Abstract
Background
CEDM has demonstrated a diagnostic performance similar to MRI and could have similar limitations in breast cancer (BC) detection.
Purpose
The aim of our study was to systematically analyze the characteristics of the lesions with the absence of enhancement with CEDMs, called false-negatives (FNs), in order to identify which clinical, radiological, histological and molecular parameters are associated with the absence of enhancement of known BCs with CEDMs, and which types of BC are most likely to cause FNs in CEDMs. We also tried to evaluate which parameters instead increased the probability of showing enhancement in the same context.
Materials and methods
Included in our study group were 348 women with 348 diagnosed BCs performing CEDM as preoperative staging. Two breast-imaging radiologists reviewed the CEDM exams. The absence of perceptible contrast enhancement at the index cancer site was indicative of an FN CEDM, whereas cases with appreciable enhancement were considered true positives (TPs). Dichotomic variables were analyzed with Fisher’s exact probability test or, when applicable, the chi-square test. Binary logistic regression was performed on variables shown to be significant by the univariate analysis in order to assess the relationship between predictors (independent variables) and TFNs (outcome).
Results
Enhancement was observed in 317 (91.1%) of the 348 BCs. From the 31 (8.9%) lesions which were FNs, we excluded 12 (38.7%) which showed an artifact generated by the post biopsy hematoma and 6 (19.4%) which were outside the CEDM field of vision. We thus obtained 13 (41.9%) BCs considered “True False Negatives” (TFNs), i.e. BCs which showed no enhancement despite being within the CEDM field of vision and failed to show post biopsy hematoma artifacts. We found that the TFNs frequently have a unifocal disease extension, diameter <10 mm, a lower number of luminal B HER2-subtypes, a higher number of DCIS, and an index lesion with microcalcifications.
Conclusions
The parameters we found to be associated with no enhancement of known BCs with CEDMs were: unifocal disease extension, DCIS histotype, lesion dimensions <10 mm, and index lesion with microcalcifications. The characteristics that instead increase the probability of showing enhancement were US mass, Luminal B HER2 negative molecular subtype, the presence of an invasive ductal component, and lesion dimensions ≥10 mm.
Keywords: Breast cancer, Contrast enhanced digital mammography, False negative, No enhancement
Highlights
-
•
The variables associated with an increased risk of no enhancement were unifocal disease extension, non-classifiable molecular subtype, DCIS histotype, lesion dimensions <10 mm, index lesion represented by microcalcifications.
-
•
A greater probability of showing enhancement entailed the presence of an invasive ductal component, index lesion represented by ultrasound mass, Luminal B HER2 negative molecular subtype, lesion dimensions ≥10 mm, multifocal disease extension.
1. Introduction
Contrast Enhanced Digital Mammography (CEDM) is the most recent diagnostic breast imaging technique that has already demonstrated a sensitivity for detecting breast cancer (BC) which is higher than digital mammography (DM) and similar to that of contrast-enhanced MRI [[1], [2], [3], [4], [5], [6], [7], [8]]. One of the indications of CEDM is the preoperative staging of patients with a recent diagnosis of BC, and also in this context it has demonstrated a high sensitivity (88%–100%) and specificity (82%–98%) with a diagnostic performance similar to MRI and superior to conventional imaging like DM, ultrasound (US), digital breast tomosynthesis (DBT) [[9], [10], [11], [12], [13], [14]].
Also in neoadjuvant chemotherapy monitoring and for screening women at increased risk of BC, the diagnostic performance of CEDM was similar to MRI and superior to conventional imaging [[15], [16], [17], [18]].
CEDM and MRI are both based on the same principle, namely, tumor angiogenesis and contrast leakage from vessels into the tumor interstitium, and they could have similar limitations in BC detection [19]. Indeed, it has been shown in literature that some malignant lesions may fail to show enhancement with MRI, including DCIS and invasive carcinomas, with a lack of enhancement in up to 12% of known malignant lesions [[19], [20], [21], [22], [23], [24], [25]].
From the analysis of several studies published in literature that evaluate the diagnostic performance of CEDM in various subgroups of patients for different purposes, an FN rate emerged between 0% and 20%. Lack of enhancement has been reported for tumors of very different sizes and for all BC types, including both invasive carcinomas and DCIS [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14],32].
To date, however, we have not identified any study published in literature that systematically analyzes the characteristics of BCs lacking in enhancement with CEDM.
The purpose of our study was to systematically analyze the characteristics of BCs without perceptible enhancement with CEDM (false negatives) in order to identify which clinical, radiological, histological and molecular parameters are associated with the absence of enhancement of known BCs with CEDMs and which types of BC are most likely to cause FNs in CEDMs and not to evaluate the diagnostic performance of CEDM in preoperative staging of BC.
We also tried to evaluate which parameters instead increased the probability of a BC showing enhancement in the same context.
2. Materials and Methods
2.1. Patients
From July 2016 to November 2019, 1736 CEDM exams were performed in our department for various indications: pre-operative staging, assessment during neoadjuvant chemotherapy, problem solving, screening of dense-breast patients and high-risk screening.
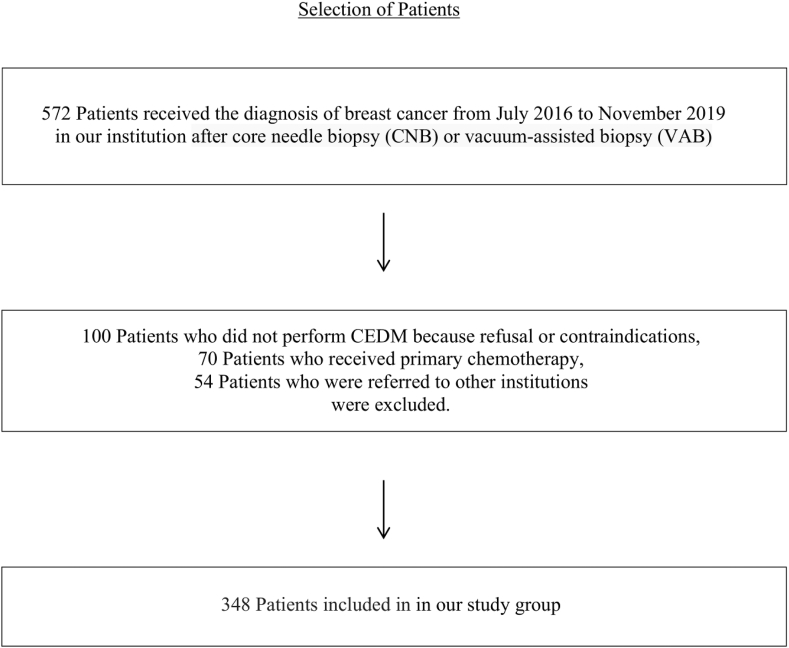
We retrospectively selected 572 CEDMs performed for preoperative staging in 572 patients with 576 newly diagnosed BCs after core needle biopsy (CNB) or stereotactic-guided vacuum-assisted biopsy (VAB) on suspicious lesions classified as BI-RADS 4 or 5 with conventional imaging [26]. The local institutional review board approved this retrospective analysis. The inclusion criteria for the study were: histological diagnosis of BC with CNB or VAB, conventional imaging (CI): DM, DBT and US performed prior to the CEDM, execution of CEDMs as preoperative staging, breast surgery performed in our center, with availability of complete histological reports of CNB, VAB and surgical specimens (SS). Of the 572 patients, we excluded 100 either because they refused it or owing to the presence of contraindications (i.e. pregnancy, renal insufficiency), 70 who received primary chemotherapy and 54 who were referred to other institutions. Therefore, 348 women aged 37–88 years, mean age 60.1 years (standard deviation [SD] 11,930) with 348 malignant lesions (index cancers) were included in our study group. Fig. 1.
Fig. 1.
Flowchart of patient enrollment.
The characteristics of each index lesion are summarized in Table 1.
Table 1.
The breast, patient and index cancer characteristics of the 348 women with 348 malignant lesions (index cancers), 317 of which showed enhancement with CEDM (TPs) and 13 of which were called “true false negative” (TFNs) included in our study group. The TFNs were the remaining cases of the group of false negatives, i.e. lesions with no perceptive contrast enhancement at the expected site of the index lesion, after excluding cases in which the cancer was outside the CEDM field of vision and those with abundant post biopsy hematoma at the site of the index lesion.
| Characteristic | Total sample (n = 348) | TP lesions (n = 317) | TFN lesions (n = 13) | |
|---|---|---|---|---|
| Breast density | BI-RADS A | 41 (11.8%) | 41 (12.9%) | – |
| BI-RADS B | 188 (54%) | 167 (52.7%) | 9 (69.2%) | |
| BI-RADS C | 81 (23.3%) | 74 (23.3%) | 3 (23.1%) | |
| BI-RADS D | 38 (10.9%) | 35 (11.1%) | 1 (7.7%) | |
| Index lesion on CI | Developing asymmetry | 3 (0.9%) | 3 (1.0%) | – |
| Architectural distortion | 9 (2.6%) | 9 (2.8%) | – | |
| Microcalcifications | 61 (17.5%) | 41 (12.9%) | 7 (53.8%) | |
| DM/DBT Mass | 2 (0.6%) | 2 (0.6%) | – | |
| US and DM/DBT Mass | 109 (31.3%) | 109 (34.4%) | 2 (15.4%) | |
| US Mass | 164 (47.1%) | 153 (48.3%) | 4 (30.8%) | |
| Palpable | Yes | 129 (37.1%) | 121 (38.2%) | 3 (23.1%) |
| No | 219 (62.9%) | 196 (61.8%) | 10 (76.9%) | |
| Personal History of BC | Yes | 45 (12.9%) | 36 (11.4%) | 3 (23.1%) |
| No | 303 (87.1%) | 281 (88.6%) | 10 (76.9%) | |
| BPE | Minimal | 137 (39.4%) | 126 (39.7%) | 5 (38.5%) |
| Mild | 133 (38.2%) | 121 (38.2%) | 5 (38.5%) | |
| Moderate | 59 (16.9%) | 53 (16.7%) | 3 (23.0%) | |
| Marked | 19 (5.5%) | 17 (5.4%) | – | |
| Biopsy | CNB | 281 (80.7%) | 270 (85.2%) | 5 (38.5%) |
| VAB | 67 (19.3%) | 47 (14.8%) | 8 (61.5%) | |
| Histology | DCIS | 35 (10.1%) | 21 (6.6%) | 8 (61.5%) |
| IDC | 186 (53.4%) | 174 (54.9%) | 4 (30.8%) | |
| ILC | 37 (10.6%) | 36 (11.4%) | – | |
| IDC/ILC | 26 (7.5%) | 25 (7.9%) | – | |
| Other invasive histotypes | 64 (18.4%) | 61 (19.2%) | 1 (7.7%) | |
| Dimensions at pathology | <10 mm | 110 (31.6) | 93 (29.3) | 9 (69.2%) |
| ≥10 mm | 238 (68.4%) | 224 (70.7%) | 4 (30.8%) | |
| Extension of the disease | Unifocal | 215 (61.8%) | 184 (58.0%) | 13 (100%) |
| Multifocal | 89 (25.6%) | 89 (28.1%) | – | |
| Multicentric | 44 (12.6%) | 44 (13.9%) | – | |
| Histological Grade | G1 | 81 (23.3%) | 74 (23.4%) | 3 (23.1%) |
| G2 | 170 (48.8%) | 150 (47.3%) | 9 (69.2%) | |
| G3 | 97 (27.9%) | 93 (29.3%) | 1 (7.7%) | |
| Estrogen Receptor Status | Positive | 281 (80.8%) | 265 (83.6%) | 4 (30.8%) |
| Negative | 32 (9.2%) | 31 (9.8%) | 1 (7.7%) | |
| Unknown | 35 (10.0%) | 21 (6.6%) | 8 (61.5%) | |
| Progesterone Receptor Status | Positive | 236 (67.8%) | 224 (70.7%) | 3 (23.1%) |
| Negative | 77 (22.2%) | 72 (22.7%) | 2 (15.4%) | |
| Unknown | 35 (10.0%) | 21 (6.6%) | 8 (61.5%) | |
| HER2 Receptor Status | Positive | 41 (11.8%) | 39 (12.3%) | 2 (15.4%) |
| Negative | 272 (78.2%) | 257 (81.1%) | 3 (23.1%) | |
| Unknown | 35 (10.0%) | 21 (6.6%) | 8 (61.5%) | |
| Ki67 | <20% | 134 (38.6%) | 82 (25.9%) | 3 (23.1%) |
| ≥20% | 179 (51.4%) | 214 (67.5%) | 2 (15.4%) | |
| Unknown | 35 (10.0%) | 21 (6.6%) | 8 (61.5%) | |
| Molecular Subtypes | Luminal A | 90 (25.9%) | 85 (26.8%) | 2 (15.4%) |
| Luminal B HER2- | 165 (47.4%) | 155 (48.9%) | 1 (7.7%) | |
| Luminal B HER2+ | 31 (8.9%) | 30 (9.5%) | 1 (7.7%) | |
| HER2 Enriched | 10 (2.9%) | 9 (2.8%) | 1 (7.7%) | |
| Triple Negative | 17 (4.9%) | 17 (5.4%) | – | |
| Not Classifiable | 35 (10.0%) | 21 (6.6%) | 8 (61.5%) | |
| Surgery | BCS | 246 (70.7%) | 215 (67.8%) | 13 (100%) |
| Mastectomy | 90 (25.9%) | 90 (28.4%) | – | |
| Bilateral BCS | 7 (2.0%) | 7 (2.2%) | – | |
| Bilateral Mastectomy | 5 (1.4%) | 5 (1.6%) | – |
All patients underwent CEDMs after the biopsy and surgical excisions were subsequently performed within thirty days after diagnosis. 129 (37.1%) patients had a palpable lesion while 219 (62.9%) were asymptomatic at the time of the biopsy. 45 (12.9%) patients also had a personal history of BC.
BI-RADS Breast Imaging Reporting and Data Systems; DM Digital Mammography; DBT Digital Breast Tomosynthesis; US Ultrasound; BC breast cancer; BPE Background Parenchymal Enhancement; CNB Core Needle Biopsy; VAB stereotactic-guided Vacuum Assisted Biopsy; DCIS Ductal Carcinoma In Situ; IDC Invasive Ductal Carcinoma; ILC Invasive Lobular Carcinoma; BCS breast-conservative surgery.
2.2. Conventional diagnostic imaging
Before the CEDM, all patients underwent a DM, DBT and US in our department to evaluate the extension of the disease. Initial DM and DBT studies were performed in the standard craniocaudal and mediolateral oblique views using a full-field digital mammography unit with tomosynthesis (Selenia Dimensions, Hologic, Bedford, USA). 3D tomosynthesis imaging, synthesized C-view images and 2D images were performed on each patient as per the standard protocol. US exams were whole breast bilateral handheld, performed by one of six radiologists in our Senology Unit using a 10–13 MHz transducer and a US unit (ESAOTE, MyLab 70 XVG, Genoa, Italy), and prior to the US the same radiologists also performed a clinical exam of both breasts. All six radiologists had 8–30 years’ experience in all methods of breast imaging and 4 years’ experience with CEDMs, plus they also performed CNBs and VABs. They specified the presumed extension of the disease in their reports based on the analysis of the CI, and also indicated the breast density evaluated on DM according to the BI-RADS criteria [26], the palpability of the index lesion, and the personal history of BC for each patient.
2.3. CEDM imaging
CEDMs were performed using a Selenia Dimensions mammography system (Hologic, Marlborough, MA). An intravenous injection of 1.5 mL/kg of body weight with an iodine-based contrast material (Ultravist 370, Bayer HealthCare LLC, Whippany, NJ) was administered with an automated bolus injection at a flow rate of 3 mL/s, followed by a 20 mL saline flush. Scanning began about 2 min after the injection, and all 4 standard mammographic views (craniocaudal and mediolateral oblique images of each breast) were obtained in sequence within 5 min. For each CEDM view, 2 acquisitions were performed serially at 26–31 kVp with rhodium and silver (Rh and Ag) filters for low-energy acquisition, and at 45–49 kVp with a copper filter for high-energy acquisition. A recombination algorithm used to subtract the unenhanced breast tissue provided a subtracted image in which only the areas of contrast enhancement were highlighted. All the CEDM views were used for our analysis [27]. The radiologists in our department evaluated CEDM images with knowledge of the biopsy results and availability of CI. They reported the presumed extension of the disease, the presence or absence of enhancement in the site of the index lesion, and also indicated the Background Parenchymal Enhancement (BPE) level, evaluated on recombined images, according to the BI-RADS criteria [26] for each patient.
2.4. Image analysis
Two radiologists of our department with knowledge of the histopathologic findings, i.e. the site of the index lesion and the CI results, retrospectively reviewed the CEDM images. The reviewers called findings true positives (TPs) when the presence of contrast medium uptake was evident at the expected site of the index lesion, whereas the absence of perceptive contrast enhancement at the expected site of the index lesion was considered to be a false-negative (FN) CEDM.
The FN cases were divided into three groups: cases in which the index lesion was outside the CEDM field of vision, cases in which an abundant post biopsy hematoma was present at the site of the index lesion, and the so-called “true false negative” (TFN) cases i.e. BCs that showed no enhancement despite being included within the CEDM field of vision and failed to show post biopsy hematoma artifacts. The TFNs were therefore the FN cases that remained after excluding the BCs outside the CEDM field of vision and those in which an abundant post biopsy hematoma, which was visible at the site of the index lesion, masked any enhancement of the BC by generating a characteristic hematoma artifact.
The index lesions outside the CEDM field of vision did not fall within the CEDM images but had been identified by US.
Some FN lesions had a copious post biopsy hematoma at the site of the index lesion which generated a characteristic artifact called “negative contrast enhancement” or “eclipse sign”, thus preventing correct visualization of the BC and interpretation of the images [28,29]. In these cases, only the artifact generated by the hematoma was visible with no other area of enhancement near it.
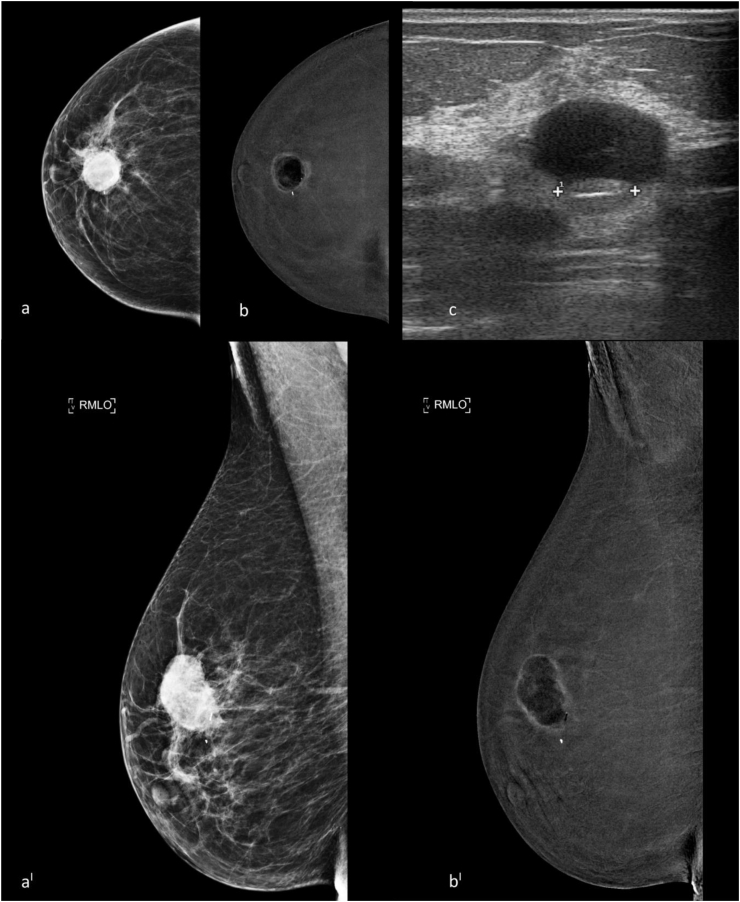
Fig. 2.
Presurgical staging with CEDM of a 60-y-old patient with an invasive ductal carcinoma in the upper-central quadrant of the right breast diagnosed by VAB. a, b, craniocaudal low energy and recombined CEDM images; aI, bI mediolateral oblique CEDM low-energy and recombined images. The CEDM examination showed the presence of a copious post biopsy hematoma at the site of the index lesion which generated a characteristic artifact called “negative contrast enhancement” or “eclipse sign”, thus preventing correct visualization of the BC and interpretation of the images. (c) US examination confirmed the presence of a copious hematoma with a post biopsy marker in the context, in the site of the index lesion. A false negative CEDM case.
Finally, those cases in which the index lesion was included within the CEDM field of vision and did not present post biopsy hematoma, were called TFNs.
2.5. Histopathologic correlation
Of the 348 malignant cases, breast-conservative surgery (BCS), i.e. wider excision and lumpectomy was performed for 246 (70.7%) cases, mastectomy for 90 (25.9%) cases, bilateral BCS for 7 (2.0%) cases, and bilateral mastectomy for 5 (1.4%) cases. The CEDMs identified 12 malignant lesions in the contralateral breast compared to the index lesion. All these contralateral lesions had been identified by second-look US or DBT after the CEDMs and subjected to CNB or VAB before surgery, which led to 5 bilateral mastectomies and 7 bilateral BCS. Moreover, the CEDMs had identified 39 additional disease foci than 10 mm away from the index lesion in the same breast. Of these 39 additional homolateral lesions, 35 had been identified by second-look US or DBT after the CEDMs and subjected to CNB or VAB before surgery while 4 had only been identified after contrast-enhanced MRI and subsequent MRI-guided biopsy. At the end of each biopsy, a clip was released and a DM was performed to evaluate the correspondence with CEDM images.
All the non-palpable lesions were marked with inert charcoal suspension before surgery under US, MRI or stereotaxic guidance. The additional malignant lesions identified after the CEDM exams were not included in this study.
Only the malignant lesions identified by DM, DBT or US before the CEDM were included for the purpose of the study.
In fact the purpose of this study is not to evaluate the diagnostic performance of CEDMs in preoperative staging of BC, but rather, to evaluate which characteristics of BCs increase the probability of showing enhancement with CEDMs, and which instead increase the probability lacking in enhancement.
The surgical specimens of all the lesions were reviewed by two pathologists with more than 25 years’ experience in breast pathology. Tumor type, grade, size, estrogen receptor (ER) status, progesterone receptor (PgR) status, human epidermal growth receptor factor 2 (HER2), Ki67 proliferative index and Molecular Subtypes were indicated in the pathology reports [30].
Four BC subtypes, classified according to the 2013 St. Gallen international expert consensus, were defined [31]: Luminal A (HR+/HER2−, Ki67 low), Luminal B HER2 negative or HER2 positive (HR+/HER2−, Ki67 high or HR+/HER2+), triple negative (HR−/HER2−), and HER2 enriched (HR−/HER2+).
The correlation between the imaging results and the pathology for each case and the therapy-management decisions were discussed at the weekly multidisciplinary breast meeting.
2.6. Statistical analysis
The histological results obtained from the surgically excised lesions were considered the gold standard for comparison with the CEDM findings. We first assessed how many index lesions showed enhancement (TPs) and how many lacked enhancement (FNs). We then removed from the FNs those in which the index lesions were outside the CEDM field of vision and those in which the post biopsy hematoma artifact masked the site of the index lesion, thus obtaining the TFNs. Therefore, in order to evaluate the characteristics of the BCs without enhancement with CEDM and understand which types of BCs are more likely to be lacking in enhancement with this diagnostic technique, we only considered the TFNs and TPs.
The statistical analysis was then performed on the TFNs and TPs.
The distribution frequencies of various clinical, radiological, histological and molecular parameters were calculated in the whole population and in each of the two subgroups (TFNs and TPs). Dichotomic variables were expressed as number and percentage and analyzed with Fisher’s exact probability test or, when applicable, the chi-square test. Binary logistic regression was performed on variables shown to be significant by the univariate analysis in order to assess the relationship between predictors (independent variables) and TFNs (outcome): results were expressed as p-value and Odds Ratios (OR) with 95% Confidence Intervals (CI). An OR interval completely above 1 indicates that the variable is associated with a TFN lesion (i.e. a risk factor of TFN with CEDM), whereas an OR interval entirely below 1 indicates that the variable is associated with reduced odds of being a TFN. Significance was defined as the value of p (alpha error) < 0.05 and OR 95% CI not including 1.
On each of the variables that were significant in the univariate analysis, we also calculated how each one influenced the probability of showing enhancement with CEDM.
The analyses were performed using IBM SPSS Statistics 23.0 (IBM SPSS Inc., Chicago, IL) and Microsoft Excel (Microsoft Corporation, Redmond, WA).
3. Results
348 women aged 37–88 years, mean age 60.1 years (standard deviation [SD] 11,930) with 348 malignant lesions were included in our study group. The characteristics of all the BCs in the study (TPs + FNs) are summarized in Table 1.
Of the 348 BCs included in the study, enhancement was observed in 317 (91.1%) lesions, namely, the TP cases.
A total of 31 (8.9%) exams showed no enhancement with CEDM, these were the FNs. Analyzing the 31 FN cases, in 12/31 (38.7%) the presence of an abundant post biopsy hematoma at the site of the index lesion was detected which generated an artifact that hindered the interpretation of the images and prevented correct visualization of the enhancement at the expected index lesion site.
All these lesions underwent VAB before CEDM and they were identified in 9 cases as microcalcifications by both DM and DBT, and in 3 cases as DM/DBT masses.
6/31 (19.4%) FNs were also represented by lesions located outside the CEDM field of vision and therefore, not visible during the exam: 2 at the sub-mammary sulcus, 2 in the high sub-clavicular site and 2 in the parasternal site. These lesions were not detected in the DMs and DBT either, however, they were observed with US and underwent CNB before CEDM.
13/31 (41.9%) cases were TFNs. Fig. 3.
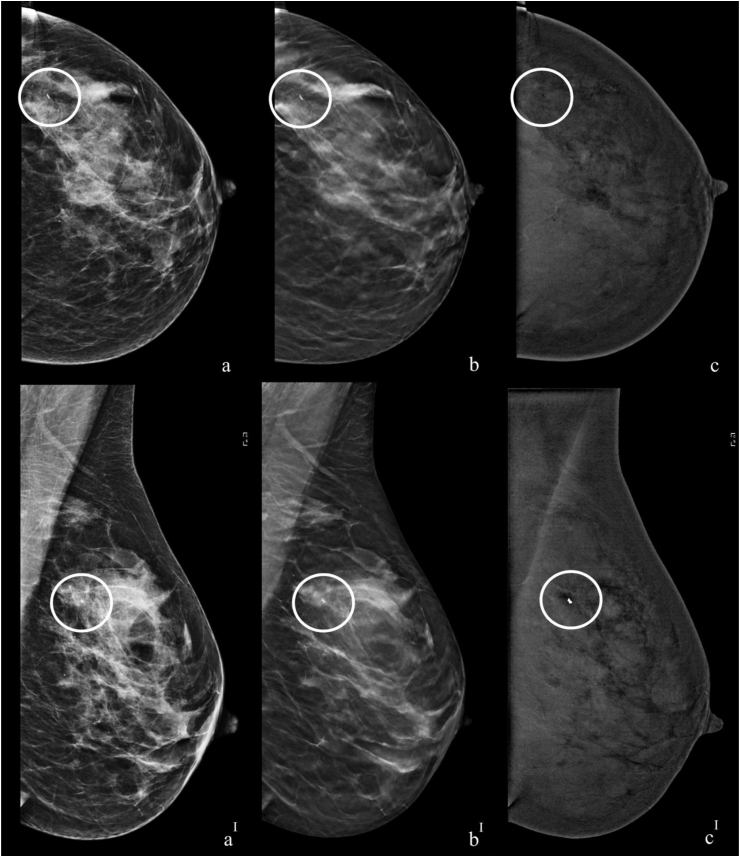
Fig. 3.
Presurgical staging with CEDM of a 49-y-old patient with a ductal carcinoma in situ in the upper-outer quadrant of the left breast diagnosed by CNB. The 6.0 mm index lesion had been identified as a mass both on US and DM/DBT. a, b, c craniocaudal DM, DBT and CEDM recombined images. aI, bI, cI mediolateral oblique DM, DBT and CEDM recombined images. BI-RADS density B. A post biopsy marker in the upper-outer quadrant of the left breast, referable to the index lesion (white circle); the CEDM examination showed the absence of perceptive contrast enhancement at the expected site of the index lesion. The index lesion in this case, which was included within the CEDM field of vision, did not present post biopsy hematoma and was considered to be a true false negative CEDM case.
Of the 13 TFNs, the majority were Ductal Carcinomas in Situ (DCIS) 8/13 (61.5%), followed by invasive ductal carcinomas (IDC) 4/13 (30.8%), and 1/13 (7.7%) was an invasive tubular carcinoma. The TFNs appeared in most cases as microcalcifications identified by DM/DBT, 7/13 (53.8%) as masses found with US exam, 4/13 (30.8%), or masses found with both US and DM/DBT exams, 2/13 (15.4%).
The characteristics of the TPs and the TFNs are summarized in Table 1, Table 2.
Table 2.
Characteristics of the 13 TFN patients.
| Patient | Age | Index lesion on CI | Palpable | Density | BPE | Dimensions at pathology (mm) | Extension of the disease | Histological Grade | Ki67 | Histology | Molecular Subtypes |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | US and DM/DBT Mass | Yes | B | Minimal | 10 | Unifocal | G2 | 0.3 | IDC∗ | Luminal B HER2- |
| 2 | 62 | US mass | No | B | Minimal | 5 | Unifocal | G1 | 0.05 | IDC∗ | Luminal A |
| 3 | 66 | Microcalcifications | No | B | Minimal | 15 | Unifocal | G2 | na | DCIS | na |
| 4 | 58 | Microcalcifications | No | B | Mild | 12 | Unifocal | G2 | na | DCIS | na |
| 5 | 60 | US and DM/DBT Mass | Yes | B | Minimal | 7 | Unifocal | G3 | 0.3 | IDC∗ | Her2-enriched |
| 6 | 44 | Microcalcifications | No | C | Mild | 8 | Unifocal | G2 | na | DCIS | na |
| 7 | 48 | DM/DBT Mass | No | D | Moderate | 10 | Unifocal | G2 | na | DCIS | na |
| 8 | 52 | Microcalcifications | No | C | Moderate | 6 | Unifocal | G1 | na | DCIS | na |
| 9 | 50 | Microcalcifications | Yes | C | Mild | 8 | Unifocal | G2 | na | DCIS | na |
| 10 | 44 | Microcalcifications | No | B | Minimal | 5 | Unifocal | G2 | na | DCIS | na |
| 11 | 49 | Microcalcifications | No | B | Mild | 5 | Unifocal | G2 | na | DCIS | na |
| 12 | 64 | DM/DBT Mass | No | B | Mild | 4 | Unifocal | G2 | 0.15 | IDC∗ | Luminal A |
| 13 | 47 | US mass | No | B | Moderate | 7 | Unifocal | G1 | 0.15 | ITC | Luminal B HER2+ |
BPE Background Parenchymal Enhancement; DM Digital Mammography; DBT Digital Breast Tomosynthesis; US Ultrasound; ∗IDC: all lesions with an invasive ductal component i.e. Invasive Ductal Carcinoma (IDC) + ILC, IDC + DCIS; DCIS Ductal Carcinoma In Situ; ITC Invasive Tubular Carcinoma.
Considering all the FNs, 91.1% (317/348) of index lesions were enhanced with CEDM, 94.57% for invasive cancer (296/313), and 60.00% for DCIS (21/35). However, by only taking the TFNs into account, 96.1% (335/348) of the index lesions were enhanced with CEDM, 98.34% for invasive cancers (296/301), and 72.41% for DCIS (21/29).
Table 3 illustrates and compares the two groups, TPs and TFNs, in terms of clinical, radiologic and pathologic variables, showing the results of univariate and logistic regression analysis.
Table 3.
Variables associated with an increased risk of TFN results with CEDMs, variables associated with a greater probability of being TPs with CEDMs and how each one affected the probability of a malignant lesion showing enhancement with CEDMs.
| Characteristic | TP (n) | TFN (n) | Univariate analysis |
Binary logistic regression |
BCs showing enhancement with CEDM (95% CI) | TFN-ir/TP-gp | |||
|---|---|---|---|---|---|---|---|---|---|
| p-value | p-value | Odds Ratio (OR) 95% CI | |||||||
| Radiological features | Breast density | BI-RADS A + BI-RADS B |
208 | 9 | – | ||||
| BI-RADS C + BI-RADS D |
109 | 4 | – | ||||||
| BPE | Minimal + Mild | 247 | 10 | – | |||||
| Moderate + Marked | 109 | 3 | – | ||||||
| Index lesion on CI | Developing Asymmetry | 3 | 0 | – | |||||
| Architectural distortion | 9 | 0 | – | ||||||
| Microcalcifications | 41 | 7 | <0.0001 | 0.0004 | 7.8537 (2.5150–24.5252) | 85.42% (72.24%–93.93%) | TFN-ir | ||
| DM/DBT Mass | 2 | 0 | – | ||||||
| US and DM/DBT Mass | 109 | 2 | – | ||||||
| US mass | 153 | 4 | – | ||||||
| All US MassΦ | 262 | 6 | 0.0010 | 0.0029 | 0.1799 (0.0582–0.5562) | 97.76% (95.19%–99.17%) | TP-gp | ||
| All DM/DBT MassΣ | 111 | 2 | – | ||||||
| Histological and molecular characteristic | Extension of the disease | Unifocal | 184 | 13 | 0.0012 | 0.0396 | 19.5366 (1.1512–331.5516) | 93,4% (88.98%–96.44%) | TFN-ir |
| Multifocal | 89 | 0 | 0.0234 | – | 100.00% (95.94%–100.00%) | TP-gp | |||
| Multicentric | 44 | 0 | – | ||||||
| Molecular Subtypes | Luminal A | 85 | 2 | – | |||||
| Luminal B HER2- | 155 | 1 | 0.0034 | 0.0197 | 0.0871 (0.0112–0.6778) | 99.36% (96.48%–99.98%) | TP-gp | ||
| Luminal B HER2+ | 30 | 1 | – | ||||||
| HER2 Enriched | 9 | 1 | – | ||||||
| Triple Negative | 17 | 0 | – | ||||||
| Not classifiable | 21 | 8 | <0.0001 | <0.0001 | 22.5524 (6.7801–75.0148) | 72.41% (52.76%–87.27%) | TFN-ir | ||
| Histology | DCIS | 21 | 8 | <0.0001 | <0.0001 | 22.5524 (6.7801–75.0148) | 72.41% (52.76%–87.27%) | TFN-ir | |
| IDC∗ | 199 | 4 | 0.0373 | 0.0293 | 0.2635 (0.0794–0.8747) | 98.03% (95.03%–99.46%) | TP-gp | ||
| ILC | 36 | 0 | – | ||||||
| Other invasive histotypes | 61 | 1 | – | ||||||
| Dimensions at pathology | ≥ 10 mm | 224 | 4 | 0.0043 | 0.0059 | 0.1845 (0.0554–0.6141) | 98.25% (95.57%–99.52%) | TP-gp | |
| < 10 mm | 93 | 9 | 0.0043 | 0.0059 | 5.4194 (1.6284–18.0356) | 91.18% (83.91%–95.89%) | TFN-ir | ||
| Histological Grade | G1 | 74 | 3 | – | |||||
| G2 | 150 | 9 | – | ||||||
| G3 | 93 | 1 | – | ||||||
| Ki67 | Ki67 ≥ 20% | 209 | 2 | – | |||||
| Ki67 < 20% | 87 | 3 | – | ||||||
| Clinical and demographic variables | Age | ≥50 | 235 | 8 | – | ||||
| <50 | 82 | 5 | – | ||||||
| Index lesion Palpability | Yes | 121 | 3 | – | |||||
| No | 196 | 10 | – | ||||||
| Personal History of BC | Yes | 36 | 3 | – | |||||
| No | 281 | 10 | – | ||||||
As indicated in Table 3, at the univariate analysis, the variables associated with an increased risk of TFN results with CEDMs were unifocal disease extension (p: 0.0012), non-classifiable molecular subtype (p < 0.0001; chi-square 46.98), DCIS histotype (p < 0.0001; chi-square 46.98), lesion dimensions <10 mm (p: 0.0043) and index lesion represented by microcalcifications on CI (p < 0,0001; chi-square 16.81). Instead, the variables associated with a greater probability of being TPs with CEDMs entailed the presence of an invasive ductal component, i.e. ductal and lobular invasive carcinoma, ductal and tubular invasive carcinoma, ductal invasive carcinoma and DCIS (p: 0.0373), index lesion represented by US mass (p: 0.0010; chi-square 10.90) on CI, i.e. US mass + US and DM/DBT mass, Luminal B HER2 negative molecular subtype (p: 0.0034), lesion dimensions ≥10 mm (p: 0.0043), and multifocal disease extension (p: 0.0234).
The binary logistic regression confirmed all the parameters identified by the univariate analysis, except for the multifocal disease extension, as significant independent risk factors for TFNs and TPs with CEDMs. Table 3.
We did not observe any statistically significant difference in the analysis of age, lesion palpability, personal history of BC, lesion grading, BPE level, Ki67 proliferative index, or breast density between the two subgroups. Table 3.
From the univariate analysis, the variables which were significant and had the greatest influence on the probability of a malignant lesion showing enhancement with CEDMs were: US mass on CI, 97.76% (95.19%–99.17%); Luminal B HER2 negative, 99,36% (96,48% - 99,98%); presence of an invasive ductal component, 98.03% (95.03%–99.46%); and dimensions ≥ 10 mm, 98.25% (95.57%–99.52%). Table 3.
BI-RADS Breast Imaging Reporting and Data Systems; BPE Background Parenchymal Enhancement; DM Digital Mammography; DBT Digital Breast Tomosynthesis; US Ultrasound; Φ All US Mass: US mass + US and DM/DBT mass; ΣAll DM/DBT Mass: DM/DBT mass + US and DM/DBT mass; ∗IDC: all lesions that had an invasive ductal component i.e. IDC + ILC, IDC + DCIS; DCIS Ductal Carcinoma In Situ; IDC Invasive Ductal Carcinoma; ILC Invasive Lobular Carcinoma; BC breast cancer; FN-ir increased risk of FN results in CEDM; TP-gp variables associated with a greater probability of being TPs with CEDMs.
As regards the evaluation of the BPE, the TFN + TP lesion group was divided into four subgroups based on the BPE level evaluated on recombined images, according to the BI-RADS criteria [26] as minimum, mild, moderate and marked. We then analyzed the subgroups, applying the exact Fisher test and/or the Chi-square test, to verify whether an increased risk of TFNs corresponded to higher levels of BPE. In particular, in the “minimum BPE” subgroup the TFN rate was 3.82% (5/131), in the “mild BPE” it was 3.97% (5/121), in “moderate BPE” 5.36% (3/53), and 0.00% (0/17) in “marked BPE”. Data show a proportional increase in the number of TFN cases as the level of BPE increases to a moderate, though not statistically significant level (Fisher exact test: 1). No TFN cases were observed among patients with marked BPE. These data, in contrast with the progressively increasing trend described above, may have been strongly influenced by the small number of cases with marked BPE (n = 17). This result is also confirmed by combining the “minimum + mild BPE” (10/257; 3.89%) and “moderate + marked BPE” (3/73; 4.11%) subgroups. In fact, also in this way there is only a slightly higher incidence of TFNs in the “moderate + marked” group (4.11%) compared to the “minimum + mild” group (3.89%), but the difference was not statistically significant (Fisher exact test: 1).
4. Discussion
In our study, we found CEDM enhancement in over 90% of malignant lesions and this was common in invasive cancer lesions compared to DCIS. After excluding the malignant lesions outside the CEDM field of vision and those with the post biopsy hematoma artifact at the site of the index lesion, and therefore only considering the TFNs, 98.34% of invasive cancers showed enhancement (296/301) and 72.41% of DCIS (21/29). These results are comparable with previously published studies evaluating the diagnostic performance of CEDMs [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18]].
From the analysis of several studies published in literature that evaluate the diagnostic performance of CEDMs in various subgroups of patients for different purposes, an FN rate emerged between 0% and 20%. Lack of enhancement has been reported for tumors of very different sizes and all BC types, including both invasive carcinomas and DCIS [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14],32].
The main cause of the FN CEDM results in our study was the “poor selection of patients” examined. In fact, of the 31 FNs observed, 6 were represented by lesions located outside the CEDM field of vision and therefore not detected during the exam. These patients would perhaps have benefited more from an exam with a wider field of vision than CEDM, such as breast MRI.
Another 12 of these 31 FNs showed an abundant post biopsy hematoma that prevented correct visualization of the enhancement at the expected site of the index lesion. All these patients had performed a VAB with an 8G needle before the CEDM, which as is well known, can give rise to the formation of abundant hematomas as a frequent complication [33,34]. In patients who have performed VAB and have to undergo CEDMs, it is therefore appropriate to monitor the evolution of the hematoma with a clinical exam and US and wait for its resorption before performing CEDMs.
In addition to the TFNs, we decided to also include the FNs in this article as due to the artifact from post biopsy hematoma or at the index lesion outside the CEDM field of vision they could be of help to others who use CEDMs. From our experience, in fact, other centers using CEDMs could learn to recognize and better manage not only post-biopsy hematoma artifacts but also patients with lesions identified by US but outside the mammography as well as the CEDM field of vision, in this way improving their clinical practice which in our experience has been burdened by these problems.
In our study we observed 13 TFN cases, mainly represented by DCIS with dimensions <10 mm and a unifocal extension. Our data were in line with those of Shimauchi et al., Teifke A. et al. and Wurdinger S et al. who analyzed the FN lesions in MRI [[21], [22], [23]]. One of the common causes of FNs identified by all the authors was in fact the small size of the index lesions. Furthermore, in our study, 9/13 TFNs had dimensions <10 mm at the final histology. Similar to the aforementioned authors, despite the two different imaging techniques used, our study showed that pure DCIS are the most frequent histotypes in the TFN group. The MRI sensitivity reported for DCIS is variable, some of these lesions, especially those with a lower pathological grade (G1) can be missed both in MRI and CEDMs, as can be assumed from our study [35,36].
Also in our study, fewer DCIS than invasive tumors showed enhancement with CEDMs, 72.41% vs. 98.34%. Of the 8 DCIS without enhancement however, it was possible to identify 7 due to the presence of suspicious microcalcifications in the low-energy images, something that is impossible with MRI.
The combined analysis of all imaging techniques is confirmed as the most appropriate approach for more effectively visualizing all the different types of BCs, as also reported by other authors [21,23,32].
We identified several variables in our study which are capable of increasing the probability of a BC showing enhancement with CEDMs, and other factors have shown significant correlation with the lack of enhancement.
These parameters can easily be obtained simply by analyzing the material obtained with CNB or VAB or from CI, and they could help distinguish those patients who could benefit less from CEDM than those in whom CEDM could be more appropriate.
Unlike reports in previous studies on both on MRI and CEDMs, in our study the BPE level did not show significant correlation with the TFN results and it cannot be considered an important factor that obscured abnormal enhancing lesions [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14],[21], [22], [23],32]. In our study this result is probably due to the very limited number of cases with a marked BPE level, only 17 cases compared to 131 with the minimal, 126 with the mild and 56 with the moderate level. In fact, if we only consider the first three groups (minimal, mild and moderate) in our work we can also observe a progressive increase in the number of TFNs as the level of BPE increases. The BPE assessment is however a subjective assessment, if two other radiologists had assessed CEDM images these results might have been different.
To try to better standardize the BPE level, it may be useful to show radiologists predefined sets of recombined images divided by levels of BPE before assessing the CEDM images. The fact that our radiologists have more than four years’ experience with CEDMs may have made them more confident and influenced the results.
The Luminal B HER2 negative molecular subtype was the subtype with the greatest probability of being TPs in the CEDMs we observed. The percentage of invasive BC resulting in TFNs in our study is too low to allow for identifying with any certainty which molecular subtype is most likely not to be visible with CEDMs, or for seeking any possible explanation. The same consideration can also be made for the analysis of the Ki67 proliferative index, which was not statistically significant given the lower number of invasive carcinomas classified as TFNs compared to the TPs.
One of the limitations of our study is represented by the small number of TFNs compared to TPs, but this can be considered a consequence of the high sensitivity that CEDMs have demonstrated to have. In our study group the most frequent molecular subtype was Luminal B, not Luminal A, as reported for the general population, which could be attributable to the group of patients enrolled all of which with a diagnosed BC, instead of a screening population [37,38]. In addition, all the analyzed data came from a single center and other limitations include the retrospective nature of the study and the fact that the radiologists who reported CEDM exams were aware of the histological diagnosis and the results of each patient’s previous examinations.
In fact, the prior knowledge of the diagnosis may have influences the radiologists’ interpretation of the CEDM images.
In conclusion, the number of BCs with a lack of enhancement with CEDMs was very low.
The parameters we found to be associated with the lack of enhancement of known Breast Cancers with CEDMs were: unifocal disease extension, DCIS histotype, lesion dimensions <10 mm and index lesion with microcalcifications on CI. Patients with these types of lesions may therefore benefit less from performing CEDMs. The characteristics that instead increase the likelihood of a BC showing enhancement are US mass on CI, Luminal B HER2 negative molecular subtype, the presence of an invasive ductal component, and lesion dimensions ≥10 mm.
Our future results could help improve daily clinical practice by enabling referral of patients to CEDMs or other exams (such as breast MRI) based on the characteristics of their tumors. For example, a patient whose index lesion is represented by an US mass on CI or (?) with an invasive ductal component at the histological examination, could be quickly referred to CEDM for preoperative staging or evaluation during neoadjuvant chemotherapy, since these characteristics endow the index lesion and also any additional foci of disease, with a greater probability of showing enhancement. This could possibly also allow for deploying fewer diagnostic resources, increasing patient compliance, and reducing waiting lists and healthcare costs.
Our results appear to be very promising, but they must be confirmed, preferably with prospective multicenter studies with larger series and randomization.
Source of funding
None.
Declaration of competing interest
None.
Footnotes
Institution from which the work originated: Diagnostic Senology Unit, Azienda Ospedaliero-Universitaria Careggi, Florence, Largo G. A. Brambilla 3, 50,134, Florence, Italy.
References
- 1.Fallenberg E.M., Schmitzberger F.F., Amer H. Contrast-enhanced spectral mammography vs. mammography and MRI - clinical performance in a multi-reader evaluation. Eur Radiol. 2017 Jul;27(7):2752–2764. doi: 10.1007/s00330-016-4650-6. [DOI] [PubMed] [Google Scholar]
- 2.Sumkin J.H., Berg W.A., Carter G.J. Diagnostic performance of MRI, molecular breast imaging, and contrast-enhanced Mammographyin women with newly diagnosed breast cancer. Radiology. 2019 Dec;293(3):531–540. doi: 10.1148/radiol.2019190887. [DOI] [PubMed] [Google Scholar]
- 3.Clauser P., Baltzer P.A.T., Kapetas P. Low-dose, contrast-enhanced mammography compared to contrast-enhanced breast MRI: a feasibility study. J Magn Reson Imag. 2020;52(2):589–595. doi: 10.1002/jmri.27079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petrillo A., Fusco R., Vallone P. Digital breast tomosynthesis and contrast-enhanced dual-energy digital mammography alone and in combination compared to 2D digital synthetized mammography and MR imaging in breast cancer detection and classification. Breast J. 2020;26(5):860–872. doi: 10.1111/tbj.13739. [DOI] [PubMed] [Google Scholar]
- 5.Kim G., Phillips J., Cole E. Comparison of contrast-enhanced mammography with conventional digital mammography in breast cancer screening: a pilot study. J Am Coll Radiol. 2019 Oct;16(10):1456–1463. doi: 10.1016/j.jacr.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Sorin V., Yagil Y., Yosepovich A. Contrast-enhanced spectral mammography in women with intermediate breast cancer risk and dense breasts. AJR Am J Roentgenol. 2018 Nov;211(5):W267–W274. doi: 10.2214/AJR.17.19355. [DOI] [PubMed] [Google Scholar]
- 7.Lee-Felker S.A., Tekchandani L., Thomas M. Newly diagnosed breast cancer: comparison of contrast-enhanced spectral mammography and breast MR imaging in the evaluation of extent of disease. Radiology. 2017 Nov;285(2):389–400. doi: 10.1148/radiol.2017161592. [DOI] [PubMed] [Google Scholar]
- 8.Jochelson M.S., Pinker K., Dershaw D.D. Comparison of screening CEDM and MRI for women at increased risk for breast cancer: a pilot study. Eur J Radiol. 2017 Dec;97:37–43. doi: 10.1016/j.ejrad.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Bicchierai G., Tonelli P., Piacenti A. Evaluation of contrast-enhanced digital mammography (CEDM) in the preoperative staging of breast cancer: Large-scale single-center experience. Breast J. 2020;26(7):1276–1283. doi: 10.1111/tbj.13766. [DOI] [PubMed] [Google Scholar]
- 10.Kim E.Y., Youn I., Ho Lee K. Diagnostic value of contrast-enhanced digital mammography versus contrast-enhanced magnetic resonance imaging for the preoperative evaluation of breast cancer. J. Breast Cancer. 2018;21:453–462. doi: 10.4048/jbc.2018.21.e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Roth R., Germaine P. Contrast-enhanced spectral mammography (CESM) versus breast magnetic resonance imaging (MRI): a retrospective comparison in 66 breast lesions. Diagn Interv Imaging. 2017 Feb;98(2):113–123. doi: 10.1016/j.diii.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 12.Fallenberg E.M., Dromain C., Diekmann F. Contrast-enhanced spectral mammography versus MRI: Initial results in the detection of breast cancer and assessment of tumour size. Eur Radiol. 2014 Jan;24(1):256–264. doi: 10.1007/s00330-013-3007-7. [DOI] [PubMed] [Google Scholar]
- 13.Amato F., Bicchierai G., Cirone D. Preoperative loco-regional staging of invasive lobular carcinoma with contrast-enhanced digital mammography (CEDM) Radiol Med. 2019 Dec;124(12):1229–1237. doi: 10.1007/s11547-019-01116-7. [DOI] [PubMed] [Google Scholar]
- 14.Zhu X., Huang J.M., Zhang K. Diagnostic value of contrast-enhanced spectral mammography for screening breast cancer: systematic review and meta-analysis. Clin Breast Canc. 2018 Oct;18(5):e985–e995. doi: 10.1016/j.clbc.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 15.Iotti V., Ravaioli S., Vacondio R. Contrast-enhanced spectral mammography in neoadjuvant chemotherapy monitoring: a comparison with breast magnetic resonance imaging. Breast Cancer Res. 2017 Sep 11;19(1):106. doi: 10.1186/s13058-017-0899-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel B.K., Hilal T., Covington M. Contrast-enhanced spectral mammography is comparable to MRI in the assessment of residual breast cancer following neoadjuvant systemic therapy. Ann Surg Oncol. 2018 May;25(5):1350–1356. doi: 10.1245/s10434-018-6413-x. [DOI] [PubMed] [Google Scholar]
- 17.Sorin V., Yagil Y., Yosepovich A. Contrast-enhanced spectral mammography in women with IntermediateBreast cancer risk and dense breast. AJR Am J Roentgenol. 2018 Nov;211(5):W267–W274. doi: 10.2214/AJR.17.19355. [DOI] [PubMed] [Google Scholar]
- 18.Sung J.S., Lebron L., Keating D. Performance of dual-energy contrast-enhanced digital mammography for screening women at increased risk of breast cancer. Radiology. 2019;293(1):81–88. doi: 10.1148/radiol.2019182660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerbel R.S. Tumor angiogenesis: past, present and the near future. Carcinogenesis. 2000 Mar;21(3):505–515. doi: 10.1093/carcin/21.3.505. [DOI] [PubMed] [Google Scholar]
- 20.Mann R.M., Cho N., Moy L. Breast MRI: state of the art. Radiology. 2019 Sep;292(3):520–536. doi: 10.1148/radiol.2019182947. [DOI] [PubMed] [Google Scholar]
- 21.Shimauchi A., Jansen S.A., Abe H. Breast cancers not detected at MRI: review of false-negative lesions. AJR Am J Roentgenol. 2010 Jun;194(6):1674–1679. doi: 10.2214/AJR.09.3568. [DOI] [PubMed] [Google Scholar]
- 22.Teifke A., Hlawatsch A., Beier T. Undetected malignancies of the breast: dynamic contrast-enhanced MR imaging at 1.0 T. Radiology. 2002 Sep;224(3):881–888. doi: 10.1148/radiol.2243010547. [DOI] [PubMed] [Google Scholar]
- 23.Wurdinger S., Kamprath S., Eschrich D. False-negative findings of malignant breast lesions on preoperative magnetic resonancemammography. Breast. 2001 Apr;10(2):131–139. doi: 10.1054/brst.2000.0232. [DOI] [PubMed] [Google Scholar]
- 24.Schnall M.D., Blume J., Bluemke D.A. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology. 2006 Jan;238(1):42–53. doi: 10.1148/radiol.2381042117. [DOI] [PubMed] [Google Scholar]
- 25.Ghai S., Muradali D., Bukhanov K. Nonenhancing breast malignancies on MRI: sonographic and pathologic correlation. AJR Am J Roentgenol. 2005 Aug;185(2):481–487. doi: 10.2214/ajr.185.2.01850481. [DOI] [PubMed] [Google Scholar]
- 26.ACR BI- RADS® Atlas breast imaging reporting and data system. American College of Radiology; Reston (VA: 2013. [Google Scholar]
- 27.Bicchierai G., Nori J., De Benedetto D. Role of contrast-enhanced spectral mammography in the post biopsy management of B3 lesions: Preliminary results. Tumor. 2018;17 doi: 10.1177/0300891618816212. 300891618816212. [DOI] [PubMed] [Google Scholar]
- 28.Bhimani C., Li L., Liao L. Contrast-enhanced spectral mammography: modality-specific artifacts and other factors which may Interfere with image quality. Acad Radiol. 2017 Jan;24(1):89–94. doi: 10.1016/j.acra.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 29.Nori J., Gill M.K., Vignoli C. Artefacts in contrast enhanced digital mammography: how can they affect diagnostic image quality and confuse clinical diagnosis? Insights Imaging. 2020 Feb 7;11(1):16. doi: 10.1186/s13244-019-0811-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meattini I., Bicchierai G., Saieva C. Impact of molecular subtypes classification concordance between preoperative core needle biopsy and surgical specimen on early breast cancer management: single-institution experience and review of published literature. Eur J Surg Oncol. 2017 Apr;43(4):642–648. doi: 10.1016/j.ejso.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 31.Goldhirsch A., Winer E.P., Coates A.S. Personalizing the treatment of women with early breast cancer: highlights of the St gallen international expert consensus on the primary therapy of early breast cancer 2013. Ann Oncol. 2013;24:2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taylor D., O’Hanlon S., Latham B. False-negative contrast-enhanced spectral mammography: use of more than one imaging modality and application of the triple test avoids misdiagnosis. BMJ Case Rep. 2017 Mar 31:2017. doi: 10.1136/bcr-2016-218556. pii: bcr2016218556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bick U., Trimboli R.M., Athanasiou A. Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights Imaging. 2020 Feb 5;11(1):12. doi: 10.1186/s13244-019-0803-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin L.L.Y., Gao Y., Lewin A.A. Overstated harms of breast cancer screening? A Large outcomes analysis of complications associated with 9-gauge stereotactic vacuum-assisted breast biopsy. AJR Am J Roentgenol. 2019 Apr;212(4):925–932. doi: 10.2214/AJR.18.20421. [DOI] [PubMed] [Google Scholar]
- 35.Kuhl C.K., Schrading S., Bieling H.B. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet. 2007 Aug 11;370(9586):485–492. doi: 10.1016/S0140-6736(07)61232-X. [DOI] [PubMed] [Google Scholar]
- 36.Neubauer H., Li M., Kuehne-Heid R. High grade and non-high grade ductal carcinoma in situ on dynamic MR mammography: characteristic findings for signal increase and morphological pattern of enhancement. Br J Radiol. 2003 Jan;76(901):3–12. doi: 10.1259/bjr/14883856. [DOI] [PubMed] [Google Scholar]
- 37.Waks A.G., Winer E.P. Breast cancer treatment: a review. J Am Med Assoc. 2019 Jan 22;321(3):288–300. doi: 10.1001/jama.2018.19323. [DOI] [PubMed] [Google Scholar]
- 38.Prat A., Cheang M.C., Martín M. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013 Jan 10;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]