Highlights
-
•
We compiled datasets of N2O emission factors (EFs) from 21 separate studies.
-
•
A Bayesian approach improves uncertainty analysis of EFs.
-
•
AN applications were lower for applications to arable fields than to grasslands.
-
•
Microbial inhibitors reduce emissions of N2O from mineral fertilisers significantly.
Keywords: N2O, Ammonium nitrate, Urea, Microbial inhibitor, National inventory, Agriculture, Greenhouse gas
Abstract
In this study, we analysed datasets of N2O emission factors (EFs) from 21 separate studies carried out on arable and managed grasslands across the UK and Ireland over the past 20 years. A total of 641 separate events were collated from 40 experimental field sites. Individual EFs ranged over an order of magnitude (0–12% of applied N) for each fertiliser type, following a log-normal distribution in all cases. Our study shows that a Bayesian approach can provide a robust statistical method that is capable of performing uncertainty analysis on log-normal distributed data in a more defensible manner than conventional statistical methods allow. This method allowed for a national scale comparison of EFs between the most commonly applied mineral fertilisers based solely on previously published data (UK and Ireland in this case). The study shows that ammonium nitrate (AN) and Calcium ammonium nitrate (CAN) are the largest emitting fertiliser types by mass across the British Isles (temperate climate zone), with EFs of 1.1 (1.0–1.2) % and 1.0 (0.7–1.3) % for all recorded events, respectively; however, emissions from AN applications were significantly lower for applications to arable fields (0.6%) than to grasslands (1.3%). EFs associated with urea (CO(NH₂)₂) were significantly lower than AN for grasslands with an EF of 0.6 (0.5–0.7) %, but slightly higher for arable fields with an EF of 0.7 (0.4–1.4) %. The study highlights the potential effectiveness of microbial inhibitors at reducing emissions of N2O from mineral fertilisers, with Dicyandiamide (DCD) treated AN reducing emissions by approximately 28% and urea treated with either DCD or N-(n)-butyl) thiophosphorictriamide (NBTP) reducing emissions by approximately 40%. Although limited by a relatively small sample size (n = 11), urea treated with both DCD and NBPT appeared to have the lowest EF of all treatments at 0.13 (0.08–0.21) %, highlighting the potential to significantly reduce N2O emissions at regional scales if applied instead of conventional nitrogen fertilisers.
1. Introduction
Modern day intensive agriculture requires the frequent application of nutrients (often in the form of mineral nitrogen (N) fertilisers) to keep productivity high, resulting in a significant distortion of the natural N cycle (Fowler et al., 2013, Reay et al., 2012). The N uptake by crops of applied mineral fertilisers (nutrient use efficiency, NUE) is frequently low, accounting for less than 50% of the total N applied (Lassaletta et al., 2014, Raun et al., 2002). Global annual application of N is in excess of 100 Tg N (Lu and Tian, 2017), resulting in > 50 Tg of N lost to the environment each year. Environmentally damaging forms of N produced as losses from these systems include nitrate (NO3−) run-off into water systems (Di and Cameron, 2002) and emissions of ammonia (NH3) (Bouwman et al., 1997), nitrogen oxides (NOx) (Davidson and Kingerlee, 1997) and nitrous oxide (N2O).
N2O is a powerful long-lived greenhouse gas (GHG) with a global warming potential over 300 times greater than an equivalent volume of carbon dioxide (CO2) (IPCC, 2014). In the stratosphere, N2O is the single largest contributor to depletion of the ozone layer, and is likely to remain so for the foreseeable future (Ravishankara et al., 2009). The majority of anthropogenic emissions of N2O are due to the intensification of the naturally occurring processes of microbial nitrification (the conversion of NH4+ to NO3−) and denitrification (the conversion of NO3− to N2), for both of which N2O is a by-product (Davidson et al., 2000, Butterbach-Bahl et al., 2013). It is estimated that agricultural sources contribute to 60 to 70% of anthropogenic N2O at a global level (Syakila and Kroeze, 2011, Tian et al., 2019), predominantly as a result of the application of mineral nitrogen fertilisers and application of livestock manures, and these emissions are set to continue increasing as the global population grows and demand for food continues to rise (Mosier and Kroeze, 2000).
Currently, the Intergovernmental Panel on Climate Change (IPCC) estimates that 1% of N applied in the form of mineral fertiliser is emitted as N2O as a result of microbial processes (the so-called “emission factor”); however, the uncertainty in this is estimated to be large (0.03 to 3%) due to the wide range of experimentally derived emission factors (EFs) observed at a global scale on which this estimate is based (0–89%; Stehfest and Bouwman, 2006, IPCC, 2014). The variability in experimental results between studies is attributed to numerous environmental and management factors which influence microbial activity in agricultural soils. Soil aerobicity is believed to be important in determining whether aerobic (nitrification) or anerobic (denitrification) processes dominate (Bouwman, 1996, Müller et al., 2004), thus the soil properties that control oxygen availability such as water filled pore space, bulk density, clay content and soil porosity are believed to play a major role in the variability of N2O EFs (Davidson et al., 2000, Flechard et al., 2007, Skiba and Ball, 2006). Other factors have been linked with N2O emissions, such as soil pH (Stevens et al., 1998), temperature (Smith et al., 1998), carbon availability (Weier et al., 1993) and presence of particular microbial species (Butterbach-Bahl et al., 2013). Although individual studies have shown the significance of each of these variables, there is still no reliable process-based model capable of replicating N2O emissions at the plot or field scale.
As most nations have made binding agreements to reduce their emissions of greenhouse gases such as N2O (i.e. the 1992 Kyoto Protocol and the 2015 Paris Agreement), the ability to accurately report emissions at national scales is of increasing importance. Over 70% of the land area in the UK and Ireland is classed as agricultural in nature, and much of this land is intensively managed with the frequent use of mineral fertilisers. The two most common synthetic N fertilizers used in the UK are ammonium nitrate (AN) and urea (CO(NH₂)₂) (approximately 40% and 10% of applied mineral N, respectively) (BSFP, 2017). The application of AN (including calcium ammonium nitrate (CAN)) and urea account for 2.2 of the 3.9 Tg of synthetic N applied annually in Great Britain (BSFP, 2017). In the Republic of Ireland, CAN accounts for approximately 30–49% of N applied (AN is commercially unavailable across the island of Ireland), with urea contributing to 10–12% (Teagasc, 2019).
Several methods have been tested to mitigate N pollution as a result of the application of mineral fertilisers. Among the most promising is the use of microbial inhibitors which directly target and slow a specific biological pathway (Abalos et al., 2014, Modolo et al., 2015). These inhibitors work by slowing the availability of N released from a fertiliser, allowing for the crop to uptake more of applied N at its own rate of growth, outcompeting other loss pathways. Microbial inhibitors have been shown to reduce Nr losses for both N2O and NH3 under laboratory conditions and in field trials, but with varying success (Sanz-Cobena et al., 2014, Ni et al., 2014, Singh et al., 2013, Rose et al., 2017, Ruser and Schulz, 2015). There are positive studies which promote the pollution reducing capabilities of these chemicals (Misselbrook et al., 2014); however, questions remain over the overall effectiveness of the inhibitors. This is especially true for nitrification inhibitors, which face claims that potential reduction of N2O emissions may increase NH3 emissions, due to the longer residence time of ammonium (NH4+) in the soil which can increase volatilization (Lam et al., 2016, Zaman et al., 2009). Although commercially available, these inhibitors are not yet used in significant amounts globally due to their higher costs. However, this can vary, and inhibitor treated urea can be competitive with AN and CAN fertilisers.
Much effort has been made in the UK and Ireland to improve N2O emission inventories through experimental studies, with a further focus on experiments that aim to find effective and applicable mitigation strategies which result in lower N2O emissions (with commitments to the delivery of the UN Sustainable Development Goals in mind, specifically SDG 13: climate action). The aim of this study is to summarise data collected from experiments over the past 20 years and apply a Bayesian statistical approach to provide the most up to date knowledge of N2O emission factors after applications of mineral nitrogen fertiliser, using data from the British Isles (UK and Ireland) as an example. We then discuss the implications of this research in terms of quantifying N2O emissions and the potential for mitigation options and future research.
2. Method
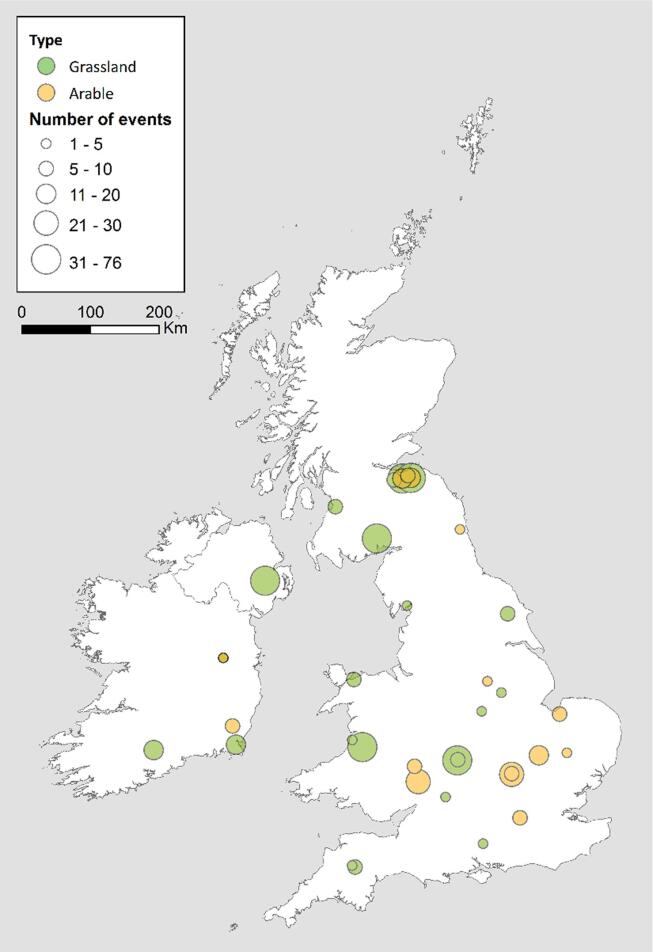
Data from 38 experimental sites across the UK and Ireland were collated in this study, providing a total of 623 separate mineral fertiliser N2O EF estimates derived from field measurements (Table 1, Fig. 1). Data were either i) extracted from published studies in which one aim of the experimentation was to explicitly measure N2O and report EFs after a mineral fertiliser application, or ii) raw data were used from the Agricultural and Environmental Data Archive (AEDA). The raw data extracted from the AEDA was used to calculate EFs for 25 days after fertiliser application using the log-normal Bayesian approach as described in Levy et al., 2017, Cowan et al., 2019a, which accounts for the log-normal spatial and temporal distribution of N2O fluxes after fertiliser events. Fertilisers treated with nitrification and urease inhibitors in these studies were included for comparison. To find the published data, a survey of literature was conducted using Google Scholar for articles considered ‘recent’ (20 years or fewer), i.e. published after January 1998 and submitted before April 2019. The following search terms and their variations were used: N2O, nitrous oxide, emission factor, mineral fertiliser, ammonium nitrate, urea, nitrification inhibitor, nitrogen use efficiency, agriculture, greenhouse gas, grassland and arable. This search based on keywords was complemented with a search through the literature cited in the articles found and known previous research.
Table 1.
All studies from which N2O EF data was extracted for use in this study.
| Reference | Site locations of fertiliser application | Field Type | Number of separate EF events |
|---|---|---|---|
| Published Studies | |||
| (Abdalla et al., 2009) | Carlow (I) | Grass & Arable | 8 |
| (Baggs et al., 2003) | Wye Estate (E) | Arable | 6 |
| (Bell et al., 2015) | Gilchriston (S), Rosemaund (E), Woburn (E) | Arable | 24 |
| (Cardenas et al., 2019) | Crichton (S), Drayton (E), Hillsborough (NI), North Wyke (E), Pwllpeiran (W) | Grass | 45 |
| (Cowan et al., 2019a) | Easter Bush (S), North Wyke (E), Abergwyngregyn (W) | Grass | 33 |
| (Cowan et al., 2019b) | Easter Bush (S) | Grass | 10 |
| (Dobbie and Smith, 2003) | Glencourse Mains (S), Bridgets (E), North Wyke (E), Aberystwyth (W), Grange-Over-Sands (E), Sutton Bonington (E), Boxwoth (E), Gleadthorpe (E), Brooms Barn (E) | Grass & Arable | 12 |
| (Harty et al., 2016) | Moorepark (I), Hillsborough (NI), Johnstown Castle (I) | Grass | 32 |
| (Hinton et al., 2015) | Gilchriston (S) | Arable | 9 |
| (Jones et al., 2005) | Glencorse (S) | Grass | 4 |
| (Krol et al., 2017) | Johnstown Castle (I) | Grass | 5 |
| (Misselbrook et al., 2014) | Gleadthorpe (E), North Wyke (E), Newark (E), Sampford Chapple (E), Boxworth (E), Cockle Park (E) | Grass & Arable | 24 |
| (Roche et al., 2016) | Marshaltown (I) | Arable | 10 |
| (Skiba et al., 2013) | Easter Bush (S) | Grass | 16 |
| (Smith et al., 2012) | Rowden (E), Crichton (S), Hillsborough (NI), High Mowthorpe (E), Bush Estate (S), Terrington (E), De Bathe (E), Boxworth (E) | Grass & Arable | 137 |
| AEDA data | |||
| (SRUC, 2017a) | Dumfries (S) | Grass | 38 |
| (SRUC, 2017b) | East Lothian (S) | Arable | 10 |
| (ADAS, 2017a) | Ceredigion (W) | Grass | 38 |
| (ADAS, 2017b) | Warwickshire (E) | Grass | 38 |
| (Rothamsted Research-North Wyke, 2017a) | Bedfordshire (E) | Arable | 29 |
| (Rothamsted Research-North Wyke, 2017b) | Devon (E) | Grass | 38 |
| (AFBI, 2017) | County Down (NI) | Grass | 46 |
| (ADAS, 2017c) | Herefordshire (E) | Arable | 29 |
(E) England, (I) Rep. of Ireland, (NI) Northern Ireland, (S) Scotland, (W) Wales
Fig. 1.
Data from 38 experimental sites across the UK and Ireland were collated in this study, providing a total of 623 separate mineral fertiliser N2O EF estimates derived from field measurements. A total of 171 EFs were measured at arable sites and 470 were measured at grassland sites.
Of the 641 events reported in these studies, 293 were of the application of AN, 63 for CAN and 118 for urea. A further 38 EFs were reported for AN (or CAN, n = 4) which had been treated with Dicyandiamide (DCD, a nitrification inhibitor) and 129 EFs are reported for urea that had been treated with either DCD or N-(n)-butyl) thiophosphorictriamide (NBTP, a urease inhibitor). There were more studies carried out at grassland field sites than under arable conditions (470 compared to 171) (Table 2).
Table 2.
A summary of data representation of the different fertiliser types and field management reported in this study.
| Fertiliser Type | All | Arable | Grassland |
|---|---|---|---|
| AN | 293 | 91 | 202 |
| AN + Inhibitor | 38 | 14 | 24 |
| CAN | 63 | 8 | 55 |
| Urea | 118 | 28 | 90 |
| Urea + Inhibitor | 129 | 30 | 99 |
| Total | 641 | 171 | 470 |
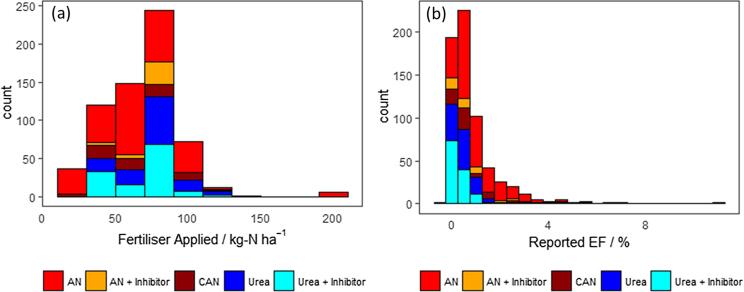
A variety of measurement methodologies and statistical analysis had been used across the different experiments to calculate N2O EFs. Fertiliser applications varied from 20 to 200 kg N ha−1 for a single event (mean values used when annual EF is reported for multiple events; Fig. 2), using a variety of application methods (e.g. farm machinery or by hand). The studies report data collected using the flux chamber approach with the exception of a single study which reports data collected using the eddy covariance method (see Cowan et al., 2019b). The flux chamber method uses an airtight enclosure placed over the soil which allows for gas emissions from the surface to accumulate (typically 30 to 60 min). This air is then extracted at timed intervals via a tap (2 to 5 samples) and stored in an airtight vial for analysis (typically gas chromatography). Fluxes are calculated using Equation (1), where F is the gas flux from the soil dC/dt is the rate of change in the concentration in time, ρ is the density of air, V is the volume of the chamber and A is the ground area enclosed by the chamber.
| (1) |
Fig. 2.
Histograms of (a) the mass of N fertiliser applied per individual event and (b) the N2O EFs reported in the experiments included in this study.
Some of the EFs in the published studies are calculated by taking a yearly average after several fertiliser applications, while others report emissions for a shorter period after the event (e.g. Skiba et al., 2013, Cowan et al., 2019a, Cowan et al., 2019b). The fluxes derived from the data taken from the AEDA archives report EFs for emissions up to 25 days after fertilisation. All of the studies measured from a “control” plot during experimentation. This is an area of the field in which no N is applied while measurements are made during fertilisation events on other experimental plots. After cumulative emissions were calculated for treated plots, the cumulative flux from the control plot was subtracted, thus the EF only represents the additional emission of N2O that occurs as a result of N addition. Based on the inclusion of control plots and the subtraction of “background” fluxes from final cumulative estimates, we can consider EFs reported from annual or per event basis as comparable in this study.
Reported N2O EFs vary from 0.3 to 11.0% of the applied nitrogen and follow a log-normal distribution (Fig. 2). Based on the log-normal distribution of the data, we report means and confidence intervals of the data using a Bayesian approach similar to that used in described in Cowan et al. (2017) to constrain the plausible range of the mean N2O flux. This allows for a more defensible statistical assessment of the means and uncertainties in lognormal datasets than the arithmetic method which is conventionally used in N2O EF studies.
The Bayesian analysis was carried out using Markov Chain Monte-Carlo (MCMC) simulations with the freely-available JAGS software (Plummer, 2016) which implements Gibbs sampling (Geman and Geman, 1984) to estimate the posterior distribution of µ, by combining the prior with the data. We used the data as reported in Stehfest and Bouwman (2006) as an informative prior with the same log-normal distribution of data. The Stehfest and Bouwman (2006) dataset is a compilation of 833 emission factors of fertiliser events reported from around the world and is the basis for the IPCC default 1% EF. We used the Bayesian approach for each estimation of the mean EF of a particular fertiliser use to calculate µ, with 95% confidence intervals from the quantiles of the posterior distribution.
3. Results
The mean EF and 95% confidence intervals (C.I.s) of all events included in this study was 0.8 (0.74–0.87) % as calculated using the Bayesian method. Overall, the mean EF of the compiled data is similar to that of the 1% estimated by the IPCC, although the majority (74%) of the individual fertiliser events report EFs below 1% due to the log-normal distribution of the data (Fig. 1b). The distribution of individual events varies widely across this range with 95 percentiles ranging from 0.02 to 3.8. However, these statistics are dominated by the large number of experiments carried out on grasslands after AN application which represent over one third of the dataset.
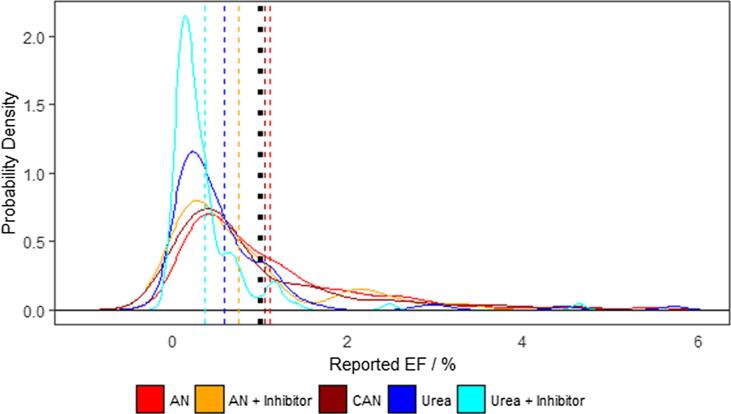
A comparison of the range of EFs reported after application of the different fertiliser types shows that emissions for all categories follow a lognormal skewed distribution, with a large overlap between the different EFs reported in the different studies (Fig. 3). This skewed distribution is similar for each of the fertilisers, including the inhibitor treated compounds. Although the majority of individual EFs for all fertiliser categories are more likely to be below 1% than above, there is a considerable difference in the distribution of EFs > 1%, with individual events considerably more likely to surpass this threshold for AN, CAN and treated AN applications than the urea fertiliser types.
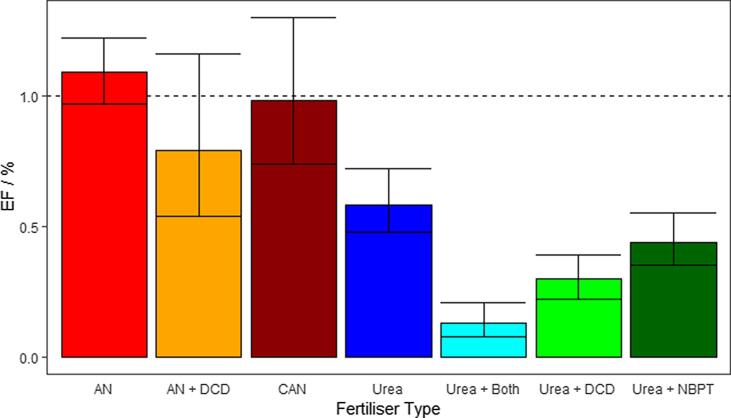
Fig. 3.
Probability densities of the reported EF data collated for this study are plotted for comparison between the five fertiliser types. Mean EF values (dashed) and IPCC EF of 1% (dotted) is added for comparison.
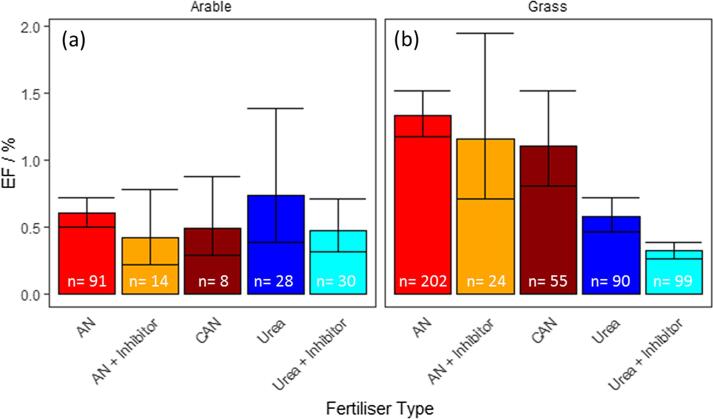
Bayesian analysis of the compiled data shows that AN and CAN application results in the largest EFs, with overall mean EFs of 1.11 and 1.04%, respectively. (Table 3). For AN, when separated between arable and grassland applications, there is a large difference between the EFs, suggesting that emissions from grassland applications of AN (EF = 1.3%) are almost double the magnitude of arable applications (EF = 0.6%) (Fig. 4). When all events are considered (arable and grassland), the EFs associated with urea applications (EF = 0.6%) were significantly lower than those for AN and CAN (t-test p < 0.01), although the number of experiments carried out in arable areas was much smaller than grassland experiments. Due to the small number of observations (n = 28), the uncertainty in EFs after urea application to arable fields (0.4 to 1.4%) is larger than those reported for grassland fields (0.5 to 0.7%) for which there are more data (n = 90).
Table 3.
Mean values and 95% confidence intervals (C.I.s) are calculated for EFs for each of the five fertiliser types for arable and grassland field types using both arithmetic and Bayesian statistical methods.
| Arithmetic | Bayesian | ||||
|---|---|---|---|---|---|
| Fertiliser | n | Mean EF | 95% C.I. | Mean EF | 95C.I. |
| All | |||||
| AN | 293 | 1.11 | (0.97–1.24) | 1.09 | (0.97–1.22) |
| AN + Inhibitor | 38 | 0.75 | (0.49–1.00) | 0.79 | (0.54–1.16) |
| CAN | 63 | 1.04 | (0.64–1.43) | 0.98 | (0.74–1.30) |
| Urea | 118 | 0.58 | (0.44–0.72) | 0.58 | (0.48–0.72) |
| Urea + Inhibitor | 129 | 0.36 | (0.28–0.45) | 0.35 | (0.29–0.41) |
| Arable | |||||
| AN | 91 | 0.61 | (0.48–0.74) | 0.60 | (0.50–0.72) |
| AN + Inhibitor | 14 | 0.37 | (0.17–0.56) | 0.42 | (0.22–0.78) |
| CAN | 8 | 0.42 | (0.29–0.55) | 0.49 | (0.29–0.88) |
| Urea | 28 | 0.51 | (0.30–0.72) | 0.74 | (0.38–1.38) |
| Urea + Inhibitor | 30 | 0.46 | (0.26–0.65) | 0.47 | (0.32–0.71) |
| Grass | |||||
| AN | 202 | 1.33 | (1.16–1.50) | 1.34 | (1.18–1.52) |
| AN + Inhibitor | 24 | 0.97 | (0.60–1.33) | 1.16 | (0.71–1.95) |
| CAN | 55 | 1.13 | (0.68–1.57) | 1.11 | (0.81–1.51) |
| Urea | 90 | 0.60 | (0.43–0.77) | 0.58 | (0.47–0.72) |
| Urea + Inhibitor | 99 | 0.34 | (0.24–0.44) | 0.32 | (0.27–0.39) |
Fig. 4.
Mean EFs are reported for different fertiliser types applied to (a) arable and (b) grassland fields. Bayesian statistical methods were used to estimate mean and 95% C.I.s for each treatment. The number of data points for each category is included (n).
EFs associated with the fertilisers treated with inhibitor compounds (DCD or NBPT) suggest that overall, emissions are lower than for their parent compound, with reductions in emissions of 28% and 40% for the treated AN and urea compounds, respectively, when compared to untreated applications. However, some of these estimates have relatively large uncertainties associated with them when separated into arable and grassland categories due to the lower number of experimental observations and wide range of observed EFs, thus limiting definitive comparisons. Due to the large number of observations made for both urea and inhibitor treated urea on grassland fields, it can be said with confidence that in these circumstances, inhibitors are reducing N2O emissions in a statistically significant manner (t-test, p < 0.01) (Table 3 & Fig. 4). A comparison of the data analysed using both arithmetic (a.k.a. naïve method, ignoring the skewed distribution) and Bayesian statistical methods shows that there is a small difference between the methods when n is large (n > 40), but larger when n is small and the mean EF is relatively large (i.e. >0.5%).
A comparison of the EFs reported in this study for AN, CAN and urea fertilisers with the most up-to-date iteration of the UK National Agricultural Emission Inventory (developed under the UK agricultural emission factor, e.g. Brown et al. 2019) is presented in Table 4. Using the different methods to calculate N2O emissions at the UK scale for the year 2017, the emission estimates are 7.07 kt N per year for the agricultural inventory and 5.34 (95% C.I. = 4.72–6.32) kt N per year using the Bayesian method. Where the methods differ most is the EFs of fertilisers applied to arable fields and the particularly high EFs used for AN and CAN in the agricultural inventory. The Bayesian method in this study predicts lower EFs for AN and CAN for arable fields, and a higher urea EF than those used in the Agricultural Inventory. In the case of urea, the C.I.s are comparable in range due to the lower number of data points (see Table 4).
Table 4.
UK scale emissions of N2O from the application of AN, CAN and urea fertilisers for the year 2017 are estimated using the agricultural inventory EFs (developed under the UK agricultural emission factor, e.g. Brown et al. 2019) and the EFs calculated by applying the Bayesian method to 623 events in this study.
| UK 2017 |
Agricultural Inventory |
Bayesian Method |
|||||
|---|---|---|---|---|---|---|---|
| Fertiliser |
Application |
Mean EF |
Emission |
Mean EF |
95% C.I. |
Emission |
95% C.I. |
| (kt N) | % | (kt N) | % | % | (kt N) | (kt N) | |
| All | |||||||
| AN | 460.1 | 1.15 | 5.29 | 1.09 | (0.97–1.22) | 5.02 | (4.49–5.61) |
| CAN | 46.9 | 1.6 | 0.75 | 0.98 | (0.74–1.30) | 0.46 | (0.35–0.61) |
| Urea | 159.9 | 0.66 | 1.06 | 0.58 | (0.48–0.72) | 0.93 | (0.76–1.14) |
| Arable | |||||||
| AN | 325.6 | 0.99 | 3.22 | 0.60 | (0.50–0.72) | 1.96 | (1.63–2.35) |
| CAN | 10.5 | 1.1 | 0.12 | 0.49 | (0.29–0.88) | 0.05 | (0.03–0.09) |
| Urea | 131.6 | 0.66 | 0.87 | 0.74 | (0.38–1.38) | 0.97 | (0.51–1.82) |
| Grass | |||||||
| AN | 134.5 | 1.54 | 2.07 | 1.34 | (1.18–1.52) | 1.80 | (1.58–2.04) |
| CAN | 36.1 | 1.7 | 0.61 | 1.11 | (0.81–1.51) | 0.40 | (0.29–0.55) |
| Urea | 28.2 | 0.62 | 0.17 | 0.58 | (0.47–0.72) | 0.16 | (0.13–0.20) |
In this study, a t-test suggests that there is no significant difference between the EF’s measured from AN and CAN (p = 0.79), and there is little difference in the mean estimates calculated using the Bayesian approach, 1.09 (0.97–1.22) % and 0.98 (0.74–1.30) % (Fig. 5). However, there is a noticeable difference between untreated urea and urea fertilisers treated with DCD (n = 87), NBPT (n = 63). There is also a relatively large difference in EFs when both DCD and NBPT are applied with urea together (n = 9), resulting in the lowest EF of any treatment at 0.13 (0.07–0.22) %. DCD treated urea is second lowest with an EF of 0.3 (0.22–0.57) %, followed by urea treated with NBPT at 0.45 (0.36–0.57) %, with untreated urea at 0.59 (0.48–0.73) %.
Fig. 5.
All different mineral fertiliser types reported in this study are presented, broken down into smaller groups to compare AN with CAN, and the effect of urea coated with the microbial inhibitors DCD and NBPT (and both DCD and NBPT applied together). The IPPC default EF of 1% is added for comparison (dashed line).
4. Discussion
This study is the largest compiled analysis of N2O EFs carried out across the British Isles to date, representing a combined total of 641 separate events from 23 studies across 40 experimental locations. These results show that there is a sizable difference in the magnitude of EFs for the different types of mineral N fertiliser applied in the UK and Ireland. Overall these events report a mean EF of 0.8 (0.74–0.87) %. This value is close to the IPCC default EF of 1 (0.3–3.0)% (IPCC, 2014) which is a skewed mean based on the compiled data of Stehfest and Bouwman (2006) which has an arithmetic mean EF of 2.0%. However, the mean EF of all events reported in this study over-represents the application of AN and CAN to grassland fields, which are also the conditions with the largest emission rates for the application of mineral fertilisers across the British Isles. The majority of EFs for all fertiliser types fall below the 1% threshold.
The breakdown of datasets into grassland and arable shows that differences in EFs are statistically significant, especially the comparison of AN with urea (p < 0.01). Overall, for both grassland and arable studies combined, AN has a mean EF of 1.1 (1.0–1.2) % compared to an estimated 0.6 (0.5––0.7) % for an equivalent application of urea. Differences are well categorised for the application of the fertilisers to grasslands (n = 470), although uncertainties remain somewhat larger in the case of arable applications, due to the smaller number of experimental studies (n = 171). This is partly due to the prevalence of studies investigating the application of animal manures to arable crops in the UK (as opposed to mineral fertilisers), the complexities of which are not discussed in this study.
EFs estimated using the Bayesian approach in this study and the values from the current UK agricultural inventory show similarities, with most values comparable in magnitude once 95% C.I.s are taken into account. Total emissions at the UK scale for AN and urea application (based on fertiliser use for 2016, (BSFP, 2017)) are estimated at 7.07 and 5.34 (95% C.I. = 4.72–6.32) kt N for the agriculture inventory and Bayesian methods, respectively. The Bayesian method generally reports smaller EFs for the different fertiliser categories, with the exception of urea application to arable fields, although uncertainty remains high for this estimate. Interestingly, this difference suggests that application of urea to arable fields emits more N2O than an equivalent application of AN, although uncertainties overlap between the values. This is in contrast to observations made on grassland fields where AN is the largest emitter.
Reasons for differences between EFs observed from grassland and arable fields are likely to be numerous, and the range of EFs observed for both range from 0 to >3% for each category; however, one plausible explanation for the change in behaviour regarding the largest emitter being AN or urea is the difference in aeration between compacted grazed fields and regularly tilled arable soils. Crops tend to be more productive on better drained soils and lower rainfall areas than grasslands (Ball et al., 2008), with much of the UK’s arable fields in the drier southern and eastern regions. By contrast, intensively grazed and upland grasslands are more likely to be damper or compacted (Sharrow, 2007), favouring N2O production. In this regard, it is not unexpected that EFs of the different forms of fertiliser may behave differently in these contrasting conditions.
Our study suggests that, based on the experimental work carried out in the UK and Ireland, microbial inhibitors have an impact on N2O emissions from both AN and urea application. In the case of the nitrification inhibitor DCD applied with AN, there is still a relatively large uncertainty associated with it and it is unclear what conditions the inhibitor is best suited for, but overall AN treated with the nitrification inhibitor DCD appears to consistently emit less N2O from both arable and grassland fields than conventional application. Further field measurements would be required to reduce the uncertainties in these values. There appears to be no statistically significant difference in EFs associated with AN and CAN, estimated at 1.09 (0.97–1.22) and 0.98 (0.74–1.30) %, respectively. AN is not commercially available in Ireland (for security reasons), while the UK predominantly applies AN. This study suggests differences between the two could justifiably be ignored at national scales and a common EF may be applied for the two treatments in national inventories.
Of the two inhibitors applied to urea fertilisers, DCD appears to reduce N2O emissions more than NBPT with EFs of 0.30 (0.22–0.39) %, and 0.44 (0.35–0.55) % for DCD treated urea and NBPT treated urea, respectively. Due to the large number of grassland experiments reported, this study can say definitively that urea treated with microbial inhibitors can significantly reduce N2O emissions at a national scale. This is especially true when urea is applied with both DCD and NBPT inhibitors, although this estimate is based on very few experimental observations (n = 11). Theoretically, if urea treated with DCD and NBPT were applied in place of all AN and untreated urea fertiliser in the UK, we can approximate using the data presented in this study that emissions could fall from our nationwide estimate of 5.34 (4.72–6.32) kt N to 0.89 (0.52–1.37) kt N, a drop of 84%. However, this approximation is based on very few experimental data points and does not represent the reality of fertiliser requirements for particular environments, or the logistics and economics of agricultural activities in the UK.
In terms of mitigation potential, the results presented in this study have mixed implications. Here we show that wide scale use of urea fertilisers would result in significantly less N2O than AN applications; however, past studies have shown that yield and quality of crops are higher when AN is applied, especially for grasslands (Chambers and Dampney, 2009, Cowan et al., 2020). Urea is also associated with much higher ammonia (NH3) emissions than AN (Burchill et al., 2016, Forrestal et al., 2016), and so an element of pollution swapping is estimated to occur if urea fertilisers were used to replace AN at a national scale. Previous studies have indicated that urea treated with microbial inhibitors may result in slightly higher yields and lower NH3 emissions than untreated urea (Cantarella et al., 2018, Misselbrook et al., 2014, Silva et al., 2017), but this is still uncertain (some experiments show reduced yield (Harty et al. 2016)) and AN (or CAN) remains the fertiliser favoured by farmers in the UK for yield and crop quality.
While the use of inhibitors appears to reduce N2O emissions significantly, these compounds incur additional cost for farmers, and may not consistently reduce N2O emissions across different environmental conditions and management practices. Without clear economic incentives, it is unlikely that the uptake of nitrification or urease inhibitors such as DCD or BTPT (or both) is likely to occur at a large scale in the current economic climate. Before further uptake of microbial inhibitors can be fully justified, more research is required to further determine the impact on agricultural practice, including yields, economic connotations and the long term ecological impacts.
A significant observation made in this study, which may not be immediately apparent to policy makers or those researching N2O emissions, is that due to the log-normal nature of the EF data, a very large number of observations are required to provide robust statistical analysis. The large variability of EFs reported in these studies (ranging from −0.28 to 10.98% of the applied nitrogen) is partly due to the environmental and management differences between field sites (as already surmised by the authors of the studies), but also the large uncertainties caused by measurement and statistical methodology deployed and the statistical means by which data are interpolated (i.e. bias and uncertainty in trapezoidal integration techniques) (Levy et al., 2017). In the studies used in this compilation, large variations in EFs are reported within the same sites under similar environmental conditions and no consistent predictive model is provided that can accurately predict N2O emissions from any given event. In some of the studies used in this compilation, rainfall and the resultant water filled pore space (WFPS) of the soil are found to correlate to some extent with the observed EFs (Bell et al., 2015, Dobbie and Smith, 2003, Skiba et al., 2013), but these comparisons are often weak and inconsistent due to the number of other influencing factors. This lack of correlation is frustratingly common for N2O studies (Butterbach-Bahl et al., 2013), and is likely to be partly a consequence of the combination of high measurement uncertainty and the large number of variables that contribute to microbial processes in heterogeneous conditions such as agricultural soils.
Our comparisons highlight that, due to the wide ranging log-normal distribution of observed EFs, comparison of different fertiliser treatments in small scale experimentation (including testing of mitigation efforts) or direct comparisons with environmental variables are unlikely to yield statistically significant comparisons that would reflect N2O emissions at regional scales without a very large number of replicates. However, the data from numerous small studies are very valuable as they can be combined to provide a more robust comparison once many have been carried out.
The Bayesian method used in this study has proven robust and capable of handling the log-normally distributed data and providing realistic uncertainties around the mean values of these data. This is an important statistical development, as conventionally used “naïve” methods (i.e. arithmetic means) should not technically be applied to data with a log-normal distribution. Due to the large number of data points, there is a similarity between values calculated using arithmetic and Bayesian methods (Table 3), although this is true of any method where the number of data points (n) is large. Where the Bayesian method is valuable is when n is small (n > 5 and n < 50), and uncertainties are more difficult to estimate using conventional means.
We decided that the most relevant prior dataset to use in this study was that of Stehfest and Bouwman, (2006), although few of these studies were carried out in the UK. The data collated for this study will provide future studies in the British Isles (or abroad) with the means by which to create more relevant priors for further use of Bayesian methods when calculating EFs. Future studies may decide to use some, or all of the available data from this study to carry out Bayesian analysis depending relevancy. Future studies may also wish to collate the raw experimental data from databases such as the Agricultural and Environmental Data Archive (AEDA) used in this study so that all events can be analysed in a similar statistical manner, based on further Bayesian approaches (Levy et al., 2017).
5. Conclusions
This study shows that Bayesian statistical methods can be used to determine means and reliable confidence intervals when applied to log-normally distributed N2O EF data. Applying a Bayesian approach to 641 fertiliser events carried out at 40 different field sites in the UK and Ireland, shows that emissions of N2O associated with AN, CAN and urea based fertilisers vary depending on fertiliser type, field use and the use of microbial inhibitors. The study highlights the large difference between observed EFs of grassland applications of AN (EF = 1.3% (1.2–1.5)) which are approximately double that of an equivalent arable application (EF = 0.6% (0.5–0.7)). The study also suggests that urea application to arable fields (EF = 0.7% (0.4–1.4)) is higher than that observed for grassland fields (EF = 0.6% (0.5–0.7)), although confidence intervals overlap in this comparison. The nitrification and urease inhibitors, DCD and NBPT, are shown to have a sizable impact on N2O emissions when applied in combination with both AN and urea fertilisers, reducing emissions considerably. In the case of urea treated with both DCD and NBPT, the reported EF in this study is estimated at 0.13% (0.08–0.21), the lowest EF of all treatments.
The study highlights, that due to the log-normal distribution of EF data, a statistical analysis is not straightforward and combined data from many studies are required to obtain sufficiently large datasets to perform a robust analysis of treatment effects. Using the Bayesian method to combine data from multiple studies to estimate EFs at national or continental scales is likely to estimate a more realistic range of uncertainties where datasets are smaller (i.e. lower income countries) than using conventional statistical methods. The study also highlights that more research is also required to calculate EFs of mineral fertilisers on arable fields in countries with established research portfolios, in order to reduce the relatively large uncertainties of the EF values. Further agronomic research with urease and nitrification inhibitors may lead to sizable reductions in agricultural N2O emissions at a national scale, however pollution swapping (i.e·NH3 emissions) and the eventual fate of the applied nitrogen should be taken account of in all experimentation.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the UK-China Virtual Joint Centre for Agricultural Nitrogen (CINAg, BB/N013468/1), which is jointly supported by the Newton Fund, via UK BBSRC and Natural Environment Research Council, and the Chinese Ministry of Science and Technology. We acknowledge funding from the NERC project “Detection and Attribution of Regional greenhouse gas Emissions in the UK (DARE-UK)” (Reference: NE/S003614/1).We thank all of the researchers in the UK and Ireland who have published their data over the past twenty years on which this study is based.
Handling Editor: Yong-Guan Zhu
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2019.105366.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abalos D., Jeffery S., Sanz-Cobena A., Guardia G., Vallejo A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agriculture, Ecosystems & Environment. 2014;189:136–144. doi: 10.1016/j.agee.2014.03.036. [DOI] [Google Scholar]
- Abdalla M., Wattenbach M., Smith P., Ambus P., Jones M., Williams M. Application of the DNDC model to predict emissions of N2O from Irish agriculture. Geoderma. 2009;151:327–337. doi: 10.1016/j.geoderma.2009.04.021. [DOI] [Google Scholar]
- ADAS, 2017a. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Manure experimental site in Ceredigion, 2011-12. https://doi.org/10.17865/ghgno621.
- ADAS, 2017b. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Dung and urine experimental site in Warwickshire, 2013. https://doi.org/10.17865/ghgno652.
- ADAS, 2017c. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Manure experimental site in Herefordshire, 2012-13. https://doi.org/10.17865/ghgno636.
- AFBI, 2017. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Manure experimental site in County Down, 2012-13. https://doi.org/10.17865/ghgno541.
- Baggs E.M., Stevenson M., Pihlatie M., Regar A., Cook H., Cadisch G. Nitrous oxide emissions following application of residues and fertiliser under zero and conventional tillage. Plant Soil. 2003;254:361–370. doi: 10.1023/A:1025593121839. [DOI] [Google Scholar]
- Ball B.C., Campbell D.J., Douglas J.T., Henshall J.K., O’Sullivan M.F. Soil structural quality, compaction and land management. Eur. J. Soil Sci. 2008;48:593–601. doi: 10.1111/j.1365-2389.1997.tb00559.x. [DOI] [Google Scholar]
- Bell M.J., Hinton N., Cloy J.M., Topp C.F.E., Rees R.M., Cardenas L., Scott T., Webster C., Ashton R.W., Whitmore A.P., Williams J.R., Balshaw H., Paine F., Goulding K.W.T., Chadwick D.R. Nitrous oxide emissions from fertilised UK arable soils: Fluxes, emission factors and mitigation. Agric. Ecosyst. Environ. 2015;212:134–147. doi: 10.1016/j.agee.2015.07.003. [DOI] [Google Scholar]
- Bouwman A.F. Direct emission of nitrous oxide from agricultural soils. Nutr. Cycl. Agroecosystems. 1996;46:53–70. doi: 10.1007/BF00210224. [DOI] [Google Scholar]
- Bouwman A.F., Lee D.S., Asman W.A.H., Dentener F.J., Van Der Hoek K.W., Olivier J.G.J. A global high-resolution emission inventory for ammonia. Glob. Biogeochem. Cycles. 1997;11:561–587. doi: 10.1029/97GB02266. [DOI] [Google Scholar]
- Brown P., Broomfield M., Cardenas L., Choudrie S., Jones L., Karagianni E., Passant N., Thistlethwaite G., Thomson A., Turtle L., Wakeling D. UK Greenhouse Gas Inventory. 1990 to 2017: Annual Report for submission under the Framework Convention on Climate Change. 2019 [Google Scholar]
- BSFP (2017): The British Survey of Fertiliser Practice. Fertiliser use on farm crops for crop year 2016. Defra, London. 99pp.
- Burchill W., Lanigan G.J., Forrestal P.J., Reville F., Misselbrook T., Richards K.G. A field-based comparison of ammonia emissions from six Irish soil types following urea fertiliser application. Ir. J. Agric. Food Res. 2016;55:152–158. doi: 10.1515/ijafr-2016-0015. [DOI] [Google Scholar]
- Butterbach-Bahl K., Baggs E.M., Dannenmann M., Kiese R., Zechmeister-Boltenstern S. Nitrous oxide emissions from soils: how well do we understand the processes and their controls? Philos. Trans. R. Soc. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2013.0122. 20130122–20130122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarella H., Otto R., Soares J.R., Silva A.G. de B. Agronomic efficiency of NBPT as a urease inhibitor: A review. J. Adv. Res. 2018;13:19–27. doi: 10.1016/j.jare.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas L.M., Bhogal A., Chadwick D.R., McGeough K., Misselbrook T., Rees R.M., Thorman R.E., Watson C.J., Williams J.R., Smith K.A., Calvet S. Nitrogen use efficiency and nitrous oxide emissions from five UK fertilised grasslands. Sci. Total Environ. 2019;661:696–710. doi: 10.1016/j.scitotenv.2019.01.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers B.J., Dampney P. Nitrogen Efficiency and Ammonia Emissions from Urea-Based and Ammonia Nitrate Fertilisers. Proceedings-International Fertiliser Society. 2009;657:1–20. [Google Scholar]
- Cowan N.J., Levy P.E., Famulari D., Anderson M., Reay D.S., Skiba U.M. Nitrous oxide emission sources from a mixed livestock farm. Agriculture, Ecosystems & Environment. 2017;243:92–102. doi: 10.1016/j.agee.2017.04.014. [DOI] [Google Scholar]
- Cowan N., Levy P., Drewer J., Carswell A., Shaw R., Simmons I., Bache C., Marinheiro J., Brichet J., Sanchez-Rodriguez A.R., Cotton J., Hill P.W., Chadwick D.R., Jones D.L., Misselbrook T.H., Skiba U. Application of Bayesian statistics to estimate nitrous oxide emission factors of three nitrogen fertilisers on UK grasslands. Environment International. 2019;128:362–370. doi: 10.1016/j.envint.2019.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan, N., Levy, P., Maire, J., Coyle, M., Leeson, S.R., Famulari, D., Carozzi, M., Nemitz, E., Skiba, U., 2020. An evaluation of four years of nitrous oxide fluxes after application of ammonium nitrate and urea fertilisers measured using the eddy covariance method. Agricultural and Forest Meteorology 280, 107812. https://doi.org/10.1016/j.agrformet.2019.107812.
- Cowan N., Levy P., Moring A., Simmons I., Bache C., Stephens A., Marinheiro J., Brichet J., Song L., Pickard A., McNeill C., McDonald R., Maire J., Loubet B., Voylokov P., Sutton M., Skiba U. Nitrogen use efficiency and N2O and NH3; losses attributed to three fertiliser types applied to an intensively managed silage crop. Biogeosciences Discussions. 2019;1–27 doi: 10.5194/bg-2019-90. [DOI] [Google Scholar]
- Davidson E.A., Keller M., Erickson H.E., Verchot L.V., Veldkamp E. Testing a Conceptual Model of Soil Emissions of Nitrous and Nitric Oxides. BioScience. 2000;50:667. [Google Scholar]
- Davidson E.A., Kingerlee W. A global inventory of nitric oxide emissions from soils. Nutr. Cycl. Agroecosystems. 1997;48:37–50. doi: 10.1023/A:1009738715891. [DOI] [Google Scholar]
- Di H.J., Cameron K.C. Nitrate leaching in temperate agroecosystems: sources, factors and mitigating strategies. Nutr. Cycl. Agroecosystems. 2002;64:237–256. doi: 10.1023/A:1021471531188. [DOI] [Google Scholar]
- Dobbie K.E., Smith K.A. Nitrous oxide emission factors for agricultural soils in Great Britain: the impact of soil water-filled pore space and other controlling variables. Glob. Change Biol. 2003;9:204–218. doi: 10.1046/j.1365-2486.2003.00563.x. [DOI] [Google Scholar]
- Flechard C.R., Ambus P., Skiba U., Rees R.M., Hensen A., van Amstel A., Dasselaar A. van den P., Soussana J.-F., Jones M., Clifton-Brown J., Raschi A., Horvath L., Neftel A., Jocher M., Ammann C., Leifeld J., Fuhrer J., Calanca P., Thalman E., Pilegaard K., Di Marco C., Campbell C., Nemitz E., Hargreaves K.J., Levy P.E., Ball B.C., Jones S.K., van de Bulk W.C.M., Groot T., Blom M., Domingues R., Kasper G., Allard V., Ceschia E., Cellier P., Laville P., Henault C., Bizouard F., Abdalla M., Williams M., Baronti S., Berretti F., Grosz B. Effects of climate and management intensity on nitrous oxide emissions in grassland systems across Europe. Agric. Ecosyst. Environ. 2007;121:135–152. doi: 10.1016/j.agee.2006.12.024. [DOI] [Google Scholar]
- Forrestal P.J., Harty M., Carolan R., Lanigan G.J., Watson C.J., Laughlin R.J., McNeill G., Chambers B.J., Richards K.G. Ammonia emissions from urea, stabilized urea and calcium ammonium nitrate: insights into loss abatement in temperate grassland. Soil Use Manag. 2016;32:92–100. doi: 10.1111/sum.12232. [DOI] [Google Scholar]
- Fowler D., Coyle M., Skiba U., Sutton M.A., Cape J.N., Reis S., Sheppard L.J., Jenkins A., Grizzetti B., Galloway J.N., Vitousek P., Leach A., Bouwman A.F., Butterbach-Bahl K., Dentener F., Stevenson D., Amann M., Voss M. The global nitrogen cycle in the twenty-first century. Philos. Trans. R. Soc. B Biol. Sci. 2013;368 doi: 10.1098/rstb.2013.0164. 20130164–20130164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geman S., Geman D. Stochastic Relaxation, Gibbs Distributions, and the Bayesian Restoration of Images. IEEE Transactions on Pattern Analysis and Machine Intelligence. 1984;PAMI-6:721–741. doi: 10.1109/tpami.1984.4767596. [DOI] [PubMed] [Google Scholar]
- Harty M.A., Forrestal P.J., Watson C.J., McGeough K.L., Carolan R., Elliot C., Krol D., Laughlin R.J., Richards K.G., Lanigan G.J. Reducing nitrous oxide emissions by changing N fertiliser use from calcium ammonium nitrate (CAN) to urea based formulations. Sci. Total Environ. 2016;563–564:576–586. doi: 10.1016/j.scitotenv.2016.04.120. [DOI] [PubMed] [Google Scholar]
- Hinton N.J., Cloy J.M., Bell M.J., Chadwick D.R., Topp C.F.E., Rees R.M. Managing fertiliser nitrogen to reduce nitrous oxide emissions and emission intensities from a cultivated Cambisol in Scotland. Geoderma Reg. 2015;4:55–65. doi: 10.1016/j.geodrs.2014.12.002. [DOI] [Google Scholar]
- IPCC, 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, R.K. Pachauri and L.A. Meyer (eds.)]. IPCC, Geneva, Switzerland, 151 pp.
- Jones S.K., Rees R.M., Skiba U.M., Ball B.C. Greenhouse gas emissions from a managed grassland. Glob. Planet. Change. 2005;47:201–211. doi: 10.1016/j.gloplacha.2004.10.011. [DOI] [Google Scholar]
- Krol D.J., Minet E., Forrestal P.J., Lanigan G.J., Mathieu O., Richards K.G. The interactive effects of various nitrogen fertiliser formulations applied to urine patches on nitrous oxide emissions in grassland. Ir. J. Agric. Food Res. 2017;56:54–64. doi: 10.1515/ijafr-2017-0006. [DOI] [Google Scholar]
- Lam S.K., Suter H., Mosier A.R., Chen D. Using nitrification inhibitors to mitigate agricultural N2O emission: a double-edged sword? Global Change Biology. 2016;23:485–489. doi: 10.1111/gcb.13338. [DOI] [PubMed] [Google Scholar]
- Lassaletta L., Billen G., Grizzetti B., Anglade J., Garnier J. 50 year trends in nitrogen use efficiency of world cropping systems: the relationship between yield and nitrogen input to cropland. Environ. Res. Lett. 2014;9 doi: 10.1088/1748-9326/9/10/105011. [DOI] [Google Scholar]
- Levy P.E., Cowan N., van Oijen M., Famulari D., Drewer J., Skiba U. Estimation of cumulative fluxes of nitrous oxide: uncertainty in temporal upscaling and emission factors: Estimation of cumulative fluxes of nitrous oxide. Eur. J. Soil Sci. 2017;68:400–411. doi: 10.1111/ejss.12432. [DOI] [Google Scholar]
- Lu, C., Tian, H., 2017. Global nitrogen and phosphorus fertilizer use for agriculture production in the past half century: shifted hot spots and nutrient imbalance 12.
- Misselbrook T.H., Cardenas L.M., Camp V., Thorman R.E., Williams J.R., Rollett A.J., Chambers B.J. An assessment of nitrification inhibitors to reduce nitrous oxide emissions from UK agriculture. Environ. Res. Lett. 2014;9 doi: 10.1088/1748-9326/9/11/115006. [DOI] [Google Scholar]
- Modolo L.V., de Souza A.X., Horta L.P., Araujo D.P., de Fátima Â. An overview on the potential of natural products as ureases inhibitors: A review. Journal of Advanced Research. 2015;6:35–44. doi: 10.1016/j.jare.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier A., Kroeze C. Potential impact on the global atmospheric N2O budget of the increased nitrogen input required to meet future global food demands. Chemosphere - Glob. Change Sci. 2000;2:465–473. doi: 10.1016/S1465-9972(00)00039-8. [DOI] [Google Scholar]
- Müller C., Stevens R.J., Laughlin R.J., Jäger H.-J. Microbial processes and the site of N2O production in a temperate grassland soil. Soil Biol. Biochem. 2004;36:453–461. doi: 10.1016/j.soilbio.2003.08.027. [DOI] [Google Scholar]
- Ni K., Pacholski A., Kage H. Ammonia volatilization after application of urea to winter wheat over 3 years affected by novel urease and nitrification inhibitors. Agriculture, Ecosystems & Environment. 2014;197:184–194. doi: 10.1016/j.agee.2014.08.007. [DOI] [Google Scholar]
- Plummer, M. 2016. Rjags: Bayesian Graphical Models Using MCMC. R Package Version 4-6 URL https://CRAN.R-project.org/package=rjags [accessed on 12th November 2018].
- Raun W.R., Solie J.B., Johnson G.V., Stone M.L., Mullen R.W., Freeman K.W., Thomason W.E., Lukina E.V. Improving Nitrogen Use Efficiency in Cereal Grain Production with Optical Sensing and Variable Rate Application. Agron. J. 2002;94:815. doi: 10.2134/agronj2002.0815. [DOI] [Google Scholar]
- Ravishankara A.R., Daniel J.S., Portmann R.W. Nitrous Oxide (N2O): The Dominant Ozone-Depleting Substance Emitted in the 21st Century. Science. 2009;326:123–125. doi: 10.1126/science.1176985. [DOI] [PubMed] [Google Scholar]
- Reay D.S., Davidson E.A., Smith K.A., Smith P., Melillo J.M., Dentener F., Crutzen P.J. Global agriculture and nitrous oxide emissions. Nat. Clim. Change. 2012;2:410. [Google Scholar]
- Roche L., Forrestal P.J., Lanigan G.J., Richards K.G., Shaw L.J., Wall D.P. Impact of fertiliser nitrogen formulation, and N stabilisers on nitrous oxide emissions in spring barley. Agriculture, Ecosystems & Environment. 2016;233:229–237. doi: 10.1016/j.agee.2016.08.031. [DOI] [Google Scholar]
- Rose T.J., Morris S.G., Quin P., Kearney L.J., Kimber S., Van Zwieten L. The nitrification inhibitor DMPP applied to subtropical rice has an inconsistent effect on nitrous oxide emissions. Soil Research. 2017;55:547. doi: 10.1071/sr17022. [DOI] [Google Scholar]
- Rothamsted Research-North Wyke, 2017a. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Fertiliser experimental site in Bedfordshire, 2011. https://doi.org/10.17865/ghgno613.
- Rothamsted Research-North Wyke, 2017b. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Fertiliser experimental site in Devon, 2013. https://doi.org/10.17865/ghgno585.
- Ruser R., Schulz R. The effect of nitrification inhibitors on the nitrous oxide (N2O) release from agricultural soils-a review. Journal of Plant Nutrition and Soil Science. 2015;178:171–188. doi: 10.1002/jpln.201400251. [DOI] [Google Scholar]
- Sanz-Cobena A., Abalos D., Meijide A., Sanchez-Martin L., Vallejo A. Soil moisture determines the effectiveness of two urease inhibitors to decrease N2O emission. Mitigation and Adaptation Strategies for Global Change. 2014 doi: 10.1007/s11027-014-9548-5. [DOI] [Google Scholar]
- Sharrow S.H. Soil compaction by grazing livestock in silvopastures as evidenced by changes in soil physical properties. Agrofor. Syst. 2007;71:215–223. doi: 10.1007/s10457-007-9083-4. [DOI] [Google Scholar]
- Silva A.G.B., Sequeira C.H., Sermarini R.A., Otto R. Urease Inhibitor NBPT on Ammonia Volatilization and Crop Productivity: A Meta-Analysis. Agron. J. 2017;109:1. doi: 10.2134/agronj2016.04.0200. [DOI] [Google Scholar]
- Singh J., Kunhikrishnan A., Bolan N.S., Saggar S. Impact of urease inhibitor on ammonia and nitrous oxide emissions from temperate pasture soil cores receiving urea fertilizer and cattle urine. Science of The Total Environment. 2013;465:56–63. doi: 10.1016/j.scitotenv.2013.02.018. [DOI] [PubMed] [Google Scholar]
- Skiba U., Ball B. The effect of soil texture and soil drainage on emissions of nitric oxide and nitrous oxide. Soil Use Manag. 2006;18:56–60. doi: 10.1111/j.1475-2743.2002.tb00050.x. [DOI] [Google Scholar]
- Skiba U., Jones S.K., Drewer J., Helfter C., Anderson M., Dinsmore K., McKenzie R., Nemitz E., Sutton M.A. Comparison of soil greenhouse gas fluxes from extensive and intensive grazing in a temperate maritime climate. Biogeosciences. 2013;10:1231–1241. doi: 10.5194/bg-10-1231-2013. [DOI] [Google Scholar]
- Smith K.A., Dobbie K.E., Thorman R., Watson C.J., Chadwick D.R., Yamulki S., Ball B.C. The effect of N fertilizer forms on nitrous oxide emissions from UK arable land and grassland. Nutr. Cycl. Agroecosystems. 2012;93:127–149. doi: 10.1007/s10705-012-9505-1. [DOI] [Google Scholar]
- Smith K.A., Thomson P.E., Clayton H., Mctaggart I.P., Conen F. Effects of temperature, water content and nitrogen fertilisation on emissions of nitrous oxide by soils. Atmos. Environ. 1998;32:3301–3309. doi: 10.1016/S1352-2310(97)00492-5. [DOI] [Google Scholar]
- SRUC, 2017a. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Fertiliser experimental site in Dumfries, 2011. https://doi.org/10.17865/ghgno599.
- SRUC, 2017b. Agricultural Greenhouse Gas Inventory Research Platform - InveN2Ory. Fertiliser experimental site in East Lothian, 2011. https://doi.org/10.17865/ghgno606.
- Stehfest E., Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: summarizing available measurement data and modeling of global annual emissions. Nutr. Cycl. Agroecosystems. 2006;74:207–228. doi: 10.1007/s10705-006-9000-7. [DOI] [Google Scholar]
- Stevens R.J., Laughlin R.J., Malone J.P. Soil pH affects the processes reducing nitrate to nitrous oxide and di-nitrogen. Soil Biol. Biochem. 1998;30:1119–1126. doi: 10.1016/S0038-0717(97)00227-7. [DOI] [Google Scholar]
- Syakila A., Kroeze C. The global nitrous oxide budget revisited. Greenh. Gas Meas. Manag. 2011;1:17–26. doi: 10.3763/ghgmm.2010.0007. [DOI] [Google Scholar]
- Tian H., Yang J., Xu R., Lu C., Canadell J.G., Davidson E.A., Jackson R.B., Arneth A., Chang J., Ciais P., Gerber S., Ito A., Joos F., Lienert S., Messina P., Olin S., Pan S., Peng C., Saikawa E., Thompson R.L., Vuichard N., Winiwarter W., Zaehle S., Zhang B. Global soil nitrous oxide emissions since the preindustrial era estimated by an ensemble of terrestrial biosphere models: Magnitude, attribution, and uncertainty. Glob. Change Biol. 2019;25:640–659. doi: 10.1111/gcb.14514. [DOI] [PubMed] [Google Scholar]
- Teagasc National Farm Survey: Fertiliser Use Survey 2005 -2015. (2019) E. Dillon, C. Buckley, B. Moran, J. Lennon and D. Wall.
- Weier K.L., Doran J.W., Power J.F., Walters D.T. Denitrification and the Dinitrogen/Nitrous Oxide Ratio as Affected by Soil Water, Available Carbon, and Nitrate. Soil Sci. Soc. Am. J. 1993;57:66. doi: 10.2136/sssaj1993.03615995005700010013x. [DOI] [Google Scholar]
- Zaman M., Saggar S., Blennerhassett J.D., Singh J. Effect of urease and nitrification inhibitors on N transformation, gaseous emissions of ammonia and nitrous oxide, pasture yield and N uptake in grazed pasture system. Soil Biology and Biochemistry. 2009;41(6):1270–1280. doi: 10.1016/j.soilbio.2009.03.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.