Abstract
For a long time, traditional purification and extraction methods for the native Torpedo californica nicotinic acetylcholine receptor in lipid-like detergent complex (nAChR-DC) have compromised its purity, functionality and X-ray structural studies possibility. The dataset presented in this article provide a characterization of the Torpedo californica nAChR-DC purified using a sequential purification processes developed in our laboratory [1]. This purification takes in consideration all of the physicochemical and functional requirements stablished by several researchers for the past three decades for the nAChR. These requirements were addressed in order to preserve the stability and functionality of nAChR-DC while ensuring the highest degree of protein purity. We focused on the effect of cholesteryl hemisuccinate (CHS) supplementation on nAChR conformational changes during the purification process. Data from the size exclusion chromatography of the nAChR-DC supplemented with CHS in concentrations ranging from 0.01 mM, 0.1 mM, 0.2 mM and 0.5 mM consistently demonstrated that 0.5 mM CHS affects receptor stability via disassemble of the pentameric oligomer. However, 0.2 mM CHS produced negligible nAChR-DC subunit disruption.
The purified nAChR-DC has been characterized by circular dichroism (CD) and fluorescence recovery after photobleaching (FRAP), in order to assess its stability. The CD data was recorded in the wavelength range of 190-250 nm, showed that CHS induce a ⍺-helix to β-sheet transition of the nAChR-DC. The nAChR-LFC-16 delipidation with Methyl-β-Cyclodextrin decreased the percentage of α-helix and increased the β-sheet antiparallel secondary structure and levels the percentage of turns to that of the nAChR-DC without CHS treatment. Additionally, the stability of the nAChR-DC supplemented with CHS and incorporated into lipid cubic phase (LCP) was monitored for a period of 30 days by means of FRAP. The LCP-FRAP data allowed to establish possible optimal crystallization conditions for the development of crystals from purified nAChR-conjugated to α-Bungarotoxin, Alexa Fluor ™ 488 (α-BTX) in order to obtain a high-resolution atomic structure by X-ray diffraction.
Keywords: Torpedo californica, Nicotinic acetylcholine receptor, Lipid-like detergent complex, Circular dichroism, Fluorescence recovery after photobleaching
Specifications Table
| Subject | Biochemistry and biophysics |
| Specific subject area | Membrane proteins, secondary structure determination and protein mobile fraction in lipidic cubic phase |
| Type of data | Figure and Table |
| How data were acquired | The purification and analytical size exclusion chromatography (SEC) was performed using a ÄKTA protein purification system. The CD spectra of thermal unfolding experiment and the secondary structure of nAChR-DC were acquired using Jasco CD J-1500 (Japan) equipped with a Julabo AWC100 temperature controller. Rectangular quartz cell with 1 mm pathlength (Jasco 0556) was used for the experiment. The FRAP data was obtained on a Zeiss Axio Observer LSM 800 confocal microscope with a Tokai hit microscope incubator temperature controller. |
| Data format | Raw, Filtered and Analyzed |
| Parameters for data collection | The nAChR-DC purification and analytical SEC elution profile was monitored at 280 nm. The nAChR-DC CD spectrometry for the secondary structure predictions were recorded in the wavelength range of 190-250 nm. The CD scale was 200 mdeg / 1.0 dOD with the digital integration time (D.I.T) of 4 seconds, a bandwidth of 1.00 nm and a data pitch of 0.1 nm. The scanning speed was 100 nm/min with 1 number of accumulations. Confocal microscopy was performed at 20°C with a 40X magnification objective. The laser bleaching intensity was set to 50% of the total power, followed by the scanning of a sequence of 568 images. Consequently, each sample was integrated within a three 14.0 μm region of interest (ROI) and one reference ROI without bleaching. |
| Description of data collection | The native nAChR was extracted from Topedo californica (Tc) electroplax tissue obtained from Aquatic Research Consultants, San Pedro CA, USA and purified using a sequential purification process according to our related research article [1]. The millidegrees values obtained from the CD instrument were converted into mean residue ellipticity using the cd capitol plotting tool program (https://capito.uni-jena.de) according to Wiedemann protocols [2]. Subsequently, the CD spectra were analyzed using the Beta Structure Selection (BeStSel) (http://bestsel.elte.hu/index.php) for the evaluation of secondary structural predictions according to Micsonai methods [3] FRAP data were collected 24 hrs. after plates were assembled. The average integrated intensity of bleached 14.0 μm ROI, was used to correct for photobleaching from irradiation during the image-acquisition sequence. Finally, the fractional fluorescence recovery, diffusion coefficient and ΔFFR value was calculated using the equations described by Padilla-Morales [4]. |
| Data source location | Institution: University of Puerto Rico, San Juan, Puerto Rico |
| Data accessibility | Raw data can be retrieved from the Mendeley data repository: http://dx.doi.org/10.17632/hjmf3tgykz.1 |
| Related research article | This data article is submitted as a companion paper to: Rafael Maldonado-Hernández, Orestes Quesada, José O. Colón-Sáez and José A. Lasalde-Dominicci, (2020) Sequential purification and characterization of Torpedo californica nAChR-DC supplemented with CHS for high-resolution crystallization studies, Analytical Biochemistry, 113887, https://doi.org/10.1016/j.ab.2020.113887. |
Value of the Data
-
•
These data provide information of the lipid requirements of the nAChR-detergent complex supplemented with cholesteryl hemisuccinate isolated and purified by sequential purification chromatography of Torpedo rich membranes. Furthermore, we used CD and an LCP-FRAP assays to examine the secondary structure and stability of the highly pure nAChR-detergent complex.
-
•
The data can be used to assess the role of cholesteryl hemisuccinate as a structural lipid modulator of nAChR-DC which is necessary to maintain the stability of the nAChR-DC structure.
-
•
The data can provide valuable information that can be used by other research groups to obtain a high-pure, functional and stable nAChR-DC or other membrane protein detergent complexes with the ultimate goal of obtaining a functional X-Ray or Cryogenic Electron Microscopy structure.
1. Data Description
1.1. CHS supplementation for nAChR-LysoFos Choline 16 (nAChR-LFC-16) preparations
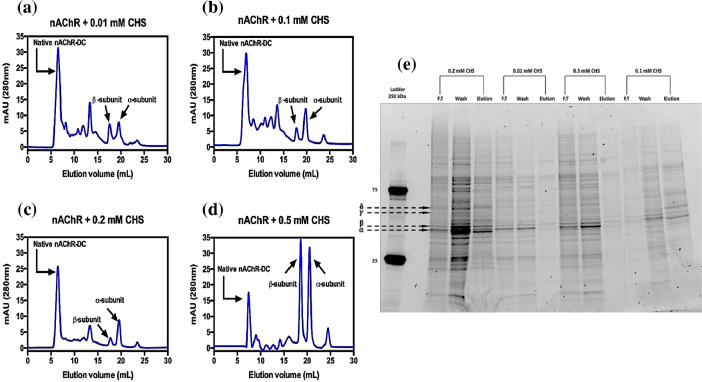
For decades, the solubilization, isolation and purification of nAChR from different sources has not been the most efficient, for this reason we supplement the processes with CHS, improving and optimizing the nAChR preparations. Fig. 1 shows the raw data of the analytical SEC for the supplementation of the samples with different CHS concentrations (0.01 mM, 0.1 mM, 0.2 mM and 0.5 mM) to supplement the solubilization, affinity purification with acetylcholine bromide coupled to Affi-Gel 15 and gel filtration chromatography steps to improve protein extraction. The SEC chromatograms of 0.01 mM and 0.1 mM CHS supplementation, presents different degrees of contamination in the eluted peaks and also shows a certain degree of disassembling of the receptor subunits (Fig. 1 a and b). Also, 0.5 mM CHS affects receptor stability by disassemble of the subunits, this can be observed in Fig. 1 d, the alpha and beta subunits peaks are significant higher than the corresponding peaks of the native nAChR-LFC-16. However, the supplementation with 0.2 mM of CHS minimized the nAChR subunit disruption (Fig. 1 c). This behavior was also observed by SDS-PAGE electrophoresis of the eluted fractions from the flow-through, wash and elution of the affinity chromatography steps (Fig. 1 e).
Fig. 1.
Analytical size exclusion chromatography of nAChR-DC supplemented with different concentrations of CHS.
1.2. Thermal unfolding experiment and the secondary structure predictions of nAChR-LFC-16
Given that nAChR activity has been found to be highly dependent on lipid composition in the vicinity of the transmembrane regions of the receptor, we wanted to know the effect of the CHS treatments that led to the purification of nAChR in its secondary structures and whether these structures would correlate with their activities. To this end, we recorded CD spectra of the nAChR-LFC-16 complexes obtained under different treatments (with and without CHS buffer supplementation and delipidation with Methyl-β-Cyclodextrin). Fig. 2 presents the CD spectrum of the nAChR-LFC-16 complex in the absence of cholesterol showing a broad minima transition between 210 nm and 222 nm (n-π*). Interestingly, the signal around 190 nm (π–π*) was very weak and similar for all conditions studied. The nAChR-LFC-16 complex purified under CHS buffer supplementation presents a spectrum with similar behavior between 190–222 nm regions, but with a slightly higher intensity and a more marked second transition. The nAChR-LFC-16 complex purified under CHS buffer supplementation followed by delipidation with Methyl-β-Cyclodextrin, presents minima at 190–222 nm regions, but with decreased intensity and flattened second transition in this region.
Fig. 2.
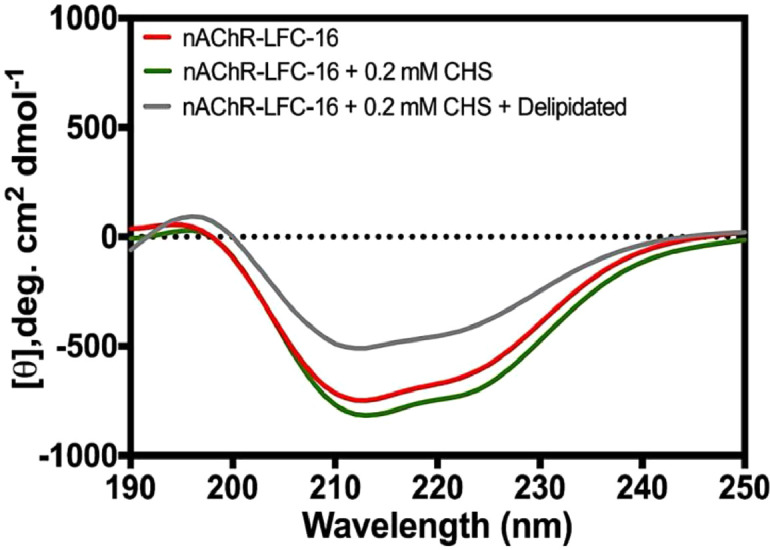
Effect of CHS buffer supplementation on secondary structure of the Purified nAChR-LFC16 using the sequential purification protocol.
Table 1 presents the percentage of secondary structure content for the nAChR-LFC-16 complex under different conditions and values of thermal denaturation at 222 nm. For the secondary structure determination we used an online algorithm named BeStSel after β-structure selection, developed by Micsonai and coworkers, (http://bestsel.elte.hu/index.php) [3]. The Jasco CD-1500 Circular Dichroism Spectrometer was set to continuously determine sample stability from 10 ˚C to 100 ˚C using the CD Temp Wavelength Scan program and monitored at 222 nm for sample denaturalization. For nAChR-LFC-16 complex samples assayed with or without CHS supplementation the α-helix percentage on an average presents slight differences 29.5% and 32.6% respectively; however the β-structure and other conformation present significant changes (Table 1). On the other hand, the thermal denaturation temperature measured at 222 nm presented very similar values for nAChR-LFC-16 complex samples. However, the secondary structure content for the delipidation with Methyl-β-Cyclodextrin of the nAChR-LFC-16 complex was completely different. The delipidation with Methyl-β-Cyclodextrin decreases the percentage of α-helix by 36% and 42% respectively, in regard to the nAChR-LFC-16 without treatment, and substantially increases the percentage of β-antiparallel secondary structure and levels the percentage of turns to that of the nAChR-LFC-16 alone (Table 1). All raw data of CD measurements is available at Mendeley data repository: http://dx.doi.org/10.17632/hjmf3tgykz.1
Table 1.
Summary of secondary structure results derived from CD spectra.
| Detergent Complex | Temperature | Helix | Antiparallel | Parallel | Turn | Other | Thermal denaturation at 222 nm |
|---|---|---|---|---|---|---|---|
| nAChR-LFC-16 | 20°C | 29.5% | 15.5% | 5.2% | 10.9% | 38.9% | 52.54 °C |
| nAChR-LFC-16 + 0.2 mM CHS | 20°C | 32.6% | 21.0% | 21.8% | 0.0% | 24.6% | 55.04 °C |
| nAChR-LFC-16 + 0.2 mM CHS + Methyl-β-Cyclodextrin delipidation | 20°C | 10.7% | 32.9% | 1.5% | 14.2% | 40.7% | 35.04 °C |
The secondary structure determination contents was done by the BeStSel (Beta Structure Selection) program according to the Micsonai protocol [3]. The Jasco CD-1500 Circular Dichroism Spectrometer allows for a continuous analysis of the secondary structure determination of a protein at variable temperature intervals according to the Jasco protocols.
1.3. Assessment of nAChR-LFC-16 stability on a lipidic matrix by FRAP
The stability of nAChR-DC is extremely important to harvest receptor crystals for X-ray diffraction purposes. Previous work in our laboratories in which fluorescence recovery assays were validated for Torpedo californica nAChR-DC purified using the original protocol and labeled α-BTX and in the LCP matrix, demonstrated the dependence of the mobile fraction of nAChR-DC regarding the physicochemical characteristics of the detergents used in the solubilization process [4,5]. With this in mind and knowing that the optimized protocol presented here uses CHS as a supplement for the stabilization of nAChR-DC, fractional fluorescence recovery assays were carried out for the purified Torpedo californica nAChR-DC labeled with α-BTX and in the LCP matrix. As described in Methods, three 14.0 μm ROI circular areas were used for FRAP experiments, where fluorescence intensities were monitored at 0.6 s intervals. With the exception of the use of temperature control in these assays, the FRAP procedure is essentially the same as that reported by our laboratory [4,5].
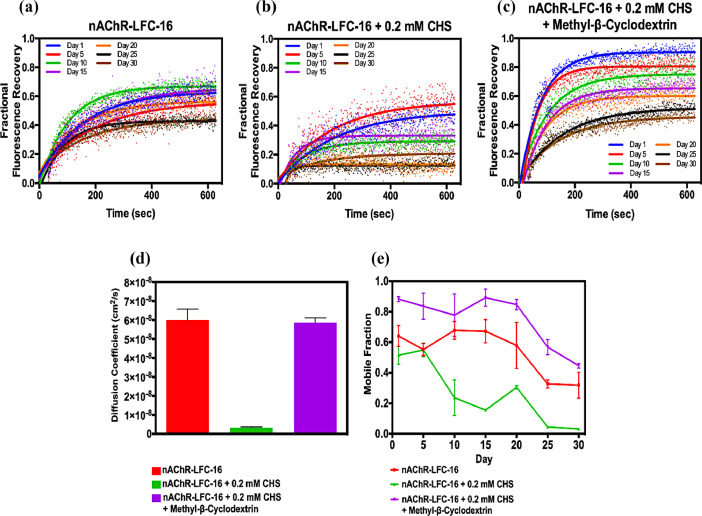
In order to evaluate the effect of CHS supplementation and CHS plus Methyl-β-Cyclodextrin treatment on the nAChR-DC stability in LCP, the fractional fluorescence recovery and diffusion coefficient were quantified for a period of 30-days (Fig. 3). For the purpose of homogeneity with previously reported works, we will use the parameter ΔFFR which refers to the difference in fractional fluorescence recovery between day 1 to 30 days. Fig. 3 a and b presents the fractional fluorescence recovery for the nAChR-LFC-16 complex without and with the CHS supplemented buffer used for the purification process. The presence of CHS increased the variability of the fractional fluorescence recovery over the 30 days assayed and lagging first order recovery signal compared to the nAChR-LFC-16 complex without CHS. The ΔFFR value for the improved protocol in the absence of CHS was 0.24 compared to the same preparation but supplemented with CHS, which decreases the ΔFFR to 0.17. Fig. 3 c presents fractional fluorescence recovery over the 30 days for nAChR-LFC-16 complex obtained with the CHS plus Methyl-β-Cyclodextrin treatment for the removal of CHS excess. A substantial recovery in fluorescence without signal lag was presented during the first 20 days compared to nAChR-LFC-16 plus CHS. However, the final 10-day analysis presented a similar behavior of the nAChR-LFC-16 without CHS treatment, almost a ΔFFR of 0.43. In terms of diffusion coefficient and mobile fraction both CHS buffer supplementation displayed a drastic drop since the first days of the 30-day period (Fig. 3 d and e). CHS buffer supplementation showed an evident reduced diffusion coefficient of ∼3.3 × 10−9 cm2/s and to ∼1.3 × 10−9 cm2/s, respectively and also decayed mobile fraction at all periods assayed (Fig. 3 d and e). Nevertheless, the nAChR-LFC-16 and CHS/ Methyl-β-Cyclodextrin treatment exhibited undistinguished differences with respect to the diffusion coefficient of ∼6.0 × 10−8 cm2/s and to ∼5.9 × 10−8 cm2/s, respectively. However, the CHS/ Methyl-β-Cyclodextrin treatment presents a notable improvement of 84% in the mobile fraction during the first 20 days over the control, non CHS supplemented purified nAChR-LFC-16 (Fig. 3 e). For more details the raw data of FRAP measurements is available at Mendeley data repository: http://dx.doi.org/10.17632/hjmf3tgykz.1
Fig. 3.
FRAP, Mobility and Diffusion measurement of nAChR with and without CHS through the lipidic cubic phase.
1.4. Crystallization of nAChR-DC into the Lipid Mesophases For X-Ray diffractions screening
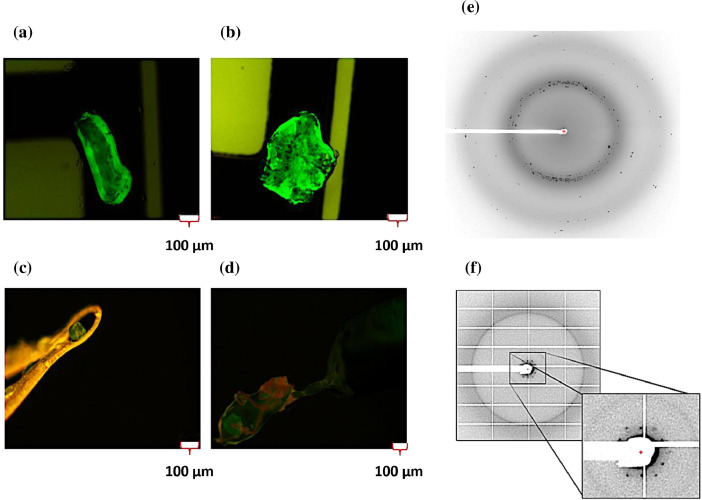
Throughout the history of nAChR, the works of Robert Stroud in 1981 and Ferdinand Hucho in 1988 paved the way for the crystallization of this important receptor. They managed to obtain crystalline arrangements or nAChR crystals prepared with traditional purification processes [6,7]. Our laboratory has greatly improved over time the strategies for crystallization of nAChR, implementing modern techniques for the development and preparation of crystals with the use of lipid matrices, producing crystals of greater size and in less preparation time [4,8]. These advances were the bases that we used for the preparation of the nAChR-DC crystals, implementing a sequential purification processes. Briefly, we purified the protein using our sequential purification method, then conjugated the nAChR-DC with α-BTX-Alexa-488 to be able to discriminate between nAChR-DC crystals with precipitant crystals. Then, we placed the samples with nAChR-DC in the presence of different precipitants and finally the samples were incubated at 18 °C and 20 °C until they were collected. To collect them, we used micro loops and quickly freezing it in liquid nitrogen for cryopreservation (Fig. 4 c and d). For the crystallization conditions we used a solution containing 0.05 M ADA pH 6.8, 12.5% PEG 1500 and 10% PEG 1000 mixed with nAChR-DC 1:1 volume ratio, and incubated in sealed wells containing 0.5 ml of the same crystallization conditions, at 20°C (Fig. 4 a–d). Furthermore, we observed that the nAChR-DC Methyl-β-Cyclodextrin treatment produced crystals formation of nAChR-DC in the LCP. The combination of purification, nucleation strategies used for these preparations and together with the use of a device created and developed in our research laboratory, (the RMP@LMx), have led to the production of crystals in a reproducible way shortening the crystal formation time. The device is capable effectively apply an electrical potential to the samples to biomimetize the action potential in the native membrane and bring the protein to a homogeneous and stable conformation to achieve constant nucleation of the crystal. In this way, nAChR, being a voltage sensitive ion channel, can be stabilized and preserved in a more natural way to its physiological membrane environment. The diffraction of these crystals has consistently produced diffraction patterns using the Advanced Photon Source (APS) 23-ID-B beamline at Argonne National Laboratory (Fig. 4 e and f).
Fig. 4.
nAChR-DC labeled with α-BTX-Alexa-488 crystals by the LCP method, using a device developed in our laboratory (RMP@LMx).
To determine the ideal concentration of CHS for the nAChR-LFC-16, analytical SEC of the affinity purification step (acetylcholine bromide coupled to Affi-Gel 15) was performed using a Superdex 200 10/300 increase Gel filtration GE column, flow rate of 0.25 ml/min. for different concentrations of CHS, (a) 0.01 mM, (b) 0.1 mM, (c) 0.2 mM and (d) 0.5 mM. The elution profile was monitored at 280 nm. (e) The SDS-PAGE was performed using denatured conditions (95 °C for 5 min). The CHS at 0.01 mM, 0.1 mM, 0.2 mM and 0.5 mM was used for nAChR-LFC-16 purification. Each row represents the steps of the acetylcholine bromide coupled to Affi-Gel 15 column (flow through (F.T), wash and elution step).
Jasco CD-1500 Circular Dichroism Spectrometer was used for the continuous analysis of the secondary structure determination of the nAChR-LFC-16 purified under different conditions at variable temperature intervals. The sample was diluted to 50 μg/μL and loaded into a 400 μL rectangular quartz cell with 1 mm pathlength. The experiment monitored the 190–250 nm wavelength range of the CD spectrum analysis. The CD scale was 200 mdeg/1.0dOD with the D.I.T of 4 s, bandwidth of 1.00 nm and data pitch of 0.1 nm. The scanning speed was 100 nm/min with 1 number of accumulations. Unit conversion was achieved by CD capitol plotting tool (https://capito.uni-jena.de) according to Wiedemann protocol [2].
The nAChR-LFC-16 samples under the different conditions studied were reconstituted into LCP as stated in the Method section. All FRAP data was collected 24 h. after plated and continued recorded every five days for 30 days. We used a ROI of 14 μm, with 70% laser intensity. Data collection was performed at 20 °C using a Zeiss Axio Observer LSM 800 confocal microscope with a 40X magnification objective. The laser bleaching intensity was set to 50% of the total power, followed by the scanning of a sequence of 568 images. All samples were performed in triplicates, Data samples collected for nAChR-LFC-16 (a) without CHS, (b) with 0.2 mM CHS and (c) with 0.2 mM CHS ± Methyl-β-Cyclodextrin. (d) Diffusion coefficient and (e) mobile fraction were collected in triplicates for each sample. The fractional fluorescence recovery curve was calculated using Eq. (1). The mobile fraction was obtained by averaging the last twenty points of the fractional recovery and finally, the diffusion coefficient was calculated using Eq (2).
Samples were prepared using the sequential purification process for X-Ray diffractions screening. (a and b) Fluorescent nAChR crystals formation at 20 °C with reservoir solution containing 0.05 M ADA pH 6.8, 12.5% PEG 1500 and 10% PEG 1000. (c and d) The nAChR crystal harvesting from LCP was performed using MiteGen loops for the flash freezing in liquid nitrogen. (e and f) Raw dataset X-Ray diffraction pattern of nAChR-DC using beamline 23-ID-B of Advanced Photon Source at Argonne National Laboratory. The samples were performed with (e) traditional and (f) sequential purification processes. X-Ray crystallography diffraction pattern was achieved using Adxv software (https://www.scripps.edu/tainer/arvai/adxv.html). The diffracted crystals of the α-BTX-Alexa-488 conjugate-bound with nAChR-DC were harvested at 20°C.
2. Experimental Design, Materials and Methods
2.1. CHS/LFC-16 detergent solubilizing and chromatography solutions
The CHS was incorporated into the LFC-16 detergent solubilizing solution used for the nAChR extraction from the Tc electroplax tissue and in each buffer used for the chromatography process. The solubilization solution contained 35 mM LFC-16 and CHS with different concentrations (0.01 mM, 0.1 mM, 0.2 mM and 0.5 mM) to a final volume of 2 mL dissolved with a solution of 40 mM Tris pH 7.4 and 300 mM NaCl. Subsequently, the solubilization solution was mixed gently, at room temperature, overnight. The crude membranes of Tc were mixed gently with 13 mL of 300 mM NaCl, 40 mM Tris pH 7.4, and the 2 mL CHS/ LFC-16 detergent solubilization solution for 1 h. at 4 °C. After incubation time, the solubilized membrane proteins were centrifuged at 162,600 g for 1 h. at 4 °C and used immediately for the purification process. For the subsequent purification steps the final concentration of the LFC-16 detergent was decreased substantially to 1.5 times its critical micelle concentration and the final concentration of CHS remained the same as the one used in the solubilization process for each concentration used (0.01 mM, 0.1 mM, 0.2 mM and 0.5 mM).
2.2. Purification and extraction of Torpedo californica nAChR-LFC-16
The native nAChR was solubilized and purified from Torpedo californica electroplax tissue, according to our related research article [1]. Briefly, we solubilized the protein using the LFC-16 which is an analogous phospholipid detergent, integrating small CHS adjustments in the preparation. Then we implemented three subsequent chromatography steps. The first purification step was adapted to an automated medium pressure system using Affi-Gel 15 affinity protein capture resin. This resin is conjugated in an anhydrous condition with Bromoacetylcholine bromide, packed and loaded in GE XK columns designed for chromatography processes under medium or low pressures. The second chromatographic step involves the use of a Capto Lentil Lectin column for the glycoprotein capture. Finally, the last step incorporates the gel filtration technique allows the separation of previously eluted glycoproteins by their hydrodynamic volume.
2.3. Circular dichroism spectroscopy (CD) of Torpedo californica nAChR-LFC-16
The CD spectra of thermal unfolding experiment and the secondary structure of nAChR-DC were acquired using Jasco CD J-1500 (Japan) equipped with a Julabo AWC100 temperature controller. To perform continuous spectra analysis according to Jasco protocols the sample was filtered with Ultrafree-MC centrifugal filter and diluted to 50 μg/μL (https://jascoinc.com/training-video/cd-temp-wavelength-scan-video/). The temperature wavelength scan program monitoring the 222 nm was set for the thermal test from 10 ˚C to 100 ˚C. The spectra for secondary structure predictions were recorded in the wavelength range of 190–250 nm at 20 ˚C. The CD scale was 200 mdeg / 1.0 dOD with the D.I.T of 4 s, a bandwidth of 1.00 nm and a data pitch of 0.1 nm. The scanning speed was 100 nm/min with 1 number of accumulations. The millidegrees values obtained from the CD instrument were converted into mean residue ellipticity using the cd capitol plotting tool program (https://capito.uni-jena.de) according to Wiedemann protocols [2]. Subsequently, the CD spectra were analyzed using the Beta Structure Selection (BeStSel) (http://bestsel.elte.hu/index.php) for the evaluation of secondary structural predictions according to Micsonai methods [3].
2.4. Assessment of nAChR-LFC-16 stability on a lipidic matrix by FRAP
LCP reconstitution was performed according to the protocols described by Liu, W. [9], with the following modifications: the monoolein were loaded into the 250 μL syringe and the purified nAChR-DC binding to α-Bungarotoxin, Alexa Fluor™ 488 conjugated (α-BTX) were loaded in a 100 μL syringe to lipid ratio 40/60 (v/v). Then FRAP experiments were performed according to the protocols described by Cherezov and Padilla-Morales with minor modifications [4,10]. Purified nAChR-DC-α-BTX in LCP were placed on 75 × 25 × 1 mm slides and washed with 1 mL of a solution containing 0.1 M sodium/potassium phosphate (pH 6.2), 21.5% polyethylene glycol (PEG) 1000, and 0.2 M sodium chloride. Finally, the LCP with protein complex was transferred into a PB600 repeating dispenser using a 10 μL Gastight Syringe (1700 Series), 0.2 μL was dispensed into wells with an incubation temperature of 20 °C. For the sample preparation with 0.5 mM Methyl-β-Cyclodextrin, the incubation time was 1 h. before reconstitution in LCP for the FRAP experiments. Subsequently SEC was performed for Methyl-β-Cyclodextrin removal. Then, the preparation for all LCP reconstitution was carried out with the same indications mentioned above. All FRAP data were collected 24 hrs. after plates were assembled. Finally, the data collection for FRAP assays were performed at 20 °C using a Zeiss Axio Observer LSM 800 confocal microscope with a 40X magnification objective. The temperature was controlled by a Tokai hit microscope incubator. Five pre-bleach images were used as a baseline. The laser bleaching intensity was set to 50% of the total power, followed by the scanning of a sequence of 568 images. Consequently, each sample was integrated within a three 14.0 μm region of interest (ROI) and one reference ROI without bleaching. The average integrated intensity of bleached 14.0 μm ROI, was used to correct for photobleaching from irradiation during the image-acquisition sequence. The data analysis of fractional fluorescence recovery curves was calculated using the following equations.
Fractional fluorescence recovery equation:
| (1) |
Where F(t) is the fractional fluorescence recovery curves, ft is the corrected fluorescence intensity of the bleached ROI, f0 is the corrected fluorescence intensity of the bleached ROI in the 600 ms after bleaching, and f∞ is the average of corrected fluorescence intensity in the five pre-bleached images. The diffusion coefficient D was calculated using the equation described by Padilla-Morales [4]. Where γ is a constant with a value of 0.88 corresponds to circular beams described by Oyola and Axelrod [11,12]. R is the radius of ROI and t1/2 is the half-time of fluorescence recovery.
Diffusion coefficient equation:
| (2) |
2.5. Crystallization of nAChR-DC into the lipid mesophases for X-ray diffractions screening
2.5.1. Reconstitution of nAChR-DC into the lipid mesophases
LCP crystallization screening was performed according to the protocols described by Liu, W. and co-workers with the following modifications [9]. Purified nAChR-DC binding to α-Bungarotoxin, Alexa Fluor™ 488 conjugated (α-BTX), was concentrated. The protein in the detergent complex was reconstituted in LCP using monoolein as follows: the monoolein was incubated at 40 °C for a few minutes until the lipid melts. The syringe coupler was attached to a 250 μL gastight syringe. Then monoolein was loaded into the syringe and protein samples were loaded in another 100 μL syringe at a protein solution to lipid ratio 40/60 v/v. Both syringes were connected through the syringe coupler. The syringe plungers were pushed alternately to move the monoolein and nAChR-DC sample through the syringes until the lipid mesophase became transparent. Finally, the crystallization trials were done: transferred the LCP nAChR-DC sample into a PB600 repeating dispenser using a 10 µL Gastight Syringe and 0.2 μL protein complex was dispensed into each well with 1 μL of corresponding crystallization screen solution with an incubation temperature of 18 °C and 20 °C.
2.5.2. LCP-nAChR-DC crystal harvesting
For the harvesting, each plate with samples was placed under a stereomicroscope equipped with a green fluorescent detector. Afterward, we used MiTeGen MicroMount for collecting the crystal directly from LCP with the loops. Finally, the flash freezing was performed, dipping the loops with the crystal in liquid nitrogen to then be shipped to APS for X-ray data collection.
Ethics statement
The electroplaque tissue from Torpedo californica electric ray was obtained from Aquatic Research Consultants, (San Pedro CA) and used according to the guidelines of Institutional Animal Care and Use Committee with the protocol number: ATP002-20-12-2017 and the Institutional Biosafety Committee with the logging number: MSRC-IBC-2018-3.
Author's contributions statement
Rafael Maldonado-Hernández, Orestes Quesada, and José A. Lasalde-Dominicci: designed the experiments and optimized the protocol. Rafael Maldonado-Hernández and Orestes Quesada: performed the experiments and contributed to the data analysis. Rafael Maldonado-Hernández, Orestes Quesada: initial draft and Orestes Quesada, and José A. Lasalde-Dominicci: edited and completed the finalized version of the manuscript.
Declaration of Competing Interest
The authors declare no competing financial interests in this paper.
Acknowledgments
This financial support was provided by; The National Institutes of Health NIGMS Grant: 1R01GM098343 (JALD, OQ) and supported by the National Institute of General Medical Sciences (NIGMS) of the National Institutes of Health (NIH) under Award Number P20GM103642, (JALD), COBRE NIEF Grant: 1P20GM103642 (JALD), Infrastructure support was provided in part by the National Institute on Minority Health and Health Disparities RCMI Grant: 2U54MD007600 (JALD), UPR- RP RISE Program Grant: 5R25GM061151(OQ, RMH) and BioXFEL STC of the National Science Foundation (NSF) Grant: 1231306 (RMH). The X-ray data was collected on the beamline 23-ID-B at Advanced Photon Source supported by U.S. Department of Energy, Office of Science, Argonne National Laboratory under Proposal ID 68121. The authors would like to thank the following students for their help: Edgardo Albino. Claude A. Maysonet-Navarro, Adriana Pastrana-González, Gloriangely Avilés-Reymundí and Manuel J. La Torre Poueymirou.
Contributor Information
Orestes Quesada, Email: quesada.orestes@gmail.com.
José A. Lasalde-Dominicci, Email: jose.lasalde@upr.edu.
References
- 1.Maldonado-Hernández R., Quesada O., Colón-Sáez J.O., Lasalde-Dominicci J.A. Sequential purification and characterization of Torpedo californica nAChR-DC supplemented with CHS for high-resolution crystallization studies. Anal. Biochem. 2020 doi: 10.1016/j.ab.2020.113887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiedemann C., Bellstedt P., Görlach M. CAPITO—a web server-based analysis and plotting tool for circular dichroism data. Bioinformatics. 2013;29:1750–1757. doi: 10.1093/bioinformatics/btt278. [DOI] [PubMed] [Google Scholar]
- 3.Micsonai A., Wien F., Kernya L., Lee Y.-H., Goto Y., Réfrégiers M., Kardos J. Accurate secondary structure prediction and fold recognition for circular dichroism spectroscopy. Proceedings of the National Academy of Sciences. 2015;112:E3095–E3103. doi: 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Padilla-Morales L.F., Colón-Sáez J.O., González-Nieves J.E., Quesada-González O., Lasalde-Dominicci J.A. Assessment of the functionality and stability of detergent purified nAChR from Torpedo using lipidic matrixes and macroscopic electrophysiology. Biochim. Biophys. Acta (BBA)-Biomembr. 2016;1858:47–56. doi: 10.1016/j.bbamem.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Padilla-Morales L.F., Morales-Pérez C.L., Pamela C., Asmar-Rovira G., Báez-Pagán C.A., Quesada O., Lasalde-Dominicci J.A. Effects of lipid-analog detergent solubilization on the functionality and lipidic cubic phase mobility of the Torpedo californica nicotinic acetylcholine receptor. J. Membr. Biol. 2011;243:47. doi: 10.1007/s00232-011-9392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kistler J., Stroud R.M. Crystalline arrays of membrane-bound acetylcholine receptor. Proc. Natl. Acad. Sci. 1981;78:3678–3682. doi: 10.1073/pnas.78.6.3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hertling-Jaweed S., Bandini G., Müller-Fahrnow A., Dommes V., Hucho F. Rapid preparation of the nicotinic acetylcholine receptor for crystallization in detergent solution. FEBS Lett. 1988;241:29–32. doi: 10.1016/0014-5793(88)81024-X. [DOI] [PubMed] [Google Scholar]
- 8.Asmar-Rovira G.A., Asseo-García A.M., Quesada O., Hanson M.A., Cheng A., Nogueras C., Lasalde-Dominicci J.A., Stevens R.C. Biophysical and ion channel functional characterization of the Torpedo californica nicotinic acetylcholine receptor in varying detergent-lipid environments. J. Membr. Biol. 2008;223:13–26. doi: 10.1007/s00232-008-9107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W., Cherezov V. Crystallization of membrane proteins in lipidic mesophases. JoVE. 2011:e2501. doi: 10.3791/2501. https://doi:10.3791/2501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cherezov V., Liu J., Griffith M., Hanson M.A., Stevens R.C. LCP-FRAP assay for pre-screening membrane proteins for in meso crystallization. Cryst. Growth Des. 2008;8:4307–4315. doi: 10.1021/cg800778j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oyola-Cintrón J., Caballero-Rivera D., Ballester L., Baéz-Pagán C.A., Martínez H.L., Vélez-Arroyo K.P., Quesada O., Lasalde-Dominicci J.A. Lateral diffusion, function, and expression of the slow channel congenital myasthenia syndrome αC418W nicotinic receptor mutation with changes in lipid raft components. J. Biol. Chem. 2015;290:26790–26800. doi: 10.1074/jbc.M115.678573. https://doi:10.1074/jbc.M115.678573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Axelrod D., Koppel D., Schlessinger J., Elson E., Webb W.W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys. J. 1976;16:1055. doi: 10.1016/S0006-3495(76)85755-4. https://10.1016/S0006-3495(76)85755-4 [DOI] [PMC free article] [PubMed] [Google Scholar]