Abstract
Introduction
Uterine sarcomas are a group of rare tumours with heterogeneous morphological and genetic features. Recent advances in the molecular characterisation of these tumours have identified a novel clinicopathological category underpinned by NTRK gene fusions.
Case report
We present the case of a 42-year-old woman with a polypoid cervical lesion formed of densely cellular, short, haphazard fascicles of monomorphic spindle cells that lacked coagulative necrosis and which showed high mitotic activity. On immunohistochemistry, the tumour was diffusely positive for pan-Trk and weakly positive for CD34 but was negative for a range of other markers, including cytokeratins, smooth muscle markers, hormone receptors and S100. FISH analysis using a NTRK1 break-apart probe was above the threshold for translocation positivity and subsequent next-generation sequencing (NGS) identified a TPM3-NTRK1 fusion.
Discussion
NTRK-rearranged uterine sarcomas are a novel subset of gynaecological mesenchymal neoplasms characterised by cytological isomorphism and fibrosarcoma-like morphology. Although distinction from more common mesenchymal neoplasms is possible on the basis of morphology and immunohistochemistry, exclusion of rare differential diagnoses, such as malignant peripheral nerve sheath tumour or the recently described COL1A1-PDGFB fusion sarcoma, requires molecular work-up with FISH or NGS. Identification of these rare tumours is clinically relevant because of their cervical location and the possible role for tropomyosin receptor kinase inhibitors in their treatment.
Keywords: NTRK, Uterus, Sarcoma
Highlights
-
•
NTRK-rearranged uterine sarcomas are a novel subset of gynaecological mesenchymal neoplasms.
-
•
They present as intramural or polypoid masses commonly involving the cervix in pre-menopausal women.
-
•
The diagnosis is clinically relevant because treatment with tropomyosin receptor kinase inhibitors may be considered.
1. Introduction
The categorisation of uterine sarcomas relies upon the recognition of characteristic morphological and immunophenotypic features with an expanding role for diagnostic molecular genetic investigations. This is illustrated by the re-emergence of high-grade endometrial stromal sarcoma as an entity in the 4th edition of the WHO classification, which was predicated upon the identification of YWHAE-FAM22 translocations in a subset of tumours with high-grade morphology and aggressive clinical behaviour [1]. The subsequent discovery of BCOR and SUZ12 gene rearrangements in high-grade endometrial stromal sarcomas has further refined our understanding of the clinico-pathological-genetic correlation of these tumours [2,3].
Undifferentiated uterine sarcomas are rare tumours showing high-grade cytological atypia and high mitotic activity which lack evidence of specific differentiation. The diagnosis is used as a ‘wastebasket’ category for high-grade tumours lacking demonstrable features of more common mesenchymal tumours, such as leiomyosarcoma or endometrial stromal sarcoma. They comprise a heterogeneous group of tumours which show complex and varied chromosomal abnormalities. With increased interrogation of the underlying molecular changes, this category is being refined [4].
Recent studies have identified NTRK gene fusions in a subset of uterine sarcomas showing fibrosarcoma-like morphology [5,6]. We present a case of a uterine sarcoma that demonstrated morphological features of an NTRK fusion-associated uterine sarcoma, confirmed on immunohistochemistry and cytogenetic investigations and next-generation sequencing.
2. Case Presentation
A 42-year-old woman presented with a lesion on the posterior cervix identified during investigation for irregular vaginal bleeding. Biopsy and subsequent hysterectomy revealed a 52 mm uterus-confined infiltrative polypoid lesion arising from the posterior cervical wall and submucosal areas of the anterior lip of the cervix which widely infiltrated cervical stroma.
On histology, the tumour was uniformly densely cellular and was formed of spindle cells with plump nuclei with even, fine chromatin. The cells were predominantly monomorphic; however, scattered individual cells with enlarged, irregular hyperchromatic nuclei were seen (Fig. 1). Many of these larger cells had copious eosinophilic cytoplasm and resembled ganglion cells. Much of the lesion was formed of short haphazard fascicles associated with pale, eosinophilic matrix. Focally, the spindle cells were set within pale myxoid matrix, imparting a tissue culture-like appearance (Fig. 2). A prominent infiltrate of lymphocytes and plasma cells was seen throughout the lesion. Occasional entrapped tubular glands were seen which were lined by bland endocervical type glandular epithelium. Scattered capillary-sized vessels were seen; however, there was no distinctive vascular architecture. There was variable mitotic activity with an average mitotic count of 8 mitoses per 10 high-power fields, including atypical forms. There was no coagulative tumour necrosis.
Fig. 1.
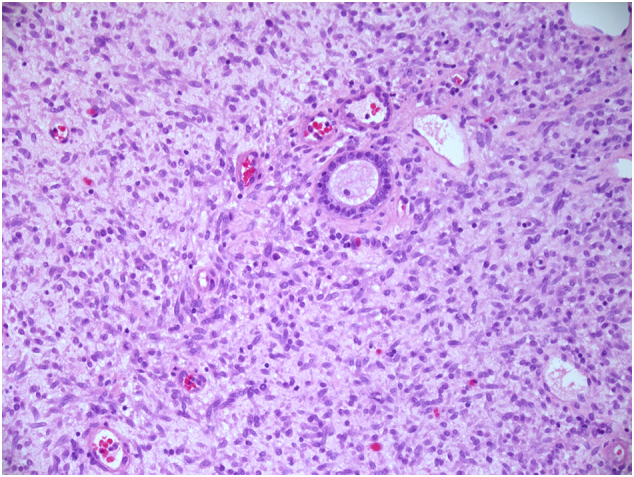
Small inactive gland entrapped by neoplastic spindle cells with hyperchromatic nuclei. There is no periglandular stromal condensation (×200 magnification).
Fig. 2.
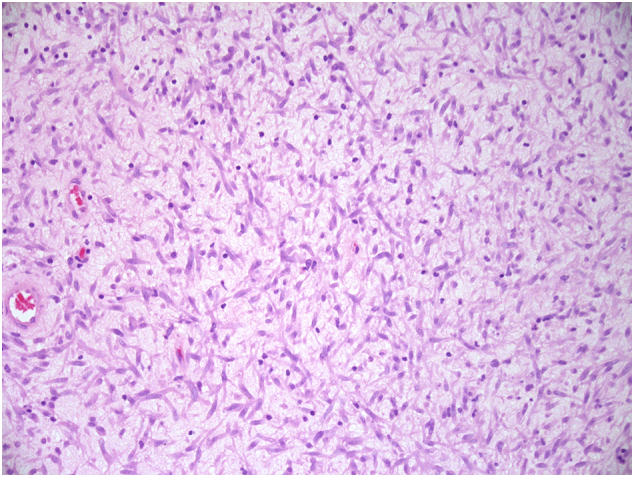
Neoplastic cells within loose myxoid matrix with scattered chronic inflammatory cells imparting a tissue culture-like appearance (×200 magnification).
A panel of immunohistochemistry revealed the tumour cells to be positive for CD10 and vimentin and negative for cytokeratins and smooth muscle markers (desmin, SMA and caldesmon). The tumour was also negative for ALK1, S100, SOX10, cKIT, DOG1, CD21, CD23 and PR and ER. A small number of cells were positive for Cyclin D1. CD34 showed weak cytoplasmic staining. Pan-TRK immunohistochemistry showed diffuse strong cytoplasmic staining.
FISH analysis of this specimen was performed using the Zytovision ZytoLight SPEC NTRK1 dual-colour break-apart probe (Z-2167-200) which detects translocations involving the chromosomal region 1q22-q23.1 harbouring the NTRK1 gene. Out of 100 cells observed, there were 16 cells that were >2 signal distance apart and 19 cells that were >1 signal distance apart. This is above the threshold for NTRK1 translocation positivity. FISH analysis performed using ZytoLight SPEC dual-colour break-apart probes for both NTRK2 and NTRK3 returned results below the threshold for translocation positivity.
Next-generation sequencing was performed using a 28-gene NGS panel. Sequencing libraries were prepared using the hybridisation-based Illumina Nextera rapid-capture enrichment protocol and sequenced on a MiSeq using V3 chemistry. Alignment and small variant calling was performed by MiSeq Reporter version 2.5 (BWA & the Somatic Variant Caller) and variants were annotated using Illumina Variant Studio 2.2. The standard protocol recommends 50 ng of DNA in a volume of 10ul. Briefly, after the tagmentation step the recovered DNA underwent a first PCR amplification (10 cycles). DNA was then hybridised with the gene- specific probes in two rounds. After the second hybridisation the DNA was amplified by PCR (18 cycles), purified, quantified (tapestation) and 13pmoles loaded onto MiSeq. Next-generation sequencing revealed the presence of a rearrangement of the NTRK1 gene (NTRK1 (exon 9)-TMP3 (intron 6)).
Following hysterectomy, the patient was treated with adjuvant pelvic radiotherapy and brachytherapy, which was completed 4 months after diagnosis. A surveillance CT scan performed 5 months after hysterectomy showed no evidence of recurrence. The patient remained clinically disease-free 11 months after surgery.
3. Discussion
We report a case of a TPM3-NTRK1 fusion in a uterine sarcoma. This tumour represents a subset of uterine sarcomas that form a novel clinicopathological category associated with activating mutations of the NTRK gene. Neurotrophic tyrosine kinase receptor (NTRK) is a group of proto-oncogenes that include NTRK1, NTRK2 and NTRK3 which encode Trk proteins that are involved in neuronal cell growth and differentiation. There is increasing recognition of the role of NTRK mutations in the pathogenesis of numerous solid tumours. NTRK rearrangements are implicated in a range of adult malignant tumours and are recognised as driver mutations in a number of paediatric soft-tissue tumours, including infantile fibrosarcomas and locally aggressive lipofibromatosis-like neural tumours [7,8]. Larotrectinib, a pan-TRK inhibitor, has demonstrated tissue type-agnostic efficacy in tumours harbouring NTRK mutations [9].
Recently discovered NTRK-rearranged uterine sarcomas present as intramural or polypoid masses involving the cervix or, less commonly, the corpus, in pre-menopausal women. In line with numerous other mesenchymal neoplasms which harbour recurrent translocations, these tumours are characterised by cytological isomorphism. Densely cellular fascicles of spindle cells with monomorphic nuclei resembling fibrosarcomas are expected. The presence of scatted pleomorphic cells has also been previously reported [5,6]. Limited data on clinical outcomes in confirmed cases are available; however, there appears to be a high frequency of early recurrence.
NTRK-rearranged uterine sarcomas have numerous fusion partners, with RBPMS-NTRK3, TPR-NTRK1, LMNA-NTRK1, TPM3-NTRK1, EML4-NTRK3 and STRN-NTRK3 translocations so far reported [5,6,10].
A number of factors may prompt consideration of a diagnosis of NTRK fusion sarcoma. NTRK rearranged uterine sarcomas present in a pre-menopausal age group, in contrast to leiomyosarcomas and endometrial stromal sarcomas, which typically present in post-menopausal women. Leiomyosarcoma remains a credible differential diagnosis because of a number of histological characteristics shared with NTRK fusion sarcomas, such as spindle cells and high mitotic count. The relative cytological uniformity seen in NTRK fusion sarcomas contrasts with haphazard architecture, high mitotic count and an absence of tumour necrosis, thus prompting ancillary testing. In contrast to uterine tumours of smooth muscle derivation, NTRK fusion sarcomas do not stain for desmin, ER and PR but may show focal positive staining for SMA. Case series have reported diffuse positivity for S100 and/or CD34 in NTRK fusion sarcomas. In keeping with our findings, reports of expression of both of these markers have not been universally corroborated. [5,6,10].
Although it may be possible to discount common differential diagnoses using simple adjuncts, confirmatory FISH or sequencing is mandatory in suspected cases of NTRK-rearranged uterine sarcomas. NTRK sarcomas are members of a recently described group of uterine fibrosarcoma-like, S100 positive mesenchymal tumours which also includes the novel COL1A1-PDGFB fusion sarcomas and fibroblastic tumours with features of malignant peripheral nerve sheath tumour [6]. Trk A and pan-Trk are the only reliable immunhistochemical investigations allowing distinction of NTRK rearranged sarcomas from these mimics. Expression of these commercially available antibodies is highly sensitive for tumours harbouring NTRK fusions [11]. Although negative in other fibrosarcoma-like, S100 positive mesenchymal tumours, Trk A or pan-Trk expression has been reported in 6% of uterine leiomyosarcomas lacking NTRK rearrangements.
In conclusion, the recently described NTRK-rearranged uterine sarcoma has emerged as a subset of undifferentiated uterine sarcoma characterised by cytological monomorphism and fibrosarcoma-like histomorphology. Whilst common immunomarkers can be used to exclude a number of common differential diagnoses, the NTRK-rearranged sarcoma lacks a consistently distinctive immunoprofile and therefore molecular testing is required for diagnosis. The accurate identification of these rare tumours is clinically relevant given the tissue type-agnostic efficacy of tropomyosin receptor kinase inhibitors in the treatment of malignant tumours with NTRK mutations.
Acknowledgments
Contributors
William Boyle contributed to conception and design of the article, analysis and interpretation of data, drafting the article, and final approval of the version submitted.
Anthony Williams contributed to analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version submitted.
Sudha Sundar contributed to the provision of clinical information.
Jason Yap contributed to the provision of clinical information.
Philippe Taniere contributed to analysis and interpretation of data, revising the article critically for important intellectual content, and final approval of the version submitted.
Pauline Rehal contributed to analysis and interpretation of data, and final approval of the version submitted.
Raji Ganesan contributed to conception and design of article, analysis and interpretation of data, drafting the article, revising the article critically for important intellectual content, and final approval of the version submitted.
Conflict of Interest
The authors declare that they have no conflict of interest regarding the publication of this case report.
Funding
No funding from an external source supported the publication of this case report.
Patient Consent
Obtained.
Provenance and Peer Review
Peer review was directed by Professor Margaret Rees, Editor in Chief. Raji Ganesan, one of the authors and an editorial board member of Case Reports in Women's Health, was blinded to the process.
References
- 1.Lee C.H., Mariño-Enriquez A., Ou W., Zhu M., Ali R.H., Chiang S., Amant F., Gilks C.B., van de Rijn M., Oliva E., Debiec-Rychter M., Dal Cin P., Fletcher J.A., Nucci M.R. The clinicopathologic features of YWHAE-FAM22 endometrial stromal sarcomas: a histologically high-grade and clinically aggressive tumor. Am. J. Surg. Pathol. 2012 May;36(5):641–653. doi: 10.1097/PAS.0b013e31824a7b1a. [DOI] [PubMed] [Google Scholar]
- 2.Hoang L.N., Aneja A., Conlon N., Delair D.F., Middha S., Benayed R., Hensley M.L., Park K.J., Hollmann T.J., Hameed M.R., Antonescu C.R., Soslow R.A., Chiang S. Novel high-grade endometrial stromal sarcoma: a morphologic mimicker of myxoid leiomyosarcoma. Am. J. Surg. Pathol. 2017 Jan;41(1):12–24. doi: 10.1097/PAS.0000000000000721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mansor S., Kuick C.H., Lim S.L., Quek R., Wong A.P.C., Lim-Tan S.K., Lim T.Y.K., Chang K.T.E. ZC3H7B-BCOR-rearranged endometrial stromal sarcomas: a distinct subset merits its own classification? Int. J. Gynecol. Pathol. 2019 Sep;38(5):420–425. doi: 10.1097/PGP.0000000000000523. [DOI] [PubMed] [Google Scholar]
- 4.Momeni-Boroujeni A., Chiang S. Uterine mesenchymal tumours: recent advances. Histopathology. 2020 Jan;76(1):64–75. doi: 10.1111/his.14008. [DOI] [PubMed] [Google Scholar]
- 5.Chiang S., Cotzia P., Hyman D.M., Drilon A., Tap W.D., Zhang L., Hechtman J.F., Frosina D., Jungbluth A.A., Murali R., Park K.J., Soslow R.A., Oliva E., Iafrate A.J., Benayed R., Ladanyi M., Antonescu C.R. NTRK fusions define a novel uterine sarcoma subtype with features of fibrosarcoma. Am. J. Surg. Pathol. 2018 Jun;42(6):791–798. doi: 10.1097/PAS.0000000000001055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Croce S., Hostein I., Longacre T.A., Mills A.M., Pérot G., Devouassoux-Shisheboran M., Velasco V., Floquet A., Guyon F., Chakiba C., Querleu D., Khalifa E., Mayeur L., Rebier F., Leguellec S., Soubeyran I., McCluggage W.G. Uterine and vaginal sarcomas resembling fibrosarcoma: a clinicopathological and molecular analysis of 13 cases showing common NTRK-rearrangements and the description of a COL1A1-PDGFB fusion novel to uterine neoplasms. Mod. Pathol. 2019 Jul;32(7):1008–1022. doi: 10.1038/s41379-018-0184-6. (Epub 2019 Mar 16) [DOI] [PubMed] [Google Scholar]
- 7.Davis J.L., Lockwood C.M., Stohr B., Boecking C., Al-Ibraheemi A., DuBois S.G., Vargas S.O., Black J.O., Cox M.C., Luquette M., Turpin B., Szabo S., Laetsch T.W., Albert C.M., Parham D.M., Hawkins D.S., Rudzinski E.R. Expanding the spectrum of pediatric NTRK-rearranged mesenchymal tumors. Am. J. Surg. Pathol. 2019 Apr;43(4):435–445. doi: 10.1097/PAS.0000000000001203. [DOI] [PubMed] [Google Scholar]
- 8.Agaram N.P., Zhang L., Sung Y.S., Chen C.L., Chung C.T., Antonescu C.R., Fletcher C.D. Recurrent NTRK1 gene fusions define a novel subset of locally aggressive lipofibromatosis-like neural tumors. Am. J. Surg. Pathol. 2016 Oct;40(10):1407–1416. doi: 10.1097/PAS.0000000000000675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan L., Zhang W. Precision medicine becomes reality-tumor type-agnostic therapy. Cancer Commun. (Lond.) 2018 Mar 31;38(1):6. doi: 10.1186/s40880-018-0274-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michal M., Hajkova V., Skalova A., Michal M. STRN-NTRK3-rearranged mesenchymal tumor of the uterus. Expanding the morphologic spectrum of tumours with NTRK fusions. Am. J. Surg. Pathol. 2019 Aug;43(8):1152–1153. doi: 10.1097/PAS.0000000000001292. [DOI] [PubMed] [Google Scholar]
- 11.Hechtman J.F., Benayed R., Hyman D.M., Drilon A., Zehir A., Frosina D., Arcila M.E., Dogan S., Klimstra D.S., Ladanyi M., Jungbluth A.A. Pan-Trk immunohistochemistry is an efficient and reliable screen for the detection of NTRK fusions. Am. J. Surg. Pathol. 2017 Nov;41(11):1547–1551. doi: 10.1097/PAS.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]


