Highlights
-
•
The mean jitter is increased in all muscles with fibrillation potentials.
-
•
The mean jitter is increased by about 75% of muscles with chronic denervation only.
-
•
Activity in chronic denervated muscles could retain immature atrophic muscle fibers.
Keywords: Single-fiber electromyography, Jitter, Concentric needle electrode, Denervation, Neuromuscular junction
Abstract
Objective
To measure the jitter parameters in muscles with denervation/reinnervation in 32 chronic radiculopathy cases.
Methods
Measurements were done in chronic denervated muscles by voluntary and electrical activation using a concentric needle electrode.
Results
Mean jitter was abnormal in 87.5% (mean 49.2 µs) and 81.25% (mean 36.8 µs), for voluntary and electrical activation. In muscles with fibrillation potentials (FPs), the mean jitter was abnormal in all cases, and impulse blocking was frequent (53.4–92.3%). In muscles without FPs, the mean jitter was abnormal in 78.9% for voluntary activation and 68.4% for electrical activation. No correlation was found between jitter and motor unit action potential amplitude.
Conclusion
The muscles with FPs were associated with the immature spread of acetylcholine receptors (AChRs) throughout the muscle membrane. Conversely, the neuromuscular junctions (NMJs) assemble may be repressed by the already reinnervated muscles. For those, higher jitter may be due to the persistence of atrophic fibers expressing neonatal myosin heavy chain (MHC) and immaturity of NMJ composting instead of the overspread of immature AChRs.
Significance
Jitter measurement must be avoided in chronic denervated muscles, regardless of FPs’ presence. The activity of reinnervated muscle could maintain neonatal MHC and repress new NMJs development.
1. Introduction
The neuromuscular junction (NMJ) comprises of three structures: the nerve terminal, the motor endplate covered in acetylcholine receptors (AChRs), and peri-synaptic Schwann cells (SCs), that sheathes the unmyelinated nerve terminal and synapse (Vannucci et al., 2019). This sophisticated machine performs the transmission of the action potentials (APs) from nerve to the muscle. Single-fiber electromyography (SFEMG) studies the neurotransmission microphysiology in vivo and is currently performed in clinical practice (Stålberg et al., 2010). The APs from the peripheral motor neuron triggers the endplate potentials (EPPs), which have some normal time variability to achieve threshold to generate muscle fiber APs. This time could be measured as the neuromuscular jitter (Juel, 2019). Accordingly, increased jitter with or without impulse blocking indicates an NMJ disfunction. After lower motor nerve damage causing Wallerian degeneration, motor axons can reinnervate to the original NMJs, either by outgrowth or collateral sprouting. In many aspects, this process seems like the NMJ formation during development (Favero et al., 2010).
During embryogenesis, immature NMJs are formed after the muscle fibers appear, and are transiently innervated by many motor neurons (Slater, 2019). At this step, all muscle fiber surface area is covered by AChRs and, the motor axons form a morphologically unspecialized but functional contact (Kandel et al., 2013). The immature AChR has a 2 to 10-fold longer mean channel opening time, lower conductance, shorter half-life (1 day), and higher sensitivity to the agonist's acetylcholine (ACh) compared to the mature form. Both the quantal content and the miniature endplate potentials (mEPPs) frequency are excessively low (Jansen and Fladby, 1990, Kaya et al., 2014, Slater, 2008, Shear and Martyn, 2009, Kandel et al., 2013, Kramer et al., 2017). At this time, the muscle fibers have a small caliber, a high input resistance and little current is required to reach the AP threshold, resulting in a more significant depolarization per unit charge. Also, the immature NaV1.5 form requires less depolarization to trigger an AP. So, even with poly-innervation and immature NMJs, motor neurons can trigger muscle fibers contraction (Slater, 2008).
Immature AChRs express α(2), β, δ and, γ subunits or α7-subunits. After birth junctional folds begin to form in the postsynaptic membrane and AChRs are clustered at the junctional area, mainly at the crests of the folds; the γ subunit is replaced by an ε subunit (Slater, 2008, Kandel et al., 2013, Bittner and Martyn, 2019). The process of AChRs clustering depends on nerve maturation and, therefore, at this stage, the “synapse elimination” takes place and, just one motor axon remains (Wilson and Deschenes, 2005, Slater, 2019). AChRs clustering depends on agrin cascade; it is released by the nerve and subsequently binds the LRP4 receptor on the muscle membrane to activate the muscle-specific receptor kinase (MuSK) (Kandel et al., 2013). The NMJs cannot be assembly if the agrin or its receptor MuSK/LRP4 complex and the intracellular adaptor proteins, Rapsyn and Dok-7, fail to express (Kandel et al., 2013, Legay and Lin, 2017). Terminal SCs are found closeness to the motor nerve terminals and are active during developmental “synapse elimination” to stimulate nerve growth. They wrap the terminal unmyelinated nerve and express trophic, adhesive, and growth-associated factors during NMJ development (Lee et al., 2017, Liu and Chakkalakal, 2018).
Immature AChRs express α(2), β, δ and, γ subunits or α7-subunits. After birth, junctional folds begin to form in the postsynaptic membrane. The terminal branches of the motor axons expand, and after the first few weeks, the NMJ changes from “plaque” (immature) to “pretzel-like” (mature) conformation. From that on, the NMJ structure remains stable (Wilson and Deschenes, 2005, Slater, 2019) and locates just in the endplate region; the pattern of single innervation persists for a lifetime (Lichtman and Sanes, 2003). The single motor neuron innervation pattern increases the quantal content (Slater, 2008). Laminins are molecules essential for fold formation, and, specifically, the laminin β2, found at the basal lamina of NMJs, plays an essential role in NMJ formation. Mice born without laminin β2 never develop folds and have a much-reduced number of active zones (Slater, 2017). The structural maturation of the NMJ is complete 3–4 weeks after birth.
SFEMG can be used to follow the course of reinnervation and is useful in detecting and quantitating axonal degeneration. The mean jitter and percent impulse blocking parameters are always abnormal and are closely related to the stage of denervation/reinnervation (Pond et al., 2014). Here, we studied jitter parameters from patients diagnosed as chronic radiculopathy with a demonstrable neurogenic pattern of the motor unit action potentials (MUAPs) with and without fibrillation potentials (FPs).
2. Methods
2.1. Patients
Thirty-two adult patients of both sexes were recruited and invited to participate in the study. All cases had previous non-quantitative electromyography (EMG) examination carried out at the Neuromuscular Diseases and Electroneuromyography Service of the São José do Rio Preto State Medical School, São Paulo, Brazil (FAMERP). The test showed MUAPs with morphological abnormality compatible with chronic reinnervation (higher amplitude and duration, decreased recruitment, and increased firing rate without full interference pattern) with or without FPs. For the practical reason for data analysis, we are going to use just the amplitude values.
A portable Keypoint electromyography machine (Medtronic, Denmark) was used in all cases. Patients were referred to the EMG evaluation with suspected chronic radiculopathy, and some had previous surgery for the cervical or lumbosacral spine. All cases are under follow-up in the specific orthopedics or neurosurgery outpatient clinics. The electrophysiological consultation was performed by a Clinical Neurophysiology and Neurology Board-Certified doctor. The clinical data were collected from the EMG report summaries. Details on surgery, medication in use, and comorbidities were obtained from the hospital's electronic medical records.
2.2. Single-fiber electromyography
The SFEMG test was done at the Neuromuscular Investigation Laboratory (LIN) at the FAMERP by the first author (JAK). Both techniques, voluntarily and electrically activation, were used to measure jitter parameters in the same muscle. A Natus™ Keypoint-Net (USA) or Natus™ UltraPro (USA) machine, which an in-built software specifically developed for SFEMG, was used. Before the SFEMG study, a conventional non-quantitative EMG was done in the chosen muscle to confirm the neurogenic abnormalities described above. When more than one muscle presented neurogenic abnormalities, the most affected was chosen, provided that there was no difficulty in voluntary activation (e.g., plegia or severe paresis).
The measurement of jitter parameters by voluntary activation was done using disposable concentric needle electrodes (CNE) of two brands: Ambu® Neuroline Concentric, 25 mm × 0.30 mm (30G), recording area 0.02 mm2 and, Natus™ Dantec® DCN Disposable Concentric Needle Electrode, 25 mm × 0.30 mm (30G), recording area 0.02 mm2. Both electrodes are routinely used and approved by the Brazilian Agency for Health Surveillance (ANVISA). The CNE was introduced into the chosen muscle. After minimal maintained voluntary contraction, the single fiber action potentials (SFAPs), or more appropriately, the “apparent single fiber action potentials” (ASFAPs) were recorded. Due to the use of disposable CNE, with a much larger recording area than the single fiber electrode, the probability of recording spikes from two or more muscle fibers increased. Thus, the term ASFAP is better for defining these potentials. The low-frequency filter was set at 1 kHz to suppress the distant APs potentials from the CNE recording area, passing only the APs with rapid rise time. Because the low-frequency filter reduces the amplitude of APs, we accepted only the ones with a minimum amplitude limit of 100 μV. Records were made after a minimum of three CNE insertions with radial advancement into the muscle. After slight movements of the electrode, a pair of ASFAPs was recorded, with both potentials belonging to the same motor unit; the trigger was put to the higher amplitude spike to get the temporal variation on the second smaller spike.
In cases where multispikes recorded, if the triggering spike has the largest jitter, this will increase all the jitter calculations, giving entirely erroneous results (Stålberg et al., 2017). So, the spike that produces the lowest summed jitter between spikes should be chosen as the time reference, and this was done during the post-processing to select the triggering signal (Stålberg et al., 2017). The jitter value was calculated by the mean consecutive differences (MCD) for each pair, ideally for 100 pairs; in some circumstances, given the difficulties in measuring jitter in neurogenic conditions, we accepted a minimum of 30 pairs (Stålberg et al., 2018). The MCD variation was calculated by the “amplitude level,” i.e., by the temporal variation of the ascending lines of depolarization of the ASFAPs. Acceptable potentials were based on the criteria established in the literature (Sanders et al., 2019), as follows. Parallel depolarization ascending lines, similar shape in the superimposition of potentials, absence of summation characterized by notches and shoulders in the APs depolarization lines, and a regular peak of the APs. Small amplitude variation was tolerated. We excluded ASFAP pairs without clear separation between them, records with less than 30 ASFAP pairs, ASFAP pairs with interpotential interval (IPI) greater than 4 ms to avoid the effect of velocity recovery function (VRF), or when the shape of potentials did not remain constant in consecutive discharges.
The classic SFAPs present rise-time <300 μs and the same shape/amplitude in consecutive discharges, reliable confirmed in the superimposition of at least ten potentials. Action potentials pairs must have a clear baseline separation. The test conclusion was defined by the average of 20 different MCDs for the reference value of 30 μs (Stålberg et al., 2016). Although the multicenter study for the new reference values for CNE jitter parameters did not contemplate large muscles other than the Extensor Digitorum, we use its reference for the Triceps, Tibialis Anterior, Medial Gastrocnemius, Vastus Lateralis, and Rectus Femoris muscles. The mean value of the sorted differences (MSD) was also obtained, which calculates the consecutive differences according to the spike pairs' frequency of discharge. When the inter-discharge interval (IDI) variation is irregular, there may be interference due to the VRF effect. When MCD/MSD ratio is higher than 1.25, MSD is used. Mean jitter values higher than 150 μs were fixed at this value to minimize the bias due to the VRF effect. The higher the jitter, the greater the impulse block's probability, consequently further artificially adding jitter. The other way for the test conclusion is the percentage of the individual MCDs above the reference limit (43 μs). When more than 10% of the ASFAP pairs, i.e., at least 3 out of 20, have values higher than 43 μs, the test was abnormal. The skin temperature was maintained at more than 30 °C.
The measurement of jitter parameters by electrical activation was done using the intramuscular microaxonal stimulation technique. Disposable monopolar needle electrode (Ambu® Monopolar Neuroline, x 0.36 mm (28G), AMBU A/S (DK-2750 Ballerup), 25 mm, was introduced into the muscle near the motor point (active). Another similar electrode (reference) was introduced 2–3 cm in any direction, to the active electrode's insertion site, following standards already described (Trontelj et al., 1986, Kouyoumdjian and Stålberg, 2008). The stimulation frequency was settled to 10 Hz. The stimulation intensity (mA), in the form of square pulses of 0.04 ms, was adjusted to produce small visible local muscle twitching, usually achieved with intensity less than two mA. The same disposable CNE described previously was inserted in the area of visible muscle twitching and a subtle manner adjusted for the registration of the ASFAPs. During the ASFAP recordings, the intensity of stimulation was finely adjusted to avoid submaximal stimulation, giving rise to jitter falsely increased with or without impulse block. Maximum stimulation was achieved when there was no further AP's latency reduction (latency stability), frequently associated with new ASFAPs recruitment, subliminal to the first one. The MCD of the latencies is ideally obtained with 100 potentials. As stressed before, in some records, 30 ASFAPs were accepted due to difficulty obtaining them in the presence of active denervation/chronic reinnervation (Stålberg et al., 2018). The frequency at 10 Hz is the one that most closely approximates the physiological one. The recording electrode was introduced into different muscle regions to minimize the recording of the same potentials. Thirty MCDs of 30 different motor endplates were measured. The mean jitter's reference limit was defined to 24 μs (Stålberg et al., 2016). The percentage of individual jitter with a value higher than 35 μs (Stålberg et al., 2016), was also calculated. When more than 10% of the individual values were greater than 35 μs, the test was abnormal. We excluded jitter values less than five µs (direct muscle fiber stimulation; no jitter), and care was taken to exclude “axon-reflex” and f-waves (Stålberg et al., 2017). Other electrophysiological parameters were the same as those already described in the previous technique.
2.3. Exclusion criteria
a. A muscle with normal non-quantitative conventional EMG at the time of SFEMG studies; b. Patients under 16 years of age; c. Patients that cannot maintain minimal voluntary contraction; d. Patients in use of anticoagulants; e. Patients with other neuromuscular comorbidities causing active denervation and chronic reinnervation (e.g., amyotrophic lateral sclerosis, polio-like sequelae, or traumatic peripheral nerve injuries).
2.4. Ethics
The study was in accord with the Helsinki Declaration of 1975 and approved by the ethics committee of the Faculdade de Medicina de Sao Jose do Rio Preto, Sao Paulo, Brazil, where the SFEMG studies were performed. All patients signed informed consent.
2.5. Statistics
Descriptive statistics: mean and standard deviation values were calculated for data with normal distribution; median and percentiles values were calculated for data with the non-Gaussian distribution. Anderson-Darling normality test was used. The comparison between the continuous parametric variables was made by Pearson correlation (Student's t-test). The comparison between the continuous nonparametric variables was made by the Mann-Whitney test (U test). To measure the linear association between two variables, a correlation of r (R-Sq.) was used, with r = 1 (R-Sq. = 1) or 100% represents the perfect linear association and r = 0 (R-Sq. = 0), represents no association between the variables. The significance level was placed at 95%. The software Minitab® was used for statistical evaluations, and p < 0.05 was accepted as the limit of significance.
3. Results
3.1. Patients
The mean age was 52.4 ± 10.6 years (32 to 74 years); the male was 17 (53.1%). The muscles studied were Tibialis Anterior (17/32), Triceps (7/32), Medial Gastrocnemius (4/32), Vastus Lateralis (3/32), and Rectus Femoris (1/32), representing mostly involvement of roots L5, C7, S1, and L3-4, respectively. In some patients, where a multiradicular abnormality was found, only one of the affected muscles was chosen. There was a prior surgery (laminectomy with discectomy) in 15 patients (46.9%), with arthrodesis (vertebral fusion) in 66.7%. Nineteen patients (59.4%) used at least one of those drugs: gabapentin, pregabalin, opioids, or other classes of continuous-use medications for chronic pain. There were 3 cases with diabetes mellitus type 2 for less than four years from diagnosis; in none of these cases, there was clinical or electrophysiological evidence of peripheral neuropathy.
3.2. Electromyography
In 13 cases (40.6%) the conventional non-quantitative EMG detected FPs subjectively ranked from 1 (rare) to 4 (profuse), reflecting active denervation. Motor unit action potentials with increased amplitude, duration, and some polyphasic were recorded in all cases, reflecting chronic reinnervation by distal or collateral sprouting. The MUAPs mean amplitude was 9.8 mV ± 3.46, ranging from 4.5 to 20 mV.
3.3. Single-fiber electromyography
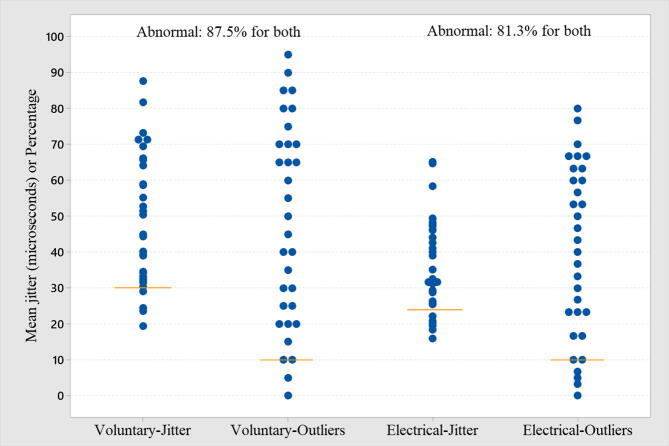
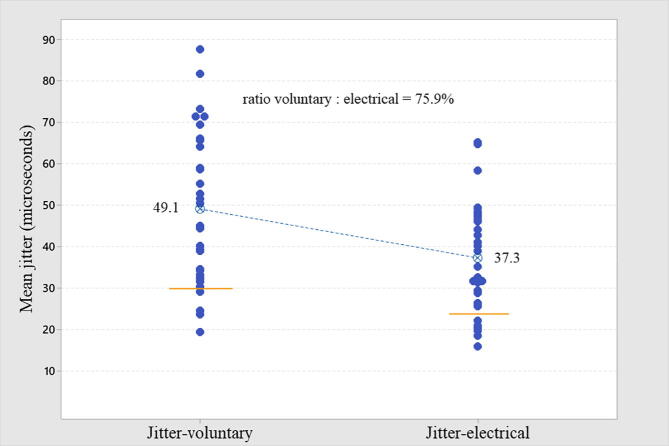
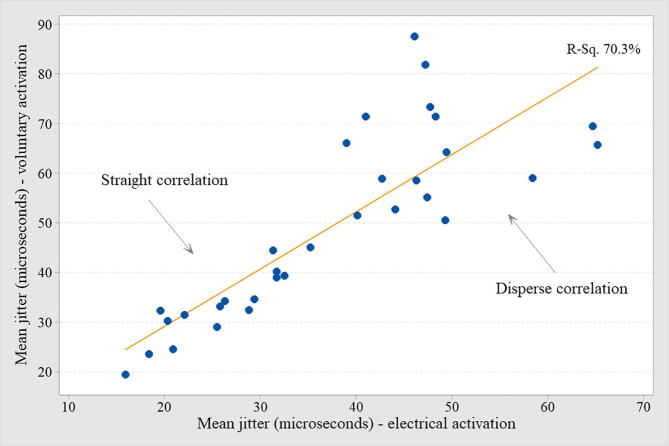
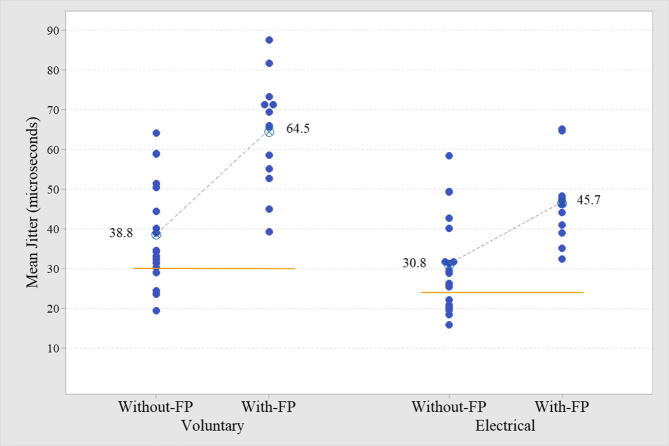
In all cases, the mean MCD value was used. The mean jitter by voluntary activation was abnormal (>30 μs) in 87.5% (Table 1, Table 2, and Fig. 1). The mean jitter by electrical activation was abnormal (>24 μs) in 81.3% (Table 1, Table 2, and Fig. 1). The comparison of the mean jitter from both activation techniques is shown in Fig. 2. In only one case (electrical activation), the recommended 30 ASFAPs were not reached due to technical difficulties. In all cases registered by voluntary activation, the target of 20 ASFAPs per patient was reached. In both forms of activation, the same abnormal proportion (87.5% for voluntary and 81.3% for electrical) was found for individual jitter, i.e., more than 10% values above 43 μs (voluntary activation) and more than 10% above 35 μs (electrical activation). The percentage of blockade found in voluntary and electrical activation was 53.1% and 37.5%, respectively. The ratio between mean jitter by voluntary/electrical activation was 75.9% (Fig. 2), and the higher the mean jitter value, the more dispersed was the correlation between the two activation techniques (Fig. 3). The jitter parameters (mean and abnormal percentage of individual values) were abnormal in all (100%) patients with FPs, regardless of the activation technique. The comparison of cases with and without FPs in either type of activation was extremely significant (Table 2, Fig. 4). The correlation between the MUAPs' amplitude values obtained in the conventional EMG and the jitter values was r (R-Sq.) = 13.8% for electrical activation and r (R-Sq.) = 8.7% for electrical activation, both non-significant. There was no statistically significant correlation between the subjective quantification (ranking) of the number of FPs and the mean jitter values. Some recordings with increased jitter by voluntary activation are shown in Fig. 5. Fig. 6 shows a typical neurogenic recording with many spikes appearing and disappearing together by increasing and decreasing the stimulus intensity.
Table 1.
Mean and individual jitter values from 32 muscles from patients with chronic radiculopathy presenting neurogenic motor action unit potentials with or without fibrillation potentials.
| Activation | n | Mean | SD | Median | Range | t-test | Mann-Whitney | |
|---|---|---|---|---|---|---|---|---|
| Mean jitter | Voluntary | 32 | 49.2 | 18.14 | 20.1–87.4 | p = 0.0023 | ||
| Electrical | 32 | 36.8 | 12.63 | 15.9–61.5 | ||||
| Individual jitter | Voluntary | 640 | 42.4 | 9.2–150 | p < 0.0001 | |||
| Electrical | 932 | 31.0 | 5.5–150 | |||||
Table 2.
Mean and individual jitter values from 32 muscles from patients with chronic radiculopathy presenting neurogenic motor action unit potentials splited into groups with and without fibrillation potentials.
| Activation | n | Mean | SD | Median | Range | t-test | Mann-Whitney | |
|---|---|---|---|---|---|---|---|---|
| Mean jitter without FPs | Voluntary | 19 | 38.8 | 12.96 | 20.1–64.2 | p < 0.0001 | ||
| Mean jitter with FPs | Voluntary | 13 | 64.5 | 13.15 | 39.7–87.4 | |||
| Mean jitter without FPs | Electrical | 19 | 30.8 | 11.57 | 15.9–56.4 | p < 0.0004 | ||
| Mean jitter with FPs | Electrical | 13 | 45.7 | 8.29 | 32.5–61.5 | |||
| Individual jitter without FPs | Voluntary | 380 | 32.8 | 9.2–150 | p < 0.0001 | |||
| Individual jitter with FPs | Voluntary | 260 | 57.9 | 9.9–150 | ||||
| Individual jitter without FPs | Electrical | 542 | 25.6 | 5.5–150 | p < 0.0001 | |||
| Individual jitter with FPs | Electrical | 390 | 37.8 | 8.2–150 | ||||
Fig. 1.
The individual jitter parameter plot for voluntary activation (87.5% abnormal), and electrical activation (81.3% abnormal) through the two criteria: mean jitter increase (line) and more than 10% abnormal for individual jitter value (line).
Fig. 2.
The individual mean jitter values plot for comparison through voluntary and electrical activation in the same muscle from 32 patients with chronic radiculopathy is shown. All muscles had motor action unit potentials with increase amplitude and long duration with or without fibrillation potentials.
Fig. 3.
The individual mean jitter correlation between voluntary and electrical activation in the same muscle from 32 chronic radiculopathy patients is shown. All muscles had motor action unit potentials with increase amplitude and long duration with or without fibrillation potentials. As long as the jitter increased, the correlation is dispersing due to the complex spikes difficult for jitter measurement.
Fig. 4.
The individual mean jitter plot for comparison in muscles with and without fibrillation potentials (FPs) after voluntary and electrical activation from 32 chronic radiculopathy patients is shown. All muscles with FPs had abnormal mean jitter.
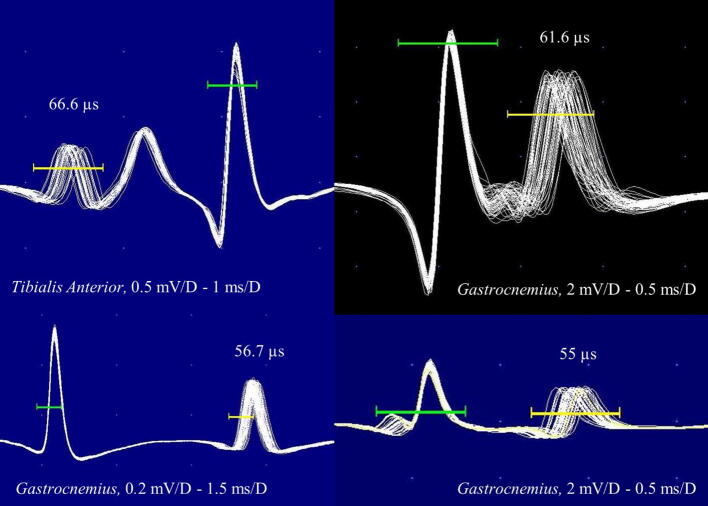
Fig. 5.
Four jitter recordings by voluntary activation and triggered by amplitude level. All jitter values are increased without impulse blocking.
Fig. 6.
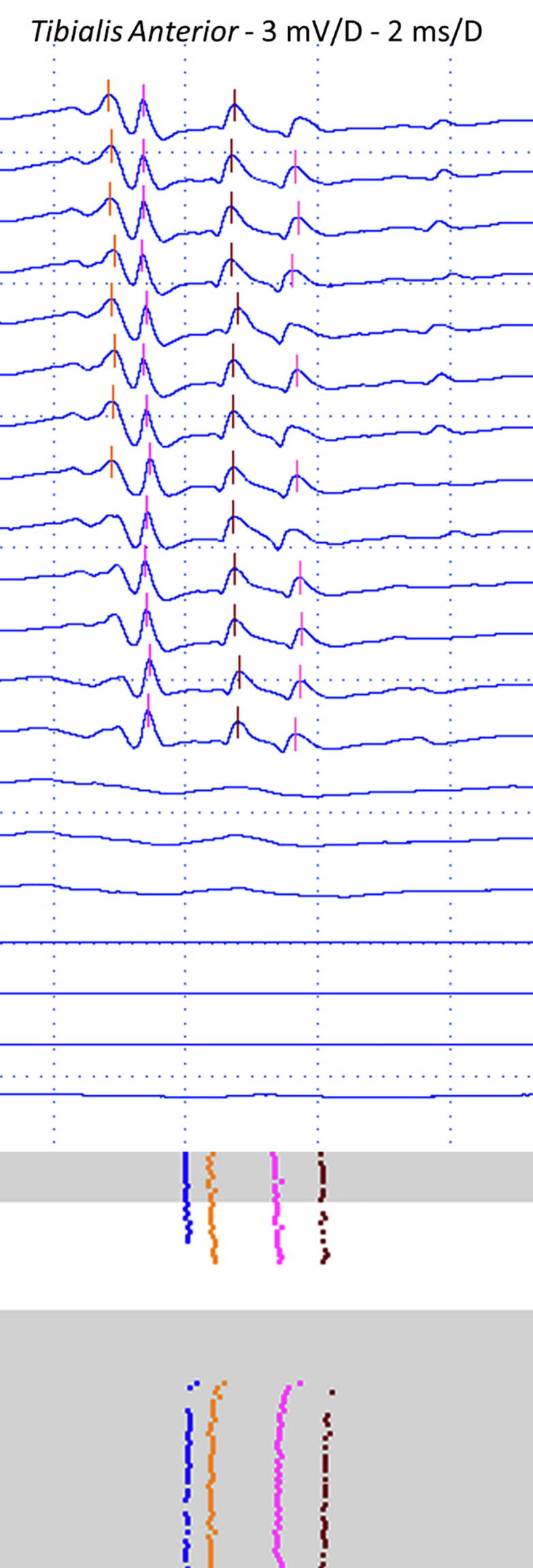
A typical neurogenic recording after collateral or distal sprouting increased the number of muscle fibers in the same motor unit. See that all four spikes appear and disappear together by increasing and decreasing the stimulus intensity.
4. Discussion
4.1. Reinnervation
Neuromuscular junctions regeneration occurs more rapidly when compared to the original development (Slater, 2008). One week after nerve injury (Wallerian degeneration), the presynaptic NMJ structures and nerve terminal axons disappear (Wu et al., 2014). Soon after denervation, ACh shifts throughout the membrane, and this extrajunctional ACh sensitivity is the most striking finding in denervation (Schiaffino et al., 1988, Wilson and Deschenes, 2005). The immature AChRs are upregulated within hours being a hallmark after denervation. In a few days, they spread throughout the muscle membrane allowing many motor axons growing (poly-innervation) to depolarize the muscle fiber membrane (Slater, 2008, Shear and Martyn, 2009, Kandel et al., 2013, Kaya et al., 2014, Wu et al., 2014, Slater, 2019). The immature NMJs structure is unstable and has a fast turnover of 1 day, characteristic of those containing a γ- rather than an ε-subunit (Wu et al., 2014). The quantal content is low, and the evoked release is sensitive to blockers of both Ca++ channels, P-type (normal), and L-type (immature). Due to the reduced acetylcholinesterase (AChE) activity, the ACh released is more effective. Few weeks after successful reinnervation, the quantal content increases to near normal (Slater, 2008). Fibrillation potentials found in EMG represent the APs of single muscle fibers that fire spontaneously in innervation absence (Willmott et al., 2012, Wu et al., 2014, Rubin, 2019). Fibrillation potentials probably are due to immature expression of AChRs along the muscle membrane and, are highly correlated to the immature atrophic fibers (Liu and Chakkalakal, 2018, Naddaf et al., 2018). Regenerating axons outgrow to the original synaptic site occurs if their endoneurial tubes are kept. When it successfully occurs, the average muscle fibers number in the motor unit is maintained (Stålberg et al., 2010, Liu and Chakkalakal, 2018). Reinnervation by sprouting occurs from the terminal axons or nodal region. Collateral neurites grow from the intramuscular intact axons. In this form of reinnervation, the average muscle fibers number in the motor unit increases considerably (Brown et al., 1981, Tam and Gordon, 2003, Stålberg et al., 2010). Axons reinnervate the original endplate sites even without muscle fibers due to signalization by the basal lamina components (Slater, 2008, Favero et al., 2010, Kandel et al., 2013). The guidance for axonal sprouting is the SCs and, its reexpression number peaks at one week after denervation (Brown et al., 1981, Slater, 2008, Wu et al., 2014, Lee et al., 2017). Due to muscle atrophy, the mean muscle fiber conduction velocity (MFCV) decreases from the mean normal 3.7–1.96 m/s (Dumitru and King, 1999, Cruz-Martínez and Arpa, 1999, Wu et al., 2014). As soon as 16 days after denervation, regenerating muscle fibers appear as small size diameter expressing neonatal myosin heavy chain (MHC) isoforms. After the muscle fiber reinnervation, repression of the immature AChRs in extrasynaptic regions and the neonatal MHC occur (Cerny and Bandman, 1987, Schiaffino et al., 1988). In neurogenic muscle biopsies, some small fibers or groups of small fibers, express neonatal MHC, probably due to lack of innervation, reflecting regeneration induced by denervation, and arrest in maturation (Dubowitz et al., 2013).
4.2. Normal adult function
As pointed out before, the motor axon loses its myelin sheath and branches to form the presynaptic nerve terminals covered and insulated by the SCs (Slater, 2008, Bittner and Martyn, 2019). The nerve terminal contains vesicles clustered in the active zones, to be released when the motor nerve AP elicit the opening of mature P-type voltage-gated calcium channels (Kaya et al., 2014, Bittner and Martyn, 2019, Juel, 2019). Exocytotic ACh release is mediated through calcium-dependent interactions between SNARE (soluble N-ethylmaleimide-sensitive factor attachment receptor) proteins, synaptobrevin on the vesicular membrane, and, syntaxin-1 and synaptic vesicle-associated protein-25 (SNAP-25) on the presynaptic nerve terminal membrane (Bittner and Martyn, 2019, Juel, 2019). The muscle surface has crowded areas of mature AChRs in the crest of the folds. Mature NaV1.4 channels are found mostly at the bottom of the synaptic clefts and all over the muscle membrane (Bittner and Martyn, 2019). A primary and secondary synaptic vesicles release pools are located near active release zone, and in the nerve terminal, respectively (Juel, 2019). Acetylcholine is synthesized from choline and acetyl Co-A via choline acetyltransferase. The amount of ACh released from one vesicle (a quantum) triggers an mEPP that discharges spontaneously at a frequency of about 1 Hz (Slater, 2008). Each vesicle contains about 5000–10,000 ACh molecules enough to bind around 500,000 AChRs at the post-synaptic membrane (Bittner and Martyn, 2019, Juel, 2019). Quanta of ACh represents the minimal number of synaptic vesicles (approximately 50) needed to trigger an EPP (Juel, 2019). As the quanta number released by each nerve impulse is about 200, the safety factor is four times (Bittner and Martyn, 2019, Juel, 2019). Mature AChRs are pentameric transmembrane proteins containing two α subunits and single β, δ, and ε subunits. Binding sites for ACh are located on the two α subunits, and AChR channel opening requires binding both subunits (Juel, 2019).
Compared to immature, mature AChRs have short open-time, higher conductance, are more stable, less susceptible to degradation, and longer half-life (10–14 days), increasing the neuromuscular transmission (Wilson and Deschenes, 2005). When EPP is elicited, it causes the voltage-gated NaV1.4 channels opening, thus initiating an AP in the muscle fiber (Slater, 2008, Juel, 2019). Acetylcholinesterase is anchored to the endplate basal lamina by the acetylcholinesterase-associated collagen (COLQ) and inactivates ACh. Approximately 50% of the released ACh is cleaved by the AChE. Some amount also diffuses out of the cleft before it reaches the AChRs. Those released after binding to the receptor are destroyed almost instantly (<1 ms) (Shear and Martyn, 2009, Bittner and Martyn, 2019, Juel, 2019).
4.3. Jitter parameters and denervation
The high jitter values in denervated muscles were caused by the immature terminal nerves/endplates (Stålberg and Thiele, 1972, Hakelius and Stålberg, 1974, Stålberg et al., 1975, Wiechers, 1990, Spaans et al., 2003, Stålberg et al., 2010, Pond et al., 2014). The presence of neurogenic blocking (“paired-blocking”), where two or more spikes block together even without high jitter, and the finding of activity-dependent conduction block, both signaling a neurogenic transmission failure not related to NMJ dysfunction, are the supporting bases to this assumption (Stålberg and Thiele, 1972, Wiechers, 1990, Noto et al., 2011, Spaans et al., 2003).
The abnormal jitter here was equally found by the mean jitter and the percentage of the abnormal individual jitter criteria, 87.5% for voluntary, and 81.25% for electrical activation. The relationship between the mean jitter obtained from voluntary to electrical activation expected to be 71% (mean voluntary jitter/√2 = mean expected stimulated jitter) was found 75.9%. In another report using CNE, the relationship reported was 79% for ED muscle. In both cases, the relationship was pretty close to our findings (Kouyoumdjian and Stålberg, 2008).
Fibrillation potentials correlate to the NMJ regeneration stage when extrajunctional acetylcholine sensitivity occurs. The overspread of immature AChRs on the muscle membrane is a cornerstone for denervated muscles (Schiaffino et al., 1988, Wilson and Deschenes, 2005, Kandel et al., 2013). The muscle fibers become atrophic and reexpress neonatal MHC and, the mEPPs are “cropping up everywhere,” waiting for innervation. After successful distal sprouting reinnervation, FPs disappear, and the MUAP morphology reshapes to the higher amplitude/long duration.
In this study, all muscles presenting FPs had abnormal jitter and impulse blocking found in 92.3% for voluntary and 53.4% for electrical activation. These findings were expected due to an NMJ machinery immaturity signaling by the FPs. On the other hand, muscles without FPs have 78.9%/69.4% abnormal mean jitter by voluntary and electrical activation. We found a significant difference between mean jitter from muscle with and without FPs; however, no correlation was found between mean jitter and the subjectively ranking of FPs activity. Finally, there was no correlation between abnormal jitter and MUAPs' amplitude.
Although the abnormal jitter values were less in muscles without FPs, we argued for its appearance in chronic reinnervated muscles. The maintaining of the muscle activity is essential to keep the AChRs' maturation and stability, to repress the expression of immature NaV1.5 channels, to limit axonal sprouting, and to repress the perisynaptic SCs processes to bridge innervated and denervated endplates (Tam and Gordon, 2003, Wilson and Deschenes, 2005, Slater, 2008, Kandel et al., 2013). On the other hand, muscle denervation and immobilization or botulinum toxin/tetrodotoxin blocking induces the formation of sprouts of the motor axon and establishes extrasynaptic contact all over the muscle membrane (Tam and Gordon, 2003, Slater, 2017). When successfully muscle reinnervation occurs, the return to almost regular activity could inhibit the reinnervation process in new denervation muscle fibers. Also, it could maintain the atrophic muscle fibers expressing neonatal MHC.
As a consequence of small diameter and immaturity, the MFCV is reduced, adding jitter, and, as a consequence of muscle activity, extrajunctional acetylcholine sensitivity and FPs do not occur. In essence, we could speculate that a mixture of active mature reinnervated muscle fibers may repress the innervation of the atrophic neonatal MHC fibers.
This study's limitation is the absence of reported CNE jitter reference for some muscles barely studied for the neuromuscular transmission suspicion disorders, such as Vastus Lateralis, Rectus Femoris, Tibialis Anterior, Medial Gastrocnemius, and Triceps muscles. We consider it not a significant problem since the parameter variation for the reference jitter values obtained through CNE is much less than for SFE.
5. Conclusion
The jitter was abnormal in all muscles presenting FPs and, in about 75% of muscles presenting neurogenic MUAPs without FPs. Neuromuscular junctions regeneration resembles embryonic development. The muscles with FPs correlated to the over-expressed immature AChRs spread throughout the muscle membrane. The already reinnervated muscles' voluntary activity could repress other NMJs assemble, and the persistence of atrophic fibers expressing neonatal MHC may add jitter.
Funding
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP), Brazil (grant number 2017/13262-4).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Bittner E.A., Martyn J.A.J. Neuromuscular physiology and pharmacology. In: Hemmings H.C., Egan T.D., editors. Pharmacology and Physiology for Anesthesia. 2nd ed. Elsevier Sanders; Philadelphia: 2019. pp. 412–427. [Google Scholar]
- Brown M.C., Holland R.L., Hopkins W.G. Motor nerve sprouting. Annu. Rev. Neurosci. 1981;4:17–42. doi: 10.1146/annurev.ne.04.030181.000313. [DOI] [PubMed] [Google Scholar]
- Cerny L.C., Bandman E. Expression of myosin heavy chain isoforms in regenerating myotubes of innervated and denervated chicken pectoral muscle. Dev. Biol. 1987;119:350–362. doi: 10.1016/0012-1606(87)90040-6. [DOI] [PubMed] [Google Scholar]
- Cruz-Martínez A., Arpa J. Muscle fiber conduction velocity in situ (MFCV) in denervation, reinnervation and disuse atrophy. Acta Neurol. Scand. 1999;100:337–340. doi: 10.1111/j.1600-0404.1999.tb00407.x. [DOI] [PubMed] [Google Scholar]
- Dubowitz V., Sewry C.A., Oldfors A. 4th ed. Elsevier Saunders; Amsterdam: 2013. Muscle Biopsy: A Practical Approach. [Google Scholar]
- Dumitru D., King J.C. Motor unit action potential duration and muscle length. Muscle Nerve. 1999;22:1188–1195. doi: 10.1002/(sici)1097-4598(199909)22:9<1188::aid-mus4>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Favero M., Buffelli M., Cangiano A., Busetto G. The timing of impulse activity shapes the process of synaptic competition. Neuroscience. 2010;167:343–353. doi: 10.1016/j.neuroscience.2010.01.055. [DOI] [PubMed] [Google Scholar]
- Hakelius L., Stålberg E. Electromyography studies of free autogenous muscle transplants in man. Scand. J. Plast. Reconstr. Surg. 1974;8:211–219. doi: 10.3109/02844317409084397. [DOI] [PubMed] [Google Scholar]
- Jansen J.K.S., Fladby T. The perinatal reorganization of the innvervation of skeletal muscle in mammals. Prog. Neurobiol. 1990;34:39–90. doi: 10.1016/0301-0082(90)90025-c. [DOI] [PubMed] [Google Scholar]
- Juel V.C. Clinical neurophysiology of neuromuscular junction disease. In: Levin K.H., Chauvel P., editors. Clinical Neurophysiology: Basis and Technical Aspects. Handbook of Clinical Neurology. 3rd series. Elsevier BV; Amsterdam: 2019. pp. 291–303. [DOI] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A., Hudspeth A.J. 5th ed. McGrawHill Medical; New York: 2013. Principles of Neural Science. [Google Scholar]
- Kaya C., Ustun Y.B., Atalay Y.O. Physiology of the neuromuscular junction and related disorders. J. Exp. Clin. Med. 2014;31:149–153. [Google Scholar]
- Kouyoumdjian J.A., Stålberg E.V. Concentric needle single fiber electromyography: comparative jitter on voluntary-activated and stimulated Extensor Digitorum Communis. Clin. Neurophysiol. 2008;119:1614–1618. doi: 10.1016/j.clinph.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Kramer C., Zoubaa S., Kretschmer A., Jordan D., Blobner M., Fink H. Denervation versus pre- and postsynaptic muscle immobilization: Effects on acetylcholine- and muscle-specific tyrosine kinase receptors. Muscle Nerve. 2017;55:101–108. doi: 10.1002/mus.25159. [DOI] [PubMed] [Google Scholar]
- Lee Y.I., Thompson W.J., Harlow M.L. Schwann cells participate in synapse elimination at the developing neuromuscular junction. Curr. Opin. Neurobiol. 2017;47:176–181. doi: 10.1016/j.conb.2017.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legay C., Lin M. Moving forward with the neuromuscular junction. J. Neurochem. 2017;142:59–63. doi: 10.1111/jnc.14028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman J.W., Sanes J.R. Watching the neuromuscular junction. J. Neurocytol. 2003;32:767–775. doi: 10.1023/B:NEUR.0000020622.58471.37. [DOI] [PubMed] [Google Scholar]
- Liu W., Chakkalakal J.V. The composition, development, and regeneration of neuromuscular junctions. Curr. Top. Dev. Biol. 2018;126:99–124. doi: 10.1016/bs.ctdb.2017.08.005. [DOI] [PubMed] [Google Scholar]
- Naddaf E., Milone M., Mauermann M.L., Mandrekar J., Litchy W.J. Muscle biopsy and electromyography correlation. Front. Neurol. 2018;9:83. doi: 10.3389/fneur.2018.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noto Y., Misawa S., Kanai K., Sato Y., Shibuya K., Isose S. Activity-dependent changes in impulse conduction of single human motor axons: A stimulated single fiber electromyography study. Clin. Neurophysiol. 2011;122:2512–2517. doi: 10.1016/j.clinph.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Pond A., Marcante A., Zanato R., Martino L., Stramare R., Vindigni V. History, mechanisms and clinical value of fibrillation analyses in muscle denervation and reinnervation by single fiber electromyography and dynamic echomyography. Eur J Transl Myol. 2014;24:3297. doi: 10.4081/ejtm.2014.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin D.I. Normal and abnormal spontaneous activity. In: Levin K.H., Chauvel P., editors. Clinical Neurophysiology: Basis and Technical Aspects. Handbook of Clinical Neurology. 3rd series. Elsevier BV; Amsterdam: 2019. pp. 257–279. [Google Scholar]
- Sanders D.B., Arimura K., Cui L., Ertaş M., Farrugia M.E., Gilchrist J., Kouyoumdjian J.A., Padua L., Pitt M., Stålberg E. Guidelines for single fiber EMG. Clin. Neurophysiol. 2019;130:1417–1439. doi: 10.1016/j.clinph.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Gorza L., Pitton G., Saggin L., Ausoni S., Sartore S. Embryonic and neonatal myosin heavy chain in denervated and paralyzed rat skeletal muscle. Dev. Biol. 1988;127:1–11. doi: 10.1016/0012-1606(88)90183-2. [DOI] [PubMed] [Google Scholar]
- Shear T.D., Martyn J.A.J. Physiology and biology of neuromuscular transmission in health and disease. J. Crit. Care. 2009;24:5–10. doi: 10.1016/j.jcrc.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Slater C.R. Reliability of neuromuscular transmission and how it is maintained. In: Engel A.G., editor. Neuromuscular junction disorders. Handbook of Clinical Neurology. 3rd series. Elsevier BV; Amsterdam, The Netherlands: 2008. [DOI] [PubMed] [Google Scholar]
- Slater C.R. The structure of human neuromuscular junctions: some unanswered molecular questions. Int. J. Mol. Sci. 2017;18:2183. doi: 10.3390/ijms18102183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slater C.R. ‘Fragmentation” of NMJs: a sign of degeneration or regeneration? A long journey with many junctions. Neuroscience. 2019;23:1. doi: 10.1016/j.neuroscience.2019.05.017. S0306-4522(19)30333-1. [DOI] [PubMed] [Google Scholar]
- Spaans F., Vredeveld J.W., Morré H.H., Jacobs B.C., De Baets M.H. Dysfunction at the motor end-plate and axon membrane in Guillain-Barré syndrome: a single-fiber EMG study. Muscle Nerve. 2003;27:426–434. doi: 10.1002/mus.10334. [DOI] [PubMed] [Google Scholar]
- Stålberg E., Thiele B. Transmission block in terminal nerve twigs: a single fibre electromyographic finding in man. J. Neurol. Neurosurg. Psychiatry. 1972;35:52–59. doi: 10.1136/jnnp.35.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stålberg E., Schwartz M.S., Trontelj J.V. Single fibre electromyography in various processes affecting the anterior horn cell. J. Neurol. Sci. 1975;24:403–415. doi: 10.1016/0022-510x(75)90166-5. [DOI] [PubMed] [Google Scholar]
- Stålberg E.V., Trontelj J.V., Sanders D.B. 3rd ed. Edshagen Publishing House Fiskebäckskil; Sweden: 2010. Single Fiber Electromyography. [Google Scholar]
- Stålberg E., Sanders D.B., Ali S., Cooray G., Leonardis L., Löseth S. Reference values for jitter recorded by concentric needle electrodes in healthy controls: A multicenter study. Muscle Nerve. 2016;53:351–362. doi: 10.1002/mus.24750. [DOI] [PubMed] [Google Scholar]
- Stålberg E., Sanders D.B., Kouyoumdjian J.A. Pitfalls and errors in measuring jitter. Clin. Neurophysiol. 2017;128:2233–2241. doi: 10.1016/j.clinph.2017.09.001. [DOI] [PubMed] [Google Scholar]
- Stålberg E., Kouyoumdjian J.A., Paiva G.P., Yanaze L.L., Sanders D.B. Problems in comparing jitter values obtained with voluntary activation and electrical stimulation. J Neuromuscul Dis. 2018;5:225–230. doi: 10.3233/JND-170289. [DOI] [PubMed] [Google Scholar]
- Tam S.L., Gordon T. Mechanisms controlling axonal sprouting at the neuromuscular junction. J. Neurocytol. 2003;32:961–974. doi: 10.1023/B:NEUR.0000020635.41233.0f. [DOI] [PubMed] [Google Scholar]
- Trontelj J.V., Mihelin M., Fernandez J.M., Stålberg E. Axonal stimulation for end-plate jitter studies. J. Neurol. Neurosurg. Psychiatry. 1986;49:677–685. doi: 10.1136/jnnp.49.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci B., Santosa K.B., Keane A.M., Jablonka-Shariff A., Yi L.C., Yan Y. What is normal? Neuromuscular junction reinnervation after nerve injury. Muscle Nerve. 2019;60:604–612. doi: 10.1002/mus.26654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiechers D. Single fiber EMG evaluation in denervation and reinnervation. Muscle Nerve. 1990;13:829–832. doi: 10.1002/mus.880130909. [DOI] [PubMed] [Google Scholar]
- Willmott A.D., White C., Dukelow S.P. Fibrillation potential onset in peripheral nerve injury. Muscle Nerve. 2012;46:332–340. doi: 10.1002/mus.23310. [DOI] [PubMed] [Google Scholar]
- Wilson M.H., Deschenes M.R. The neuromuscular junction: anatomical features and adaptations to various forms of increased, or decreaded neuromuscular activity. Int. J. Neurosci. 2005;115:803–828. doi: 10.1080/00207450590882172. [DOI] [PubMed] [Google Scholar]
- Wu P., Chawla A., Spinner R.J., Yu C., Yaszemski M.J., Windebank A.J. Key changes in denervated muscles and their impact on regeneration and reinnervation. Neural Regen. Res. 2014;15:1796–1809. doi: 10.4103/1673-5374.143424. [DOI] [PMC free article] [PubMed] [Google Scholar]