Summary
RAS proteins function as highly regulated molecular switches that control cellular growth. In addition to regulatory proteins, RAS undergoes a number of posttranslational modifications (PTMs) that regulate its activity. Lysine 104, a hot spot for multiple PTMs, is a highly conserved residue that forms key interactions that stabilize the RAS helix-2(H2)/helix-3(H3) interface. Mutation at 104 attenuates interaction with guanine nucleotide exchange factors (GEFs), whereas ubiquitination at lysine 104 retains GEF regulation. To elucidate how ubiquitination modulates RAS function, we generated monoubiquitinated KRAS at 104 using chemical biology approaches and conducted biochemical, NMR, and computational analyses. We find that ubiquitination promotes a new dynamic interaction network and alters RAS conformational dynamics to retain GEF function. These findings reveal a mechanism by which ubiquitination can regulate protein function.
Subject Areas: Structural Biology, Biochemistry, Biocomputational Method
Graphical Abstract

Highlights
-
•
Ubiquitination at K104 modulates the conformation and dynamics of Switch II in KRAS
-
•
RAS K104 ubiquitination modulates GEF interactions
-
•
K104 is a unique site modulating KRAS structure and function
Structural Biology, Biochemistry; Biocomputational Method
Introduction
KRAS is the most frequently mutated oncogene in human cancer, with mutations most prevalent in lung, colorectal, and pancreatic cancers (Waters and Der, 2018). Although inhibitors that target a cysteine 12 oncogenic mutant of KRAS are now in phase 1 clinical trials, inhibitors that target the most prevalent KRAS mutations have yet to be identified (Hansen et al., 2018; Janes et al., 2018). As such, identification of novel regulatory mechanisms may facilitate development of anti-KRAS drugs and treatment options (Cox et al., 2014).
RAS proteins function as binary on-off molecular switches that cycle between inactive GDP-bound and active GTP-bound states and transduce signals through multiple pathways to regulate cellular growth. Nucleotide cycling requires the action of regulatory proteins. In the unstimulated cell, RAS proteins are populated in their inactive GDP-bound state. However, in response to growth-stimulatory signals, guanine nucleotide exchange factors (GEFs) co-localize and activate RAS by facilitating exchange of GDP for GTP. Inactivation is achieved through GTPase-activating proteins (GAPs) that bind to GTP-bound RAS and promote GTP hydrolysis (Geyer and Wittinghofer, 1997; Vigil et al., 2010). RAS contains two dynamic regions termed switch I (SWI; residues 30–37) and switch II (SWII; residues 60–76 with 66–74 corresponding to H2). These switch regions populate distinct conformations when the protein is bound to GDP versus GTP. Effectors and GAP proteins recognize specific conformations of the switch regions and bind with preferential affinity to the active GTP-bound state. Activated GTP-bound RAS can interact with multiple effectors (e.g., RAF kinase, RAL exchange factors, Phosphoinositol 3-Kinase [PI3K], the RAC-selective GEF TIAM1, phospholipase C, NORE1) to promote downstream signaling pathways that control cell growth, differentiation, and apoptosis (Stephen et al., 2014).
Post-translational modifications (PTMs) within the hypervariable carboxyl-terminal domain of RAS are critical for membrane localization and RAS activation (Ahearn et al., 2011). However, less well characterized are PTMs in the core G-domain. These include distinct lysine modifications, such as acetylation, ubiquitination, and most recently methylation (Ahearn et al., 2011; Yoshino et al., 2019). Notably, KRAS monoubiquitination at lysine 147 up-regulates RAS activity, signaling, and tumorigenesis (Baker et al., 2013a; Sasaki et al., 2011). In contrast, monoubiquitination at a minor site, lysine 104 (Sasaki et al., 2011), does not alter the intrinsic biochemical properties or regulation by GEFs and GAPs (Baker et al., 2013b). Lysine 104 is proximal to the GEF binding surface and can also undergo acetylation, yet the consequence of this modification on RAS activity is controversial. Lysine 104 acetylation (Ac-K104) was initially reported to disrupt activation by GEFs and result in down-regulation of KRAS G12V-driven effector signaling and growth transformation in NIH3T3cells (Yang et al., 2012). These studies were conducted in the context of a KRAS K104Q mutation, which was introduced to mimic constitutive acetylation. However, KRAS K104Q was later shown to have a small yet compensatory GEF/GAP defect (Yin et al., 2017), consistent with findings that KRAS G12V/K104Q retains steady-state GTP-bound levels and the ability of the oncogenic KRAS G12V mutant to cause morphologic transformation of NIH 3T3 mouse fibroblasts (Yin et al., 2017). The differential downstream signaling capabilities of KRAS G12V, K104Q in NIH3T3cells by two distinct groups suggests context dependence associated with this KRAS variant. Notably, KRAS acetylated at lysine 104 in vitro using genetic expansion approaches was found to retain GEF regulation (Knyphausen et al., 2016). Hence in this context, K104Q does not serve as an acetylation mimetic.
Lysine 104 appears to be a hot spot for RAS PTMs. This residue plays a key role in regulating SWII conformation, as it forms stabilizing contacts with R73 and G75 in H2. We have previously shown that the K104Q mutation disrupts these electrostatic interactions and causes partial unfolding at this helical interface, resulting in a partial defect in GEF and GAP interactions. To delineate how GEF interactions are retained upon disruption of this electrostatic interaction by ubiquitination, we employed structural, computational, and biochemical approaches. Using real-time NMR, we find the ubiquitination at K104 retains GEF-mediated GDP-GTP exchange, in contrast to mutations at this position (Yin et al., 2017). However, our NMR data indicate that ubiquitination at lysine 104 causes partial disruption of the H2/H3 interface, similar to the K104Q mutation. We next conducted unbiased Rosetta modeling and observed that ubiquitin samples multiple orientations and forms transient contacts with the end of H2 in RAS. We further assessed the conformation and dynamic properties of monoubiquitinated RAS at 104 (mUbKRAS104) using NMR and MD simulations and observe that ubiquitin transiently interacts with H2 and loop 7 to promote formation of a new contact between D69 and R102. This in turn, reduces RAS dynamics, stabilizes the H2/H3 interface, and facilitates GEF binding. Consistent with these predictions, addition of trimethylamine-N-oxide (TMAO) as a cosolute (Su et al., 2017), stabilizes this interface and restores GEF-mediated nucleotide exchange for lysine 104 mutations. Taken together, our findings uncover a novel mechanism of protein regulation by monoubiquitination whereby ubiquitin conjugation dynamically modulates protein recognition.
Results
Monoubiquitination of KRAS at 104 Retains Regulatory and Effector Interactions
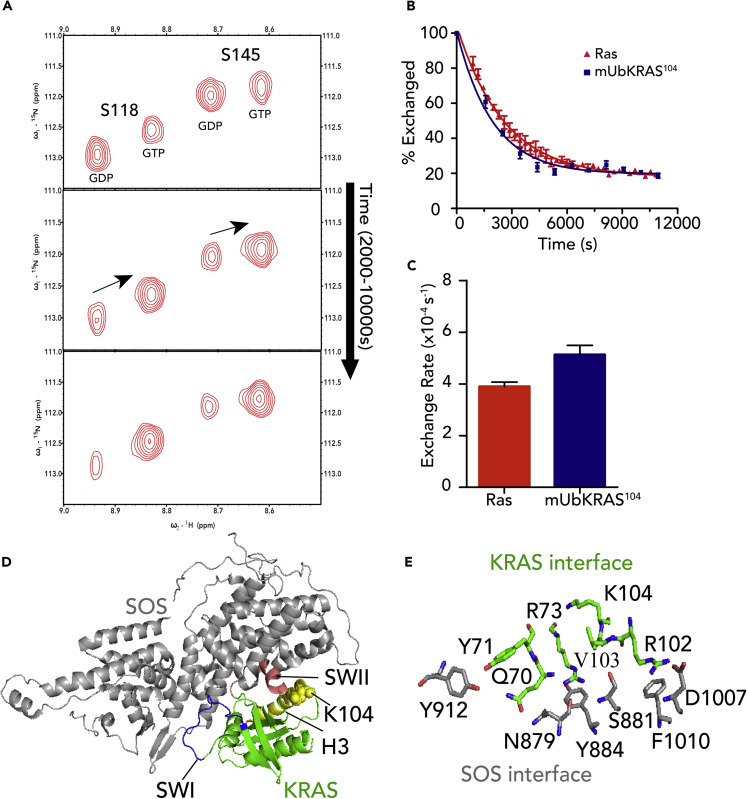
The side chain of lysine 104 in KRAS-GDP (PDB 4LPK) forms key interactions with the backbone carbonyl group of R73 and G75 in H2. This interface lies in close proximity to residues R102 and V103, which make direct contacts with the GEF, Son of Sevenless (SOS) (PDB: 1NVW) (Margarit et al., 2003) (Figures 1D and 1E), which plays a key role in RAS activation. Lysine 104 in RAS contacts SWII. These contacts appear key for stabilizing the H2/H3 interface as mutations (K104A, K104Q, K104R, K104C) at this position perturb SOS regulation (Yin et al., 2017) (Table S1, adapted from Yin et al., 2017). In contrast to mutations at 104, mUbKRAS104, generated by conjugating ubiquitin to a RAS K104C variant using disulfide chemistry, retains both SOS- and GAP-mediated regulation (Baker et al., 2013b). The KRAS K104C mutant mUbKRAS104 retains thermal stability and the global fold of WT RAS, whereas the K104C mutant shows defects in GEF regulation due to loss of contacts with Switch II (Baker et al., 2013b). To better characterize SOS-mediated guanine nucleotide exchange of mUbKRAS104, we applied real-time NMR. This approach has been used to assay the kinetics of guanine nucleotide exchange by quantitatively monitoring nucleotide-dependent changes in peak intensity and is not complicated by alterations in exchange due to introduction of guanine nucleotide fluorophores (Smith et al., 2015). We then incubated unmodified and monoubiquitinated 15N-enriched KRAS-GDP with native GTP and the SOS catalytic domain (SOScat) and collected a series of 1H-15N 2D NMR heteronuclear single quantum coherence (HSQC) spectra as a function of time (Figure 1). Two-dimensional 1H-15N HSQC spectra allow for the detection of protons directly bonded to a 15N nucleus, including both backbone and side-chain resonances. Because an NH resonance can be detected for every residue with the exception of proline, the spectrum contains a “fingerprint” of the protein backbone. Upon the addition of GTP and SOS, new peaks corresponding to the GTP-bound state appear. The time course of GDP- and GTP-dependent resonance changes can be fit to obtain nucleotide exchange rate. Depicted in Figure 1B are NH resonances associated with S118 and S145. These peaks are well resolved and sensitive to GDP-GTP exchange. The GDP/GTP ratio was plotted as a function of time (Figure 1A) and fitted by exponential decay to determine the exchange rate (Figure 1B). Similar to our previous observation using fluorescence-based approaches, monoubiquitination at position 104 retains SOS-mediated GDP-GTP exchange (Figure 1C).
Figure 1.
mUbKRAS104 Retains Sensitivity to SOS-Mediated Guanine Nucleotide Exchange
(A) Nucleotide exchange rate of WT and mUbKRAS104 was determined using real-time NMR. 1H-15N HSQC of 15N-enriched RAS in the presence of the GEF catalytic domain of SOS (SOScat) was used to monitor amide peaks associated with either the GTP- or GDP-bound state of KRAS. Zoom of HSQC highlighting change over time of S118 and S145 NH resonances resulting from exchange of GDP for GTP. WT KRAS and mUbKRAS104 bound to GDP (150 μM) incubated with excess GTP in the presence of SOScat at a 1,700:1 ratio of RAS to SOScat. SOS-mediated exchange rates for WT and mUbKRAS104 were measured by real-time 1H-15N HSQC NMR over a period of 3 h. Experiments were conducted on a Bruker Avance 850 MHz NMR spectrometer at 20°C in NMR buffer (20 mM Tris-Maleate, 50 mM NaCl, 5 mM MgCl2 pH 7.0).
(B) The percent exchange of GDP for GTP was determined by GDP intensity/(GDP + GTP intensity) for each spectra with respect to time of acquisition. Results were fitted to a single-phase dissociation decay. The errors were determined by SE of the duplicate.
(C) Quantification of relative nucleotide exchange rates. mUbKRAS104 shows slightly higher rates of nucleotide exchange relative to WT KRAS. Results are reported as rates of exchange ±SE (n = 2).
(D) Ribbon diagram highlighting the RAS-SOS interface (PDB:1NVW). KRAS is shown in green, SWI in blue, SWII in red, and the K104 side chain in yellow.
(E) Zoom of the RAS-SOS interface highlighting residues proximal to K104 that contribute to complex formation.
To assess whether binding of RAS to a key downstream effector was altered by KRAS monoubiquitination at 104, we compared the relative binding affinity of GMPPNP-bound mUbKRAS104 and WT RAS with the RAF kinase. Using a fluorescence-based assay monitoring Mant-GMPPNP dissociation as a function of CRAF RAS-binding domain (RBD) concentration, we observed that mUbKRAS104 reduces affinity for the CRAF-RBD by ∼3-fold relative to WT KRAS (Figure S1).
Monoubiquitination of KRAS-GDP at 104 Causes Structural Perturbations in Helix 2 and Helix 3
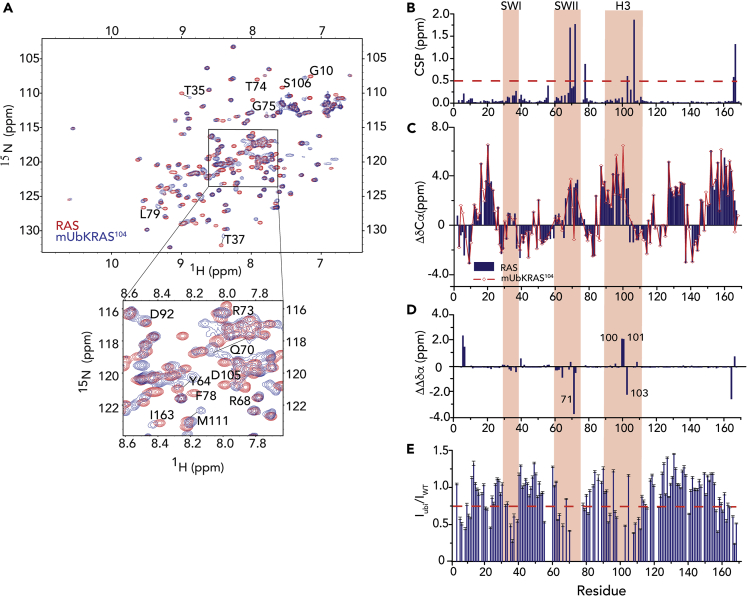
To investigate how mUbKRAS104 retains SOS regulation despite loss of key contacts with SWII, we conducted NMR studies to characterize structural alterations resulting from mUbKRAS104. We first assigned 141 of 167 non-proline backbone NH and Cα resonances of mUbKRAS104 bound to Mg2+ and GDP by acquiring 3D HNCA triple resonance data on 13C,15N-enriched protein, using known WT KRAS assignments as a starting point (Yin et al., 2017). A 2D 1H-15N HSQC spectral overlay of mUbKRAS104 and WT KRAS bound to GDP is shown in Figure 2A. Inspection of chemical shift differences between KRAS WT and mUbKRAS104 bound to GDP show that ∼40% of the backbone NH peaks undergo chemical shift changes upon ubiquitin modification. Using chemical shift perturbation (CSP) analyses (Figure 2B), the largest CSPs (>0.5 ppm) occur in three regions of the protein: residues proximal to the site of the ubiquitination (positions 103 and 107), residues within SWII, and residues at the C terminus. These findings are consistent with perturbation of contacts between K104, R73, and G75 at the end of SWII. In contrast, a few residues in SWI show mild CSP (35 and 36).
Figure 2.
mUbKRAS104 Alters the Local Conformation and Dynamics of H2 (SWII) and H3
(A) 1H-15N 2D NMR HSQC overlay comparing 15N-enriched mUbKRAS104 (blue) to WT KRAS (red).
(B) NMR analyses of GDP-bound mUbKRAS104 relative to WT KRAS shows large chemical shift perturbation (CSPs) in SWII and neighboring residues in H3 but minor changes in β1 within SW1. Chemical shift perturbations were calculated based on weighted average chemical shift (square root of ((Δσ 1H)2 + (Δσ 15N)2/25)) of WT and mUbKRAS104 NH peaks in 1H-15N 2D HSQC NMR spectra of 15N-enriched KRAS-GDP.
(C) Differences in secondary structure between GDP-bound WT KRAS and mUbKRAS104 determined from Cα and Cβ chemical shift indexing indicate that mUbKRAS104 perturbs the local conformation surrounding the modification site at 104 in H3 and the H2 (residues 71–74) within SWII.
(D) Difference in chemical shift indexing between GDP-bound mUbKRAS104 and WT KRAS highlighting perturbations in secondary structure in H3 and the H2 (residues 71–74) in SWII resulting from KRAS monoubiquitination at K104.
(E) Changes in backbone dynamics obtained from 2D 1H-15N HSQC NH peak intensity plot of mUbKRAS104 compared with WT KRAS. Plot shows pattern of broadening in β1, SWI and II, as well as residues neighboring the modification site at 104. Residues within SWII (residues 73–76) experience significant broadening and were undetectable in the mUbKRAS104 spectrum. Spectra were recorded using Bruker Avance III 700 and 850 spectrometers at 25°C on a 0.1 mM 15N-enriched samples of GDP-bound KRAS WT and mUbKRAS104. The errors were determined by the signal-to-noise ratio of the peak.
As carbon Cα chemical shifts can be used to measure secondary structure propensity (Wishart and Sykes, 1994), we employed chemical shift indexing (CSI) to compare secondary structural differences between mUbKRAS104 and WT KRAS (Figure 2C). We find that, overall, the secondary structure of mUbKRAS104 is similar to WT KRAS with the exception of H2 and H3. We calculated the CSI difference between mUbKRAS104 and WT KRAS. Results shown in Figure 2D indicate a loss of secondary structure for residues 71–75 at the C-terminal end of H2 and alteration of helical content for residues 100–103 in H3 near the mutation site. These secondary structure changes correlate with the large CSP observed for these residues (Figure 2B). Small distortions in secondary structure were also observed in the C-terminal residues (166–167). As the NH peak intensity in the 1H-15N HSQC spectrum is dependent on the relaxation properties of amides, changes in the peak intensity can be used to assess the changes in local motion. Peak broadening was observed in SWII as well as in the region neighboring the site of modification at 104, including the loop 7 between H3 and β5 (Figure 2E). These observations indicate that ubiquitination of KRAS at 104 not only perturbs H2/H3 secondary structure but also modulates RAS dynamics. Peak broadening was also observed for residues V8 in β1 and T35 and I36 in SWI. The perturbed regions revealed by NMR are highlighted on the 3D structure of WT KRAS bound to GDP [PDB: 4LPK] (Figure 3).
Figure 3.
Ribbon Diagram Highlighting NMR Spectral Changes Resulting from mUbKRAS104
NMR spectral perturbations (CSP, broadening) resulting from mUbKRAS104 mapped on the 3D structure of KRAS-GDP (PDB code 4LPK). Resonances associated with residues that display chemical shift changes (CSP >0.5 ppm) are shown in orange, whereas residues that undergo broadening (ImUbKRAS104/IWT < 0.75) are shown in blue. Resonances associated with residues that under both broadening and shifts are shown in red. Unassigned peaks are colored in gray.
Ubiquitin Forms Dynamic Contacts with Switch II and Stabilizes the RAS-SOS Interface
Our NMR analyses indicate that GDP-bound mUbKRAS104 perturbs the conformation and dynamics of the RAS H2/H3 interface. Hence, it is unclear how SOS sensitivity is retained despite the observed structural and dynamic perturbations at this key SOS binding interface. To investigate the underlying molecular mechanism, we conducted both Rosetta modeling and MD simulations. Previously, we employed unbiased Rosetta modeling to investigate whether native versus chemical ligation alters conformational sampling of ubiquitin when ligated to KRAS (Saha et al., 2011). We found that chemical ligation of RAS at 147 mimics conformational sampling of the native linkage, as well as the GAP deficiency associated with occlusion effects by ubiquitin modification at K147, consistent with experimental data (Baker et al., 2013a). Using a similar approach, we generated 5,000 models of mUbKRAS104. Notably, the lowest 0.1% of scoring models indicates that ubiquitin does not adopt a single conformation when ligated to KRAS at 104 (Figure 4A). These models show little restriction of ubiquitin orientation, with the low scoring models occupying a disc-like shape above KRAS with a diameter of roughly ∼65Å (Figure 4A; right). Given the ambiguities associated with precision of the Rosetta score function when modeling conjugated ubiquitin, we selected lowest 10% of scoring structures as an approximation for the conformational ensemble of mUbKRAS104. We then calculated the contact frequency for each residue of RAS that comes in proximity to ubiquitin (Figure 4B). These analyses indicate that the primary regions of KRAS that ubiquitin contacts are at the end of H2 in SWII, loop 7 between H3 and βI and C terminus. These contacts are consistent with NMR CSPs and peak broadening reported in Figures 2B and 2E, respectively. Residues with significant CSP and broadening at the ubiquitin-KRAS interface of the Rosetta models are highlighted in Figure 4A. We also performed the Rosetta modeling of mUbKRAS104 bound to the SOScat to investigate whether ubiquitin occludes the RAS binding site. We observe that ubiquitin freely samples multiple orientations in the space similar to the mUbKRAS104 alone and the majority ubiquitin conformers do not share contacts with SOS (Figure S2). Only a minor subset of ubiquitin conformers contact regions (884–909) and (1,019 and 1,021) of SOS, which are not involved in the RAS-SOS interactions. Additionally, those regions of SOS are highly charged and do not possess the typical hydrophobic character that ubiquitin typically needs to interact with the canonical binding site on ubiquitin (Harrison et al., 2016). Collectively, our findings indicate that ubiquitin is capable of making direct contacts with the KRAS at the H2/H3 SOS interface but does not occlude RAS-SOS interactions.
Figure 4.
Unbiased Rosetta Modeling of mUbKRAS104
Ubiquitin, when ligated to KRAS at 104 in H3, samples multiple orientations and forms transient contacts with RAS at the end of H2 in SWII and loop connecting H3 and H4.
(A) Top 20 Rosetta models of mUbKRAS104. mUbKRAS104 residues displaying CSP greater than 0.5 ppm or peak broadening greater than 50% have Cα carbons shown as a sphere. Purple residues show ubiquitin contacts, whereas gray residues do not contact ubiquitin in the ensemble. Left: side view of the mUbKRAS104 conformational ensemble; right: view from the top. Ubiquitin occupies roughly 65Å area when conjugated to K104 and with little conformational restriction. SWI residues are colored in green and residues within SW II (residues 61–74) are colored in gold.
(B) Frequency plot of 580 top-scoring Rosetta models that have a KRAS residue within 8Å of ubiquitin by Rosetta Energy Units (~%10 of models). The ubiquitin contact surfaces on RAS mapped by the contact frequency is consistent with peak broadening in the 1H-15N HSQC NMR spectrum of mUbKRAS104-GDP.
Since the ubiquitin modeling used is primarily a rigid body, this approach is unable to provide information about the dynamics of the protein. Accordingly, we used molecular dynamics simulations to characterize dynamic changes resulting from ubiquitin ligation at position 104. We performed four 1-μs all-atom MD simulations, two on GDP-bound mUbKRAS104 and two on WT KRAS (for reference). As each simulation of mUbKRAS104 started from two different initial conformations (see Transparent Methods), we checked whether the two simulations converged to a single ensemble using the time evolution of the radius of gyration, the relative orientation of KRAS and ubiquitin, and contacts between KRAS and ubiquitin. As shown in Figure S3, the two simulations do not converge to a single conformation. Rather, they evolved into two distinct ensembles characterized by distinct relative orientations and KRAS-ubiquitin contact profiles. Although there were several attempts at convergence, both the angle and radius gyration plateaued at different values, with one of the simulations sampling a more compact conformation in which ubiquitin forms a larger interface with KRAS. This is illustrated more clearly in the normalized distribution of the angle and radius of gyration using the data in the better-equilibrated last 700 ns of each trajectory (Figure S4). Our observations that the two runs do not sample the same region of configurational space are not entirely unexpected since the large-scale domain motions required for convergence likely occur at timescales longer than a microsecond. Therefore, we checked whether the configurations sampled by the combined trajectory or separately by each simulation are consistent with the wide range of ubiquitin orientations suggested by the proximity analysis of the Rosetta-predicted models and the structural perturbations observed by NMR. As shown in Figure 5A, KRAS-ubiquitin contacts and fluctuations averaged over the two trajectories, but not each trajectory separately, consistent with the data from Rosetta and NMR. This suggests that both trajectories can be regarded as sampling relevant conformations accessible to the complex.
Figure 5.
Unbiased Atomistic MD Simulations of mUbKRAS104 Bound to GDP Suggest that Ubiquitin Forms Dynamic Interactions with the KRAS Switch Regions
(A) The relative orientation of ubiquitin and KRAS derived from two independent MD runs of mUbKRAS104 bound to GDP. KRAS is shown in gray with SWI and SWII in green and yellow, respectively. Representative structures of ubiquitin from simulation 1 and 2 are depicted in red and blue, respectively. The nucleotide is shown as licorice structure at the bottom of the image.
(B) The difference of residue root-mean-square fluctuations (δRMSF) between WT KRAS and mUbKRAS104 averaged over the last 700 ns of simulation 1 (red), simulation 2 (blue), and their average (black dashed line). SWI and SWII regions are highlighted in yellow, and H2 and H3 in black slanted lines.
(C) Normalized contact frequencies of KRAS with ubiquitin residues in simulation 1 (red) and 2 (blue) of mUbRAS104-GDP.
(D) Normalized frequency of contacts for residues in H2 and H3 in ensembles of mUbKRAS104 and WT KRAS bound to GDP. In (C) and (D), values averaged over the two simulations are shown as black dashes.
(E) Snapshots between mUbKRAS104 and WT KRAS bound to GDP highlighting differences in interactions between select H2 (yellow) and H3 (pink) residues. Hydrogen bonds are depicted as white dashes. KRAS and ubiquitin are depicted as black and orange surfaces, respectively.
To further analyze the simulation results and evaluate the effect of ubiquitination on KRAS dynamics, we calculated average backbone RMSFs (Figure 5B) and KRAS-ubiquitin residue contacts (Figure 5C) for each simulation. Also shown are RMSFs and contacts averaged over the two trajectories. In one of the simulations, KRAS interacts with ubiquitin primarily via its N terminus, loop 3, and SWII. In the other, ubiquitin interacts with SWII, loop 7, and the C terminus of KRAS (Figures 5C, S3C, and S3D). The combined analysis (average over the two trajectories) indicates that contacts via the N/C termini, loop 3, and loop 7 are transient and occur ∼20%–70% of the time, except for E76 (near the C-terminal end of SWII), R164 (C terminus), and K104 (ubiquitination site), which stably persist for >80% of the time. This is consistent with the NMR and Rosetta results described above. Furthermore, from the changes in the average RMSF values per residue, which measure changes in conformational fluctuations due to the ubiquitination, we observe a decrease in SWI and SWII dynamics in trajectory 1. In contrast, the switch regions are, on average, somewhat more dynamic in trajectory 2. The absence of loop 3-ubiquitin interaction in the latter appears to account for the increased dynamics. Ubiquitin stabilized loop 7 in both simulations, which can be explained by the proximity of the loop to the ubiquitination site. Combining the two trajectories, we observe a small but significant dampening of conformational fluctuations in KRAS upon 104 monoubiquitination, especially at SWII, consistent with the broadening observed in the 1H-15N 2D HSQC of mUbKRAS104 (Figure 2E).
Since the interface between H2 and H3 in KRAS is important for recognition of SOS, we investigated the distances and interaction patterns between the residues in these two helices of mUbKRAS104 and compared the results with WT KRAS. We observed a mostly conserved pattern of H2-H3 contact (Figure 5D) in the two simulations of mUbKRAS104 as well as between mUbKRAS104 and WT KRAS. However, there are some differences. In WT KRAS, the H2/H3 interface is stabilized primarily by interactions involving the side chains of K104, R73, and G75. Ubiquitination at 104 disrupts these interactions. Instead, in both simulations of mUbKRAS104, we observe a new hydrogen bond between side chains of R102 in H3 and D69 in H2 (Figure 5E), stabilizing the H2/H3 interface and thereby maintaining a site for SOS recognition. In the absence of the D69-R102 hydrogen bond, the H2/H3 interface in mUbKRAS104 is stabilized by dynamic contacts between the R68 and H95 side chains. Although the loss of interaction involving Lys104 causes some perturbation in the H2/H3 interface (Figure 2D), the helical content of H3 remains intact during the simulations while some loss is observed for H2 compared with WT KRAS by two amino acids in one of the two runs of mUbKRAS104 (Figure S5). In addition, the helical content of H2 is lost in about 20% of the conformers in simulation 2 of mUbKRAS104, suggesting a significant impact of ubiquitination on the structure of the H2 in SWII.
TMAO Restores the K104C Sensitivity to SOS-Mediated Guanine Nucleotide Exchange
Our computational and NMR analyses indicate that ubiquitin ligation to KRAS at position 104 retains SOS regulation by stabilizing the H2/H3 interface. To provide further experimental support, we investigated whether osmolytes can similarly restore SOS activity from disruption of the H2/H3 interface by point mutation. We employed the osmolyte, TMAO, as this cosolute can stabilize proteins in solution by enhancing its compact folded conformation even under various denaturing stresses (Guseman et al., 2018; Su et al., 2017). KRAS mutations at position 104, including K104Q, K104C, K104R, and K104A, impair SOS upregulation of RAS activity by disrupting the H2/H3 interface (Baker et al., 2013b; Yin et al., 2017). As such, we investigated whether TMAO can stabilize mutational perturbation at the H2/H3 interface and restore SOS activity. As a cysteine mutation at position 104 was used to generate a disulfide linkage between RAS and ubiquitin, we investigated the effects of TMAO on SOS-mediated KRAS104C guanine nucleotide dissociation. For these experiments, we loaded KRASK104C with MANT-GDP and determined the rate of MANT-GDP nucleotide dissociation in the absence and presence of SOScat. Consistent with our previous findings that mutations at 104 perturb SOS regulation, KRASK104C reduces SOS-mediated nucleotide dissociation (Figure 6). However, the presence of 500 mM TMAO significantly restores the SOS-mediated GDP dissociation rate. These data indicate that, like ubiquitination, TMAO can partially restore SOS activity by stabilizing RAS conformation and dynamics.
Figure 6.
The Osmolyte TMAO Enhances SOScat-mediated RAS-GDP Nucleotide Dissociation for WT and KRASK104C
(A) The relative rates of SOS-mediated GDP dissociation for WT KRAS and KRASK104C in the absence and presence of the osmolyte TMAO were measured using a fluorescence-based assay.
(B) Quantification of SOS-mediated GDP dissociation. KRASK104C shows reduced SOScat-mediated GDP dissociation relative to WT KRAS. Addition of 0.5 M Trimethylamine N-Oxide (TMAO) stimulates SOS-mediated nucleotide exchange for both WT and KRASK104C. All the measurements were performed in duplicate; results are the mean ± SD (n = 2).
Discussion
Post-translational modifications within the G-domain of RAS proteins present an additional layer of complexity in modulation of RAS activity and may provide novel avenues to target RAS-driven tumorigenesis (Ahearn et al., 2018, 2011). To date, PTMs such as ubiquitination, acetylation ,and methylation have been identified in G domain of RAS at multiple sites, including K104, K117, and K147 (Ahearn et al., 2018). The modulatory effects of PTMs on RAS are dependent on the site and type (Ahearn et al., 2018). Although a number of PTMs in RAS have been reported, deciphering molecular mechanisms associated with modulation of RAS activity and tumorigenesis are often hampered by limited methods available to authentically install the type of PTM at the specific site of RAS both in vivo and in vitro. As such, proper installation of the PTM on RAS and characterization at the molecular level will aid in understanding the functional and structural consequences. Previously we found that ubiquitination at K147 of KRAS promotes activation of RAS by abrogating GAP-mediated hydrolysis (Baker et al., 2013a). We also observed that ubiquitination of KRAS at 104 retains SOS regulation. In contrast, mutations at this site disrupt SOS-mediated guanine dissociation (Baker et al., 2013b). In characterizing the effects of mUbKRAS104, we uncovered a novel mechanism by which conjugating ubiquitin to RAS modulates RAS dynamics and RAS/GEF interactions.
By employing real-time NMR, we find that mUbKRAS104 retains SOS-mediated GDP-GTP exchange similar to WT KRAS, consistent with our previous findings (Baker et al., 2013b). Lysine 104 lies in H3. The K104 side chain interacts with backbone carbonyl oxygens of R73 and G75 in H2 and is important for maintaining the integrity of H2 in SWII. Moreover H2 and H3 provide key contacts for SOS recognition based on crystal structures of RAS proteins bound to SOS1cat (PDB codes 1BKD, 1NVW, and 1XD2) (Margarit et al., 2003; Sondermann et al., 2004). Specifically, R102 in H3 makes contacts with Phe1010 and Asp1007 in SOScat. Valine103, adjacent to the modification site, interacts with Ser881 in SOScat. Moreover, RAS residues Gln70, Tyr71, and R73 at the end of H2 form additional interactions with SOScat (Figures 1D and 1E) (Hall et al., 2001). Therefore, the structural integrity of the H2/H3 interface is important for SOS recognition. Consistent with these observations, mutations at 104 perturb SOS-mediated nucleotide exchange. NMR analyses indicate that ubiquitination at 104 perturbs regions in H2 and H3, including residues 70–78 and residues 100–108. However, although ubiquitination results in loss of these native interactions, new interactions are generated. Rosetta modeling shows that ubiquitin, when ligated at 104 to RAS, can sample conformations in proximity to regions that show NMR perturbations. This observation was further corroborated by MD simulations, which yielded two major ensembles of mUbKRAS104 conformers wherein the ubiquitin-KRAS interface involves both H2 and H3.
We also set out to quantitatively assess changes in dynamic properties of SWII at atomic resolution using NMR spin relaxation experiments, but the sample stability was not sufficient to carry out the intensity modulation experiments for measuring the relaxation rates. In lieu of quantifying mUbKRAS104 dynamics by NMR, we employed MD simulations. The MD simulations complement our experimental data and show a reduction in RMSD fluctuations (Figure 5B), consistent with the NMR peak broadening observed in SWI and SWII (Figure 2E). For the majority of the mUbKRAS104 conformers, the H2/H3 interface is stabilized through altered dynamics of H2 and formation of a dynamic interaction network that compensates for loss of native H2-H3 interactions. The D69-R102 interaction observed in some crystal structures (Johnson et al., 2019) and previous simulations (Rambahal, 2013) stabilizes a portion of SWII in a more GTP-like conformation in which H2 orients toward H3. Given these observations, we analyzed the existing PDB structure of RAS proteins for D69 and R102 interactions and found hydrogen bonds in 20.4% of the GDP-bound structures and 46.1% of the GTP-bound structures (Table S2). This is consistent with peak broadening observed at the end of H2 and residues proximal to K104 (Figure 2E). Moreover, our findings that addition of the osmolyte TMAO restores SOS deficiency caused by mutation at 104 provides additional evidence that stabilizing interactions can compensate for loss of 104 contacts with H2. To date, TMAO has not been reported in the studies of RAS. TMAO is a natural osmolyte that stabilizes a wide spectrum of proteins against hydrostatic pressure, heat, and denaturing agents like urea (Walker et al., 2019). A widely accepted theory is that TMAO stabilizes proteins by increasing the free energy of the unfolded state through solvent exclusion and hydrogen bonding of water and thus shifts the equilibrium toward the native state (Ma et al., 2014). In a recent paper, TMAO was postulated to stabilize charge interactions between residues (Su et al., 2017).
Our computational analyses indicate that ubiquitin does not dock or form strong molecular interactions that anchor ubiquitin at the RAS surface, yet forms transient interactions with RAS and alters RAS dynamics in the proximity of the KRAS H2-H3 interface. Consistently, we have previously titrated 15N-enriched KRAS with a large excess of free ubiquitin and did not observe the significant interactions by NMR. Hence, ubiquitin modulates RAS structure and dynamics, by dynamically sampling the RAS surface. This novel mechanism of RAS regulation adds to accumulating evidence that dynamic protein complexes (Hopper and Robinson, 2014; Lobingier et al., 2017) together with inherent protein dynamics, play important roles in modulating multiple cellular processes such as signaling pathways, chaperon-assistant folding and transcriptional regulation (Csizmok et al., 2016; Sandikci et al., 2013; Stein et al., 2009; Tompa et al., 2015).
Furthermore, although ubiquitin conjugation to RAS at 104 dynamically samples multiple orientations with a large amplitude at the KRAS surface, our Rosetta modeling (Figure S2) and MD simulations (Figure S6) indicate that the ubiquitin does not occlude the RAS-SOS interface. Therefore, our finding that the ubiquitin modulates the dynamics of SWII to stabilize the H2/H3 interface to restore SOS-mediated activation represents a new mechanism for modulation of RAS signaling. It is worth noting that this mechanism has been proposed in a computational study for kinases (Ball et al., 2016). Ubiquitin PTMs are generally thought to function as recognition elements that bind ubiquitin recognition motifs. Although it is unclear whether mUbKRAS104 promotes new interactions in this manner, our data indicate that ubiquitin conjugation can alter the conformational ensemble of a dynamic proteins. This represents a paradigm for ubiquitin signaling and provides a potential mechanism for how ubiquitin conjugation can alter protein function.
Lysine 104 of KRAS is a hotspot for PTMs; however, the physiological roles of lysine modifications at this site and interplay between the different PTMs are unclear. Moreover, investigation of the interplay between the different PTMs is needed. As monoubiquitination and acetylation are mutually exclusive, mechanisms may exist to choose between different modifications at this site in response to changes in the cellular environment or signaling events. It will be interesting to characterize these two PTMs at K104 in the context of the oncogenic mutations of KRAS and the potential interplay. Our findings indicate that RAS lysine 104 monoubiquitination does not alter intrinsic or regulator-mediated biochemical function. Therefore, the biological role of ubiquitination at this site is still unclear. Ubiquitination at K104 may create a new docking site to promote or interfere with RAS interacting proteins or modulate other lysine modifications. However, identification of enzymes that control RAS ubiquitination at this site is needed to dissect the role of this modification and assess whether this mechanism of regulation presents new opportunities to target aberrant RAS signaling and tumorigenesis.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Rachael Baker, Alex J. Guseman, and Robert McGinty for discussion on experimental strategies. We thank Greg Young and Min-Qi Lu for assistance in NMR data collection and sample preparation, respectively. We thank Woonghee Lee for support in the use of NMRFRAM-Sparky. S.L.C. is supported by NIH P01CA203657 and NIH R35 GM134962. A.A.G. is supported by NIH R01GM124233. V.N. is supported by UTHealth Innovation for Cancer Prevention Research Training Program Pre-Doctoral Fellowship (Cancer Prevention and Research Institute of Texas grant RP160015). A.A.G. and V.N. thank the Texas Advanced Computing Center (TACC) and the Extreme Science and Engineering Discovery Environment (XSEDE Grant No. MCB150054) for computational resources.
Author Contributions
G.Y., J.Z., A.C., and J.P. produced the protein samples. G.Y. and J.Z. recorded the NMR spectra. J.Z. performed biochemical assays. V.N., V.T., and J.H. performed the MD simulations. G.Y., J.Z., and V.N. analyzed the data. S.L.C., G.Y., and A.A.G. designed this study. G.Y., S.L.C., V.N., and J.H. wrote the manuscript. All authors commented on the manuscript.
Declaration of Interests
The authors declare that they have no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101448.
Supplemental Information
References
- Ahearn I., Zhou M., Philips M.R. Posttranslational modifications of RAS proteins. Cold Spring Harb. Perspect. Med. 2018;8:a031484. doi: 10.1101/cshperspect.a031484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahearn I.M., Haigis K., Bar-Sagi D., Philips M.R. Regulating the regulator: post-translational modification of RAS. Nat. Rev. Mol. Cell Biol. 2011;13:39–51. doi: 10.1038/nrm3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Lewis S.M., Sasaki A.T., Wilkerson E.M., Locasale J.W., Cantley L.C., Kuhlman B., Dohlman H.G., Campbell S.L. Site-specific monoubiquitination activates Ras by impeding GTPase-activating protein function. Nat. Struct. Mol. Biol. 2013;20:46–52. doi: 10.1038/nsmb.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker R., Wilkerson E.M., Sumita K., Isom D.G., Sasaki A.T., Dohlman H.G., Campbell S.L. Differences in the regulation of K-Ras and H-Ras isoforms by monoubiquitination. J. Biol. Chem. 2013;288:36856–36862. doi: 10.1074/jbc.C113.525691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball K.A., Johnson J.R., Lewinski M.K., Guatelli J., Verschueren E., Krogan N.J., Jacobson M.P. Non-degradative ubiquitination of protein kinases. PLoS Comput. Biol. 2016;12:e1004898. doi: 10.1371/journal.pcbi.1004898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox A.D., Fesik S.W., Kimmelman A.C., Luo J., Der C.J. Drugging the undruggable RAS: Mission possible? Nat. Rev. Drug Discov. 2014;13:828–851. doi: 10.1038/nrd4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csizmok V., Follis A.V., Kriwacki R.W., Forman-Kay J.D. Dynamic protein interaction networks and new structural paradigms in signaling. Chem. Rev. 2016;116:6424–6462. doi: 10.1021/acs.chemrev.5b00548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M., Wittinghofer A. GEFs, GAPs, GDIs and effectors: taking a closer (3D) look at the regulation of Ras-related GTP-binding proteins. Curr. Opin. Struct. Biol. 1997;7:786–792. doi: 10.1016/s0959-440x(97)80147-9. [DOI] [PubMed] [Google Scholar]
- Guseman A.J., Perez Goncalves G.M., Speer S.L., Young G.B., Pielak G.J. Protein shape modulates crowding effects. Proc. Natl. Acad. Sci. U S A. 2018;115:10965–10970. doi: 10.1073/pnas.1810054115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall B.E., Yang S.S., Boriack-Sjodin P.A., Kuriyan J., Bar-Sagi D. Structure-based mutagenesis reveals distinct functions for Ras switch 1 and switch 2 in Sos-catalyzed guanine nucleotide exchange. J. Biol. Chem. 2001;276:27629–27637. doi: 10.1074/jbc.M101727200. [DOI] [PubMed] [Google Scholar]
- Hansen R., Peters U., Babbar A., Chen Y., Feng J., Janes M.R., Li L.-S., Ren P., Liu Y., Zarrinkar P.P. The reactivity-driven biochemical mechanism of covalent KRASG12C inhibitors. Nat. Struct. Mol. Biol. 2018;25:454–462. doi: 10.1038/s41594-018-0061-5. [DOI] [PubMed] [Google Scholar]
- Harrison J.S., Jacobs T.M., Houlihan K., Van Doorslaer K., Kuhlman B. UbSRD: the ubiquitin structural relational database. J. Mol. Biol. 2016;428:679–687. doi: 10.1016/j.jmb.2015.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper J.T.S., Robinson C.V. Mass spectrometry quantifies protein interactions--from molecular chaperones to membrane porins. Angew. Chem. Int. Ed. 2014;53:14002–14015. doi: 10.1002/anie.201403741. [DOI] [PubMed] [Google Scholar]
- Janes M.R., Zhang J., Li L.-S., Hansen R., Peters U., Guo X., Chen Y., Babbar A., Firdaus S.J., Darjania L. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell. 2018;172:578–589.e17. doi: 10.1016/j.cell.2018.01.006. [DOI] [PubMed] [Google Scholar]
- Johnson C.W., Lin Y.-J., Reid D., Parker J., Pavlopoulos S., Dischinger P., Graveel C., Aguirre A.J., Steensma M., Haigis K.M. Isoform-specific destabilization of the active site reveals a molecular mechanism of intrinsic activation of KRas G13D. Cell Rep. 2019;28:1538–1550.e7. doi: 10.1016/j.celrep.2019.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knyphausen P., Lang F., Baldus L., Extra A., Lammers M. Insights into K-Ras 4B regulation by post-translational lysine acetylation. Biol. Chem. 2016;397:1071–1085. doi: 10.1515/hsz-2016-0118. [DOI] [PubMed] [Google Scholar]
- Lobingier B.T., Hüttenhain R., Eichel K., Miller K.B., Ting A.Y., von Zastrow M., Krogan N.J. An approach to spatiotemporally resolve protein interaction networks in living cells. Cell. 2017;169:350–360.e12. doi: 10.1016/j.cell.2017.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Pazos I.M., Gai F. Microscopic insights into the protein-stabilizing effect of trimethylamine N-oxide (TMAO) Proc. Natl. Acad. Sci. U S A. 2014;111:8476–8481. doi: 10.1073/pnas.1403224111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margarit S.M., Sondermann H., Hall B.E., Nagar B., Hoelz A., Pirruccello M., Bar-Sagi D., Kuriyan J. Structural evidence for feedback activation by Ras.GTP of the Ras-specific nucleotide exchange factor SOS. Cell. 2003;112:685–695. doi: 10.1016/s0092-8674(03)00149-1. [DOI] [PubMed] [Google Scholar]
- Rambahal N. 2013. Conformational Dynamics of K-RAS and H-RAS Proteins: Is There Functional Specificity at the Catalytic Domain? UT GSBS Dissertations and Theses (Open Access)https://digitalcommons.library.tmc.edu/utgsbs_dissertations/380/ [Google Scholar]
- Saha A., Lewis S., Kleiger G., Kuhlman B., Deshaies R.J. Essential role for ubiquitin-ubiquitin-conjugating enzyme interaction in ubiquitin discharge from Cdc34 to substrate. Mol. Cell. 2011;42:75–83. doi: 10.1016/j.molcel.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandikci A., Gloge F., Martinez M., Mayer M.P., Wade R., Bukau B., Kramer G. Dynamic enzyme docking to the ribosome coordinates N-terminal processing with polypeptide folding. Nat. Struct. Mol. Biol. 2013;20:843–850. doi: 10.1038/nsmb.2615. [DOI] [PubMed] [Google Scholar]
- Sasaki A.T., Carracedo A., Locasale J.W., Anastasiou D., Takeuchi K., Kahoud E.R., Haviv S., Asara J.M., Pandolfi P.P., Cantley L.C. Ubiquitination of K-Ras enhances activation and facilitates binding to select downstream effectors. Sci. Signal. 2011;4:ra13. doi: 10.1126/scisignal.2001518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M.J., Marshall C.B., Theillet F.-X., Binolfi A., Selenko P., Ikura M. Real-time NMR monitoring of biological activities in complex physiological environments. Curr. Opin. Struct. Biol. 2015;32:39–47. doi: 10.1016/j.sbi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sondermann H., Soisson S.M., Boykevisch S., Yang S.-S., Bar-Sagi D., Kuriyan J. Structural analysis of autoinhibition in the Ras activator son of sevenless. Cell. 2004;119:393–405. doi: 10.1016/j.cell.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Stein A., Pache R.A., Bernadó P., Pons M., Aloy P. Dynamic interactions of proteins in complex networks: a more structured view. FEBS J. 2009;276:5390–5405. doi: 10.1111/j.1742-4658.2009.07251.x. [DOI] [PubMed] [Google Scholar]
- Stephen A.G., Esposito D., Bagni R.K., McCormick F. Dragging ras back in the ring. Cancer Cell. 2014;25:272–281. doi: 10.1016/j.ccr.2014.02.017. [DOI] [PubMed] [Google Scholar]
- Su Z., Mahmoudinobar F., Dias C.L. Effects of trimethylamine-N-oxide on the conformation of peptides and its implications for proteins. Phys. Rev. Lett. 2017;119:108102. doi: 10.1103/PhysRevLett.119.108102. [DOI] [PubMed] [Google Scholar]
- Tompa P., Schad E., Tantos A., Kalmar L. Intrinsically disordered proteins: emerging interaction specialists. Curr. Opin. Struct. Biol. 2015;35:49–59. doi: 10.1016/j.sbi.2015.08.009. [DOI] [PubMed] [Google Scholar]
- Vigil D., Cherfils J., Rossman K.L., Der C.J. Ras superfamily GEFs and GAPs: validated and tractable targets for cancer therapy? Nat. Rev. Cancer. 2010;10:842–857. doi: 10.1038/nrc2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker E.J., Bettinger J.Q., Welle K.A., Hryhorenko J.R., Ghaemmaghami S. Global analysis of methionine oxidation provides a census of folding stabilities for the human proteome. Proc. Natl. Acad. Sci. U S A. 2019;116:6081–6090. doi: 10.1073/pnas.1819851116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters A.M., Der C.J. KRAS: the critical driver and therapeutic target for pancreatic cancer. Cold Spring Harb. Perspect. Med. 2018;8:a031435. doi: 10.1101/cshperspect.a031435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart D.S., Sykes B.D. The 13C chemical-shift index: a simple method for the identification of protein secondary structure using 13C chemical-shift data. J. Biomol. NMR. 1994;4:171–180. doi: 10.1007/BF00175245. [DOI] [PubMed] [Google Scholar]
- Yang M.H., Nickerson S., Kim E.T., Liot C., Laurent G., Spang R., Philips M.R., Shan Y., Shaw D.E., Bar-Sagi D. Regulation of RAS oncogenicity by acetylation. Proc. Natl. Acad. Sci. U S A. 2012;109:10843–10848. doi: 10.1073/pnas.1201487109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin G., Kistler S., George S.D., Kuhlmann N., Garvey L., Huynh M., Bagni R.K., Lammers M., Der C.J., Campbell S.L. A KRAS GTPase K104Q mutant retains downstream signaling by offsetting defects in regulation. J. Biol. Chem. 2017;292:4446–4456. doi: 10.1074/jbc.M116.762435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshino H., Yin G., Kawaguchi R., Popov K.I., Temple B., Sasaki M., Kofuji S., Wolfe K., Kofuji K., Okumura K. Identification of lysine methylation in the core GTPase domain by GoMADScan. PLoS One. 2019;14:e0219436. doi: 10.1371/journal.pone.0219436. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.