Abstract
Background
Kidney pathologies often result in change in renal size. Knowledge of normal kidney sizes is important for screening, diagnosis, prognosis and follow-up management of paediatric renal diseases.
Objectives
The aim of this study was to establish the age-, height- and weight-matched kidney dimensions in apparently healthy Nigerian children.
Method
A descriptive, cross-sectional study of right and left kidney parameters (length, width, thickness and volume) of 1315 school-aged Nigerian children was conducted over 8 months. Ages ranged from 5 to 17 years. Parameters were obtained using a General Electric (GE) LOGIC 400CL ultrasound machine. Kidney dimensions were correlated with age, sex and anthropometric measurements.
Results
Normative values for all the kidney parameters for each age, height and weight groups and also gender were established for the study population. The left kidneys were noted to be longer and thicker, and of more volume than the right kidneys. The right kidneys were seen to be wider (p < 0.01). Length of the left kidneys in females was noted to be more than those of the males in the age- and weight-matched categories (p < 0.05). The width of both kidneys was higher in the males in all the categories (p < 0.05). Males showed higher values of thickness and volume in the height category. All the renal parameters significantly correlated with body size indicators, except for body mass index.
Conclusion
This study has established gender-, age-, weight- and height-specific range of values of the kidney parameters of apparently healthy children together with regression models.
Keywords: Kidney, Renal sizes, Children, Ultrasound, Nigeria
Introduction
Anomalies of renal sizes are associated with and are manifestations of diseases involving the kidneys.1 The importance of accurate reference values of children’s renal sizes measured by ultrasonography cannot be overemphasised.2 Ultrasonography is without risk of ionising radiation and is therefore safe in the evaluation of growing children. It also provides a quick and accurate assessment of other visceral organ dimensions.3 Reports have demonstrated that renal length differs from race to race.4,5,6 The size and weight of an organ have also been shown to be influenced by environmental variations, ethnicity, hereditary components, routine diet, water intake7,8 and high altitudes, where atmospheric pressure is reduced, with the partial pressure of oxygen altering the physiology of the kidneys.9
The kidneys can be affected by congenital or acquired diseases, either localised or systemic. Examples are solitary kidney, renal hypoplasia,10 multicystic and polycystic kidneys, acute malaria because of plasmodium falciparum,11 auto-immune diseases such as Kawasaki disease,12 recurrent urinary tract infection, neoplasms, urolithiasis, trauma, drugs, ingestion of native concoctions, diabetes, hypertension, renal artery stenosis and so on. Knowledge of renal size helps in differentiating acute from chronic kidney diseases (CKD).2,13 Chronic diseases include diseases that reduce the size of the kidneys such as chronic glomerulonephritis, nephrosclerosis and diabetic nephropathy, and those that increase its sizes such as multi- or polycystic kidney diseases and so on. In addition, renal length and volume are very important parameters for numerous purposes such as the assessment of candidates for/with kidney transplant, decision in obtaining renal biopsies and follow-up of patients with end-stage liver disease in which nephromegaly and increased echogenicity of renal cortex can be associated with pathological findings (renal size usually reverses after liver transplant). Kidney size is an important parameter used for the clinical evaluation of renal abnormalities, such as atrophy, hypoplasia and hypertrophy in children. Sonography is used to monitor the kidneys of children before and after liver transplants with sizes compared with published normative values.14 Renal involvement can be a part of a syndrome such as Beckwith–Wiedemann syndrome (BWS). This syndrome is reported to have a high risk of development of embryonic tumours such as Wilm’s tumour.15 Screening protocols with ultrasonography have been implemented in some countries for the early detection of these tumours16 because a criterion for its diagnosis is evidence of renal enlargement.
A study by Jones et al.17 demonstrated that renal volume, which correlates better with renal mass, is a more sensitive means of diagnosing kidney abnormality than any single linear measurement. Also at autopsy, renal volume has been reported to correlate well but indirectly with the number of functioning nephrons,18 hence its inclusion in this study.
A Nigerian study on paediatric hospital admissions by Esezobar et al.19 showed that acute renal disease accounted for up to 82.9% of admissions. It is invaluable to have a more comprehensive, standardised, sonographic measurement for use in the course of the renal assessment of a child, hence this study.
Materials and methods
This was a descriptive, cross-sectional study of the kidney parameters of apparently healthy, school-aged, Nigerian children without any known renal disease. Whilst informed consent was received from the parents of the children, child assent was also obtained from each child involved in this study. Detailed medical history of each child was acquired from the parents including pre-existing diseases that could affect the kidneys, past urologic surgeries and known history of chronic diseases. After clinical examination of each child by one of the authors who is a clinician, only apparently healthy children were recruited for the study.
Clinical exclusion criteria were fever, periorbital or pedal edema, macular or maculopapular rashes and sickle cell anaemia. Imaging exclusion criteria were altered echogenicity, presence of renal cysts, urolithiasis, unilateral kidney, hydronephrosis, ureterocele, renal ectopia, horseshoe kidneys and other developmental anomalies, and neoplasm.
A total of 1315 children (633 boys and 682 girls) between the ages of 5 and 17 years were selected for this study using a random selection method. Age, sex and anthropometric measurement of the body size indicators such as weight (WT), height (HT), body surface area (BSA) and body mass index (BMI) were obtained for each subject. Using the vertical scale of a portable stadiometer, each participant was placed, without shoes, in an upright position with the head held in the Frankfort plane and the height measured to the nearest 0.5 cm. With each participant lightly clothed, weight was measured with a weighing scale to the nearest 0.1 kg.
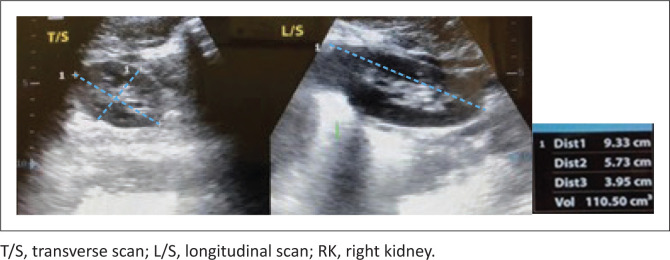
Kidney parameters were obtained using a GE LOGIC 400CL ultrasound machine made by GE medical systems with a 3.5 MHz curvilinear probe. Renal sizes by ultrasound were obtained by one of the authors who is a radiologist. To achieve greater accuracy, two sequential measurements were taken and the mean calculated. Kidney measurements were obtained with subjects in a prone position20 and in quiet respiration. The bipolar length of each kidney was measured from the highest to its lowest point. The width and thickness were obtained in the transverse plane in an orthogonal direction, near the renal hilum but free of the pelvis. The renal thickness or anterioposterior (AP) diameter, was measured in the same transverse plane with a line perpendicular to the width (at its central highest point), as shown in Figure 1. The probe therefore was not exactly perpendicular to the skin. No subject was included more than once. No sedation nor any preparation was used. The mean renal length and 5th and 95th percentiles were determined for each age. The BSA and BMI were calculated using the respective formulas:
FIGURE 1.
Showing points of measurements of a kidney.
BSA = (weight (kg) × height (m)/3600)1/2 (Mosteller formula).
BMI = weight (kg)/height2 (m).
Renal volume = length (cm) × width (cm) × thickness (cm) × 0.523.
Ethical considerations
Ethical approval to conduct the study was obtained from the College of Medicine Research Ethics Committee, University of Nigeria Enugu Campus (Reference no. 070/06/2019).
Results
The mean values of the left and right renal dimensions of the various age groups of the studied population have been presented in Table 1. Table 2 presents a height-matched comparison, whilst Table 3 shows a weight-matched comparison. Table 4 presents the renal correlation. The regression formula for the various renal dimensions is presented in Table 5.
TABLE 1.
Distribution of mean renal dimensions (cm) based on various age categories (years).
| Parameters | Sex | 6 years | 7 years | 8 years | 9 years | 10 years | 11 years | 12 years | 13 years | 14 years | 15 years | 16 years | 17 years |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right kidney length | M | 8.2 ± 0.6 | 8.6 ± 0.6 | 8.5 ± 0.6 | 8.9 ± 0.8 | 8.9 ± 0.7 | 9.0 ± 0.7 | 9.0 ± 0.9 | 9.7 ± 0.7 | 10.0 ± 0.8 | 10.2 ± 0.7 | 10.4 ± 0.9 | 10.1 ± 0.8 |
| F | 8.08 ± 0.6 | 8.5 ± 0.6 | 8.6 ± 0.9 | 8.8 ± 0.7 | 9.0 ± 0.8 | 9.0 ± 0.9 | 9.6 ± 0.7 | 9.5 ± 1.8 | 10.0 ± 1 | 10.0 ± 0.7 | 10.6 ± 1 | 10.2 ± 0.7 | |
| Left kidney length | M | 8.3 ± 0.6 | 8.6 ± 0.8 | 8.6 ± 0.7 | 9.0 ± 0.7 | 8.8 ± 1.1 | 9.1 ± 0.8 | 9.3 ± 1.0 | 9.4 ± 1.9 | 10.2 ± 0.8 | 10.6 ± 1.0 | 10.6 ± 0.8 | 10.3 ± 0.8 |
| F | 8.4 ± 0.7 | 8.5 ± 0.6 | 8.7 ± 0.6 | 9.0 ± 0.8 | 9.1 ± 0.9 | 9.1 ± 1 | 9.7 ± 0.8 | 10.0 ± 1.0 | 10.5 ± 0.9 | 10.5 ± 0.8 | 10.9 ± 0.9 | 10.2 ± 0.9 | |
| Right kidney width | M | 5.7 ± 0.7 | 6.1 ± 0.5 | 6.1 ± 0.6 | 6.3 ± 0.8 | 6.3 ± 0.6 | 6.4 ± 0.5 | 6.5 ± 0.8 | 7.1 ± 0.7 | 7.3 ± 0.7 | 7.2 ± 0.7 | 7.3 ± 0.6 | 7.3 ± 0.7 |
| F | 5.7 ± 0.6 | 6.0 ± 0.5 | 6.2 ± 0.7 | 6.2 ± 0.6 | 6.3 ± 0.6 | 6.3 ± 0.6 | 6.8 ± 0.7 | 6.9 ± 0.8 | 7.1 ± 0.7 | 7.1 ± 0.6 | 7.4 ± 0.8 | 7.5 ± 0.8 | |
| Left kidney width | M | 5.7 ± 0.5 | 6.2 ± 0.5 | 6.2 ± 0.5 | 6.3 ± 0.6 | 6.3 ± 0.6 | 6.3 ± 0.5 | 6.4 ± 0.8 | 7.0 ± 0.7 | 7.4 ± 0.7 | 7.2 ± 0.8 | 7.3 ± 0.6 | 7.5 ± 0.7 |
| F | 5.7 ± 0.6 | 6.0 ± 0.4 | 6.1 ± 0.6 | 6.1 ± 0.6 | 6.3 ± 0.6 | 6.2 ± 0.7 | 6.8 ± 0.7 | 7.1 ± 0.6 | 7.1 ± 0.6 | 7.3 ± 0.5 | 7.4 ± 0.7 | 7.3 ± 0.6 | |
| Right kidney thickness | M | 3.2 ± 0.4 | 3.4 ± 0.3 | 3.4 ± 0.4 | 3.5 ± 0.4 | 3.5 ± 0.4 | 3.6 ± 0.3 | 3.7 ± 0.6 | 3.8 ± 0.6 | 4.0 ± 0.5 | 4.0 ± 0.5 | 4.1 ± 0.4 | 4.0 ± 0.3 |
| F | 3.1 ± 0.4 | 3.4 ± 0.4 | 3.3 ± 0.4 | 3.5 ± 0.4 | 3.6 ± 0.4 | 3.6 ± 0.5 | 3.8 ± 0.0.8 | 3.9 ± 0.4 | 4.0 ± 0.4 | 4.7 ± 0.5 | 4.1 ± 0.5 | 4.0 ± 0.5 | |
| Left kidney thickness | M | 3.7 ± 0.5 | 4.0 ± 0.4 | 4.0 ± 0.4 | 4.2 ± 0.5 | 4.1 ± 0.7 | 4.1 ± 0.3 | 4.1 ± 0.5 | 4.5 ± 0.7 | 4.7 ± 0.6 | 4.7 ± 0.6 | 4.7 ± 0.5 | 4.9 ± 0.4 |
| F | 3.7 ± 0.4 | 3.8 ± 0.4 | 3.9 ± 0.4 | 4.0 ± 0.5 | 4.2 ± 0.5 | 4.1 ± 0.6 | 4.6 ± 0.6 | 4.5 ± 0.5 | 4.6 ± 0.6 | 4.7 ± 0.6 | 4.8 ± 0.5 | 4.8 ± 0.6 | |
| Right kidney volume | M | 75.8 ± 20.1 | 88.3 ± 16.7 | 90.2 ± 19.3 | 100.3 ± 20.4 | 98.2 ± 22.0 | 103.3 ± 19.5 | 110.5 ± 36.7 | 132.5 ± 31.1 | 148.7 ± 32.8 | 148.9 ± 32.0 | 155.9 ± 30.1 | 146.7 ± 17.5 |
| F | 72.9 ± 15.2 | 85.5 ± 17.0 | 90.6 ± 21.8 | 95.5 ± 20.1 | 102.4 ± 23.7 | 103.5 ± 27.7 | 124.7 ± 32.6 | 131.3 ± 32.6 | 144.6 ± 34.1 | 168.0 ± 34.3 | 164.4 ± 47.5 | 152.6 ± 28.4 | |
| Left kidney volume | M | 89.2 ± 20.6 | 106.4 ± 21.4 | 106.7 ± 23.4 | 119.1 ± 25.8 | 111.7 ± 27.8 | 116.7 ± 22.0 | 127.1 ± 38.7 | 146.8 ± 45.4 | 178.2 ± 40.3 | 181.2 ± 51.4 | 188.8 ± 37.0 | 190.7 ± 41.5 |
| F | 88.4 ± 15.4 | 97.4 ± 14.2 | 104.2 ± 22.5 | 111.8 ± 29.0 | 120.5 ± 28.2 | 118.6 ± 35.7 | 151.4 ± 38.3 | 160.9 ± 40.5 | 173.8 ± 41.3 | 176.5 ± 34.8 | 195.1 ± 44.1 | 182.5 ± 43.2 |
M, male; F, female.
TABLE 2.
Distribution on mean renal dimensions (cm) based on various height categories (cm).
| Parameters | Sex | ≤ 105 cm | 106–110 cm | 111–115 cm | 116–120 cm | 121–125 cm | 126–130 cm | 131–135 cm | 136–140 cm | 141–145 cm | 146–150 cm | ≥ 151 cm |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right kidney length | M | 7.20 | 7.58 | 7.94 | 8.08 | 8.39 | 8.59 | 8.73 | 8.80 | 9.00 | 9.09 | 9.65 |
| F | 6.90 | 7.69 | 7.93 | 8.02 | 8.21 | 8.48 | 8.73 | 8.79 | 8.95 | 9.14 | 9.69 | |
| Left kidney length | M | 7.17 | 7.67 | 8.13 | 8.23 | 8.44 | 8.52 | 8.78 | 8.85 | 9.08 | 9.08 | 9.69 |
| F | 7.97 | 7.89 | 8.21 | 8.32 | 8.35 | 8.61 | 8.78 | 9.01 | 9.05 | 9.34 | 9.63 | |
| Right kidney width | M | 5.00 | 5.11 | 5.45 | 5.64 | 6.08 | 6.11 | 6.15 | 6.26 | 6.41 | 6.60 | 6.92 |
| F | 5.47 | 5.39 | 5.49 | 5.71 | 5.77 | 6.09 | 6.14 | 6.23 | 6.48 | 6.36 | 6.71 | |
| Left kidney width | M | 4.53 | 5.36 | 5.60 | 5.64 | 6.05 | 6.06 | 6.20 | 6.29 | 6.32 | 6.43 | 6.82 |
| F | 5.23 | 5.15 | 5.40 | 5.69 | 5.80 | 5.90 | 6.05 | 6.14 | 6.33 | 6.33 | 6.75 | |
| Right kidney thickness | M | 2.70 | 3.06 | 3.10 | 3.17 | 3.37 | 3.43 | 3.41 | 6.26 | 3.59 | 3.74 | 3.73 |
| F | 3.13 | 2.95 | 3.11 | 3.22 | 3.19 | 3.31 | 3.39 | 3.50 | 3.60 | 3.62 | 3.85 | |
| Left kidney thickness | M | 2.83 | 3.58 | 3.52 | 3.75 | 3.88 | 4.10 | 4.04 | 6.29 | 4.13 | 4.24 | 4.42 |
| F | 3.33 | 3.50 | 3.61 | 3.65 | 3.80 | 3.84 | 3.91 | 4.03 | 4.22 | 4.26 | 4.54 | |
| Right kidney volume | M | 48.54 | 60.30 | 67.43 | 73.16 | 86.96 | 90.46 | 92.35 | 96.54 | 104.04 | 113.25 | 125.48 |
| F | 59.28 | 61.35 | 68.33 | 74.20 | 76.02 | 86.24 | 91.50 | 96.58 | 104.89 | 106.72 | 128.01 | |
| Left kidney volume | M | 46.71 | 72.75 | 80.46 | 87.09 | 100.00 | 105.41 | 110.70 | 113.57 | 119.34 | 125.34 | 148.18 |
| F | 69.82 | 71.40 | 80.60 | 86.16 | 92.14 | 97.70 | 104.68 | 112.58 | 121.94 | 127.54 | 149.79 |
M, male; F, female.
TABLE 3.
Distribution of mean renal dimensions (cm) based on various weight categories (kg).
| Parameters | Sex | 15–20 kg | 21–25 kg | 26–30 kg | 31–35 kg | 36–40 kg | 41–45 kg | 46–50 kg | 51–55 kg | 56–60 kg | 61–65 kg | 66–70 kg | ≥ 70 kg |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right kidney length | M | 7.66 | 8.22 | 8.67 | 8.87 | 9.18 | 9.47 | 9.95 | 10.15 | 9.96 | 10.27 | 10.74 | 10.64 |
| F | 7.78 | 8.20 | 8.69 | 8.84 | 9.07 | 9.65 | 9.53 | 10.02 | 10.41 | 10.61 | 10.59 | 10.47 | |
| Left kidney length | M | 7.81 | 8.29 | 8.67 | 8.93 | 9.36 | 9.60 | 10.02 | 10.11 | 10.04 | 10.47 | 10.98 | 10.84 |
| F | 8.13 | 8.40 | 8.77 | 8.97 | 9.28 | 9.86 | 9.86 | 10.46 | 10.52 | 10.74 | 11.09 | 10.67 | |
| Right kidney width | M | 5.37 | 5.77 | 6.13 | 6.31 | 6.63 | 6.83 | 6.94 | 7.11 | 7.25 | 7.39 | 7.66 | 7.43 |
| F | 5.44 | 5.82 | 6.10 | 6.29 | 6.50 | 6.79 | 6.76 | 7.09 | 7.25 | 7.46 | 7.47 | 7.68 | |
| Left kidney width | M | 5.46 | 5.79 | 6.11 | 6.26 | 6.64 | 6.70 | 6.83 | 7.09 | 7.23 | 7.44 | 7.47 | 7.63 |
| F | 5.34 | 5.77 | 5.98 | 6.20 | 6.35 | 6.87 | 6.83 | 7.24 | 7.14 | 7.46 | 7.54 | 7.83 | |
| Right kidney thickness | M | 3.01 | 3.21 | 3.42 | 3.53 | 3.66 | 3.82 | 3.91 | 3.98 | 4.02 | 4.06 | 4.11 | 4.16 |
| F | 3.05 | 3.21 | 3.34 | 3.53 | 3.56 | 3.88 | 3.93 | 3.97 | 4.06 | 5.60 | 4.30 | 4.40 | |
| Left kidney thickness | M | 3.49 | 3.75 | 4.02 | 4.10 | 4.19 | 4.37 | 4.43 | 4.64 | 4.70 | 4.89 | 5.01 | 4.93 |
| F | 3.55 | 3.75 | 3.90 | 4.03 | 4.25 | 4.48 | 4.55 | 4.76 | 4.61 | 4.72 | 4.95 | 5.12 | |
| Right kidney volume | M | 62.67 | 77.07 | 91.29 | 103.74 | 116.46 | 130.18 | 142.17 | 151.41 | 152.92 | 161.63 | 176.79 | 171.72 |
| F | 65.08 | 77.18 | 89.01 | 98.50 | 105.87 | 127.66 | 129.50 | 141.66 | 154.79 | 217.68 | 172.29 | 178.05 | |
| Left kidney volume | M | 74.76 | 90.67 | 106.49 | 121.02 | 137.67 | 148.62 | 160.71 | 174.88 | 179.40 | 199.96 | 216.94 | 215.32 |
| F | 77.60 | 91.23 | 103.00 | 112.76 | 126.34 | 153.32 | 154.21 | 180.87 | 174.93 | 189.83 | 209.24 | 215.08 |
M, male; F, female.
TABLE 4.
Correlation matrix coefficients of renal dimensions with age and body size indicators.
| Parameters | Age | Height | Weight | Body Mass Index | Body Surface Area | Right Kidney Length | Right Kidney Thickness | Right Kidney Width | Right Kidney Volume | Left Kidney Length | Left Kidney Thickness | Left Kidney Width | Left Kidney Volume |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | 1 | 0.371** | 0.747** | 0.052 | 0.785** | 0.583** | 0.289** | 0.568** | 0.503** | 0.565** | 0.473** | 0.578** | 0.617** |
| Height | 0.371** | 1 | 0.413** | −0.101** | 0.955** | 0.331** | 0.158** | 0.280** | 0.267** | 0.315** | 0.263** | 0.313** | 0.339** |
| Weight | 0.747** | 0.413** | 1 | 0.049 | 0.979** | 0.709** | 0.388** | 0.671** | 0.646** | 0.690** | 0.626** | 0.694** | 0.790** |
| Body Mass Index | 0.052 | −0.101** | 0.049 | 1 | −0.061* | 0.051 | 0.010 | 0.069* | 0.040 | 0.054 | −0.002 | 0.043 | 0.033 |
| Body Surface Area | 0.785** | 0.955** | 0.979** | −0.061* | 1 | 0.719** | 0.384** | 0.676** | 0.639** | 0.701** | 0.628** | 0.699** | 0.788** |
| Right Kidney Length | 0.583** | 0.331** | 0.709** | 0.051 | 0.719** | 1 | 0.323** | 0.642** | 0.658** | 0.715** | 0.513** | 0.633** | 0.714** |
| Right Kidney Thickness | 0.289** | 0.158** | 0.388** | 0.010 | 0.384** | 0.323** | 1 | 0.373** | 0.890** | 0.317** | 0.338** | 0.360** | 0.396** |
| Right Kidney Width | 0.568** | 0.280** | 0.671** | 0.069* | 0.676** | 0.642** | 0.373** | 1 | 0.693** | 0.571** | 0.562** | 0.679** | 0.698** |
| Right Kidney Volume | 0.503** | 0.267** | 0.646** | 0.040 | 0.639** | 0.658** | 0.890** | 0.693** | 1 | 0.566** | 0.516** | 0.598** | 0.659** |
| Left Kidney Length | 0.565** | 0.315** | 0.690** | 0.054 | 0.701** | 0.715** | 0.317** | 0.571** | 0.566** | 1 | 0.465** | 0.647** | 0.817** |
| Left Kidney Thickness | 0.473** | 0.263** | 0.626** | −0.002 | 0.628** | 0.513** | 0.338** | 0.562** | 0.516** | 0.465** | 1 | 0.642** | 0.820** |
| Left Kidney Width | 0.578** | 0.313** | 0.694** | 0.043 | 0.699** | 0.633** | 0.360** | 0.679** | 0.598** | 0.647** | 0.642** | 1 | 0.885** |
| Left Kidney Volume | 0.617** | 0.339** | 0.790** | 0.033 | 0.788** | 0.714** | 0.396** | 0.698** | 0.659** | 0.817** | 0.820** | 0.885** | 1 |
, p < 0.05;
, p < 0.01.
TABLE 5.
Distribution of the regression formula for the various renal dimensions using age and body size indicators.
| Dependent variable | Regression formula | p-value |
|---|---|---|
| Right Kidney Length | (5.91) + Age (0.04) + HT (0.01) +WT (0.03) | < 0.0001 |
| Right Kidney Thickness | (2.43) + Age (0.004) + HT (0.002) + WT (0.02) | < 0.0001 |
| Right Kidney Width | (4.48) + Age (0.04) + HT (0.004) + WT (0.02) | < 0.0001 |
| Left Kidney Length | (5.58) + Age (0.05) + HT (0.01) + WT (0.021) | < 0.0001 |
| Left Kidney Thickness | (2.68) ‒ Age (0.004) + HT (0.005) + WT (0.03) | < 0.0001 |
| Left Kidney Width | (4.23) + Age (0.043) + HT (0.005) + WT (0.03) | < 0.0001 |
| Right Kidney Volume | (30.67) + Age (0.64) – HT (0.003) + WT (2.06) | < 0.0001 |
| Left Kidney Volume | (33.91) + Age (0.079) + HT (0.02) + WT (2.39) | < 0.0001 |
Significant asymmetry was noted in all the measured renal parameters. Whilst the left renal length, thickness and volume were greater than those of the right in all the age-, weight- and height-matched categories (p < 0.01), the reverse was noted for the right renal width. Significant sexual dimorphism was observed in the kidney dimensions (p < 0.05). The left kidney was significantly longer in females (p < 0.05) in the age and weight categories, whilst the males showed significantly wider kidneys in all the categories (age, height and weight). Females were also noted to have thicker kidneys in most of the categories (age and weight) and larger volume in all the categories than males, though without statistical significance (p > 0.05).
Of those who did not meet the imaging inclusion criteria, six subjects were found to have some congenital renal pathologies. Two of these six children had unilateral kidneys, one subject had a left ureterocele with gross left-sided hydronephrosis whilst the three remaining subjects had ectopic kidneys visualised in the pelvis. These abnormal findings were communicated to the parents of each of these children. The final study sample size was 1315.
Discussion
Several studies on renal sizes have been reported in neonates/infants,15,21,22,23 children,14,24,25,26,27,28 adults4,6,29,30,31 and geriatric subjects.5,32 This study shows that the left kidney is longer, thicker and more voluminous than the right kidney. This is consistent with previous reports22,26,33,34 but contrary to research that noted that there is no statistical difference between the left and right kidneys.20,35
Sexual dimorphism was observed in our study – the left kidney in females was longer than the left kidney of males when age- and weight-matched. This is in agreement with studies conducted in New York by Chen et al.24 In addition, sexual dimorphism was noted in another study in infants.22 However, some other researchers showed no sexual dimorphism in their reports, although some commented that the rate of general somatic growth and body proportion are different between boys and girls.2,25,26,28,33,36
Studies carried out on Indian and Chinese children25,37 showed a progressive increase in renal length and volume with age. This increase with age was not consistent in our study population until 9 years of age and beyond. The difference in the number of participants within each of the groups and/or variations in the nutritional levels of the children in these particular age groups may be contributory factors to this observation.
The renal parameters in our study correlated best with BSA and weight p < 0.05. This is in agreement with some reports38,39 but contrary to other studies10,34,36,38,40,41 in which there was best correlation with height. Yet, other researchers have reported correlation with both height and weight.28,42,43,44 Oh et al.2 observed that height is the most influencing factor amongst the somatic variables in children < 2 years of age, whilst weight and age have good correlation with renal length from 2 to 12 years of age. A study by Pantoja et al.42 also noted that the kidneys were significantly larger in the obese subjects than in children with normal weights. Previous reports in low birth weight infants and premature deliveries have observed that these children have low nephron number and therefore reduced renal volumes45,46,47 and discovered they have a related risk of hypertension and renal diseases.45 One can speculate that the bigger the body size of a person, the higher the nephron number to take care of the body’s metabolic needs.
Previous Nigerian-based results on renal parameters of adults30,48 demonstrated that the renal parameters correlated best with weight compared with the current study where BSA had the strongest correlation followed by weight. We have demonstrated that age and all the body size indicators significantly correlate (p < 0.01) with all the renal dimensions with the exception of BMI which is in agreement with reports by Younus et al.28 that BMI may not be a good predictor of renal measurement. However, this is contrary to another report where BMI was demonstrated to significantly relate to renal length.42
The prevalence of congenital anomaly as revealed in this study was 0.46% which is slightly lower than the 0.89% recorded by Scott et al.22 in a similar study carried out in infants.
Conclusion
We have established age-, weight- and height-specific normal values of the kidneys in apparently healthy Nigerian children and developed regression equations for adequate evaluation and follow-up of renal diseases in clinical radiology and general medicine. We also noted significant sexual dimorphism and bilateral asymmetries in the kidney parameters of the studied population.
Limitation of the study
Urinalysis, serum electrolyte, urea and creatinine or glomerular filtration rate tests were not carried out for the study population, which may have further eliminated possibilities of including children with renal diseases. These investigations are more specific for kidney function than they are for renal morphology, which is the focus of this work.
Acknowledgements
Competing interests
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Authors’ contributions
All authors contributed equally to this work.
Funding information
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Data availability statement
Data may be made available by the corresponding author, on request.
Disclaimer
The views and opinions expressed in this article are those of the authors and do not necessarily reflect the official policy or position of any affiliated agency of the authors.
Footnotes
How to cite this article: Ezeofor SN, Anyanwu GE, Obikili EN. Reference indices for evaluating kidney dimensions in children using anthropometric measurements. S Afr J Rad. 2019;24(1), a1882. https://doi.org/10.4102/sajr.v24i1.1882
Note: Special Collection: Paediatric Radiology.
References
- 1.Kadioglu A. Renal measurements, including length, parenchymal thickness, and medullary pyramid thickness, in healthy children: What are the normative ultrasound values? Am J Roentgenol. 2010;194(2):509–515. 10.2214/AJR.09.2986 [DOI] [PubMed] [Google Scholar]
- 2.Oh M-S, Hwang G, Han S, et al. . Sonographic growth charts for kidney length in normal Korean children: A prospective observational study. J Korean Med Sci. 2016;31(7):1089–1093. 10.3346/jkms.2016.31.7.1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buchholz NP, Abbas F, Biyabani SR, Javed Q, Talati J. Ultrasonographic renal size in individuals without known renal disease. J Pak Med Assoc. 2000;50(1):12–16. [PubMed] [Google Scholar]
- 4.Kang K-Y, Lee YJI, Park SC, et al. . A comparative study of methods of estimating kidney length in kidney transplantation donors. Nephrol Dial Transplant. 2007;22(8):2322–2327. 10.1093/ndt/gfm192 [DOI] [PubMed] [Google Scholar]
- 5.Van Den Noortgte N, Velghe A, Petrovic M, et al. . The role of ultrasonography in the assessment of renal function in the elderly. J Nephrol. 2003;16(5):658–662. [PubMed] [Google Scholar]
- 6.Miletic D, Fuckar Z, Sustic A, Mozetic V, Stimac D, Zauhar G. Sonographic measurement of absolute and relative renal length in adults. J Clin Ultrasound. 1998;26(4):185–189. [DOI] [PubMed] [Google Scholar]
- 7.Sheikhazadi A, Sadr SS, Ghadyani MH, et al. . Study of the normal internal organ weights in Tehran’s population. J Forensic Leg Med. 2010;17(2):78–83. 10.1016/j.jflm.2009.07.012 [DOI] [PubMed] [Google Scholar]
- 8.Caglar V, Kumral B, Uygur R, Alkoc OA, Ozen OA, Demirel H. Study of volume, weight and size of normal pancreas, spleen and kidney in adults autopsies. FMAR. 2014;2(1):63–69. 10.4236/fmar.2014.23012 [DOI] [Google Scholar]
- 9.Musa MJ, Abukonna A. Sonographic measurement of renal sizes in normal high altitude populations. J Radiat Res Appl Sci. 2017;10(3):178–182. 10.1016/j.jrras.2017.04.004 [DOI] [Google Scholar]
- 10.Cain JE, Di Giovonni V, Smeeton J, Rosenblun ND. Genetics of renal hypoplasia: Insights into the mechanism of controlling nephron endowment. Pediatr Res. 2010;68(1):91–98. 10.1203/PDR.0b013e3181e35a88 [DOI] [PubMed] [Google Scholar]
- 11.Atalabi OM, Orimadegun A, Adekanmi AJ, Akinyinka O. Ultrasonography renal sizes, cortical thickness and volume in Nigerian children with acute falciparum malaria. Malar J. 2013;12(1):92 10.1186/1475-2875-12-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang H-P, Lai Y-C, Tsai I-J, Chen S-Y, Cheng C-H, Tsau Y-K. Nephromegaly in children with Kawasaki disease: New supporting evidence for diagnosis and its possible mechanism. Pediatric Res. 2008;63(2):207–210. 10.1203/PDR.0b013e31815ef737 [DOI] [PubMed] [Google Scholar]
- 13.Faubel S, Patel NU, Lockhart ME, Cadnapaphornchai MA. Renal relevant radiology: Use of ultrasonography in patients with AKI. Clin J Am Soc Nephrol. 2014;9(2):382–394. 10.2215/CJN.04840513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Currarino G, Williams B, Dana K. Kidney length correlated with age: Normal values in children. Radiology. 1984:150(3);703–704. 10.1148/radiology.150.3.6695070 [DOI] [PubMed] [Google Scholar]
- 15.De Baun MR, Siegal MJ, Choyke PL. Nephromegaly in infancy and early childhood: A risk factor for Wilm’s tumour in Beckwith–Weideman Syndrome. J Pediatr. 1992;132(3Pt1):401–404. 10.1016/s0022-3476(98)70009-5 [DOI] [PubMed] [Google Scholar]
- 16.Ortiz-Neira CL, Traubici J, Alan D, et al. . Sonographic assessment of real growth in patients with Beckwith – Weidemann syndrome: The Beckwith – Weidemann syndrome real nomogram. Clinics. 2009;64(1):41–44. 10.1590/s1807-59322009000100008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones T, Riddick L, Harpen M, Dubusisson R, Smuels D. Ultrasonographic determination of renal mass and renal volume. J. Ultrasound Med. 1983;2(1):151–154. [DOI] [PubMed] [Google Scholar]
- 18.Nyengaard J, Bendtsen T. Glomerular number and size in relation to age, kidney weight and body surface in normal man. Anat Rec. 1992;232(2):194–201. 10.1002/ar.1092320205 [DOI] [PubMed] [Google Scholar]
- 19.Esezobor CI, Ladapo TA, Osinaite B, Lesi FE. Paediatric acute kidney injury in a tertiary hospital in Nigeria; prevalence, causes and mortality rate. PLoS One. 2012;7(12):e51229 10.1371/journal.pone.0051229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emamian SA, Nielson MB, Pederson JF, Ytte L. Kidney dimensions at sonography; correlation with age, sex and habitus in 665 adult volunteers. AJR. 1993;160(1):83–86. 10.2214/ajr.160.1.8416654 [DOI] [PubMed] [Google Scholar]
- 21.Zerin JM, Meuer RD. Sonographic assessment of renal length in the first year of life: The problem of ‘spurious nephromegaly’. Pediatr Radiol. 2000;30(1):52–57. 10.1007/s002470050014 [DOI] [PubMed] [Google Scholar]
- 22.Scott JES, Hunter EW, Lee REJ, Mathews JNS. Ultrasound measurement of renal size in new born infants. Arch Dis Childhood. 1990;65(4):361–364. 10.1136/adc.65.4_spec_no.361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Soyupak SK, Narli N, Yapicioglu H, Satar M, Sungur EH. Sonographic measurements of the liver, spleen and kidney dimensions in the healthy term and preterm newborns. Eur J Radiol. 2002;43(1):73–78. 10.1016/s0720-048x(01)00466-1 [DOI] [PubMed] [Google Scholar]
- 24.Chen JJ, Pugach J, Patel M, Luisiri A, Steinhardt GF. The renal length Normogram: A multivariable approach. J Urol. 2002:168(5);2149–2152. 10.1016/S0022-5347(05)64339-X [DOI] [PubMed] [Google Scholar]
- 25.Otiv A, Mehta K, Ali U, Nadkarni M. Sonographic measurement of renal size in normal Indian children. Indian Pediatrics. 2012;49(7):533–536. 10.1007/s13312-012-0120-7 [DOI] [PubMed] [Google Scholar]
- 26.Dinkel E, Erter M, Peters H, Berres M, Schulte-Wissermenn H. Kidney size in childhood: Sonographic growth charts for kidney length and volume. Pediatr Radiol. 1985;15(1):38–43. 10.1007/BF02387851 [DOI] [PubMed] [Google Scholar]
- 27.Safak AA, Simek E, Talat B. Sonographic assessment of the normal limits and percentile curves of liver, spleen and kidney dimensions in healthy school-aged children. J Ultrasound Med. 2005;24(10):1359–1364. 10.7863/jum.2005.24.10.1359 [DOI] [PubMed] [Google Scholar]
- 28.Younus N, Raza F, Bhugio S, et al. . Sonographic measurement of normal renal size and correlation with somatic variables in subset of Karachi pediatric population. Pakistan J Med Dent. 2015;4(2):24–29. [Google Scholar]
- 29.Njeze NR, Okeke DO, Ezeofor SN, Onodugo OD. The effect of advancing age on absolute renal length. West Africa J Radiol. 2011;18(1):16–18. [Google Scholar]
- 30.Okoye IJ, Agwu KK, Idigo FU. Normal sonographic renal length in southeast Nigeria. Afr J Med Sci. 2005;34(2):129–131. [PubMed] [Google Scholar]
- 31.Oyuela-Carrasco J, Rodriguez-Castellanos F, Kimura E, Delgado-Hemandez R, Herrera-Felix JP. Renal length measured by ultrasound in adult Mexican population. Nefrologia. 2009;29(1):30–34 10.3265/Nefrologia.2009.29.1.30.1.en.full.pdf [DOI] [PubMed] [Google Scholar]
- 32.Akpainar IN, Altun E, Avcus S, Tuney D, Ekinci G, Biren T. Sonographic measurement of kidney size in geriatric patients. J Clin Ultrasound. 2003;31(6):315–318. 10.1002/jcu.10178 [DOI] [PubMed] [Google Scholar]
- 33.Han BK, Babcock DS. Sonographic measurement and appearance of normal kidney in children. AJR. 1985;145(3):611–616. 10.2214/ajr.145.3.611 [DOI] [PubMed] [Google Scholar]
- 34.Eze CU, Agwu KK, Ezeasor DN, Agwuna KK, Aronu AE, Mba EI. Sonographic biometry of normal kidney dimensions among school-aged children in Nsukka, Southeast Nigeria. West Indian Med J. 2014;63(1):46–53. 10.7727/wimj.2013.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abdoerlrahman HAB, Mansour AA, Gar-elnabli MEM, Saeed EA. Ultrasound renal length and parenchymal thickness in normal Sudanese population. Int J Sci Res. 2016;5(1):623–625. [Google Scholar]
- 36.Konus OL, Ozdemic A, Allaya A, Erbas G, Celik H, Isik S. Normal liver, spleen and kidney dimensions in neonates, infants and children: Evaluation with sonographic. ARJ. 199;171(6):1639–1698. 10.2214/ajr.171.6.9843315 [DOI] [PubMed] [Google Scholar]
- 37.Loftus WK, Gent RJ, LeQuesne GW, Metreweli C. Renal length in Chinese children: Sonographic measurement and comparison with western data. J Clin Ultrasound. 1998;26(7):349–352. [DOI] [PubMed] [Google Scholar]
- 38.Kim J-H, Kim M-J, Sok HL, Jieun K, Lee M-J. Length and volume of morphologically normal kidneys in Korean children: Ultrasound measurement and estimation using body size. Korean J Radiol. 2013;14(4):677–682. 10.3348/kjr.2013.14.4.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saeed Z, Mirza W, Sayami R, Sheikh A, Yazdani I, Hussain SA. Sonographic measurement of renal dimensions in adults and its correlates. Int J Collab Res Intern Med Public Health. 2012;4(9):1626–1641. [Google Scholar]
- 40.Rousan LA, Fataftah J, Al-Omari MH, Hayajneh WA, Miqdady M, Khader YS. Sonographic assessment of kidney length in Jordanian children: Results from a tertiary hospital in the North of the Kingdom. J Med J. 2015;49(2):101–107. [Google Scholar]
- 41.Kim BW, Song MK, Chung S, Kim KS. Evaluation of kidney size in children: A pilot study of renal length as a surrogate of organ. Korean J Pediatr. 2012;55(2):54–57. https://doi.org/0.3345/kjp.2012.55.2.54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pantoja ZJR, Mallios R, Murphy J. The effect of obesity on kidney length in a healthy pediatric population. Pediatr Nephrol. 2009;24(10):2023–2027. 10.1007/s00467-009-1202-1 [DOI] [PubMed] [Google Scholar]
- 43.Glodny B, Unterholzner V, Taferner B, et al. . Normal kidney size and its influencing factors-a 64-slice MDCT study of 1.040 asymptomatic patients. BMC Urol. 2009;9(1):19 10.1186/1471-2490-9-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JH, Kim MJ, Lim SH, Kim J, Lee MJ. Length and volume of morphologically normal kidneys in Korean children: Ultrasound measurement and estimation using body size. Korean J Radiol. 2013;14(4):677–682. 10.3348/kjr.2013.14.4.677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luyckx VA, Shukha K, Brenner BM. Low nephron number and its clinical consequences. Rambam Maimonides Med J. 2011;2(4):e0061 10.5041/RMMJ.10061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer J, Wang Z, Hoy W. Low birth weight and reduced renal volume in aboriginal children. Am J Kidney Dis. 2001;37(5):915–920. 10.1016/s0272-6386(05)80006-x [DOI] [PubMed] [Google Scholar]
- 47.Kandasamy Y, Smith R, Wright IM, Lumbers ER. Relationships between glomerular filtration rate and kidney volume in low birth weight neonates. J Nephrol. 2013;26(5):894–898. 10.5301/jn.5000220 [DOI] [PubMed] [Google Scholar]
- 48.El-Reshaid W, Abdul-Fattah H. Sonographic assessment of renal size in healthy adults. Med Princ Pract. 2014;23(5):432–434. 10.1159/000364876 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data may be made available by the corresponding author, on request.