Abstract
Nemaline myopathy is a heterogeneous disorder of skeletal muscle, and histologically characterized by the presence of nemaline bodies in muscle fibers. Patients with typical congenital form of nemaline myopathy initially present with proximal but later also distal muscle weakness, mostly involving facial and respiratory muscle. Cardiac involvement has been rarely observed especially in nebulin-related nemaline myopathy and there have been only two reports about nebulin-related nemaline myopathy patients with cardiac involvement. We present here the case of a 65-year-old woman manifesting slowly progressive distal myopathy with respiratory and heart failure. She harbored two variants in the nebulin gene, c.20131C > T (p.Arg6711Trp) and c.674C > T (p.Pro225Leu), and one of them, c.674C > T, was a novel variant. In this report, we discuss the pathogenicity of the novel variant and its association with clinical phenotypes including cardiac involvement.
Keywords: Nemaline myopathy, Congenital, Heart failure, Respiratory failure, Nebulin (NEB)
Abbreviations: NM, nemaline myopathy; CK, creatine kinase; ECG, electrocardiography; bpm, beats per minute; TTE, transthoracic echocardiography; LVOT, left ventricular outflow track; LVOT-VTI, LVOT-velocity time integral.
Highlights
-
•
A NEB-related nemaline myopathy patient developed distal myopathy, respiratory and heart failure.
-
•
Two variants in NEB were noted and one of them was a novel variant.
-
•
The novel variant might be associated with the clinical symptoms including heart failure.
1. Introduction
Nemaline myopathy (NM) is a clinically and genetically heterogeneous disorder of skeletal muscle, and histologically characterized by the presence of rod-like structures, known as nemaline bodies, in muscle fibers [1]. NM has been divided into various categories based on its onset and/or severity [1,2]. Patients with typical congenital form of NM initially present with proximal but later also distal muscle weakness, mostly involving facial and respiratory muscle [1]. However, cardiac involvement has been rarely observed [2]. Genetically, twelve genes and numerous mutations have been identified, and NEB are one of the most common causes of NM [2]. Here, we describe a novel NEB variant detected in a Japanese patient with distal myopathy, respiratory and heart failure.
2. Case report
A 65-year-old woman presented a week history of heart and respiratory failure. She was born at term without complications and presented normal motor milestones in childhood. There was no obvious family history of neuromuscular diseases (Fig. 1A). At the age of 33, she noted muscle atrophy predominantly seen in the right lower extremity. She gradually felt difficulty in walking due to right drop foot. She needed a peroneal orthosis at the age of 42. On admission, she complained of dyspnea in a supine position and showed edema on bilateral lower extremities. Her oxygen saturation was less than 90% on room air, and arterial blood gas analysis revealed hypercapnia and hypoxemia with increased alveolar-arterial gradient. Then, she needed a ventilator due to her state of CO2 narcosis. Two weeks after admission, she was referred to the neurological department to investigate an underlying neuromuscular disease leading to respiratory and heart failure.
Fig. 1.
(A) Pedigree chart of the patient. An arrow indicates the proband. (B-D) Systemic CT scan showed muscle atrophy and fatty replacement of the lower extremity muscles. Of note, asymmetric atrophy in the soleus muscle and anterior tibial muscle is observed (C) (arrowheads). (D, E) Paraspinal muscle atrophy was also noted. (F—I) Representative pathological findings of the right biceps brachii muscle: marked variation in fiber size on hematoxylin and eosin (H&E) staining (F), scattered fibers with nemaline bodies (arrows) on modified Gomori-trichrome staining (H, I), and type 1 fiber predominance on ATPase staining at pH 10.6 (G). (J) A schematic presentation of the nebulin protein structure and its protein interaction partners. (K,L) Identified variants, c.20131C > T (K) and c.674C > T (L), were confirmed by performing Sanger sequencing of the PCR fragments (red arrows). (M) Alignment of the nebulin amino acid sequence in different species. The substitution sites of the novel variant (Pro225) is highly evolutionarily conserved. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Neurological examinations revealed facial muscle weakness including narrow high-arched palate, and generalized but distal dominant muscle weakness and atrophy. Scoliosis was not observed. Severe muscle weakness was observed in ankle plantar flexion predominantly in the right side while mild weakness was observed in upper extremities.
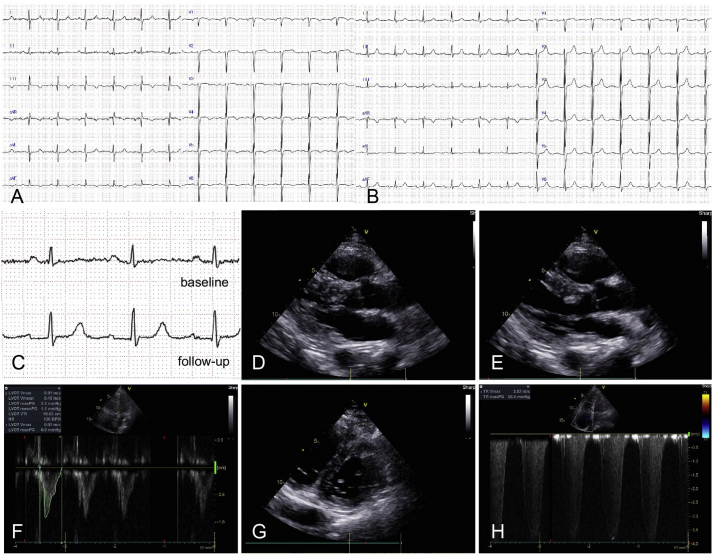
In lower extremities, distribution of muscle atrophy was asymmetric (right > left) (Fig. 1B, C) but pes cavus was observed bilaterally. Deep tendon reflexes were decreased in all extremities. Laboratory tests revealed elevated serum level of brain natriuretic peptide (992.9 pg/mL; reference range, <18.4 pg/mL) with normal serum creatinine level (0.63 mg/dL; reference range, 0.46–0.79 mg/dL). Serum level of creatine kinase (CK) was not elevated (69 U/L; reference range, 41–153 U/L) and CK-MB level was almost normal (26 U/L; reference range, <20 U/L). Follow-up laboratory tests 17 h after the first tests revealed normal CK (39 U/L) and CK-MB (16 U/L) levels. Electrocardiography (ECG) revealed a regular sinus rhythm with a heart rate of 76 beats/min (bpm) and right axis deviation (Fig. 2A). An SI/QIII pattern, that is indicative of right ventricular strain, was observed (Fig. 2A). Inverted T wave was observed in lead aVF, lead V1 and V4–6 (Fig. 2A). Transthoracic echocardiography (TTE) demonstrated dilatation of both right atrium and right ventricle while left ventricular function was preserved (Fig. 2D-G). Tricuspid regurgitation was observed and tricuspid regurgitation pressure gradient was 58 mmHg (Fig. 2H), which indicated the presence of pulmonary hypertension. A computed tomography showed bilateral pleural fluids and generalized muscular atrophy including paraspinal muscle (Fig. 1D, E), especially in lower extremities with asymmetry (Fig. 1B, C). Her percent vital capacity measured after weaning from the ventilator was decreased (54%). An electromyogram showed decreased amplitude of the interference wave in lower extremities. A biopsy of the left biceps brachii muscle showed marked variation in the fiber size and scattered fibers with nemaline bodies on modified Gomori trichrome staining (Fig. 1F, H and I). Type 1 fiber predominance was observed on ATPase staining at pH 10.6 (Fig. 1G). After admission, she recovered from CO2 narcosis, and was gradually weaned from the respirator. TTE performed a month after admission revealed normal left ventricular function without any regional wall motion abnormalities. After a 9-month follow-up, she started to use noninvasive positive pressure ventilation during the nighttime and follow-up ECG showed no ST change noted at baseline (Fig. 2B) while slightly prolonged PR interval was observed (Fig. 2C).
Fig. 2.
(A) ECG at baseline: a regular sinus rhythm with 76 bpm and right axis deviation. A large S wave in lead I, a Q wave in lead III and inverted T wave in lead III were observed. Inverted T wave was also observed lead V1 and V4–6. (B) ECG at a 16-month follow-up: a regular sinus rhythm with 76 bpm. Prolonged PR interval (0.24 s) was observed. (C) Comparison between lead II ECG at baseline and at a 16-month follow-up. Although PR interval was normal at baseline, slightly prolonged PR interval (0.24 s) was observed at a 16-month follow-up. (D, E) Echocardiogram at baseline in systolic (D) and diastolic phases (E). No regional wall motion abnormalities were observed and visual evaluation of ejection fraction was above 60%. (F) Cardiac output was 5.3 L/min which was calculated by multiplying left ventricular outflow track (LVOT)-cross sectional area (data not shown), LVOT-velocity time integral (VTI) and heart rate, suggesting that left ventricular function was preserved. (G) Parasternal short axis view showed that the right ventricle was dilated resulting in a D-shaped distortion of the left ventricle. (H) Tricuspid regurgitation was observed and estimated tricuspid regurgitation pressure gradient was 58 mmHg.
Using the Ion PGM sequencing system coupled with the original targeted panels for known causative genes in congenital muscular diseases [3], we identified a compound heterozygous variant in NEB, c.20131C > T:p.Arg6711Trp and c.674C > T:p.Pro225Leu, being.
not listed up in any of the following public databases: Exome Aggregation Consortium, Exome Sequencing Project v. 6500 and 1000 Genomes (Fig. 1J). One of them, c.20131C > T, was a known variant while the remaining variant, c.674C > T, was a novel. The novel missense variant, c.674C > T, was predicted as “deleterious variants” by SIFT, PolyPhen-2, and Mutation Taster. These variants were confirmed by Sanger sequencing: these sequences were in our patient by using a next-generation sequencer using the following primers: 5’-GCAATTTTTCAAATAAAGGTGACA-3′ and 5’-TCAGTATCAAAATGTGACAATAAGGA-3′ for c.20131C > T:p.Arg6711Trp (Fig. 1K), and 5’-AATGACTTGGGTTGGATGGA-3′ and 5’-TCCTCTGAAATACAACATTTAGTCT-3′ for c.674C > T:p.Pro225Leu (Fig. 1L). The substitution sites of the novel variant (Pro225) is highly evolutionarily conserved (Fig. 1M). Based on these clinical features, histopathological findings and DNA analysis, we diagnosed her as having NM with two variants in NEB.
3. Discussion
We report here a NEB-related NM patient presenting slowly progressive distal myopathy with respiratory and heart failure. She had a known missense variant of c.20131C > T [4,5], and a novel variant of c.674C > T in NEB. Intriguingly, the novel missense variant, c.674C > T, is located at the tropomodulin binding site (Fig. 1J). As known, tropomodulin maintains the thin filament length by blocking actin polymerization at the pointed end. This missense variant might develop NM through the misinteraction between nebulin and tropomodulin like as previous cases [6].
Heart failure is rarely observed in patients with NM [2], especially in NEB-related NM. It can be explained by lower NEB expression in heart [7]. To date, there have been only two patients with NEB-related NM presenting heart failure (Table 1) [4,5]. In the reported two NEB-related NM patients and our case, ECG and echocardiography were not indicative of any underlying ischemic heart disease or valvular disease, which can be causative of heart failure. Another possible cause of heart failure was cor pulmonale or cardiomyopathy associated with NM [8]. These three patients including the reported two cases demonstrated respiratory failure in addition to heart failure (Table 1), indicating that cor pulmonale was associated with their heart failure. Although the variant of c.20131C > T is common in these patients with heart failure, the variant does not affect the binding of actin, suggesting that the pathogenicity of the variant of c.20131C > T is not confirmed [4]. Indeed, the carriers of a single c.20131C > T variant do not demonstrate any abnormal phenotype including myopathy or heart failure [4]. In contrast, patients with cardiac involvement had another variant which can affect the binding of tropomodulin (our case) or desmin (reported two cases) [4,5]. Previous in vivo/vitro studies suggested that tropomodulin or desmin might be related to cardiac involvement [9,10]. Taken together, it is possible that the combination of the c.20131C > T variant and another variant which can affect the interaction between nebulin and tropomodulin or desmin is related to develop cor pulmonale in NEB-related NM. On the contrary, the presence of cardiomyopathy was not confirmed in our patient and the reported two patients as no cardiac MRI study or pathological examination was performed. However, the patient reported by Mizuno et al. demonstrated left ventricular hypertrophy and reduced ejection fraction during the disease course (Table 1). Furthermore, our patient showed that prolongation of PR interval on ECG was observed over the disease progression. One possible cause of the ECG change is conduction delay associated with NM. This phenomenon should be further investigated in future studies.
Table 1.
Clinical and genetic features of patients with NEB-related nemaline myopathy demonstrating heart failure.
| Age/Sex | Age of the onset | Cardiac function |
Respiratory failure (VC) | Gene mutation |
References | |||
|---|---|---|---|---|---|---|---|---|
| Cardiac marker | electrocardiogram | echocardiogram | Homozygous or heterozygous | Variant/ mutation | ||||
| 63/M | 46 | NA | No abnormal change | Dilation of both RA and RV | + (1.0 L) | Heterozygous | c.20131C > T | 4 |
| c.22924 del T | ||||||||
| 39/M | 32 | NA | NA | LVH and decreased EF (53.1%) | + (0.8 L) | Heterozygous | c.20131C > T | 5 |
| c.23161A > T | ||||||||
| 65/F | 33 | Elevated BNP | S1Q3T3 pattern⁎/ inverted T wave in lead aVF, V1 and V4–6 | Normal LV function/ severe TR | + (1.4 L) | Heterozygous | c.20131C > T | Our case |
| c.674C > T | ||||||||
M: male, F: female, NA: not available, BNP: brain natriuretic peptide, LVH: left ventricular hypertrophy, EF: ejection fraction, RA: right atrium, RV: right ventricle, LV: left ventricle, TR: tricuspid regurgitation VC: vital capacity. ⁎S wave in lead I, Q wave and inverted T wave in lead III.
A limitation of this study is that we could not confirm whether these variants were bi-allelic or not. We did not evaluate DNA of her parents, and the distance between two alleles in our patient was too separated to evaluate using PCR method. Moreover, the diagnosis of left ventricular involvement was based on clinical findings, such as pleural effusions or a serum BNP elevation. Our patient developed not only left heart failure but right heart failure because of longstanding respiratory muscle weakness, which can also cause these clinical findings. Further studies are necessary to confirm the pathogenicity of the novel variant and the mechanism of cardiac involvement in NEB-related NM.
Declaration of competing interest
none.
Funding
This study was supported by the Intramural Research Grant for Neurological and Psychiatric Disorders of the National Center of Neurology and Psychiatry under grant numbers 30–9 (Dr. Aritoshi Iida); Japan Agency for Medical Research and Development grant numbers JP18ek0109285h0002 (Dr. Ichizo Nishino and Dr. Aritoshi Iida), and JP18kk0205001s0203 (Dr. Ichizo Nishino); Joint Usage and Joint Research Programs, the Institute of Advanced Medical Sciences, Tokushima University grant number 2019, A9 (Dr. Aritoshi Iida).
References
- 1.Wallgren-Pettersson C., Pelin K., Hilpelä P., Donner K., Porfirio B., Graziano C. Clinical and genetic heterogeneity in autosomal recessive nemaline myopathy. Neuromuscul Disord. 1999;9(8):564–572. doi: 10.1016/s0960-8966(99)00061-9. [DOI] [PubMed] [Google Scholar]
- 2.Sewry C.A., Laitila J.M., Wallgren-Pettersson C. Nemaline myopathies: a current view. J Muscle Res Cell Motil. 2019;40:111–126. doi: 10.1007/s10974-019-09519-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishikawa A., Mitsuhashi S., Miyata N., Nishino I. Targeted massively parallel sequencing and histological assessment of skeletal muscles for the molecular diagnosis of inherited muscle disorders. J Med Genet. 2017;54(2):104–110. doi: 10.1136/jmedgenet-2016-104073. [DOI] [PubMed] [Google Scholar]
- 4.Tsunoda K., Yamashita T., Motokura E., Takahashi Y., Sato K., Takemoto M. A patient with slowly progressive adult-onset nemaline myopathy and novel compound heterozygous mutations in the nebulin gene. J Neurol Sci. 2017;373:254–257. doi: 10.1016/j.jns.2016.12.069. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno Y., Mori-Yoshimura M., Oya Y., Nishikawa A., Nishino I., Takahashi Y. Two cases of nemaline myopathy presenting with hypertrophy of distal limbs with prominent asymmetry. Rinsho Shinkeigaku. 2017;57(11):691–697. doi: 10.5692/clinicalneurol.cn-001024. [DOI] [PubMed] [Google Scholar]
- 6.Lehtokari V.L., Pelin K., Sandbacka M., Ranta S., Donner K., Muntoni F. Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum Mutat. 2006;27(9):946–956. doi: 10.1002/humu.20370. [DOI] [PubMed] [Google Scholar]
- 7.Bang M.L., Chen J. Roles of Nebulin family members in the heart. Circ J. 2015;79(10):2081–2087. doi: 10.1253/circj.CJ-15-0854. [DOI] [PubMed] [Google Scholar]
- 8.Finsterer J., Stöllberger C. Review of cardiac disease in Nemaline myopathy. Pediatr Neurol. 2015;53(6):473–477. doi: 10.1016/j.pediatrneurol.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 9.Sussman M.A., Welch S., Walker A., Klevitsky R., Hewett T.E., Witt S.A. Hypertrophic defect unmasked by calcineurin expression in asymptomatic tropomodulin overexpressing transgenic mice. Cardiovasc Res. 2000;46(1):90–101. doi: 10.1016/s0008-6363(99)00422-8. [DOI] [PubMed] [Google Scholar]
- 10.Baker L.K., Gillis D.C., Sharma S., Ambrus A., Herrmann H., Conover G.M. Nebulin binding impedes mutant desmin filament assembly. Mol Biol Cell. 2013;24(12):1918–1932. doi: 10.1091/mbc.E12-11-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]