكلي، ومحددات موت خلايا الكبد المبرمج، والنشاط المناعي، والسيتوكينات المسببة للالتهابات (الأنترلويكينات ١، و٤، وعامل نخر الورم الفا) وتغيرات نسيج الكبد.
النتائج
حَسَّن استخدام حمض أورسودويكسيكوليك علامات الناقلات الكيميائية للكبد (ناقلات جاما غلوتاميل، الفوسفاتيز القاعدي، ناقلات الاسبارتات)، في كل من مجموعات الوقاية والعلاج. كما نتج عن ذلك زيادة في عدد الخلايا المناعية في مجموعة العلاج بحمض أورسودويكسيكوليك مقارنة بمجموعة عديد السكاريد الدهني. وتسبب العلاج بحمض أورسودويكسيكوليك في انخفاض مستوى عامل نخر الأورام الفا في البلازما في مجموعة الوقاية ولكن دون حدوث أي تأثير على مجموعة العلاج بالمقارنة بمجموعة عديد السكاريد الدهني. وبالمثل، لا يوجد تأثير لحمض أورسودويكسيكوليك على مستوى الأنترلويكينات. كما نتج عن استخدام حمض أورسودوكسيكوليك تحسنا في ميزات الأنسجة الكبدية وانخفاض في محددات موت الخلايا المبرمج في أنسجة الكبد في كل من مجموعة العلاج والوقاية بالمقارنة بمجموعة عديد السكاريد الدهني.
الاستنتاجات
اظهرت هذه الدراسة دليلا على فاعلية حمض أورسودويكسيكوليك في العلاج والوقاية من حدوث الركود الصفراوي الناتج عن استخدام عديد السكاريد الدهني.
لكلمات المفتاحية: موت الخلايا المبرمج, السيتوكين, ركود صفراوي ناتج عن عديد السكاريد الدهني, النشاط المناعي, حمض أورسودويكسيكوليك, موت الخلايا المبرمج للخلايا الكبدية
Introduction
Cholestasis is a condition caused by disruption of bile formation due to impaired secretion by hepatocytes or blockage of the flow of bile through intrahepatic or extrahepatic bile ducts.1 Infections may induce cholestasis via bacterial endotoxins, such as lipopolysaccharides (LPS), which are released by gram-negative bacteria from sites of bacterial infection into the circulation. These endotoxins provoke hepatic Kupffer cells to release inflammatory cytokines, such as tumour necrosis factor-α (TNF-α) and interleukin-6 (IL-6), which can lead to the failure of bile secretion.2,3 Sepsis-induced cholestasis is an early complication in 20% of patients hospitalized with sepsis.4 Severe sepsis may result in life-threatening organ failure due to dysregulated host immune response against infection.5 Sepsis-associated hepatic damage may result from cholestasis caused by the impairment of hepatocellular and bile duct formation induced by circulating pro-inflammatory cytokines.2 Apart from treating sepsis with appropriate antibiotics and supportive care, there are currently no specific treatments available for cholestasis.6
Ursodeoxycholic acid (UDCA) (3, 7-dihydroxy-5-cholanic acid) is a hydrophilic bile acid that is commonly used for the treatment of various cholestatic disorders,7,8 including primary biliary cholangitis (PBC), an autoimmune disease of the liver characterized by progressive cholestasis that often leads to biliary fibrosis and liver cirrhosis.9 UDCA treatment has been shown to be very effective in about two thirds of patients with PBC, with a survival rate similar to the general population.10 Additionally, UDCA has also been beneficial in the treatment of gallbladder and biliary stones (cholelithiasis). UDCA is one of the main constituents of bile acid in black bear bile, which has historically been used in Chinese traditional medicine for treating various liver diseases. It is normally present in human bile in low concentrations within total bile acids. Its first reported efficacy in the treatment of hepatic diseases appeared in early Japanese medical literature.11
The mechanisms responsible for the therapeutic effect of UDCA in liver disease are not well understood. Three mechanisms have been suggested, based on evidence from animal studies. These include (1) cellular protection of cholangiocytes (cryoprotection) from the harmful effect of bile acids, (2) augmentation of bile flow and secretion, and (3) protection of hepatocytes from apoptosis that cause loss of cellular elements.12 A previous study using an animal model of sepsis-induced cholestasis with sepsis-induced cholestasis in mice found that UDCA can stabilize hepatocyte membranes and enhance the synthesis of membrane transporters, including the bile salt export pump (BSEP).13
The aims of this study are to explore the effect of UDCA in the treatment and prevention of bacterial endotoxin sepsis-induced cholestasis and to examine the underlying mechanisms, including liver tissue apoptosis, inflammatory mediators, and any possible immunomodulatory mechanisms.
Materials and Methods
Chemicals
LPS (L-2880-10 mg) was obtained from Sigma Chemical Co. (St. Louis, MO). This was supplied as a lyophilized powder for reconstitution as a 5 ml sterile saline solution (NaCl 0.9%). UDCA was provided in suspension form by Ursofalk (Dr Falk Pharma GmbH, in Freiburg, Germany). Aspartate aminotransferase (AST), alkaline phosphatase (ALP), alanine aminotransferase (ALT), and total bilirubin (TBIL) kits were purchased from Wako Pure Chemical Industries (Osaka, Japan). Annexin V-FITC apoptosis detection kits were purchased from Aldrich Chem. Corp. (USA). TNF-alpha kits and interleukin kits (IL1, IL4) were obtained from the ASSAYPRO Co. (Universal Biologicals Ltd., Cambridge, UK). Enzyme-linked immunosorbent assay (ELISA) kits and T/B/NK kits were purchased from BD Biosciences Co. (Franklin Lakes, New Jersey, US).
Animals
60 male albino rats, weighing 100–150 g, were obtained from King Fahd Medical Research Center (King Abdulaziz University, Jeddah, KSA). The animals were housed in plastic cages with free access to water and standard chow. They were placed at an adjusted temperature of 20–25 °C with a controlled humidity of 50 ± 5% and a 12 h dark/light cycle. They were acclimatized for one week before starting the experimental work. This study was conducted according to the guidelines of the Research Animal Care Committee-approved protocol at the King Fahd Medical Research Center. All studies conducted on animals were approved by the Bioethical Research Unit of the Faculty of Medicine, King Abdulaziz University (Ref: 597-17).
Experimental design
Rats were randomly classified into five groups (12 rats each). The control (untreated) group received saline (0.5 ml/kg, i.p.) and water (0.5 ml/kg, p.o.)– the vehicles for delivering LPS and UDCA, respectively– for 10 days. The UDCA-treated group received a daily dose of UDCA (100 mg/kg, p.o.) via gastric gavage for 10 days.14 In the sepsis-induced cholestasis group, rats were injected with a single sub-lethal dose of LPS (5 mg/kg, i.p.).3,15 In the treatment group, rats were injected with LPS; 24 h later, these rats received 100 mg/kg UDCA p.o. for 10 days.16 In the preventive group, rats received a daily dose of UDCA for 10 days before LPS injection; the rats were sacrificed 24 h later. At the end of the experiment, rats were euthanized using 50 mg/kg i.p. pentobarbital sodium. Blood samples were collected from the retro-orbital venous plexus with heparinized sterile capillary tubes. The blood samples were allowed to clot and then centrifuged at 3000 rpm for 10 min for further analysis.
Liver enzyme analysis
Liver injury was estimated via biochemical serum markers. Sera were collected and stored in a deep freezer at −20 °C until the time of analysis to measure the activities of the following liver enzymes: gamma-glutamyl transferase (GGT), aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), and total bilirubin (TBIL). These were analysed using the Transaminase Cii Test Kit (Wako Chemicals). The serum AST:ALT ratio was computed from the results of the serum AST and ALT levels. The levels of clinical biochemistry for GGT, AST, ALT, ALP, and TBIL were evaluated to determine the enzymatic activities of the livers in the control and experimental group animals. The activity of all serum enzymes was measured using commercially available kits according to the manufacturer's instructions (Transaminase CII-test Wako, Japan). 0.25 ml of buffer/substrate solution was added to 0.05 ml of each serum sample in a test tube. This was incubated at 37 °C for 30 min in a water bath followed by the addition of 0.25 ml of chromogen solution. The content was mixed and allowed to stand for 20 min at 25 °C. Following this, 2.5 ml of sodium hydroxide (0.4 N) was added and mixed. The absorbance was read against the blank after 5 min at 540 nm. Liver enzyme activities in IU L-1 were read from the standard curve. For computation of the serum AST:ALT ratio, a 0.05 ml aliquot of serum was used to assay AST activity, which was measured by monitoring the concentration of oxaloacetate hydrazone formed with 2,4 dinitrophenyl hydrazine at 530–550 nm. ALT activity in the serum was similarly measured using the end point colorimetric assay method with a kit obtained from Randox Laboratories, UK.
Flow cytometric analysis of apoptosis
Livers and spleens were perfused with phosphate buffer saline (PBS), then removed, cut into small pieces, and stored in a deep freezer at −20 °C until use. The antiapoptotic effect was measured using the Annexin V-FITC Apoptosis Detection Kit (eBioscience, San Diego, CA, US) following the manufacturer's instructions. Flow cytometric analysis was performed on a BD FACSCalibur using Cell Questpro software version 6.0 (BD Biosciences).
Immunological assays
Spleens were used to evaluate the effects of T and B lymphocytes and natural killer (NK) cells. Part of the liver tissue was immersed in buffered formalin and the sections were stained with haematoxylin & eosin (H&E) for routine histology. The Rat T/B/NK Cocktail (BD Biosciences), a three-color reagent cocktail designed to identify rat T, B, and NK lymphocyte populations via direct immunofluorescence staining, was used for flow cytometric analysis.
Cytokine quantification
Tumour necrosis factor alpha (TNF-α), interleukin-1 (IL-1), and interleukin-4 (IL-4) concentrations in tissue homogenates were determined using H CUSABIO® following the manufacturer's instructions. Moreover, the Assay Max Rat TNF-α ELISA kit was used to detect TNF-α expression in rat cell culture supernatants using ASSAYPRO® following the manufacturer's instructions.
Histopathology
The liver was cut into small pieces (2 mm × 2 mm), fixed with 10% neutral-buffered formalin, processed for paraffin embedding, sectioned (5-microns thick), and stained with H&E. (The nuclei were stained a deep blue-black with haematoxylin, and the cytoplasm was stained pink with eosin.) For histopathology, stained sections were examined using a microscope (SPI Supplies®) connected to a digital camera. (The camera fits eyepieces 23–30 mm in size).
Statistical analysis
The Statistical Package for the Social Sciences, Version 22 (SPSS, Inc, Chicago, IL, USA) was used for data analysis. Data are expressed as mean ± standard deviation (SD). A one-way analysis of variance (ANOVA) was used, followed by Tukey's post hoc test for multiple comparisons. A P value of <0.05 was considered statistically significant.
Results
The following variables demonstrate the effect of UDCA on the prevention and treatment groups of sepsis-induced cholestasis:
Effect of UDCA on biochemical parameters of liver function
To verify the efficacy of UDCA treatment, liver function parameters in the serum were measured as indicators of liver-injury status. The results for each group are listed in Table 1. The rats in the LPS group had significantly higher values for GGT, ALP, TBIL, and AST compared to the control group and the UDCA group (P < 0.05). The UDCA treatment group only had significantly lower values for GGT, ALP, and AST (P < 0.05). However, in the UDCA pretreatment group, the values for GGT, ALP, TBIL, and AST were significantly lower than the corresponding values in the LPS group (P < 0.05). Moreover, the ALP and TBIL values were significantly lower than those in the treatment group (P < 0.05).
Table 1.
Effect of UDCA on liver enzymes activities in the prevention and treatment groups of sepsis-induced cholestasis in rats.
| Group | Parameters |
|||||
|---|---|---|---|---|---|---|
| GGT(IU/L) | ALP(IU/L) | TBIL(umol/L) | AST(IU/L) | ALT(IU/L) | AST/ALT Ratio |
|
| Control group (n=12) %change |
1.75 ± 0.50 (−46.1) | 89.67 ± 23.30b (−14.6) | 1.75 ± 0.5b,c (−30) | 58.5 ± 8.18b (−22.13) | 23.61 ± 0.65 (−14.1) | 2.47 ± 0.32 (−9.5) |
| UDCA group (n=12) %change |
1.67 ± 0.52b (−48.6) | 83.62 ± 21.29b (−20.3) | 1.67 ± 0.52b,c (−33.2) | 62.05 ± 6.72b (−17.4) | 24.18 ± 0.53 (−12) | 2.50 ± 0.43 (−8.4) |
| LPS group (n=12) | 3.25 ± 0.50a | 105.64 ± 15.48a | 2.5 ± 0.58a | 75.13 ± 24.89a | 27.50 ± 4.25 | 2.73 ± 1.01 |
| Treatment group (n=12) %change |
2.2 ± 0.10b (−32.3) | 99.26 ± 26.27b (−5.4) | 2.5 ± 0.53 (0) | 60.60 ± 11.32b (−16.6) | 24.18 ± 4.76 (−12.3) | 2.50 ± 0.52 (−8.4) |
| Prevention group (n=12) %change |
2.1 ± 0.20b (−35.3) | 50.90 ± 23.08b,c (−22.9) | 1.14 ± 38b,c (−54.4) | 62.11 ± 6.27b (−17.3) | 24.84 ± 7.07 (−9.67) | 2.50 ± 0.3 (−8.4) |
Significant change compared to control group at P < 0.05.
Significant change compared to LPS group at P < 0.05.
Significant change compared to treatment group at P < 0.05.
Effect of UDCA on hepatocyte apoptosis
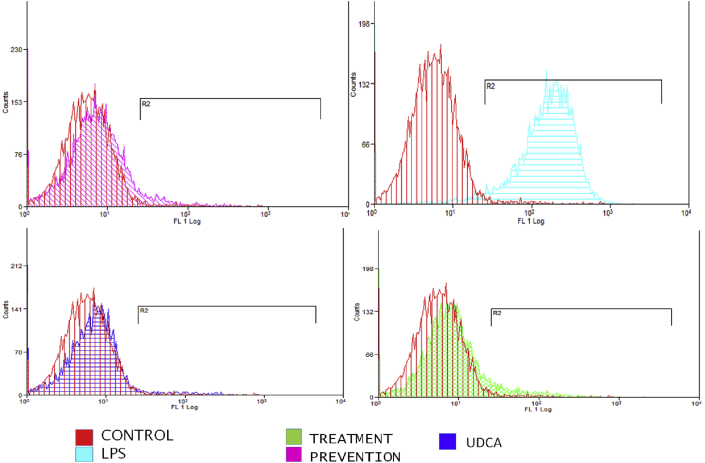
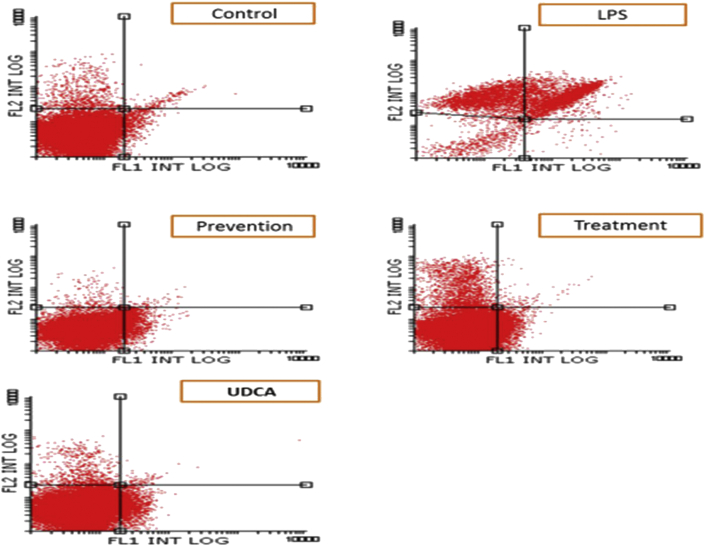
Liver tissue was isolated and examined using flow cytometric analysis, as shown in Figure 1, Figure 2. The results indicate that the LPS-injected group had significantly increased apoptotic activity compared to the control group (P = 0.003). Both treatment and pretreatment groups demonstrated reduced apoptosis compared to the LPS group (P = 0.013 and P = 0.002, respectively). There was no significant difference between the control group and the UDCA group (P = 0.63), between the control group and the treatment group (P = 0.44), and between the control group and the prevention group (P = 0.9) (Table 2). To further investigate the immunomodulatory properties of UDCA, liver tissues were isolated from the five rat groups.
Figure 1.
The effects of UDCA on the percentages of apoptotic cells in rat liver tissue in the control, LPS, treatment, and prevention groups.
Figure 2.
Flow cytometry dot plot showing the effects of UDCA on LPS-induced apoptosis in all groups compared to the control group. Apoptotic cells were identified and quantified using the Annexin V-FITC apoptosis detection kit. The dot-plot shows early apoptotic cells in the lower right quadrant, late apoptotic cells in the upper right quadrant, dead cells in the upper left quadrant and living cells in the lower left quadrant.
Table 2.
Effect of UDCA on percentage of apoptosis of liver tissue in the prevention and treatment groups of sepsis-induced cholestasis in rats.
| Group | Percentage of Apoptosis Mean ± SD |
|---|---|
| Cotrol group (n=12) | 4.33 ± 2.7b |
| UDCA group (n=12) | 5.37 ± 1.1b |
| LPS group (n=12) | 10.75 ± 4.93a,c |
| Treatment group (n=12) | 5.89 ± 2.19b |
| Prevention group (n=12) | 4.58 ± 3.9b |
Data are presented as mean ± SD.
Significant change compared to control group at P < 0.05.
Significant change compared to LPS group at P < 0.05.
Significant change compared to treatment group at P < 0.05.
Immunoregulatory properties of UDCA
A decrease in the activity of NK cells was observed in the LPS group in comparison with the control group, but the decline was not statistically significant (P = 0.14) (Table 3). Similarly, no statistical significant difference in NK cell activity was detected between the LPS and UDCA treatment groups, nor between the LPS and pretreatment groups (P = 0.12 and P = 0.74, respectively). The effect of UDCA on T-lymphocyte (CD3) activity in the different groups was examined. The percentage of CD3 when UDCA was used as treatment was higher compared to the LPS group (P < 0.001), but the pretreatment group showed that the proportion of CD3 cells was decreased significantly compared to both the LPS group and the control group (P < 0.05); the distinction was not statistically significant between the treatment and pretreatment groups (P = 0.17).
Table 3.
Effect of UDCA on the percentage of natural killer (NK) cells & cluster of differentiation (CD3) in preventive and treated groups of LPS- induced cholestasis in rats.
| Groups | Percentage of NK cells Mean ± SD |
Percentage CD3 (T cells co-receptor protein ) Mean ± SD |
|---|---|---|
| Cotrol group (n=12) | 8.63 ± 2.35 | 62.14 ± 3.20c |
| UDCA group (n=12) | 8.63 ± 2.35 | 62.14 ± 3.20 |
| LPS group (n=12) | 5.26 ± 2.9 | 57.86 ± 9.83c |
| Treatment group (n=12) | 8.58 ± 4.12 | 37.29 ± 4.30a,b |
| Prevention group (n=12) | 5.9 ± 4.5 | 68.03 ± 5.42b |
Data are presented as mean ± S.D.
Significant change compared to control at P < 0.05.
Significant change compared to LPS group at P < 0.05.
Significant change compared to treatment group at P < 0.05.
Effect of UDCA on proinflammatory cytokines
The effect of UDCA on inflammatory mediators in the treatment and prevention groups was also investigated, as shown in Table 4. The concentration of TNF-α in the LPS-injected group was significantly increased compared to the control group. Treatment with UDCA decreased the concentration of TNF-α, with no significant difference compared to the LPS group, although the use of UDCA as a pretreatment decreased the concentration of TNF-α significantly (P < 0.05). There was no significant difference between the treatment and pretreatment groups (P = 0.5). The concentration of the inflammatory cytokine IL-1α in the LPS group was significantly increased compared to the control group (P < 0.05), with no significant difference detected between the LPS and UDCA treatment groups or between the LPS and pretreatment groups (P = 0.7). In addition, the concentration of IL-4 in the LPS group was significantly higher compared to the control group (P < 0.05), while no difference was observed between the LPS group and either the treatment or pretreatment group. The IL-4 concentration in the pretreatment group was significantly less than that in the treatment group (P = 0.032).
Table 4.
Effect of UDCA on the concentration of inflammatory mediators in liver tissue in the prevention and treatment of cholestasis-induced by LPS in rats.
| Groups | TNF-α Mean ± SD |
IL-1α Mean ± SD |
IL-4 Mean ± SD |
|---|---|---|---|
| Control group (n=12) | 37.40 ± 2.47b,c | 714.60 ± 73.32b | 625.60 ± 124.25 |
| UDCA group (n=12) | 39.60 ± 7.42b | 700.90 ± 54.25b | 600.80 ± 100.57 |
| LPS group (n=12) | 75.63 ± 3.72a | 898.50 ± 63.56 | 804.25 ± 61.01 |
| Treatment group (n=12) | 62.83 ± 9.20a | 851.33 ± 130.38 | 851.67 ± 51.00 |
| Prevention group (n=12) | 55.38 ± 13.15b | 832.63 ± 80.01 | 749.63 ± 89.55c |
Data are presented as mean ± S.D.
Significant change compared to control at P < 0.05.
Significant change compared to LPS group at P < 0.05.
Significant change compared to treatment group at P < 0.05.
The effect of UDCA on liver histology changes
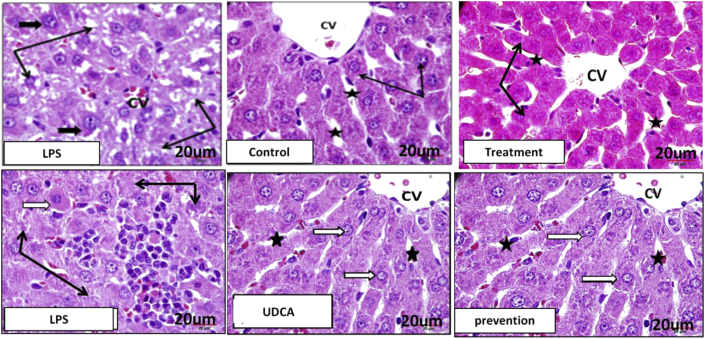
The histopathological features are depicted in Figure 3. The hepatocytes in the control rat liver are arranged around central veins and extend in branched plates towards the portal area. They are polyhedral in shape with acidophilic stained cytoplasm. Their nuclei are vesicular, central, and rounded, with dispersed chromatin. Portal tracts showed branches of the portal vein, hepatic artery, and bile duct. They are surrounded by fine connective tissue fibres with few cells. Administering UDCA to control rats did not alter the normal structure of the liver parenchymatous tissue. The liver parenchyma of the LPS group demonstrated congested blood sinusoids, hepatocytes with unstained cytoplasmic degenerated regions, and small degenerated or lost nuclei. In the LPS group, in the region of necrotic inflammatory infiltrate, apoptotic, degenerated, and inflammatory cells were observed around bile ducts.
Figure 3.
Liver histology in all groups: Control & UDCA: normal hepatocytes with acidophilic cytoplasm and large nuclei (thin black arrows), with intervening blood sinusoids (stars). LPS: hepatocytes with degenerated cytoplasm, and small nuclei (black arrows), and large size nuclei (small black arrows). Treatment: marked improvement of changes induced by LPS in hepatocytes (black arrow) & blood sinusoid (black stars). Prevention: normal hepatocytes with no degenerative changes (white arrows), and no blood sinusoidal congestion (stars).
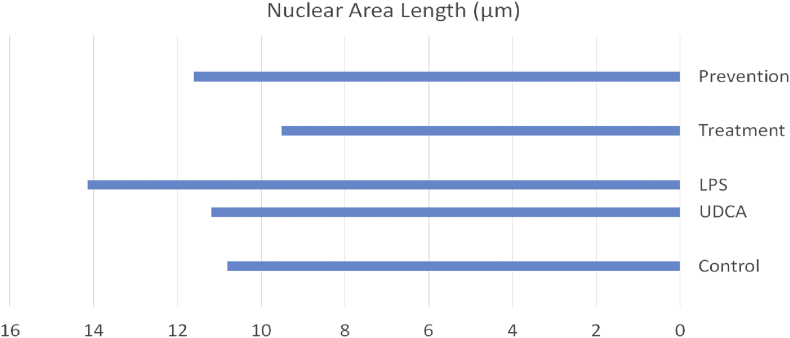
The liver of the pretreatment group that received UDCA for 10 days before LPS administration exhibited apparent protection against the histopathological changes found in the LPS group. Hepatocytes looked normal, with no degenerative changes in their cytoplasm or nuclei; sinusoids between cells were not congested and were similar to those of the control group. Regions near the portal area showed normal hepatocytes and few degenerated inflammatory cell aggregates. In this group, administration of UDCA as a treatment caused marked histological changes compared to those induced by LPS. Hepatocytes, sinusoids, and portal areas looked normal without any degenerative, necrotic, or apoptotic changes. No inflammatory cells were observed in the portal tracts. The effect of UDCA on the nuclear area length (measured in μm) in the liver tissue was also investigated. In both the treatment and pretreatment groups, the administration of UDCA caused a significant reduction in the nuclear area length compared to the LPS group (Figure 4). Taken together, these results indicate the cytoprotective effect of UDCA on hepatocytes.
Figure 4.
Effect of UDCA on the nuclear area length in liver tissue in the prevention and treatment of cholestasis induced by LPS in rats.
Discussion
Sepsis-induced cholestasis is a type of inflammatory cholestasis in which pro-inflammatory cytokines, produced by hepatic Kuepfer cells due to the effects of LPS endotoxins, induce impairment of bile production.3 These pro-inflammatory cytokines cause transcriptional and post-transcriptional impairment of hepatocellular expression of bile acids carriers.11,17
In this study, the intra-peritoneal injection of LPS induced cholestasis in LPS-treated rats, resulting in a significant rise in the TBIL and liver enzymes, including GGT, ALP, and AST (Table 1), similar to what was observed during other investigations of sepsis-induced cholestasis in animal models.15,18 The liver enzyme ALP is a cellular membrane-bound enzyme that is expressed and released into the circulation in response to cellular membrane damage caused by endogenous bile acids, which lead to hepatocyte damage and lysis.19 The efficacy of UDCA in reversing the effect of cholestasis in LPS-induced rats in the present experiment was reflected by the significant reduction of the liver enzymes AST, GGT, and ALP following treatment with UDCA. The protective effect of UDCA in the present study was seen when mice were treated with UDCA prior to LPS-induction, which resulted in mean values of TBIL and liver enzymes (GGT, ALP, and AST) comparable to those in the control group. Such a protective effect is thought to occur due to the formation of a complex between the negatively-charged UDCA and cholesterol in the hepatocyte membrane, causing repulsion of the negatively-charged bile acids.20 The protective effect of UDCA has also been reported in relation to total parenteral nutrition (TPN)-induced cholestasis in humans21 and in the prevention of PBC recurrence after liver transplantation.22
The accumulation of endogenous bile acids in hepatocytes results in cellular damage and hepatocyte death. The primary suggested mechanism underlying hepatocyte death is LPS-induced apoptosis.23 In our study, LPS-induced hepatocyte death was evident via the higher percentage of apoptosis compared to the control group; UDCA resulted in a lower percentage of apoptosis comparable to that of the control group, suggesting a positive effect of UDCA in minimizing or preventing hepatocellular damage and death. The effect of UDCA on apoptosis inhibition has been reportedly mediated by the upregulation of various anti-apoptotic proteins, such as FLICE-inhibitory protein, myeloid leukaemia sequence-1 protein, and cellular inhibitor of apoptosis-2 protein.24
The immunomodulatory effect of UDCA in sepsis-induced cholestasis was demonstrated in our study via the reduction of the percentage of CD3 T-cell co-receptor protein in the treatment group compared to the LPS-induced group. These findings corroborate the results reported by Manousou et al., in which a reduction in chemokine receptor (CXRR3) expression on T lymphocytes was found in the group treated with UDCA compared to the untreated group,25 providing evidence for the immunomodulatory effect of UDCA. We did not find any difference between the study groups in the percentage of natural killer (NK) lymphocytes. The immunomodulatory effect of UDCA has been reported in patients with cholestasis secondary to PBC, in which UDCA was found to suppress immunoglobulin, IL-2, IL-4, and interferon-gamma production.26
In the present study, inflammatory cytokines such as TNF-α, IL-1α, and IL-4 were induced in response to LPS administration, corroborating the findings of Tanasescu et al.27 Treatment with UDCA resulted in a significant reduction in TNF-α, but not IL-1α or IL-4, expression in the prevention group, with no significant effect on the treatment group. Similarly, no significant differences in the TNF-α and IL-6 cytokines mean values were found between LPS-induced animals and controls.13 UDCA did not reduce the expression of inflammatory cytokines in rat hepatocytes.28 The lack of effect of UDCA on the expression of cytokines may be explained by the important role played by the cytokines in the mechanisms of liver cell regeneration.29
The histopathological examination of the livers of animals that received the UDCA treatment showed significant improvement of histopathological features of inflammation induced by LPS compared to the LPS group. These findings were consistent with the findings reported by Buko et al., in which treatment with UDCA resulted in histopathological improvement and reversal of liver fibrosis in an animal model of liver fibrosis.16 The assessment of apoptosis in liver tissue is an important tool to evaluate the amount of liver tissue damage. We found significantly low liver tissue apoptosis in the UDCA-treated group, which supports the idea of the protective effect of UDCA against liver damage. The effect of UDCA on the inhibition of apoptosis may manifest even if combined with other apoptosis-inducing agents; Rodrigues et al. found ∼50–100% apoptotic change inhibition in different cell types when UDCA was combined with apoptosis-inducing agents.30
Our study lacks molecular and genetic analysis, which could have highlighted the mechanisms by which UDCA exerts its therapeutic and preventive effect on sepsis-induced cholestasis. In a recent study, the effect of UDCA was attributed to the impaired gene expression of some canalicular membrane transporters, including the bile salt export pump (BSEP) and multi-drug resistance-associated protein 2.3
Conclusion
Our findings support the concept that UDCA is beneficial for the treatment and prevention of sepsis-induced cholestasis in experimental animals, an effect that was observed via improvements in liver enzyme expression levels and histopathological abnormalities.
Recommendations
We recommend conducting further studies to address the underlying molecular basis of the mechanism of action of UDCA in cholestatic disorders.
Source of funding
This study was funded by a grant from King Abdulaziz City for Science and Technology (KACST), KSA (Ref. No. 34-35-216).
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
All studies conducted on animals were approved by the Bioethical Research Unit of the Faculty of Medicine, King Abdulaziz University, Jeddah (Ref: 597-17).
Authors contributions
Guarantor of the article: OS. Development of study concept and design: RA, MH, and OS. Acquisition, analysis, and interpretation of the data, along with approval of the final paper, was performed by all authors. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The team would like to thank the King Fahd Medical Research Center for providing the necessary research facilities to carry out this work. We also acknowledge Dr. Trevor Rawbone, Cardiff, UK, for English editing and proofreading of the manuscript.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.De Bruyne R., Van Biervliet S., Vande Velde S., Van Winckel M. Clinical practice: neonatal cholestasis. Eur J Pediatr. 2011;170:279–284. doi: 10.1007/s00431-010-1363-8. [DOI] [PubMed] [Google Scholar]
- 2.Geier A., Fickert P., Trauner M. Mechanisms of disease: mechanisms and clinical implications of cholestasis in sepsis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:574–585. doi: 10.1038/ncpgasthep0602. [DOI] [PubMed] [Google Scholar]
- 3.Trauner M., Fickert P., Stauber R.E. Inflammation-induced cholestasis. J Gastroenterol Hepatol. 1999;14:946–959. doi: 10.1046/j.1440-1746.1999.01982.x. [DOI] [PubMed] [Google Scholar]
- 4.Whitehead M.W., Hainsworth I., Kingham J.G. The causes of obvious jaundice in South West Wales: perceptions versus reality. Gut. 2001;48:409–413. doi: 10.1136/gut.48.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer M., Deutschman C.S., Seymour C.W., Shankar-Hari M., Annane D., Bauer M. The third international consensus definitions for sepsis and septic shock (Sepsis-3) J Am Med Assoc. 2016;315:801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hao H., Cao L., Jiang C., Che Y., Zhang S., Takahashi S. Farnesoid X receptor regulation of the NLRP3 inflammasome underlies cholestasis-associated sepsis. Cell Metabol. 2017;25:856–867 e5. doi: 10.1016/j.cmet.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beuers U., Boyer J.L., Paumgartner G. Ursodeoxycholic acid in cholestasis: potential mechanisms of action and therapeutic applications. Hepatology. 1998;28:1449–1453. doi: 10.1002/hep.510280601. [DOI] [PubMed] [Google Scholar]
- 8.Lazaridis K.N., Gores G.J., Lindor K.D. Ursodeoxycholic acid 'mechanisms of action and clinical use in hepatobiliary disorders. J Hepatol. 2011;35:134–146. doi: 10.1016/s0168-8278(01)00092-7. [DOI] [PubMed] [Google Scholar]
- 9.Carey E.J., Ali A.H., Lindor K.D. Primary biliary cirrhosis. Lancet. 2015;386:1565–1575. doi: 10.1016/S0140-6736(15)00154-3. [DOI] [PubMed] [Google Scholar]
- 10.Beuers U., Gershwin M.E., Gish R.G., Invernizzi P., Jones D.E.J., Lindor K. Changing nomenclature for PBC: from 'cirrhosis' to 'cholangitis'. J Hepatol. 2015;63:1285–1287. doi: 10.1016/j.jhep.2015.06.031. [DOI] [PubMed] [Google Scholar]
- 11.Hagey L.R., Crombie D.L., Espinosa E., Carey M.C., Igimi H., Hofmann A.F. Ursodeoxycholic acid in the Ursidae: biliary bile acids of bears, pandas, and related carnivores. J Lipid Res. 1993;34:1911–1917. [PubMed] [Google Scholar]
- 12.Paumgartner G., Beuers U. Ursodeoxycholic acid in cholestatic liver disease: mechanisms of action and therapeutic use revisited. Hepatology. 2002;36:525–531. doi: 10.1053/jhep.2002.36088. [DOI] [PubMed] [Google Scholar]
- 13.Razori M.V., Maidagan P.M., Ciriaci N., Andermatten R.B., Barosso I.R., Martin P.L. Anticholestatic mechanisms of ursodeoxycholic acid in lipopolysaccharide-induced cholestasis. Biochem Pharmacol. 2019;168:48–56. doi: 10.1016/j.bcp.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Buko V.U., Kuzmitskaya-Nikolaeva I.A., Naruta E.E., Lukivskaya O.Y., Kirko S.N., Tauschel H.D. Ursodeoxycholic acid dose-dependently improves liver injury in rats fed a methionine- and choline-deficient diet. Hepatol Res. 2011;41:647–659. doi: 10.1111/j.1872-034X.2011.00820.x. [DOI] [PubMed] [Google Scholar]
- 15.Konno A., Enomoto N., Takei Y., Hirose M., Ikejima K., Sato N. Oral contraceptives worsen endotoxin-induced liver injury in rats. Alcohol Clin Exp Res. 2002;26 doi: 10.1097/01.ALC.0000026980.53519.20. 70S-4S. [DOI] [PubMed] [Google Scholar]
- 16.Buko V.U., Lukivskaya O.Y., Zavodnik L.V., Sadovnichy V.V., Petushok N.E., Tauschel N.D. Antioxidative effect of ursodeoxycholic acid in the liver of rats with oxidative stress caused by gamma-irradiation. Ukr Biokhim Zh (1999) 2002;74:88–92. [PubMed] [Google Scholar]
- 17.Hartmann G., Cheung A.K., Piquette-Miller M., de Vincente F., Corcuera M.T., Gómez-Aguado F. Inflammatory cytokines, but not bile acids, regulate expression of murine hepatic anion transporters in endotoxemia. J Pharmacol Exp Therapeut. 2002;303:273–281. doi: 10.1124/jpet.102.039404. [DOI] [PubMed] [Google Scholar]
- 18.Garcia-Dominguez J., Aller M.A., Garcia C., de Vicente F., Corcuera M., Gómez-Aguado F. Splanchnic Th(2) and Th(1) cytokine redistribution in microsurgical cholestatic rats. J Surg Res. 2010;162:203–212. doi: 10.1016/j.jss.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Kaplan M.M., Righetti A. Induction of rat liver alkaline phosphatase: the mechanism of the serum elevation in bile duct obstruction. J Clin Invest. 1970;49:508–516. doi: 10.1172/JCI106260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basiglio C.L., Mottino A.D., Roma M.G. Tauroursodeoxycholate counteracts hepatocellular lysis induced by tensioactive bile salts by preventing plasma membrane-micelle transition. Chem Biol Interact. 2010;188:386–392. doi: 10.1016/j.cbi.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Simic D., Milojevic I., Bogicevic D., Miodrag M., Vladimir R., Biljana D. Preventive effect of ursodeoxycholic acid on parenteral nutrition-associated liver disease in infants. Srp Arh Celok Lek. 2014;142:184–188. doi: 10.2298/sarh1404184s. [DOI] [PubMed] [Google Scholar]
- 22.Bosch A., Dumortier J., Maucort-Boulch D., Scoazec J.Y., Wendum D., Conti F. Preventive administration of UDCA after liver transplantation for primary biliary cirrhosis is associated with a lower risk of disease recurrence. J Hepatol. 2015;63:1449–1458. doi: 10.1016/j.jhep.2015.07.038. [DOI] [PubMed] [Google Scholar]
- 23.Ponzetti K., King M., Gates A., Anwer M.S., Webster C.R. Cyclic AMP-guanine exchange factor activation inhibits JNK-dependent lipopolysaccharide-induced apoptosis in rat hepatocytes. Hepat Med. 2010;2010:1–11. doi: 10.2147/HMER.S7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellinger M., Xu W., Pathil A., Stremmel W., Chamulitrat W. Ursodeoxycholyl lysophosphatidylethanolamide inhibits cholestasis- and hypoxia-induced apoptosis by upregulating antiapoptosis proteins. Exp Biol Med (Maywood) 2015;240:252–260. doi: 10.1177/1535370214547157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Manousou P., Kolios G., Drygiannakis I., Koulentaki M., Pyrvolaki K., Voumvouraki A. CXCR3 axis in patients with primary biliary cirrhosis: a possible novel mechanism of the effect of ursodeoxycholic acid. Clin Exp Immunol. 2013;172:9–15. doi: 10.1111/cei.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa M., Tsujii T., Matsumura K., Yamao J., Matsumura Y., Kubo R. Immunomodulatory effects of ursodeoxycholic acid on immune responses. Hepatology. 1992;16:358–364. doi: 10.1002/hep.1840160213. [DOI] [PubMed] [Google Scholar]
- 27.Tanasescu C. Correlation between cholestasis and infection. Rom J Gastroenterol. 2004;13:23–27. [PubMed] [Google Scholar]
- 28.Diamond T., Dolan S., Thompson R.L., Rowlands B.J. Development and reversal of endotoxemia and endotoxin-related death in obstructive jaundice. Surgery. 1990;108:370–374. discussion 4-5. [PubMed] [Google Scholar]
- 29.Fausto N., Campbell J.S., Riehle K.J. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues C.M., Fan G., Ma X., Kren B.T., Steer C.J. A novel role for ursodeoxycholic acid in inhibiting apoptosis by modulating mitochondrial membrane perturbation. J Clin Invest. 1998;101:2790–2799. doi: 10.1172/JCI1325. [DOI] [PMC free article] [PubMed] [Google Scholar]