Abstract
Purpose
Over the past decades, a variety of biomaterials have been investigated in terms of their suitability for oral mucosa tissue engineering. The aim of this study was to compare collagen and GelMA hydrogels as connective tissue scaffolds for fibroblasts and as substrates for seeding and culture of oral epithelial keratinocyte cells.
Methods
Human primary oral fibroblast and keratinocyte cells were isolated from gingival biopsies. The mixture of fibroblasts with GelMA or collagen gel were aliquoted within six-well tissue culture plate inserts and cross-linked using visible light or reconstitution buffer/heat, respectively. The viability of fibroblasts in the hydrogels was investigated after one and three days of cultivation using the PrestoBlue assay. Following the addition and culture of oral keratinocytes onto the connective tissue constructs, the tissue-engineered oral mucosa was assessed histologically.
Results
The tissue viability assay shows that collagen hydrogels encapsulating fibroblasts displayed significantly higher cell viability than cell-laden GelMA constructs after 24 and 72 h (p < 0.05). A stratified and differentiated epithelium has formed on the surface of cell-laden collagen hydrogel but not on the surface of the GelMA-based substrate.
Conclusion
Collagen-based scaffold offers superior biological properties compared to GelMA hydrogel in terms of oral fibroblast growth, as well as epithelial cell adhesion and differentiation. Therefore, collagen-based hydrogels remain the preferred choice for oral mucosa tissue engineering.
Keywords: Cell encapsulation, Collagen, GelMA, Three-dimensional culture, Hydrogel, Oral mucosa, Tissue engineering
1. Introduction
Oral mucosa has certain self-healing potential in small defect sizes; however, the reconstruction of large defects requires autologous grafts. The insufficient available oral mucosa for intra-oral grafting and donor site morbidity encouraged investigators to introduce engineered oral mucosa models. In addition to intra-oral applications for the healing of oral tissues, extra-oral applications of engineered oral mucosa equivalents in urethroplasty, ocular surface reconstruction, and treatment of a burn wound have been reported in the literature. Furthermore, these models can be used in vitro for assessment of the biocompatibility of dental materials and oral care products, drug delivery studies, simulation of oral diseases and investigation of tissue invasion by oral microorganisms. Tissue-engineered models of the oral mucosa have also the potential to reduce the need for animal testing. Frequent applications of oral mucosa models have resulted in the introduction of various types of commercial brands of oral mucosa into the market like RHOE (SkinEthic) and EpiOral (MaTek).1, 2
The first step in oral mucosal tissue engineering is to prepare the lamina propria by loading fibroblasts into a suitable scaffold material that can act as a substrate for the subsequent seeding of epithelial cells.3 The material selected as the fibroblast carrier must meet several requirements including the ability to mimic the extracellular matrix of connective tissues, allow fibroblast proliferation, and support epithelial cell adhesion and differentiation.4 Various types of natural and synthetic biomaterials have been used previously for this purpose including fibrin-agarose, collagen–GAG–chitosan, fibrin glue, collagen–elastin matrix, de-epidermis dermis (DED), silk fibroin, poly-l-lactic acid (PLA)-poly (3-hydroxybutyrate-co-3-hydroxy valerate) (PHBV) and collagen.1.
High water content, resemblance to natural tissues, and the possibility of cell encapsulation make the hydrogels promising candidates for oral mucosa tissue engineering.5 Collagen is a natural polymer with excellent cytocompatibility and is the major component of the natural lamina propria. This biomaterial is the most popular candidate for the engineering of skin and oral mucosa and has been extensively used by clinicians under different commercial brands.1 Collagen can be extracted from natural sources —like rat tail— and ready to be used for tissue engineering applications.6 In addition, it is possible to cross-link collagen with non-toxic substances.3 Besides these advantages, some limitations, such as poor mechanical properties, lack of suturability and fast degradation rate, have led researchers to explore the possibility of using other materials in oral mucosa engineering.7
Denaturation and hydrolysis of collagen can lead to the production of another material, known as gelatin. This product is also biodegradable, biocompatible, can form a hydrogel, and has the ability to form three-dimensional (3D) cell culture models.8 Using unsaturated methacrylamide groups, Van Den Bulcke et al. described the functionalization of gelatin and production of gelatin-methacrylamide (GelMA).9 In contrast to gelatin, this semi-synthetic hydrogel has better mechanical properties, while still keeping its biological properties. GelMA has been used in the engineering of different tissues, like bones, myocardium, cardiac tissues, cartilage, vascular networks, skeletal muscle and more.10
Comparison of different biomaterials can help in standardization and better development of engineered oral mucosa models for the potential laboratories and clinical applications. Although the suitability of some collagen-based and synthetic scaffolds for engineering human oral mucosa have been successfully examined in the past,11 the possibility of using GelMA for this purpose has not been studied so far. The aim of this study was to compare collagen and GelMA hydrogels as carriers for fibroblasts and as connective tissue substrate for seeding of oral epithelial cells.
2. Materials and methods
2.1. Isolation and expansion of oral fibroblast and keratinocyte cells
Gingival fibroblasts were isolated from human gingival biopsies upon gaining approval by the Institutional Review Board of Marquette University (Milwaukee, USA) and according to our previous work.11 After overnight incubation in 4 mg/mL dispase (Sigma, USA), and separation of the connective tissue from the epithelium using two sterile forceps, the de-epithelialized lamina propria was minced, and fibroblasts were isolated by incubation of minced tissues in collagenase type I (0.05% w/v) for 1 h at 37 °C. Then, the digested tissue was centrifuged, thoroughly washed, and transferred to a tissue culture flask containing Dulbecco's modified Eagle's medium (DMEM; Corning, Mediatech Inc., USA), 10% v/v fetal bovine serum (FBS, Sigma), and 1% antibiotic/antimycotic (Sigma), then incubated at 37 °C in 5% CO2. Gingival keratinocytes were separated from the epithelial layer by cutting the tissue into small pieces and using trypsin–ethylenediaminetetraacetic acid (EDTA) for 20 min at 37 °C. Isolated cells were then cultured in Green's medium containing DMEM/Ham's F12 (3/1) (Gibco) supplemented with 10% v/v FBS, 1% antibiotic/antimycotic, 25 mg/mL adenine, 0.4 μg/mL hydrocortisone, 5 μg/mL insulin, 5 μg/mL transferrin, 1.3 ng/mL triiodothyronine, 1.3 ng/mL triiodothyronine and 5 ng/mL epidermal growth factor (EGF; Sigma). After reaching confluency, fibroblast and keratinocyte cells were expanded, and the three first cell subcultures were used in the following experiments.
2.2. Preparation of cell-embedded collagen hydrogel
Cellular collagen was developed following the protocol described by Dongari-Bagtzoglou and Kashleva.3 Briefly, the mixture of 10 × DMEM 13.8 mg/mL, FBS 8.5% (v/v), l-glutamine 2 mM, reconstitution buffer (22 mg mL/1 sodium bicarbonate and 20 mM HEPES) and 5 mg/mL rat-tail type I collagen (Nutragen, Advanced BioMatrix, San Diego, CA) was prepared on ice and neutralized to pH 7.4. The isolated gingival fibroblast cells, at a density of 1 × 106 cells/ml, were then mixed properly with the abovementioned solution. After the distribution of the mixture into six-well tissue culture inserts (0.4 μm pore size, 24 mm diameter, VWR), cell-laden collagens were incubated at 37 °C for 2 h to complete the collagen gelation. Finally, the regular medium was added inside and outside the inserts.
2.3. Preparation of cell-embedded GelMA hydrogel
To prepare cellular GelMA according to the manufacturer's reconstitution protocol (Cellink, Ref No: REP-VL-350000), lithium acylphosphinate photoinitiator (LAP; Cellink, US) was dissolved in DMEM at 60 °C for 5–10 min and sterilized using the 0.22 μm sterile filter. The sterilized solution was added to the sterile freeze-dried GelMA powder (Cellink). The mixture was stirred for 30 min at 70 °C to ensure dissolution. After checking the pH (7.0–7.5), fibroblast cell suspension prepared in FBS, and antibiotic/antimycotic was dispersed in GelMA solution so that the final solution contained 0.3% w/v LAP, 10% w/v GelMA, 10% v/v FBS, 1% antibiotic/antimycotic and 1 × 106 cells/ml. After thoroughly mixing, the warm solution (37 °C) was aliquoted into six-well tissue culture plate inserts. The cell-laden hydrogels were then exposed for 30 s to LED light (395 nm, 3 W) for cross-linking. The resulting polymerized hydrogels were washed with PBS to remove unreacted residues, submerged in the regular medium (inside and outside the insert) and transferred to an incubator.
2.4. Assessment of viability of encapsulated fibroblasts in GelMA and collagen
At 24 and 72 h after preparation of cell-laden hydrogels, the viability of gingival fibroblast cells within the collagen or GelMA constructs was investigated using the PrestoBlue (Invitrogen, USA) assay. For this purpose, the constructs were washed with PBS, and a solution containing 10% v/v of PrestoBlue reagent in phenol red-free DMEM (HyClone Laboratories, Inc., Logan, UT, USA) was applied to each sample. After a 3-h incubation at 37 °C and 5% CO2, the fluorescence intensity of triplicate aliquots of each sample, which was transferred to a 96-well plate, was measured by a microplate reader (Synergy HTX, BioTEK) at excitation/emission wavelengths of 540/590 nm. The percentage of the fluorescence intensity in all groups over that in the collagen at day 1 was used for calculating the cell viability. At each time point, samples were submerged in the fresh medium after measurements.
2.5. Engineering of the full-thickness oral mucosa
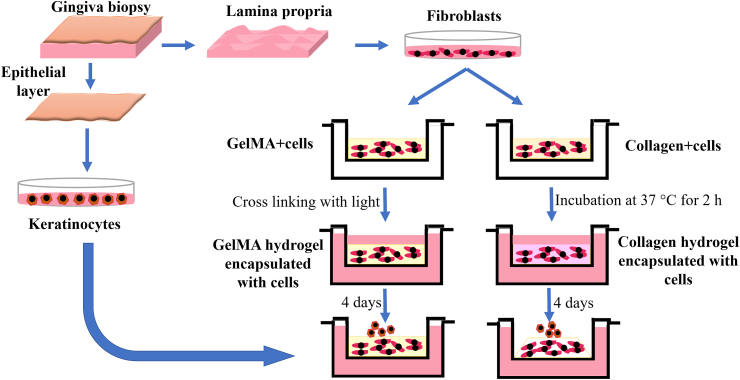
Four days after solidification of hydrogels, the isolated oral keratinocytes (50 μL of cell suspension containing 1 × 106 cells) were seeded onto the surface of each construct. Fig. 1 shows the different steps of isolation of fibroblast and keratinocyte cells from gingiva biopsies, cell encapsulation in collagen and GelMA hydrogels, and keratinocyte seeding.
Fig. 1.
Workflow chart showing the different steps of isolation of cells, cell encapsulation in collagen and GelMA hydrogels and keratinocyte seeding.
After 2 h of incubation to promote adhesion of keratinocytes to the substrate, Green's medium was added inside the inserts. All samples were incubated for 4 days, then were raised to the air-liquid interface for 10 more days.
2.6. Histology
For histological examination, samples were fixed in 10% v/v buffered formalin for 24 h, processed overnight with an automatic tissue processor (KEDEE, China) and embedded in paraffin wax. After cutting 5 μm sections, the samples were stained with hematoxylin and eosin (H&E) and examined under a light microscope (Evos Fluorescent, life technologies).
2.7. Statistical analysis
To analyze the differences between cell-laden collagen and GelMA, a one-way analysis of variance (ANOVA) was performed with Tukey's test using GraphPad Prism (GraphPad, CA, USA). Data were represented as mean ± standard deviation, considering p < 0.05 statistically relevant.
3. Results
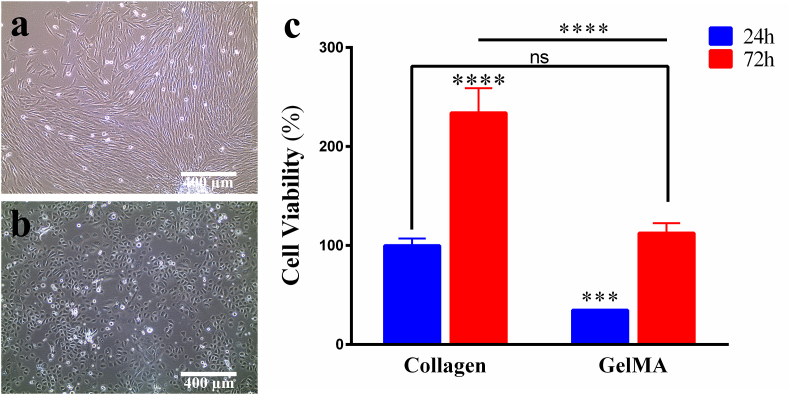
The isolated fibroblasts show a spindle-shaped appearance, while epithelial cells have a cuboidal shape (Fig. 2a and b). The cytotoxicity of GelMA and collagen was assessed at day 1 and day 3 by using PrestoBlue assay (Fig. 2c). The percentage of cell viability in GelMa tested 24 h after cell encapsulation was 34.78% and increased to 112.5% after 3 days of culture. The cell viability assay demonstrates that collagen hydrogels encapsulating fibroblasts display significantly higher cell viability than cell-laden GelMA constructs after 24 h (p < 0.05) (Fig. 2c). The cell viability increases significantly in both groups after 72 h (p < 0.05). However, lower cell survival in GelMA and a greater level of cell viability in collagen hydrogels is evident at both time points (p < 0.05).
Fig. 2.
Morphology of the isolated (a) fibroblasts and (b) keratinocytes. (c) The viability of fibroblasts encapsulated into collagen or GelMA analyzed by PrestoBlue assay after one day and three days of cultivation. Cell viability was expressed as percentages derived from the fluorescence intensity of each sample divided by the mean fluorescence intensity of the collagen group at 24 h *p < 0.05, ns: non-significant.
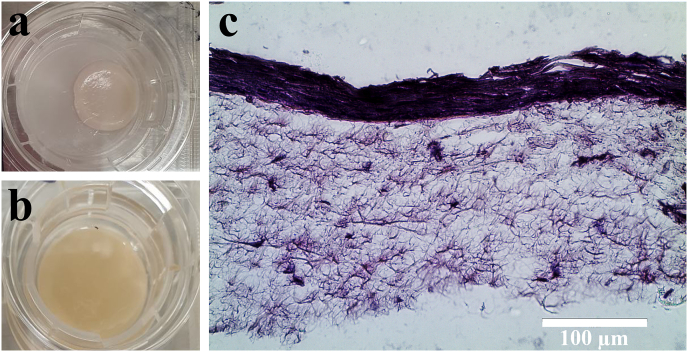
As shown in Fig. 3a and b, while collagen hydrogels are considerably contracted in 4 days, cross-linked GelMA hydrogels maintain their initial shape and size without any noticeable contraction. The histological evaluation confirms the adherence of the seeded keratinocytes onto the fibroblast-populated collagen gel and development of a multilayered stratified epithelium on its surface (Fig. 3d). The engineering of oral mucosa using GelMA was unsuccessful due to the lack of adhesion and proliferation of epithelial cells.
Fig. 3.
Images of (a) contracted cell-laden collagen gel and (b) GelMA hydrogel containing fibroblasts after 4 days of cultivation. (c) H&E stained histological section of tissue engineered oral mucosa based on collagen hydrogel (magnification: 40×).
4. Discussion
Over the last few years, a variety of biomaterials have been investigated in terms of suitability for encapsulation of the fibroblasts in oral mucosa engineering. The aim of this study was to investigate the viability of fibroblasts encapsulated into collagen or GelMA, as the first step in the engineering of the oral mucosa.
The concentration of GelMA used in our study was 10%. The reason for choosing this concentration was that more cell spreading and survival rate, along with less degradation and mass swelling of this concentration have been previously demonstrated compared to a lower concentration of GelMA.12 Researchers also confirmed the decrease of cell viability with increasing concentration of GelMA.13, 14, 15
We used LAP as the water-soluble photocrosslinking agent. Although 2-hydroxy-1-[4-(2-hydroxyethoxy) phenyl]-2-methyl-1-propanone (Irgacure 2959) can also be used for this purpose, its low solubility in water and the need for UV exposure, which can damage cells have reduced its use.16,17 In the study by Monteiro et al. more detrimental effect of UV polymerization on cells in comparison to visible wavelengths have been confirmed.18 On the other hand, LAP is a visible light photoinitiator that can be used at lower concentrations compared to Irgacure 2959.19,20
In this study, the viability of fibroblasts in the collagen and GelMA hydrogels was investigated after one and three days of cultivation, demonstrating lower cell viability in GelMA than that in collagen at both time points. The denser network properties and reduced transportation of cell nutrients could be a possible reason for this decreased viability in GelMA.21 In a study by Krouwels et al. who compared the human nucleus pulposus cells performances in collagen, GelMA, alginate, agarose, fibrin, and hyaluronic acid-poly (ethylene glycol) hydrogels, more DNA content was observed in fibrin and collagen hydrogels in comparison to other groups.22 Similarly, the study of Ma et al. showed lower cell viability and lower expression of the proliferation marker gene in the cells encapsulated in GelMA scaffolds in comparison to those in the decellularized extracellular matrix (dECM)-based and collagen I-based scaffolds.23
At day 4, we detected the obvious contraction of collagen with a concave surface, which allowed keratinocyte seeding. However, no contraction was observed in the cell-laden GelMA constructs. The dense network of GelMA is likely to prevent its contraction. It was not possible to create an epithelial layer on the surface of the GelMA, while a complete epithelium had been formed on the surface of the cell-laden collagen. Zhao et al. in their study, reported that to produce an organized epidermis on the surface of GelMA, its thickness should be less than 200 μm.24 We aliquoted 2 mL of the cell-hydrogel mixture in each well, which resulted in a thickness of about 0.5 mm. It seems that the high thickness of GelMA and its low-permeable structure prevent sufficient nutrient transport to the epithelial cells.
Although fibroblast viability and epithelial cell adhesion were not supported by GelMA in our study, the type of cells loaded in the hydrogel can lead to a different result. Lin et al. prepared a 5% w/v GelMA containing 0.5% w/v Irgacure 2959. After encapsulation of the endothelial colony-forming cells and mesenchymal stem cells in this hydrogel, they compared the extent of vascular network formation in this construct to that of bovine type-I collagen. The result of their study showed higher vascular network formation in GelMA.25
Encapsulation of fibroblasts in GelMA can be further optimized by changing different parameters, such as the methacrylation degree, cross-linking density, the thickness of samples, and reducing LAP concentration. Further studies are required to explore the potential approaches in modifying GelMA to improve its properties in terms of epithelial cell adhesion. Development and evaluation of hydrogel scaffolds based on a combination of GelMA with collagen or other natural scaffolds, as well as surface coating with bio-active materials, could be the potential avenues to overcome the challenges observed in this study.
5. Conclusions
Based on the results of this study, it can be concluded that collagen-based hydrogel offers superior biological properties compared to GelMA hydrogel in terms of oral fibroblast growth within the scaffold and epithelial cell adhesion and differentiation on the surface of the engineered substrate. Therefore, collagen-based hydrogels remain the preferred choice of the connective tissue scaffold for oral mucosa tissue engineering.
Declaration of competing interest
None.
Acknowledgement
This study was supported by National Institute of Dental & Craniofacial Research of the National Institutes of Health (NIH) [grant number R15DE027533.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Moharamzadeh K. Oral mucosa tissue engineering. In: Tayebi L., Moharamzadeh K., editors. Biomaterials for Oral and Dental Tissue Engineering. 2017. pp. 223–244. [Google Scholar]
- 2.Tabatabaei F., Moharamzadeh K., Tayebi L. Three-dimensional in vitro oral mucosa models of fungal and bacterial infections. Tissue Eng Part B: Rev. 2020 doi: 10.1089/ten.teb.2020.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dongari-Bagtzoglou A., Kashleva H. Development of a highly reproducible three-dimensional organotypic model of the oral mucosa. Nat Protoc. 2006;1:2012–2018. doi: 10.1038/nprot.2006.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kinikoglu B., Damour O., Hasirci V. Tissue engineering of oral mucosa: a shared concept with skin. J Artif Organs. 2015;18:8–19. doi: 10.1007/s10047-014-0798-5. [DOI] [PubMed] [Google Scholar]
- 5.Nuttelman C.R., Rice M.A., Rydholm A.E., Salinas C.N., Shah D.N., Anseth K.S. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog Polym Sci. 2008;33:167–179. doi: 10.1016/j.progpolymsci.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajan N., Habermehl J., Cote M.F., Doillon C.J., Mantovani D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat Protoc. 2006;1:2753–2758. doi: 10.1038/nprot.2006.430. [DOI] [PubMed] [Google Scholar]
- 7.Moharamzadeh K., Brook I., Van Noort R., Scutt A., Thornhill M. Tissue-engineered oral mucosa: a review of the scientific literature. J Dent Res. 2007;86:115–124. doi: 10.1177/154405910708600203. [DOI] [PubMed] [Google Scholar]
- 8.Jaipan P., Nguyen A., Narayan R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017;7:416–426. [Google Scholar]
- 9.Van Den Bulcke A.I., Bogdanov B., De Rooze N., Schacht E.H., Cornelissen M., Berghmans H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules. 2000;1:31–38. doi: 10.1021/bm990017d. [DOI] [PubMed] [Google Scholar]
- 10.Van Hoorick J., Tytgat L., Dobos A. Photo-)crosslinkable gelatin derivatives for biofabrication applications. Acta Biomater. 2019;97:46–73. doi: 10.1016/j.actbio.2019.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Moharamzadeh K., Brook I.M., Van Noort R., Scutt A.M., Smith K.G., Thornhill M.H. Development, optimization and characterization of a full-thickness tissue engineered human oral mucosal model for biological assessment of dental biomaterials. J Mater Sci Mater Med. 2008;19:1793–1801. doi: 10.1007/s10856-007-3321-1. [DOI] [PubMed] [Google Scholar]
- 12.Athirasala A., Lins F., Tahayeri A. A novel strategy to engineer pre-vascularized full-length dental pulp-like tissue constructs. Sci Rep. 2017;7:3323. doi: 10.1038/s41598-017-02532-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colosi C., Shin S.R., Manoharan V. Microfluidic bioprinting of heterogeneous 3D tissue constructs using low-viscosity bioink. Adv Mater. 2016;28:677–684. doi: 10.1002/adma.201503310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W., Heinrich M.A., Zhou Y. Extrusion bioprinting of shear-thinning gelatin methacryloyl bioinks. Adv Healthc Mater. 2017;6 doi: 10.1002/adhm.201601451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang L., Highley C.B., Sun W., Burdick J.A. A Generalizable strategy for the 3D bioprinting of hydrogels from nonviscous photo-crosslinkable inks. Adv Mater. 2017;29:1604983. doi: 10.1002/adma.201604983. [DOI] [PubMed] [Google Scholar]
- 16.Kappes U.P., Luo D., Potter M., Schulmeister K., Rünger T.M. Short- and long-wave UV light (UVB and UVA) induce similar mutations in human skin cells. J Invest Dermatol. 2006;126:667–675. doi: 10.1038/sj.jid.5700093. [DOI] [PubMed] [Google Scholar]
- 17.Williams C.G., Malik A.N., Kim T.K., Manson P.N., Elisseeff J.H. Variable cytocompatibility of six cell lines with photoinitiators used for polymerizing hydrogels and cell encapsulation. Biomaterials. 2005;26:1211–1218. doi: 10.1016/j.biomaterials.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 18.Monteiro N., Thrivikraman G., Athirasala A. Photopolymerization of cell-laden gelatin methacryloyl hydrogels using a dental curing light for regenerative dentistry. Dent Mater. 2018;34:389–399. doi: 10.1016/j.dental.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benton J.A., DeForest C.A., Vivekanandan V., Anseth K.S. Photocrosslinking of gelatin macromers to synthesize porous hydrogels that promote valvular interstitial cell function. Tissue Eng. 2009;15:3221–3230. doi: 10.1089/ten.tea.2008.0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fairbanks B.D., Schwartz M.P., Bowman C.N., Anseth K.S. Photoinitiated polymerization of PEG-diacrylate with lithium phenyl-2,4,6-trimethylbenzoylphosphinate: polymerization rate and cytocompatibility. Biomaterials. 2009;30:6702–6707. doi: 10.1016/j.biomaterials.2009.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billiet T., Gevaert E., De Schryver T., Cornelissen M., Dubruel P. The 3D printing of gelatin methacrylamide cell-laden tissue-engineered constructs with high cell viability. Biomaterials. 2014;35:49–62. doi: 10.1016/j.biomaterials.2013.09.078. [DOI] [PubMed] [Google Scholar]
- 22.Krouwels A., Melchels F.P.W., van Rijen M.H.P. Comparing hydrogels for human nucleus pulposus regeneration: role of osmolarity during expansion. Tissue Eng C Methods. 2018;24:222–232. doi: 10.1089/ten.TEC.2017.0226. [DOI] [PubMed] [Google Scholar]
- 23.Ma X., Yu C., Wang P. Rapid 3D bioprinting of decellularized extracellular matrix with regionally varied mechanical properties and biomimetic microarchitecture. Biomaterials. 2018;185:310–321. doi: 10.1016/j.biomaterials.2018.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao X., Lang Q., Yildirimer L. Photocrosslinkable gelatin hydrogel for epidermal tissue engineering. Adv Healthc Mater. 2016;5:108–118. doi: 10.1002/adhm.201500005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin R.Z., Chen Y.C., Moreno-Luna R., Khademhosseini A., Melero-Martin J.M. Transdermal regulation of vascular network bioengineering using a photopolymerizable methacrylated gelatin hydrogel. Biomaterials. 2013;34:6785–6796. doi: 10.1016/j.biomaterials.2013.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]