Abstract
Objectives
Non-alcoholic fatty liver disease (NAFLD) is a multisystem disease which can affect the cardiovascular system as well. We conducted this study to determine the cardiac effects of NAFLD such as conduction of impulse and ventricular repolarisation on electrocardiography (ECG).
Methods
In this study, we recruited patients with risk factors for NAFLD (group I; n = 23) and NAFLD patients (group II; n = 74) from Shar Hospital in Sulaimani City, Iraq. We analysed anthropometric measurements, serum fasting lipid profile, glucose levels, liver enzymes, and ECG recordings.
Results
ECG recordings showed significantly longer PR intervals, significantly shorter QTcB and JTc intervals, and a higher Tp-e/QTcB ratio in group II patients than in group I patients. These abnormalities were not associated with risk factors for diabetes. The TQ duration was significantly correlated with serum alanine aminotransferase (r = 0.411, p < 0.001) and aspartate aminotransferase (r = 0.272, p = 0.019) levels.
Conclusion
In our study, the presence of significant abnormalities in ventricular repolarisation suggests that patients with newly diagnosed NAFLD have subclinical cardiac stress and a higher risk of ventricular arrhythmias.
Keywords: Anthropometry, Electrocardiography, Non-alcoholic liver disease, Ventricular arrhythmias, Ventricular repolarisation
Highlights
-
•
Non-alcoholic fatty liver disease patients are at risk of developing ventricular repolarization.
-
•
Abnormal electrocardiograph changes are related to the serum levels of liver enzymes.
-
•
People at risk of developing non-alcoholic liver disease are also have abnormal ventricular repolarization indices.
الملخص
أهداف البحث
مرض تشحم الكبد اللاكحولي هو مرض متعدد الأسباب، ويمكن أن يؤثر في الجهاز القلبي الوعائي. ولأجل تقييم تأثيراته في الجهاز القلبي الوعائي، تم التقصي عن إيصال النبضة القلبية وإعادة استقطاب البطين الأيسر في تخطيط القلب الكهربائي.
طرق البحث
أجريت هذه الدراسة من آب ٢٠١٨ مرورا آب ٢٠١٩ في قسم الأدوية السريرية / كلية الطب، في جامعة السليمانية. تم إخضاع ٢٣ مريضا يتصفون بعوامل الخطورة لمرض تشحم الكبد اللاكحولي (المجموعة ١)، و٧٤ مريضا يشخص لأول مرة بمرض تشحم الكبد اللاكحولي (المجموعة ٢) في مستشفى شار التعليمي في مدينة السليمانية بالعراق. تم تحليل نتائج القياسات البشرية، ومستويات الشحوم والسكر الصائم، وتخطيط القلب الكهربائي في هذه الدراسة.
النتائج
أظهرت مخططات القلب الكهربائي زيادة ذات دلالة نوعية في طول فترة PR وقصر ذا دلالة نوعية في فترة QTcB ، وJTc مع زيادة نسبة Tp-e/QTcB عند مرضى المجموعة ٢ مقارنة بالمجموعة ١ ولم يلاحظ علاقة هذه التغيرات مع مرض السكري كعامل خطورة. كما تم ملاحظة علاقة طردية بين مستويات أنزيمات الكبد وطول الفترة TQ.
الاستنتاجات
وجود تغيرات نوعية في إعادة استقطاب البطين الأيسر يدلناعلى أن مرضى تشحم الكبد اللاكحولي حديثي التشخيص يعانون من إجهاد قلبي غير ظاهر سريريا، وهم أكثر تعرضا لخطورة عدم انتظام ضربات القلب.
الكلمات المفتاحية: قياسات انثروبومترية, تخطيط القلب الكهربائي, تشحم الكبد اللاكحولي, عدم انتظام ضربات البطين, اعادة اسنقطاب البطين
Introduction
Non-alcoholic fatty liver disease (NAFLD) is a multisystem disease which induces insulin resistance, low-grade inflammation, and oxidative stress syndrome.1 It is associated with multiple diseases which affect the cardiovascular system, including type 2 diabetes (T2D), hypertension, dyslipidaemia, and metabolic syndrome. Most of the clinical burden of NAFLD is related to cardiovascular disease,2, 3, 4, 5 and NAFLD status is strongly associated with the outcomes of cardiovascular events. Accumulating evidence indicates that NAFLD is independently positively associated with heart block and cardiac arrhythmia in persons with components of metabolic syndrome.6,7 NAFLD patients with T2D presented higher risks of premature ventricular arrhythmias and atrial fibrillation,8,9 and NAFLD was strongly associated with prolonged corrected QT (QTc) interval.10 Moreover, Hung et al. (2015) reported that severe NAFLD increased the risk of prolonged QTc interval, regardless of diabetes status.11
Patients with newly diagnosed NAFLD typically present no signs or symptoms of cardiovascular disease. Electrocardiography (ECG) is a simple, non-invasive test which can show subclinical abnormalities in heart rhythm and conduction. This cross-sectional study investigated ECG findings to identify abnormalities in ventricular repolarisation, which are associated with increased risk of cardiovascular events.
Materials and Methods
This study was approved by the Ethical Committee of the Faculty of the University of Sulaimani (No. 7-29-2894). All procedures were conducted in accordance with the Declaration of Helsinki, and the patients were informed that they were free to withdraw from the study at any time. The researchers explained the study design, and all participants provided informed consent for an ECG study before the study began.
This cross-sectional observational study investigated ECG abnormalities in patients with newly diagnosed NAFLD. It was conducted from August 2018 through August 2019 in the Department of Pharmacology, College of Medicine, University of Sulaimani, in cooperation with Shar Hospital in Sulaimani City. Eligible participants were men and women aged 30–50 years. All patients were recruited from private clinics in Sulaimani City, and they had been referred to Shar Hospital for further evaluation. NAFLD diagnosis was based on ultrasonographic detection of fatty infiltration and significantly elevated levels of liver enzymes (>40 IU/L). The inclusion criteria were a new diagnosis of NAFLD, confirmed by ultrasonographic evidence of liver fatty infiltration and an abnormally high serum alanine aminotransferase enzyme level. Participants with a history of coronary artery disease or cardiovascular events and those currently receiving medical treatment (e.g. macrolides, antipsychotics, antidepressants, antihistamines, and antiarrhythmics) were excluded, as were those who consumed alcohol (previously or currently) and pregnant and nursing women.
Anthropometric data collected included body weight (kg), height (m), and waist circumference (cm). Body mass index (BMI; kg/m2) was calculated as body weight divided by the square of the height (m2), and waist-to-height ratio (WHeR) was calculated as waist circumference (cm) divided by height (cm).
ECG assessment
All participants underwent 12-lead ECG with 12 standard leads, a sensitivity of 10 mm/mV, and a recording speed of 25 mm/s. Participants with normal sinus rhythm and no evidence of ventricular hypertrophy, ischemic changes, atrial/ventricular arrhythmias, or bundle-branch block were included in the analysis. ECG recordings were scanned, after which the scans were magnified with Windows Photo Viewer and the following data were obtained: heart rate (beats/min), RR interval (s), PR interval (ms), QRS wave duration (ms), QRS dispersion (ms), measured QT (QTm) interval (ms), QTc interval (ms), measured JT (JTm) interval (ms), corrected JT (JTc) interval (ms), TQ interval (ms), R-wave amplitude at lead V5 (mV), S-wave amplitude (mV), and the summation of S (v1) and R (v5). QTc interval (ms) was calculated using Bazett's formula: QTcB = QTm/.12
Prolongation of QTcB was defined as a value ≥440 ms (for men) or ≥460 ms (for women). JTm was defined as QT interval − QRS complex, and corrected (JTc) was defined as QTcB − QRS complex. QTcB/TQ ratio was calculated by dividing QTcB interval by TQ. This ratio represents cardiac restitution (i.e. the duration of action potential and the conduction velocity depending on the previous diastole) and it shows the relationship between the duration of action potential (represented by QT duration) and the diastolic interval (represented by TQ duration). A ratio of ≥1.0 indicates an increased risk of cardiac arrhythmia (from impulse re-entry), notably torsade de pointes.
Tp-e interval is the interval from the peak to the end of the T-wave, as measured by precordial leads.13 The ratios of Tp-e to QTm and to QTB were calculated using the relevant measurements. ECG measurement and interpretation was performed by two cardiologists.
Laboratory variables
After an overnight fast, venous blood was drawn from each patient and centrifuged at 3000 rpm for 15 min, after which the sera were separated for determination of lipid profile (including triglyceride, total cholesterol), serum glucose, and liver enzymes (including alanine [ALT] and aspartate aminotransferase [AST] levels) in the clinical laboratories of Shar Hospital. Triglyceride index was defined as the serum triglyceride (mg/dl) level divided by 160, and cholesterol index was defined as the serum total cholesterol (mg/dl) level divided by 200. ALT/AST and AST/ALT ratios were also calculated.
Twenty-five patients (12 men and 13 women; group I) with risk factors (including obesity, dyslipidaemia, and diabetes) commonly present in NAFLD patients and 76 NAFLD patients (46 women and 30 men; group II) were enrolled in the study. Two patients in each group had abnormal ECG findings and were excluded.
Statistical analysis
The results are presented as numbers, percentages, and means ± standard deviation. The data were analysed using the two-tailed independent two-sample t-test (for continuous data), the chi-square test (for categorical data), or simple (Pearson) correlation. All statistical analyses were performed with Excel 2007 (Microsoft Corporation, Redmond, WA, USA), and a P value of ≤0.05 was considered a statistical significance in all analyses.
Results
Table 1 shows the selected characteristics and laboratory results of the participants. Groups I and II did not differ significantly in sex distribution or any anthropometric variable. Group II was significantly younger than group I. Cholesterol index, triglyceride index, and fasting serum glucose values did not differ significantly between groups (Table 1). ALT, AST, and their ratios were significantly higher in group II (Table 1).
Table 1.
Characteristics of participants.
| Variables | Group I (n = 25) | Group II (n = 76) | P value |
|---|---|---|---|
| Sex | |||
| Male: Female | 12:13 | 30:46 | 0.453 |
| Age (years) | 51.0 ± 10.9 | 43.4 ± 8.9 | 0.003 |
| Body mass index (kg/m2) | 31.5 | 33.7 ± 5.7 | 0.072 |
| Waist circumference (cm) | 106.5 ± 12.3 | 107.1 ± 9.6 | 0.810 |
| Waist-to-height ratio | 0.656 ± 0.070 | 0.651 ± 0.086 | 0.814 |
| Triglyceride index | 1.256 ± 0.650 | 1.057 ± 0.521 | 0.171 |
| Cholesterol index | 0.880 ± 0.196 | 0.929 ± 0.159 | 0.260 |
| Diabetes (no.) | 10 | 17 | 0.084 |
| Fasting serum glucose (non-diabetic) | 118.2 ± 30.1 | 107.6 ± 16.3 | 0.203 |
| Fasting serum glucose (diabetic) | 146.3 ± 69.6 | 156.7 ± 42.7 | 0.841 |
| Alanine aminotransferase (U/L) | 19.1 ± 5.7 | 42.4 ± 21.3 | <0.001 |
| Aspartate aminotransferase (U/L) | 18.9 ± 4.3 | 28.5 ± 13.5 | <0.001 |
| Alanine/aspartate aminotransferase ratio | 1.053 ± 0.383 | 1.593 ± 0.582 | <0.001 |
| Aspartate/alanine aminotransferase ratio | 1.075 ± 0.389 | 0.720 ± 0.279 | <0.001 |
The results are presented as mean ± SD. P values were calculated using the two-tailed independent two-sample t-test for continuous data, and the Chi-square test for discrete data. Group I: patients with risk factors, Group II: patients with non-alcoholic fatty liver disease.
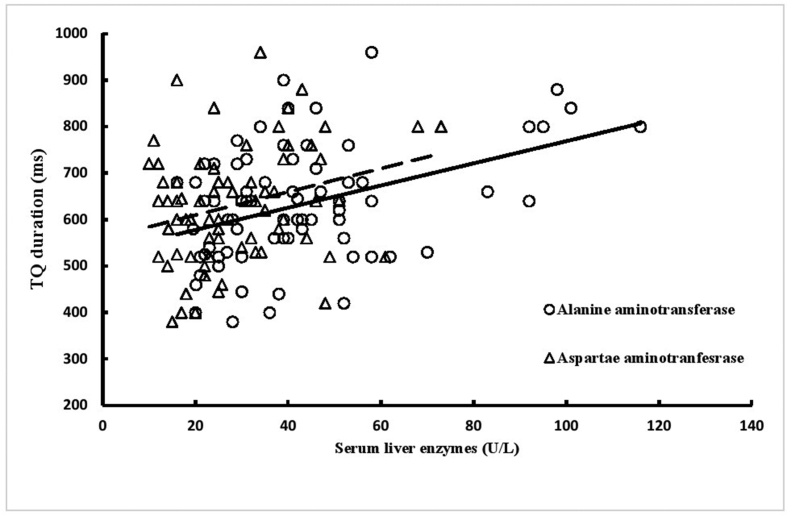
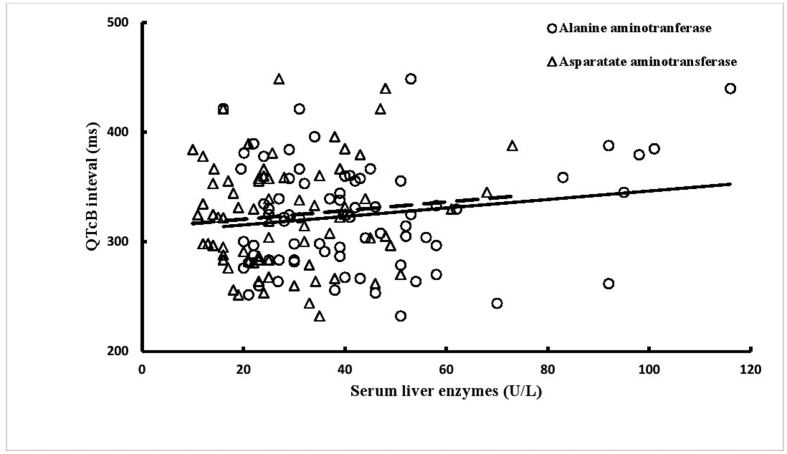
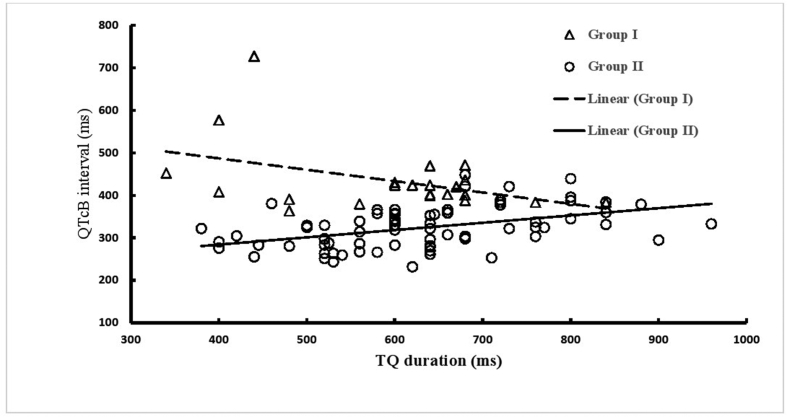
Analysis of ECGs showed that QTcB and JTc intervals were shorter and PR interval and Tp-e/QTcB were longer in group II patients than in group I patients (Table 2). ECG findings of group II patients did not differ significantly with respect to diabetes status (Table 3). ALT and AST were significantly positively correlated with TQ interval (Figure 1), but not with QTcB interval (Figure 2). Five patients in group I and one patient in group II had prolonged QTcB intervals (≥440 ms for men; ≥460 ms for women). The JTc-index was ≥112 for 4 (17.4%) patients in group I and 26 (35.1%) patients in group II (p = 0.108). Four patients in group I had a QTcB/TQ ratio of >1.0, but none in group II. The QTcB/QT slope (β-coefficient) was −0.267 in group I (r = −0.421) and 0.171 in group II (r = 0.443), which indicates a significant difference between groups in beat recovery (Figure 3).
Table 2.
Electrocardiographic findings of patients with risk factors (Group I) and patients with non-alcoholic fatty liver disease (Group II).
| Electrocardiographic variables | Group I (n = 23) | Group II (n = 74) | P value |
|---|---|---|---|
| Heart rate (beats per minute) | 81.4 ± 14.5 | 79.1 ± 12.9 | 0.486 |
| RR interval (ms) | 745.7 ± 115.1 | 761.1 ± 116.8 | 0.577 |
| PR interval (ms) | 146.3 ± 25.3 | 161.4 ± 29.8 | 0.021 |
| QRS duration (ms) | 58.4 ± 9.1 | 59.±16.4 | 0.725 |
| QRS dispersion (ms) | 14.3 ± 4/3 | 13.1 ± 5.9 | 0.289 |
| QTm interval (ms) | 371.7 ± 58.2 | 371.5 ± 39.2 | 0.987 |
| QTcB interval (ms) | 434.2 ± 77.5 | 324.0 ± 48.4 | <0.001 |
| QT index | 102.7 ± 17.6 | 101.1 ± 10.5 | 0.691 |
| TQ interval | 579.8 ± 122.1 | 631.3 ± 125.2 | 0.261 |
| JTm duration (ms) | 313.4 ± 60.1 | 312.2 ± 42.8 | 0.932 |
| JTc duration (ms) | 375.9 ± 80.0 | 264.7 ± 52.0 | <0.001 |
| JT index | 109.6 ± 22.6 | 107.5 ± 13.8 | 0.674 |
| Tp-e duration (ms) | 82.0 ± 18.9 | 77.7 ± 16.6 | 0.339 |
| Tp-e/QTm | 0.224 ± 0.055 | 0.211 ± 0.049 | 0.338 |
| Tp-e/QTcB | 0.193 ± 0.051 | 0.245 ± 0.062 | <0.001 |
| Voltage criteria | |||
| R1+S5 (mV) | 4.4 ± 2.4 | 5.4 ± 3.2 | 0.193 |
| S1+R5 (mV) | 16.3 ± 4.8 | 17.3 ± 5.5 | 0.433 |
The results are expressed as mean ± SD. P values were calculated using the two-tailed independent two-sample t-test.
Table 3.
Electrocardiographic findings of non-alcoholic fatty liver disease presenting with/without diabetes.
| Electrocardiographic measures | Group II without diabetes (n = 59) | Group II with diabetes (n = 15) | P value |
|---|---|---|---|
| Heart rate (beat per minute) | 79.1 ± 13.3 | 78.8 ± 11.7 | 0.928 |
| RR interval (ms) | 764.4 ± 120.5 | 748.5 ± 103.4 | 0.614 |
| PR interval (ms) | 159.0 ± 30.7 | 170.7 ± 24.7 | 0.134 |
| QRS duration (ms) | 60.0 ± 17.6 | 56.7 ± 10.2 | 0.358 |
| QRS dispersion (ms) | 13.00 ± 5.9 | 13.3 ± 6.1 | 0.866 |
| QTm interval (ms) | 369.7 ± 41.1 | 378.7 ± 30.7 | 0.353 |
| QTcB interval (ms) | 323.2 ± 50.7 | 327.3 ± 39.3 | 0.734 |
| QT index | 100.6 ± 10.7 | 103.1 ± 9.6 | 0.391 |
| TQ interval | 635.5 ± 126.5 | 614.7 ± 122.9 | 0.566 |
| JTm duration (ms) | 309.7 ± 44.9 | 322.0 ± 33.1 | 0.246 |
| JTc duration (ms) | 263.2 ± 54.3 | 270.6 ± 42.6 | 0.577 |
| JT index | 106.6 ± 14.2 | 111.0 ± 11.9 | 0.240 |
| Tp-e duration (ms) | 77.1 ± 16.7 | 80.0 ± 16.9 | 0.560 |
| Tp-e/QTm | 0.211 ± 0.050 | 0.212 ± 0.046 | 0.937 |
| Tp-e/QTcB | 0.244 ± 0.064 | 0.247 ± 0.059 | 0.881 |
| Voltage criteria | |||
| R1+S5 (mV) | 5.41 ± 3.27 | 5.49 ± 3.25 | 0.939 |
| S1+R5 (mV) | 17.43 ± 5.75 | 16.6 ± 4.47 | 0.563 |
The results are expressed as mean ± SD. P value was calculated using two-tailed independent two-sample t-test.
Figure 1.
Correlations of serum liver enzymes with TQ interval in patients with non-alcoholic fatty liver disease (group II): correlation coefficient (r) and probability (p) for alanine aminotransferase (r = 0.411, p < 0.001) and aspartate aminotransferase (r = 0.272, p = 0.019).
Figure 2.
Correlations of TQ duration with QTcB interval (ms) in patients with non-alcoholic fatty liver disease (group II): correlation coefficient (r) and probability (p) for alanine aminotransferase (r = 0.172, p = 0.142) and aspartate aminotransferase (r = 0.110, p = 0.350).
Figure 3.
Steepness restitution shows slopes for group I (−0.267) and group II (0.171). Group I: patients with risk factors; group II: patients with non-alcoholic fatty liver disease.
Discussion
The present results showed significant abnormalities in the ECG records of NAFLD patients, indicating that these abnormalities were unrelated to risk factors, including diabetes. The significant positive correlations between TQ interval (an indicator of restitution steepness) and liver enzyme levels indicate that abnormalities in ECG records reflect disease severity. The characteristics of groups I and II did not differ significantly. Although the patients in group II was significantly younger, the age difference was not associated with ECG findings.14 Ultrasonography findings, serum levels of liver enzymes, and the derived enzyme ratios were used to confirm NAFLD diagnoses in group II patients. These ECG findings can specifically be attributed to NAFLD.15 Previous studies reported that ECG abnormalities in NAFLD were limited to QTc interval, bundle-branch block, and other ECG findings related to coronary artery disease.16
However, previous studies did not compare ECG findings of persons presenting with risk factors for NAFLD. In the present study, PR interval was longer in group II than in group I, which is consistent with a previous study reporting that NAFLD patients had first-degree heart block.6 The ECG records of the present NAFLD patients showed significant shortening of the QTcB interval, which is inconsistent with previous findings. This discrepancy is explained by the fact that the present patients had newly diagnosed NAFLD. Previous studies reported that QTcB prolongation was 2.55 ms in mild NAFLD and 12.13 ms in severe NAFLD.10,11 Moreover, indices of ventricular repolarisation, including JTc interval and Tp-e/QTcB index, were significantly worse in NAFLD patients, which indicates that these patients have a significantly higher risk of ventricular arrhythmias.17,18
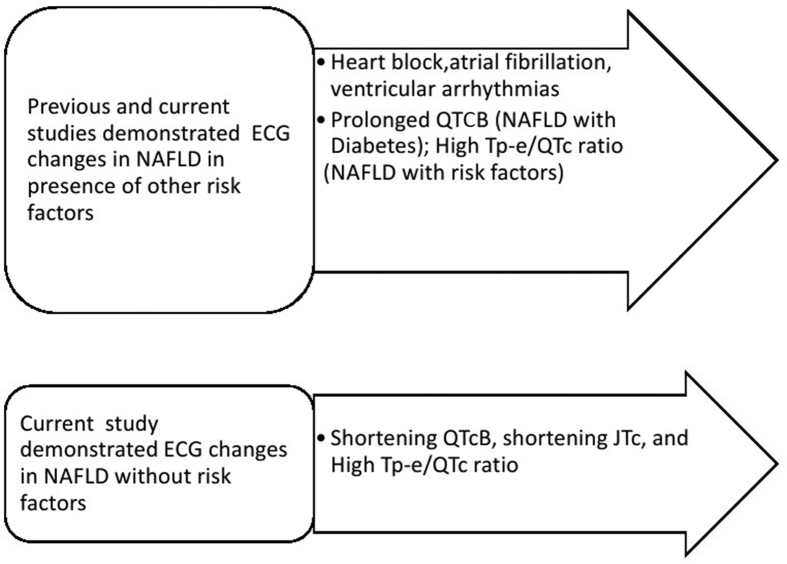
We noted a positive correlation between levels of hepatic enzymes and QTcB interval, which suggests that QTcB duration reflects disease severity. However, the correlation was insignificant because our patients had newly diagnosed NAFLD. TQ index is a measure of the relationship between duration of action potential and ventricular diastole, and it was positively correlated with QTcB interval in group II and inversely correlated with QTcB interval in group I. This suggests that ventricular steepness restitution (i.e. the slope of the action potential duration in the restitution curve) in NAFLD patients is abnormal and could result in cardiac strain and ventricular arrhythmias.19 Ismaiel et al. (2019) concluded in their systematic review conducted on 20 studies that NAFLD is independently associated with prolonged QTc interval, atrial fibrillation, and bundle-branch block.20 Sudden cardiac death is one clinical manifestation which is observed in patients with congestive heart failure presenting with a high NAFLD fibrosis score.21 Therefore, our findings are consistent with others that poor cardiovascular prognosis is a feature of NAFLD as abnormal ventricular repolarisation is linked with sudden death. This study adds new information that NAFLD patients have new ECG changes which are unrelated to the coexisting risk factors and can predict the development of arrhythmias (Figure 4).
Figure 4.
Schematic diagram showing the ECG changes associated with non-alcoholic fatty liver disease.
The strengths of this study are that our participants presented with newly diagnosed NAFLD (Group II) and risk factors for NAFLD (Group I). Therefore, the cause of ECG changes (QTcB, JTc, and Tp-e/QTcB) can be concluded to be NAFLD rather than risk factors (e.g. obesity, dyslipidaemia, and diabetes).
The limitations of the study include small sample size and the variability in metabolic derangement between groups I and II.
Conclusion
In conclusion, the presence of significant abnormalities in ECG indices and intervals indicates that newly diagnosed NAFLD patients have higher risks of cardiac stress and ventricular arrhythmias. Simple ECG studies are useful in identifying subclinical abnormalities and determining the risk of ventricular arrhythmia in NAFLD patients.
Recommendations
The results of this study lead us to recommend a further study with a large sample size, adjusting the risk factors in patients with and without NAFLD. Moreover, routine ECG investigations of all NAFLD patients are important to identify patients at risk of potential cardiac arrhythmias.
Source of funding
University of Sulaimani supported this study (No. 7-29-2894).
Conflict of interest
The authors have no conflict of interest to declare.
Ethical approval
The Ethical Committee of the Faculty of the University of Sulaimani approved this study (No.7-29-2894).
Consent
Consent form obtained which included investigation with ECG.
Authors contributions
M.S.M.A-N. Contributed to study conception and design, statistical analysis, final interpretation of data, and drafting and revising of the article, and gave final approval. V.A.W.E. Contributed to study design, acquisition, interpretation of data, and revision of the article, and gave final approval. D.S.H. Contributed to concept, study design, and acquisition of data, and gave final approval. M.O.M. Contributed to concept, study design, and acquisition of data, and gave final approval. All authors have critically reviewed and approved the final draft and are responsible for the content and similarity index of the manuscript.
Acknowledgment
The authors thank the staff and patients of Shar Hospital for their assistance. We are also grateful to the Faculty of Medicine, University of Sulaimani, for supporting this research.
Footnotes
Peer review under responsibility of Taibah University.
References
- 1.Han E., Lee Y.H. Non-alcoholic fatty liver disease: the emerging burden in cardiometabolic and renal diseases. Diabetes Metab J. 2017;41(6):430–437. doi: 10.4093/dmj.2017.41.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yilmaz Y., Kurt R., Yonal O., Polat N., Celikel C.A., Gurdal A. Coronary flow reserve is impaired in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Atherosclerosis. 2010;211(1):182–186. doi: 10.1016/j.atherosclerosis.2010.01.049. [DOI] [PubMed] [Google Scholar]
- 3.Bonapace S., Perseghin G., Molon G., Canali G., Bertolini L., Zoppini G. Nonalcoholic fatty liver disease is associated with left ventricular diastolic dysfunction in patients with type 2 diabetes. Diabetes Care. 2012;35(2):389–395. doi: 10.2337/dc11-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Targher G., Valbusa F., Bonapace S., Bertolini L., Zenari L., Rodella S. Non-alcoholic fatty liver disease is associated with an increased incidence of atrial fibrillation in patients with type 2 diabetes. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0057183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mellinger J.L., Pencina K.M., Massaro J.M., Hoffmann U., Seshadri S., Fox C.S. Hepatic steatosis and cardiovascular disease outcomes: an analysis of the Framingham Heart Study. J Hepatol. 2015;63(2):470–476. doi: 10.1016/j.jhep.2015.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mantovani A., Rigolon R., Pichiri I., Bonapace S., Morani G., Zoppini G. Nonalcoholic fatty liver disease is associated with an increased risk of heart block in hospitalized patients with type 2 diabetes mellitus. PloS One. 2017;12(10) doi: 10.1371/journal.pone.0185459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Usman M.S., Siddiqi T.J. Emerging evidence for the association between non-alcoholic fatty liver disease and cardiac arrhythmias. Dig Liver Dis. 2017;49(10):1166. doi: 10.1016/j.dld.2017.07.013. [DOI] [PubMed] [Google Scholar]
- 8.Mantovani A., Rigamonti A., Bonapace S., Bolzan B., Pernigo M., Morani G. Nonalcoholic fatty liver disease is associated with ventricular arrhythmias in patients with type 2 diabetes referred for clinically indicated 24-hour Holter monitoring. Diabetes Care. 2016;39(8):1416–1423. doi: 10.2337/dc16-0091. [DOI] [PubMed] [Google Scholar]
- 9.Käräjämäki A.J., Pätsi O.P., Savolainen M., Kesäniemi Y.A., Huikuri H., Ukkola O. Non-alcoholic fatty liver disease as a predictor of atrial fibrillation in middle-aged population (OPERA Study) PloS One. 2015;10(11) doi: 10.1371/journal.pone.0142937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Targher G., Valbusa F., Bonapace S., Bertolini L., Zenari L., Pichiri I. Association of nonalcoholic fatty liver disease with QTc interval in patients with type 2 diabetes. Nutr Metabol Cardiovasc Dis. 2014;24(6):663–669. doi: 10.1016/j.numecd.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 11.Hung C.S., Tseng P.H., Tu C.H., Chen C.C., Liao W.C., Lee Y.C. Nonalcoholic fatty liver disease is associated with QT prolongation in the general population. J Am Heart Assoc. 2015;4(7) doi: 10.1161/JAHA.115.001820. pii: e001820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bazett H.C. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 13.Karaagac K., Emul A., Tenekecioglu E., Agca F.V., Ozluk O.A., Tutuncu A. The effects of metabolic syndrome on tpte interval and TpTe/Qt ratio in patients with normal coronary arteries. Eurasian J Med. 2014;46(3):182–186. doi: 10.5152/eajm.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van den Berg E.H., Gruppen E.G., Ebtehaj S., Bakker S.J.L., Tietge U.J.F., Dullaart R.P.F. Cholesterol efflux capacity is impaired in subjects with an elevated fatty liver index, a proxy of non-alcoholic fatty liver disease. Atherosclerosis. 2018;277:21–27. doi: 10.1016/j.atherosclerosis.2018.07.028. [DOI] [PubMed] [Google Scholar]
- 15.Stefan N., Häring H.U., Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7(4):313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 16.İşcen S. RBBB is associated with an increased risk of NAFLD in young healthy individuals. Int J Cardiol. 2013;168(4):4056–4057. doi: 10.1016/j.ijcard.2013.07.035. [DOI] [PubMed] [Google Scholar]
- 17.Al-Nimer M.S.M., Hussein Subclinical ventricular repolarization abnormality in uncontrolled compared with controlled treated hypertension. Indian Heart J. 2017;69(2):136–140. doi: 10.1016/j.ihj.2016.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yilmaz Coşkun F., Elboğa G., Altunbaş G., Vuruşkan E., Uğur B.K., Sucu M. Evaluation of ventricular repolarization features with Tp-e, Tp-e/QTc, JTc and JTd during electroconvulsive therapy. J Electrocardiol. 2018;51(3):440–442. doi: 10.1016/j.jelectrocard.2018.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Fossa A.A. Beat-to-beat ECG restitution: a review and proposal for a new biomarker to assess cardiac stress and ventricular tachyarrhythmia vulnerability. Ann Noninvasive Electrocardiol. 2017;22(5) doi: 10.1111/anec.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ismaiel A., Colosi H.A., Rusu F., Dumitrașcu D.L. Cardiac arrhythmias and electrocardiogram modifications in non-alcoholic fatty liver disease. A systematic review. J Gastrointestin Liver Dis. 2019;28(4):483–493. doi: 10.15403/jgld-344. [DOI] [PubMed] [Google Scholar]
- 21.Takahashi T., Watanabe T., Shishido T., Watanabe k, Sugai T., Toshima T. The impact of non-alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels. 2018;33(7):733–739. doi: 10.1007/s00380-017-1113-1. [DOI] [PubMed] [Google Scholar]