Abstract
Proline dehydrogenase (oxidase, PRODH/POX), the first enzyme in the proline degradative pathway, plays a special role in tumorigenesis and tumor development. Proline metabolism catalyzed by PRODH/POX is closely linked with the tricarboxylic acid (TCA) cycle and urea cycle. The proline cycle formed by the interconversion of proline and P5C between mitochondria and cytosol interlocks with pentose phosphate pathway. Importantly, by catalyzing proline to Δ1-pyrroline-5-carboxylate (P5C), PRODH/POX donates electrons into the electron transport chain to generate ROS or ATP. In earlier studies, we found that PRODH/POX functions as a tumor suppressor to initiate apoptosis, inhibit tumor growth and block the cell cycle, all by ROS signaling. It also suppresses hypoxia inducible factor (HIF) signaling by increasing α-ketoglutarate. During tumor progression, PRODH/POX is under the control of various tumor-associated factors, such as tumor-suppressor p53, inflammatory factor peroxisome proliferator-activated receptor gamma (PPARγ), onco-miRNA miR-23b* and oncogenic transcription factor c-MYC. Recent studies revealed the two-sided features of PRODH/POX-mediated regulation. Under metabolic stress such as oxygen and glucose deprivation, PRODH/POX can be induced to serve as a tumor survival factor through ATP production or ROS-induced autophagy. The paradoxical roles of PRODH/POX can be understood considering the temporal and spatial context of the tumor. Further studies will provide additional insights into this protein and on its metabolic effects in tumors, which may lead to new therapeutic strategies.
Keywords: proline dehydrogenase/oxidase, reactive oxygen species (ROS), tumor suppressor, oncogene, metabolic stress
Introduction
Proline dehydrogenase, a.k.a. proline oxidase (PRODH/POX), catalyzing the first step in proline catabolism is widely distributed in living organisms. PRODH/POX possesses a variety of regulatory functions, such as redox homeostasis, osmotic adjustment, protection against metabolic stress and providing signaling in a variety of organisms, including bacteria, plants and mammals etc [1–4]. In humans, PRODH/POX and proline metabolism have been correlated with various physiologic and pathologic situations, such as in familial hyperprolinemias [5, 6] and neuropsychiatric disorders [7, 8]. During the last decade, intensive investigation of PRODH/POX in human cancers has made significant advances, which will be the focus in this review.
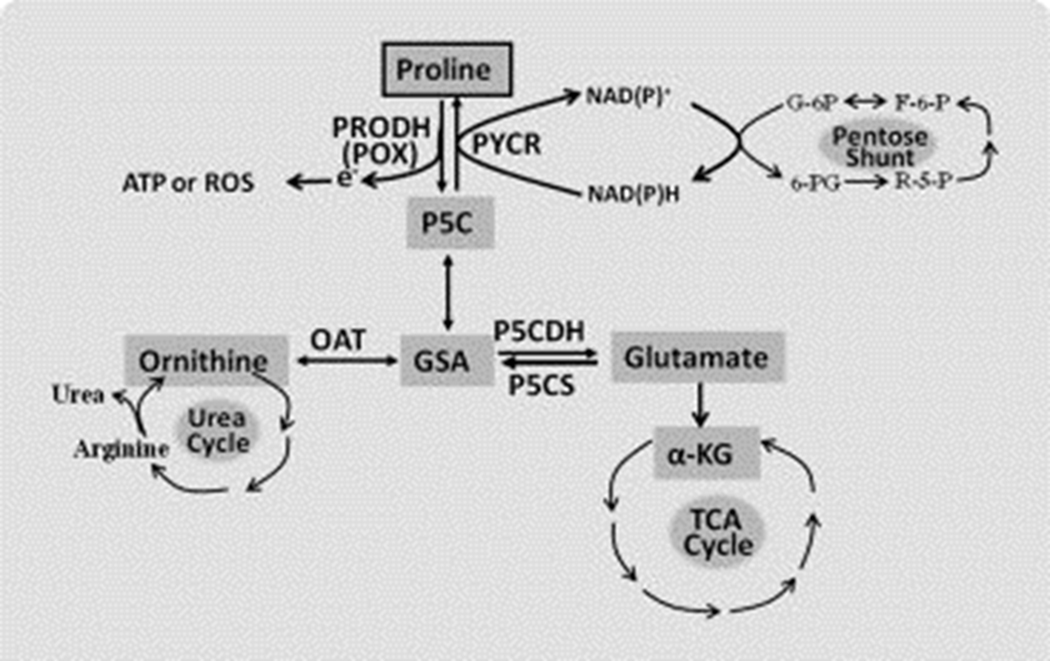
PRODH/POX is a flavin adenine dinucleotide-containing enzyme tightly bound to mitochondrial inner membranes which catalyzes the oxidation of L-proline to Δ1-pyrroline-5-carboxylate (P5C). Proline is one of the most abundant amino acids in the cell microenvironment. Together with hydroxyproline, proline accounts for 25% of collagen, a predominant protein in the extracellular matrix (ECM). As the only proteinogenic secondary amino acid, proline has special metabolic features [3, 6, 9, 10]. Not only does proline have its own family of enzymes, but also its catabolism initiated by PRODH/POX is metabolically linked with several other core metabolic pathways in human body. As shown in Figure 1, proline is sequentially converted by PRODH/POX and P5C dehydrogenase (P5CDH) to glutamate, a precursor of α-ketoglutarate (α-KG), thereby proline serves an anaplerotic function for the tricarboxylic acid (TCA) cycle. Through the sequential activities of PRODH/POX and ornithine aminotransferase (OAT), proline is converted to ornithine entering the urea cycle. Importantly, PRODH/POX and P5C reductase (PYCR) mediate the interconversion between proline and P5C to form a “proline cycle” between cytosol and mitochondria (Figure 2). Proline cycle transfers reducing and oxidizing potentials to maintain redox homeostasis. In the mitochondria, PRODH/POX oxidizes proline to P5C and donates electrons through its flavine adenine dinucleotide into the electron transport chain (ETC) to generate ATP or reactive oxygen species (ROS) depending on context [11, 12]. The ROS generated by PRODH/POX participates in signaling not only to regulate tumor cell death, but also to modulate various survival signals and pro-survival autophagy, contributing to tumorigenesis and tumor progression. The generated P5C is exported from mitochondria to the cytosol, and then is converted back to proline by PYCR using NADPH or NADH as a cofactor. Previous studies have shown that by oxidizing NADPH to NADP+, the proline cycle forms a metabolic interlock with the pentose phosphate pathway, thereby linking proline to glucose metabolism [13, 14]. Reducing potential as NADPH produced by the pentose phosphate pathway could be transferred to the ETC for ATP generation by the oxidation of proline. Moreover, NADPH could also be used for redox defense, and the generated pentoses are used for nucleotide synthesis. All of these prerequisites allow PRODH/POX and proline metabolism to play versatile roles in tumor metabolic regulation, including the role of PRODH/POX as a tumor suppressor and survival factor.
Figure 1.
Proline metabolic links in human body. Proline metabolism is closely related with TCA cycle, urea cycle, and pentose phosphate pathway (pentose shunt). Abbreviations: GSA, glutamic‐gamma‐semialdehyde; OAT, ornithine aminotransferase; P5C, Δ1‐pyrroline‐5‐carboxylate; P5CDH, P5C dehydrogenase; P5CS, P5C Synthase; PRODH (POX), proline dehydrogenase (oxidase); PYCR, P5C reductase. The interconversion between P5C and GSA is spontaneous.
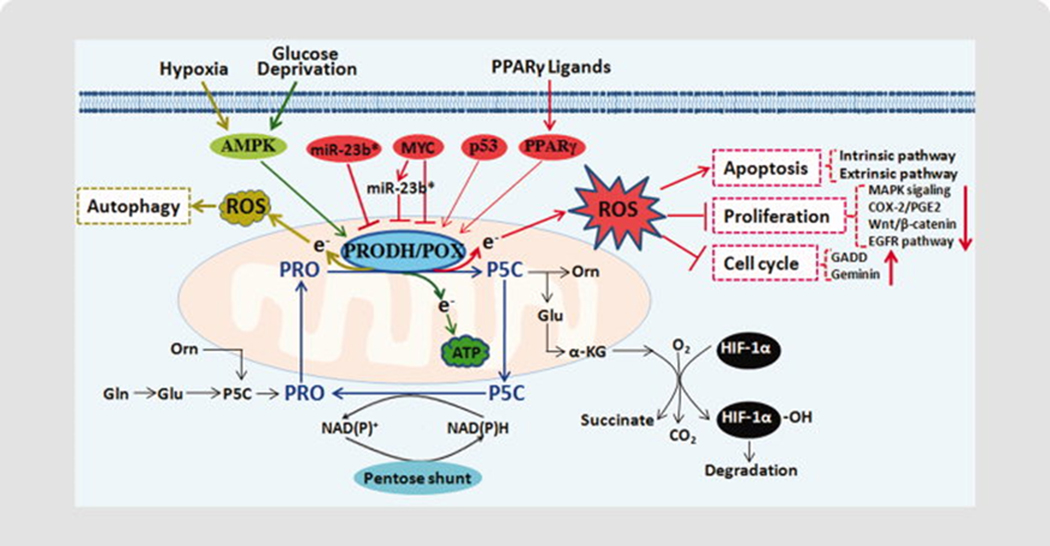
Figure 2.
Regulation of PRODH/POX in cancer. 1) Proline cycle. Interconversion of proline and P5C forms the proline cycle in the cytosol and mitochondria. Proline cycle acts as a redox shuttle transferring reducing potential generated by the pentose phosphate pathway (pentose shunt) into mitochondria for the production of either ROS or ATP responding to different stresses. 2) PRODH/POX is induced by p53, PPARγ, and its ligands, suppressed by miR‐23b* and oncogenic protein MYC, and functions as a mitochondrial tumor suppressor. As a tumor suppressor, PRODH/POX can initiate apoptosis, inhibit proliferation and induce G2 cell cycle arrest through ROS generation, and suppress HIF‐1 signaling through increasing α‐ketoglutarate (α‐KG) production. Under nutrient stress, such as glucose or oxygen deprivation, PRODH/POX is upregulated to act as a survival factor, producing ATP or ROS for prosurvival autophagy. Abbreviations: Glu, glutamate; Orn, ornithine; Pro, proline
1. PRODH/POX as a mitochondrial tumor suppressor
1.1. PRODH/POX induces apoptosis and inhibits tumor growth
Apoptosis is a multi-step, multi-pathway process for programmed cell death that is inherent in every cell and tissue of the human body. It contributes to embryonic development and self-renewal and maintenance of adult tissues. Defective apoptosis plays an important role in the development and progression of cancer. Cancer treatment by chemotherapy and irradiation kills cancer cells primarily by inducing apoptosis. Serial functional analysis on PRODH/POX indicated that PRODH/POX had tumor-suppressing roles, one of which is PRODH/POX-induced apoptosis in a variety of cancer cells [15, 16, 12, 17]. Both intrinsic and extrinsic apoptotic pathways are involved in PRODH/POX-induced apoptosis [18]. By generating ROS, PRODH/POX induces the release of cytochrome c, and activation of caspase-9 to mediate mitochondrial apoptosis (intrinsic pathway). On the other hand, PRODH/POX stimulates the expression of tumor necrosis factor-related apoptosis inducing ligand (TRAIL) and death receptor 5 (DR5), resulting in the cleavage of caspase-8 and thus death receptor-mediated apoptosis (extrinsic pathway) [18]. Several laboratories independently showed that the apoptosis initiated by PRODH/POX depended on ROS generation [15, 16, 19, 18]. N-acetyl cysteine (NAC), a widely used antioxidant agent, and ectopic expression of manganese superoxide dismutase (MnSOD), a specific antioxidant enzyme locating to mitochondria dramatically reduced PRODH/POX-induced apoptosis [12, 18].
In addition to initiating apoptosis, PRODH/POX inhibits tumor cell growth and proliferation by modulating several proliferative signaling pathways and cell cycle regulators [20]. PRODH/POX suppresses the mitogen-activated protein kinase (MAPK) pathway, cyclooxygenase-2 (COX-2)/prostaglandin E2 (PGE2) signaling, epidermal growth factor receptor (EGFR) pathway, and Wnt/β-catenin signaling [18, 21]. Cell cycle regulators, geminin and DNA damage inducible proteins (GADDs) are induced by PRODH/POX, resulting in cell cycle arrest [20]. All these effects of PRODH/POX were confirmed to be dependent on PRODH/POX-mediated ROS signaling, as shown in Figure 2.
Extensive investigation in a mouse xenograft model showed that ectopic expression of PRODH/POX significantly reduced tumor formation in nude mice [20]. DLD1-1 tet-off POX cells are DLD-1 colorectal cancer cells stably transfected with the PRODH gene under the control of a tetracycline-inhibitable promoter [15]. When injected into nude mice, the expression of PRODH/POX in the explant in vivo can be manipulated by the administration of doxycycline in the drinking water. When mice were given doxycycline, PRODH/POX expression was suppressed and tumors readily formed in all the mice within a few days. By contrast, when mice received no doxycycline, overexpression of PRODH/POX dramatically reduced tumor development. Thus, these results corroborate the above effects of PRODH/POX in vitro and further suggest the tumor suppressor function of PRODH/POX.
1.2. PRODH/POX suppresses hypoxia-inducible factor 1 (HIF-1) signaling
PRODH/POX downregulates HIF-1 signaling including its downstream gene VEGF under both normoxic and hypoxic conditions [20]. HIF-1 as a transcriptional factor regulates the expression of a variety of genes in response to hypoxia. It plays an important role in tumor development, including angiogenesis, tumor growth and invasion, and thus is an attractive cancer therapeutic target. HIF-1 is composed of HIF-1α and HIF-1β. HIF-1α determines the formation and transcriptional activity of HIF-1. It has been known that the stability and transcriptional activity of HIF-1α is tightly controlled by cellular oxygen tension, and intratumoral hypoxia can lead to its accumulation. Under nomoxia, prolyl hydroxylases (PHD), members of the 2-oxoglutarate(also called as α-KG)-dependent dioxygenase enzyme family, hydroxylate specific prolyl residues of HIF-1α, resulting in its binding to Von-Hippel Lindau (VHL) protein, ubiquitination and finally proteosomal degradation [22]. Under hypoxia, HIF-1 is not hydroxylated because of the unavailability of dioxygen, a critical substrate, and is thereby stabilized. Studies have shown that the activity of PHDs is increased by increasing concentrations of α-KG, an important co-substrate [22]. Furthermore, several TCA cycle intermediates, such as succinate and fumarate, inhibit PHD activity and stabilize HIF-1 signaling [22, 23].
As shown in Figure 2, when proline is available, high expression of PRODH/POX increases the degradation of proline sequentially to glutamate and α-KG. Supporting this hypothesis, HPLC analysis showed that PRODH/POX overexpression did increase the level of α-KG [20]. Moreover, consistent with the observations of others [22], dimethyloxalylglycine, the widely used α-KG analogue, could block the inhibition of HIF-1 signaling induced by PRODH/POX, suggesting the crucial role of α-KG in the down-regulation of HIF by PRODH/POX. Furthermore, PRODH/POX decreased the levels of succinate, fumarate and lactate [20]. Further studies should elucidate whether this regulation also contributes to HIF-1 signaling.
1.3. PRODH/POX is downregulated in human tumors
Immunohistochemical detection of PRODH/POX in different kinds of cancer tissues with corresponding normal tissues as controls showed that PRODH/POX protein was frequently absent or significantly down-regulated in tumors, especially kidney, bladder, stomach, colon and rectum, liver, and pancreas [20, 24]. This result suggested that accompanying tumor development, the tumor suppressive activity of PRODH/POX was eliminated by the downregulation of PRODH/POX. Importantly, PRODH/POX protein and transcript levels were found to have a differential expression in kidney tumor. PRODH/POX protein levels showed more striking decreases than transcript levels [20, 24], indicating that PRODH/POX may be regulated at the post-transcriptional level.
Several genetic and epigenetic processes account for the silencing of tumour suppressors, such as genetic mutations, promoter methylation, histone acetylation and post-transcriptional regulation by microRNAs (miRNAs), etc. PRODH gene sequencing did not reveal any somatic mutation or single nucleotide polymorphisms (SNP) in tumor tissues. Also, no hypermethylation in PRODH promoter was found. The differential expression pattern of PRODH/POX transcript and protein pointed to a possible regulation of miRNAs at the post-transcriptional level, which will be discussed below.
2. Regulation of PRODH/POX as a tumor suppressor
2.1. Upregulation of PRODH/POX by tumor suppressor p53
Tumor suppressor p53 is one of the most important factors defending against tumor progression, which controls the cell cycle and apoptosis of tumor cells. P53 and its interacting molecules have been the focus of thousands of studies in laboratories around the world. P53 is activated due to DNA damage or genotoxic stress and is known to be mutated in more than half of human tumors. In 1997, Polyak K. et al. screened 7202 genes using serial analysis of gene expression to identify the genes responding to p53, and found that PRODH gene encoding PRODH/POX was one of 14 genes most strongly induced by p53 [25]. In fact, it was this finding which opened a new era in the research of PRODH/POX and proline metabolism in human tumors, including all of the functions and regulations of PRODH/POX independent of p53 discussed in this review. Subsequent investigation of the regulation of PRODH/POX by p53 demonstrated the existence of a p53-response element in the PRODH promoter, which may confer a direct transcriptional regulation of PRODH by p53 [26]. Thus, defective p53 regulation may serve as one of the mechanisms for loss of PRODH/POX in some tumors. Functional studies then revealed that PRODH/POX was a critical contributing factor in the apoptosis mediated by p53 [27, 9].
Recent studies from other laboratories demonstrated that p53 also regulates metabolic pathways to inhibit oncogenic transformation and promote tumor cell survival [28, 29]. The activation and function of p53 in response to metabolic stress, such as nutrient deprivation or energy depletion, is linked with the AMP-activated protein kinase (AMPK)/mTOR pathway. This pathway also mediates p53-induced cell growth and autophagy. Interestingly, PRODH/POX performs a similar function under metabolic stress (see the following sections). The linkage of p53 and PRODH/POX in these areas deserves further investigation.
2.2. Stimulation of PRODH/POX by PPARγ
In screening studies to identify regulatory factors for PRODH/POX other than p53, our laboratory co-transfected a variety of transcription factors together with a luciferase reporter construct containing PRODH promoter into various colorectal cancer cell lines. These studies revealed that peroxisome proliferator activated receptor gamma (PPARγ) was the most potent activator of the PRODH promoter, while several other transcription factors such as c-Jun, c-Fos, and p-65 of NF-κB showed only modest effects [17]. A PPAR response element (PPRE) exists in the PRODH promoter, and the binding of ligand-activated PPARγ to the PRODH promoter was confirmed by electrophoretic mobility shift assay (EMSA) and chromotin immunoprecipitation assay (ChIP) [17].
PPARγ belongs to the nuclear hormone receptor superfamily and functions as a ligand-dependent transcription factor [30]. The ligands of PPARγ can be naturally-occurring, such as prostaglandins and oxidized lipids, or synthetic and pharmacologic, i.e., the thiazolidinediones (TZDs). Modulation of PPARγ-regulated pathways plays an important role in metabolism and inflammatory responses, especially in glucose homeostasis. TZDs have been used as a hypoglycemic agent to treat type 2 diabetes. It is noteworthy that PPARγ and its ligands also play an important role in cancer. PPARγ is widely expressed in many malignant tissues. Serial studies showed that its ligands can induce differentiation, apoptosis, autophagy, and inhibition of cell growth in a variety of cancer cells [31, 32], although PPARγ ligands may also contribute to cell survival in certain context for specific cancer cells [33, 34]. Epidemiologic studies in the patients with type 2 diabetes indicate that TZDs significantly decreased the risk of lung cancer [35]. However, the mechanism of these effects is not well established. The finding of the regulation of PPARγ on PRODH/POX provides a new clue for understanding its effects in tumors.
Pharmacologic TZDs not only activated PRODH promoter activity, but also upregulated PRODH/POX protein expression [17]. Furthermore, studies from different groups showed that TZDs troglitazone and rosiglitazone markedly increased the generation of ROS in several cancer cell lines, including colorectal cancer cells and nonsmall cell lung cancer cells [17, 36, 37]. Both groups showed that these TZDs induced apoptosis through ROS signaling, and PRODH/POX knockdown by siRNA significantly inhibited the ROS production and apoptosis stimulated by TZDs. These findings suggest that PRODH/POX plays a critical role in the anticancer effect of PPARγ. Additional studies are needed to determine whether PRODH/POX also mediates other effects of PPARγ and its ligands, and whether these effects can be extended to other cancer cells and tumor models in vivo. Indeed, our recent study showed that PRODH/POX served as a survival factor in PPARγ-induced autophagy in tumor cells exposed to oxidized low density lipoprotein (ox-LDL) [34], whose lipid components are PPARγ ligands [38]. This point will be discussed later.
2.3. Downregulation of PRODH/POX by miR-23b*
MiRNAs are conserved, endogenously expressed, non-coding small RNAs of 18–25 nucleotides in length. They are a new class of post-transcriptional regulators discovered during the last decade, which inhibit protein translation or induce mRNA degradation through interacting with target mRNA 3’ untranslated region (UTR) [39]. Up to now, over 700 miRNAs have been identified in humans. They regulate the expression of thousands of genes, and thus are critical regulators of cellular function, including proliferation, differentiation, apoptosis and metabolic signalings. The aberrant expression or alteration of miRNAs has been linked to various human diseases, especially cancer. The control of gene expression by miRNAs likely occurs in all cancer cells. Their targets are usually important proteins such as oncoproteins (i.e., c-MYC, RAS), tumor suppressor proteins (i.e., p53), or proteins regulating the cell cycle. Depending on the targets they regulate, miRNAs can act as oncogenes or tumor suppressor genes in tumorigenesis. Thus, whether tumor suppressor protein PRODH/POX could be targeted by miRNAs becomes an interesting question. The aforementioned inconsistency between PRODH/POX transcript and protein expression in tumors further motivated us to explore the regulation of PRODH/POX by miRNAs.
Using target-prediction algorithms, 91 potential miRNAs were predicted to target PRODH/POX mRNA 3’UTR [24]. The following miRNA microarray showed that 10 miRNAs had an increased expression in renal cancer cells relative to normal cells. However, when the 10 mimic miRNAs were transfected into normal renal epithelial cells (ECs), only miR-23b* significantly inhibited PRODH/POX protein expression, but not mRNA level. In contrast, in renal cancer cells, the inhibitory antagomir against miR-23b* could reverse the decreased PRODH/POX expression. Subsequently, miR-23b* direct binding to PRODH/POX mRNA 3’UTR was experimentally confirmed by co-transfecting the mimic miR-23b* and a luciferase reporter containing 3’UTR of PRODH/POX mRNA into cells.
Ectopic expression of miR-23b* in normal renal ECs impaired the function of PRODH/POX, including PRODH/POX-induced ROS generation, apoptosis, and PRODH/POX-inhibited HIF-1 signaling [24]. On the other hand, when miR-23b* was inhibited in renal cancer cells, ROS production and the percentage of cells undergoing apoptosis increased, and HIF-1 signaling decreased. These findings provide a therapeutic strategy to inhibit tumor cell growth by decreasing the levels of miR-23b* or by blocking its effect on PRODH/POX.
The data obtained from human renal carcinoma tissues and matched normal counterparts in vivo showed significant differences in both miR-23b* and PRODH/POX protein levels, and a negative correlation between miR-23b* and PRODH/POX protein, substantiating the in vitro findings described above [24]. These findings suggest that the increased miR-23b* may contribute to renal oncogenesis and progression by downregulating tumor suppressor PRODH/POX.
2.4. Suppression of PRODH/POX by oncogenic transcription factor c-MYC
Our most recent study investigating the effect of oncogenic transcription factor c-MYC (termed as MYC) on PRODH/POX further emphasized the importance of PRODH/POX as a tumor suppressor [40]. MYC gene was first discovered in Burkitt’s lymphoma patients. Later on, this oncogenic protein was found to be overexpressed in many kinds of human tumors. As a transcriptional activator or suppressor, overexpression of MYC in tumors leads to the dysregulated expression of a large number of genes including miRNAs, which are involved in cell proliferation, apoptosis, differentiation, and cell metabolism. Using the Burkitt’s lymphoma cell model P493 MYC tet-off cells which bear a tetracycline-repressible MYC construct, we found that PRODH/POX protein increased in a time-dependent fashion with MYC expression diminished by tetracycline, and consequently decreased on MYC recovery by tetracycline removal [40]. PRODH/POX mRNA expression also showed a significant increase with suppressed MYC expression, but the increase is far less than that of protein levels, implicating the regulation of MYC on PRODH/POX at both of transcriptional and post-transcriptional level, especially the latter. This finding was further confirmed in the PC3 human prostate cancer cells which have high expression of MYC, suggesting the suppression of PRODH/POX by MYC may be a common event in different kinds of human tumors.
Analysis of PRODH promoter nucleotide sequence revealed one canonical MYC binding site (E-box) and one noncanonical binding site in the PRODH promoter region. However, chromatin immunoprecipitation (ChIP) assay did not show evidence for MYC binding to any of these two PRODH promoter regions, suggesting that the decreased PRODH/POX mRNA expression may be mediated secondarily through other transcription factors regulated by MYC [40]. Furthermore, to clarify the potential role of miR-23b* in the post-transcriptional suppression of PRODH/POX protein by MYC, the effect of MYC on the expression of miR-23b* was examined. Not surprisingly, MYC markedly upregulated miR-23b* expression. When miR-23b* was inhibited by its antagomirs in the P493 cells with MYC overexpression, PRODH/POX protein level increased [40]. By contrast, ectopic expression of miR-23b* under MYC suppression led to a marked decrease of PRODH/POX protein expression. Moreover, the luciferase assays in PC3 prostate cancer cells, which were transfected with the luciferase reporter containing PRODH/POX mRNA 3’UTR, confirmed that MYC inhibited the expression of PRODH/POX primarily through increasing miR-23b*.
Further studies indicated that PRODH/POX suppression is essential for MYC-mediated cancer cell proliferation and survival. Knockdown of PRODH/POX in P493 cells with suppressed MYC expression consistently reduced the production of ROS at different time points [40]. As a response, the percentage of apoptotic and dead cells occurring with MYC suppression was decreased by PRODH/POX knockdown, and the diminished cell growth was significantly reversed by suppressing PRODH/POX [40]. These results were confirmed in PC3 cells [40]. To summarize the above findings, oncogenic transcription factor MYC inhibited PRODH/POX expression and thereby inhibited its tumor suppressor function, which may be an important step in tumorigenesis and tumor progression. When MYC is suppressed, the increase of PRODH/POX promotes proline catabolism to generate ROS, leading to the initiation of apoptosis and the decrease of cell proliferation and growth. This may provide a novel strategy in tumor treatment.
3. PRODH/POX as a survival factor
Increasing tumor-suppressor proteins have been found to be two-sided in their effects. One is certainly the function of suppressing tumor formation, but the other is promoting tumor survival. For example, the first discovered tumor-suppressor protein Retinoblastoma protein (pRb), could also act as a survival factor [41]. P53 is another important example. As described above, p53 protects cells from DNA damage by activating repair or initiating apoptosis, but under energetic stress, p53 contributes to cell adaptation and survival [28, 29]. Therefore, it is not surprising that PRODH/POX is upregulated to contribute to cell survival under metabolic stresses in tumor cells. The recent finding that PRODH/POX may be involved in mitochondrial hormesis (mitohormesis), a process described in C. elegans in which apoptotic signals may also trigger a survival response is another interesting model confirming the pro-survival role of PRODH/POX and proline metabolism [42].
3.1. PRODH/POX under glucose deprivation
It is known that tumor cells primarily utilize glucose and glutamine as their main energy sources, but such addiction requires an adequate blood supply. With rapid growth, tumors cells will enter a phase where vascularization is insufficient, and even with neovascularization blood is delivered unevenly and many tumor cells not only are hypoxic but also will be starved of nutrients, including glucose and glutamine. At this point, to meet their bioenergetic demands, it is necessary for tumor cells to seek alternative energy source. The availability of proline in cellular microenvironment, its unique metabolism and its response to different stresses ensure the possibility of proline as an energy source. Indeed, proline concentration is increased in various tumors [43, 44]. The upregulation of metalloproteinases (MMPs) degrading ECM, the main reservoir of proline in tumor microenvironment, was observed in tumors, which was a critical step for tumor progression and metastasis [45, 46].
Pandhare et al. showed that even with glucose limitation intracellular ATP levels were maintained in RKO colorectal cancer cells [13]. Upon glucose depletion, the expression and activities of MMP-2 and MMP-9 increased, accompanied by an increase of intracellular proline levels. Consistent with these findings, PRODH/POX activities and expression were dramatically upregulated. Using DLD1-POX tet-off cells exposed to low-glucose concentration, induction of PRODH/POX by tetracycline removal led to a significant increase of ATP. Due to the interlock between proline cycle and pentose phosphate pathway, we tested the bioenergetic contribution of this interlock by comparing the activity of pentose phosphate pathway in DLD1-POX tet-off cells with and without PRODH/POX induction through the measurement of 14CO2 production from 1-14C-glucose [47]. As expected, induction of PRODH/POX increased the pentose phosphate pathway more than 5-fold, and the increase was seen over a range of low-glucose concentrations. To further confirm that the increased activity of pentose phosphate pathway is coupled to the ATP increase, we treated cells with dehydroepiandrosterone (DHEA), a well-known inhibitor of G6PDH [48], the rate-limiting enzyme of the pentose phosphate pathway. The result showed that DHEA did significantly decrease the PRODH/POX-dependent increase of the cellular ATP [49]. Thus, by cycling of P5C to proline, pentose phosphate pathway could contribute, at least in part, to PRODH/POX-induced ATP production. In addition, the aneplerotic role of proline-derived α-KG for TCA cycle may also contribute to PRODH/POX-induced ATP production.
The mammalian target of rapamycin (mTOR) pathway is an important signaling pathway responding to the availability of extracellular nutrients [50]. mTOR belongs to the phosphatidylinositol 3-kinase-related enzyme family and is a crucial regulator of cell growth, proliferation and energy metabolism. It can coordinate anabolic and catabolic processes with the availability of nutrients, and thus its inhibition could mimic a condition of nutrient or energy stress [13, 51]. Therefore, as a support to the above findings, we mimicked a nutrient starvation signal in RKO colorectal cells using the inhibitor of mTOR, rapamycin, which directly binds to mTOR to prevent the formation of TOR complex 1. As a result, PRODH/POX was activated to generate proline-dependent ATP. Dehydroproline, the inhibitor of PRODH/POX enzymatic activity, could block the bioenergenic response of PRODH/POX [13]. Concomitant with the increases of PRODH/POX activity and ATP production, the activity of pentose phosphate pathway increased 2-fold in RKO cells treated with rapamycin. It seems that the downregulation of mTOR from glucose deprivation serves as a mechanism for the upregualtion of PRODH/POX.
AMP-activated protein kinase (AMPK) is a critical energy sensor for tumor cells to adapt to nutrient or oxygen deprivation. Importantly, AMPK is also an upstream inhibitor of mTOR activity. So, as a parallel of the above studies, we investigated the involvement of AMPK in the induction of PRODH/POX by glucose deprivation [13]. The activation of AMPK by phosphorylation markedly increased in RKO cells following glucose withdrawal. A synthetic AMPK activator, 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) induced a dose- and time-dependent increase in PRODH/POX activity. Thus AMPK activation may be a pathway mediating PRODH/POX induction under conditions of low-glucose stress to maintain cellular energy levels. And mTOR is one of the downstream signalings of activated AMPK, which may contribute to this regulation.
3.2. PRODH/POX under hypoxic tumor microenvironment
Although oxygen diffuses through tissues more readily than nutrients like glucose, hypoxia is a well-recognized and important feature of the microenvironment during tumor development, and oxygen distribution in tumors follows a pattern similar to that of glucose. Thus, we extended our studies of PRODH/POX to conditions of hypoxia or hypoxia combined with low-glucose [52]. Through transfecting the PRODH promoter luciferase construct into a variety of cancer cell lines, we found hypoxia dramatically increased the activity of PRODH promoter. And real-time PCR confirmed this upregulation of PRODH/POX in most of the cancer cell lines, including those of colon, kidney, breast, prostate, melanoma, lung and ovary. The time- and dose-dependent increase of PRODH/POX protein with hypoxia was then shown in HT29 colorectal cancer cells. Combined oxygen and glucose deprivation resulted in an additive increase in PRODH/POX protein levels.
Using MDA-MB-231 HRE-EGFP breast tumor xenografts, we further showed the upregulation of PRODH/POX in the hypoxic microenvironment in vivo [52]. MDA-MB-231 HRE-EGFP is a human breast cancer cell line which expresses the EGFP reporter gene under the control of a promoter containing hypoxia-response elements (HREs) that could bind to and are induced by HIF-1 [46]. The immunohistochemistry of PRODH/POX and EGFP, and co-localization analysis showed that PRODH/POX positively correlated with EGFP expression which responded to hypoxia, suggesting the upregulation of PRODH/POX by hypoxia. Surprisingly, further exploration showed that hypoxia-induced PRODH/POX expression was dependent on neither HIF-1α or HIF-2α, the critical transcription factors mediating hypoxic regulation of target genes. Consistent with AMPK-mediated PRODH/POX induction under glucose starvation, a specific inhibitor of AMPK activation, compound C, blocked the hypoxia-induced increases of PRODH/POX expression.
Additional functional analysis revealed that increased PRODH/POX contributed to the survival of cancer cells in response to both oxygen and glucose deprivation [52]. Furthermore, using either dehydroproline or PRODH/POX siRNA, we showed that under hypoxia the survival contribution of PRODH/POX resulted from its induction of ROS, but not ATP. And ROS here were used for inducing pro-survival autophagy, but not apoptosis and cell growth inhibition mentioned above. Compound C, the AMPK inhibitor could inhibit autophagy, suggesting AMPK-PRODH/POX-ROS-autophagy serves as a survival pathway under hypoxia. However, the downstream factor of AMPK, mTOR was irrelevant to the PRODH/POX-induced autophagic response, although AMPK was shown to induce autophagy by suppressing mTOR activity. On the other hand, under glucose deprivation either with or without hypoxia, PRODH/POX contributed to ATP production by degrading proline. These results suggest that the catabolism of proline by PRODH/POX provides a switching point between ATP and ROS production according to the specific microenvironmental stress. The mechanism mediating this switch is an interesting question and needs to be further studied.
3.3. Regulation of PRODH/POX by oxLDL, a physiologic/pathophysiologic PPARγ ligand
As mentioned previously, PPARγ and its ligands not only induced apoptosis, but also contribute to survival [33]. This was also reflected by their regulation on PRODH/POX and the pro-survival function of PRODH/POX. OxLDL can function as a PPARγ ligand, activating PPARγ signaling [53]. Epidemiological studies revealed the association of high levels of oxLDL with increased cancer risk [54, 55], but the mechanism remained unclear. Using physiologic concentrations of oxLDL, we examined its effects on the expression of PRODH/POX and its underlying mechanism. We showed that oxLDL upregulated PRODH/POX in different kinds of cancer cells. And it was 7-ketocholesterol (7KC), a major oxysterol of oxLDL, but not other constituents of oxLDL which activated PRODH/POX. Knockdown of PPARγ significantly decreased oxLDL- and 7KC-induced PRODH/POX expression, suggesting an important role of PPARγ in the oxLDL-induced PRODH/POX expression.
Although oxLDL was cytotoxic to cancer cells, knockdown of PRODH/POX by siRNA further decreased cell viability. Thereby, in contrast to the effects of other pharmacologic TZDs, the effects of oxLDL on PRODH/POX are not for apoptosis, but for survival. Since oxLDL could activate both apoptosis and autophagy, we assessed induction of autophagy by PRODH/POX in HT29 colorectal cancer cells exposed to oxLDL. Beclin-1 is one of the central regulators of autophgy in mammalian cells [56]. The conversion of light-chain 3 protein (LC3)-I into LC3-II is an important hallmark for augophagosome accumulation. By measuring beclin-1 protein and the LC3-I to LC3-II conversion, we showed that PRODH/POX knockdown inhibited oxLDL-induced autophagy. We used DLD1-POX tet-off cell model, in which overexpression of PRODH/POX, itself, independent of any stimulation, was sufficient to activate autophagy. On the other hand, ROS assays showed that oxLDL significantly increased intracellular ROS production, and nearly two-thirds of oxLDL-induced ROS was dependent of PRODH/POX. Further investigation of the mechanism established the link of PRODH/POX-generated ROS and its induction of autophagy. The infection of adenoviral vector containing MnSOD into the DLD1-POX cells reduced the expression of beclin-1 under conditions of PRODH/POX overexpression. These results not only elucidated the association between oxLDL and its function in carcinogenesis, but also provided additional evidence for PRODH/POX as a survival factor.
4. Understanding PRODH/POX in the context of temporal and spatial tumorigenesis
Taken together, PRODH/POX is a two-edged sword under regulation of different tumor-associated factors. On the one side, PRODH/POX acts as a tumor suppressor for initiation of apoptosis and inhibition of tumor growth etc. On the other, under certain conditions, it functions as a survival factor by producing either ATP or ROS for pro-survival autophagy. At first glance, these functions seem paradoxical and are confusing. However, as we now know, cancer develops over long periods by accumulating complex, successive pro-survival changes. Understanding that cancer is a time-dependent multistep process across time helps us to explain this paradox. Considering the temporal and spatial stresses in tumor development, we have proposed a timeline for PRODH/POX functional involvement in tumors, including the initial evasion of tumor cells from apoptosis, tumor cell survival metabolic stress and finally mechanisms for rapid growth [9].
Pre-transformation stress such as DNA damage activates p53 and thereby PRODH/POX. The activated p53 and PRODH/POX may blockade the cell cycle for DNA repair or to initiate apoptosis. Only small numbers of cells evade apoptosis to display characteristics of malignant transformation. Later on, the release from growth constraints and loss of suppressor activities allow these cells to proliferate, followed by nutrient or hypoxic stress due to detachment from blood supply. Under nutrient or energy stress, AMPK is activated and mTOR is downregulated which will upregulate PRODH/POX as a survival factor to provide alternative energy source or to induce pro-survival autophagy. When neoangiogenesis restores the blood supply with adequate nutrients and oxygen, tumor cells will enter a phase of rapid growth under the stimulation of growth signals, such as oncogenic protein MYC, RAS, and onco-miRNAs. At this phase, the expression of PRODH/POX will be suppressed by these factors.
Of course, this is only a proposed regulatory pattern for PRODH/POX in tumor development. Tumor progression is a constantly changing network of various oncogenic proteins, tumor-suppressor proteins and different signaling pathways. Some of the molecular changes may be tissue specific. Therefore, it’s still necessary to discover all of the relevant links and functions of PRODH/POX to thoroughly understand its roles in tumor development.
5. Conclusion
In summary, PRODH/POX and its induction of proline metabolism play a critical role in tumor initiation and development, either as a tumor suppressor or as a survival factor, which is summarized in Figure 2. As a tumor suppressor, PRODH/POX mediates various signaling pathways including the induction of apoptotic signaling and the suppression of HIF-1 and proliferative signaling. It is induced by p53, PPARγ and its ligands, and suppressed by onco-miR-23b* and oncogenic protein MYC. As a survival factor, PRODH/POX is induced to protect against nutrient or hypoxic stress. And on such occasions, PRODH/POX is channeled into maintain or produce energy either inducing ROS-mediated autophagy or providing ATP. Its various regulatory mechanisms and functions emphasize the complexity of tumor development.
Acknowlegements
The work was supported by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. This project also has been funded in part with Federal funds from the National Cancer Institute, NIH, under contract no. HHSN27612080001. The content of this review does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. government. We thank Dr. Ziqiang Zhu for his reading of the manuscript.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- [1].Phang JM, Downing SJ, Yeh GC, Smith RJ, Williams JA, Hagedorn CH (1982) Stimulation of the hexosemonophosphate-pentose pathway by pyrroline-5-carboxylate in cultured cells. J Cell Physiol. 110, 255–261. [DOI] [PubMed] [Google Scholar]
- [2].Yeh GC, Roth EF Jr., Phang JM, Harris SC, Nagel RL, Rinaldi A. (1984) The effect of pyrroline-5-carboxylic acid on nucleotide metabolism in erythrocytes from normal and glucose-6-phosphate dehydrogenase-deficient subjects. J Biol Chem. 259, 5454–5458. [PubMed] [Google Scholar]
- [3].Phang JM (1985) The regulatory functions of proline and pyrroline-5-carboxylic acid. Curr Top Cell Regul. 25, 91–132. [DOI] [PubMed] [Google Scholar]
- [4].Peng Z, Lu Q, Verma DP (1996) Reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol Gen Genet. 253, 334–341. [DOI] [PubMed] [Google Scholar]
- [5].Schafer IA, Scriver CR, Efron ML (1962) Familial hyperprolinemia, cerebral dysfunction and renal anomalies occuring in a family with hereditary nephropathy and deafness. N Engl J Med. 267, 51–60. [DOI] [PubMed] [Google Scholar]
- [6].Phang JM, Hu CA, Valle D. (2001) Disorders of proline and hydroxyproline metabolism In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. In Metabolic and Molecular Bases of Inherited Disease, New York: McGraw-Hill; pp. 1821–1838. [Google Scholar]
- [7].Bender HU, Almashanu S, Steel G, Hu CA, Lin WW, Willis A, Pulver A, Valle D. (2005) Functional consequences of PRODH missense mutations. Am J Hum Genet. 76, 409–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Willis A, Bender HU, Steel G, Valle D. (2008) PRODH variants and risk for schizophrenia. Amino Acids. 35, 673–679. [DOI] [PubMed] [Google Scholar]
- [9].Phang JM, Liu W, Zabirnyk O. (2010) Proline metabolism and microenvironmental stress. Annu Rev Nutr. 30, 441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Phang JM, Liu W. (2012) Proline metabolism and cancer. Front Biosci. 17, 1835–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Adams E, Frank L. (1980) Metabolism of proline and the hydroxyprolines. Annu Rev Biochem. 49, 1005–1061. [DOI] [PubMed] [Google Scholar]
- [12].Liu Y, Borchert GL, Donald SP, Surazynski A, Hu CA, Weydert CJ, Oberley LW, Phang JM (2005) MnSOD inhibits proline oxidase-induced apoptosis in colorectal cancer cells. Carcinogenesis. 26, 1335–1342. [DOI] [PubMed] [Google Scholar]
- [13].Pandhare J, Donald SP, Cooper SK, Phang JM (2009) Regulation and function of proline oxidase under nutrient stress. J Cell Biochem. 107, 759–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Phang JM, Liu W, Hancock C, Christian KJ (2012) The proline regulatory axis and cancer. Front Oncol. 2, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Donald SP, Sun XY, Hu CA, Yu J, Mei JM, Valle D, Phang JM (2001) Proline oxidase, encoded by p53-induced gene-6, catalyzes the generation of proline-dependent reactive oxygen species. Cancer Res. 61, 1810–1815. [PubMed] [Google Scholar]
- [16].Maxwell SA, Rivera A. (2003) Proline oxidase induces apoptosis in tumor cells, and its expression is frequently absent or reduced in renal carcinomas. J Biol Chem. 278, 9784–9789. [DOI] [PubMed] [Google Scholar]
- [17].Pandhare J, Cooper SK, Phang JM (2006) Proline oxidase, a proapoptotic gene, is induced by troglitazone: evidence for both peroxisome proliferator-activated receptor gamma-dependent and -independent mechanisms. J Biol Chem. 281, 2044–2052. [DOI] [PubMed] [Google Scholar]
- [18].Liu Y, Borchert GL, Surazynski A, Hu CA, Phang JM (2006) Proline oxidase activates both intrinsic and extrinsic pathways for apoptosis: the role of ROS/superoxides, NFAT and MEK/ERK signaling. Oncogene. 25, 5640–5647. [DOI] [PubMed] [Google Scholar]
- [19].Lu M, Kwan T, Yu C, Chen F, Freedman B, Schafer JM, Lee EJ, Jameson JL, Jordan VC, Cryns VL (2005) Peroxisome proliferator-activated receptor gamma agonists promote TRAIL-induced apoptosis by reducing survivin levels via cyclin D3 repression and cell cycle arrest. J Biol Chem. 280, 6742–6751. [DOI] [PubMed] [Google Scholar]
- [20].Liu Y, Borchert GL, Donald SP, Diwan BA, Anver M, Phang JM (2009) Proline Oxidase Functions as a Mitochondrial Tumor Suppressor in Human Cancers. Cancer Res. 69, 6414–6422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Liu Y, Borchert GL, Surazynski A, Phang JM (2008) Proline oxidase, a p53-induced gene, targets COX-2/PGE2 signaling to induce apoptosis and inhibit tumor growth in colorectal cancers. Oncogene. 27, 6729–6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Verma A. (2006) Oxygen-sensing in tumors. Curr Opin Clin Nutr Metab Care. 9, 366–378. [DOI] [PubMed] [Google Scholar]
- [23].Koivunen P, Hirsila M, Remes AM, Hassinen IE, Kivirikko KI, Myllyharju J. (2007) Inhibition of hypoxia-inducible factor (HIF) hydroxylases by citric acid cycle intermediates: possible links between cell metabolism and stabilization of HIF. J Biol Chem. 282, 4524–4532. [DOI] [PubMed] [Google Scholar]
- [24].Liu W, Zabirnyk O, Wang H, Shiao YH, Nickerson ML, Khalil S, Anderson LM, Perantoni AO, Phang JM (2010) miR-23b targets proline oxidase, a novel tumor suppressor protein in renal cancer. Oncogene. 29, 4914–4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Polyak K, Xia Y, Zweier JL, Kinzler KW, Vogelstein B. (1997) A model for p53-induced apoptosis. Nature. 389, 300–305. [DOI] [PubMed] [Google Scholar]
- [26].Maxwell SA, Kochevar GJ (2008) Identification of a p53-response element in the promoter of the proline oxidase gene. Biochem Biophys Res Commun. 369, 308–313. [DOI] [PubMed] [Google Scholar]
- [27].Rivera A, Maxwell SA (2005) The p53-induced gene-6 (proline oxidase) mediates apoptosis through a calcineurin-dependent pathway. J Biol Chem. 280, 29346–29354. [DOI] [PubMed] [Google Scholar]
- [28].Bensaad K, Vousden KH (2005) Savior and slayer: the two faces of p53. Nat Med. 11, 1278–1279. [DOI] [PubMed] [Google Scholar]
- [29].Vousden KH, Prives C. (2009) Blinded by the Light: The Growing Complexity of p53. Cell. 137, 413–431. [DOI] [PubMed] [Google Scholar]
- [30].Willson TM, Brown PJ, Sternbach DD, Henke BR (2000) The PPARs: from orphan receptors to drug discovery. J Med Chem. 43, 527–550. [DOI] [PubMed] [Google Scholar]
- [31].Phang JM, Pandhare J, Zabirnyk O, Liu Y. (2008) PPARgamma and Proline Oxidase in Cancer. PPAR Res. 2008, 542694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Robbins GT, Nie D. (2012) PPAR gamma, bioactive lipids, and cancer progression. Front Biosci. 17, 1816–1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Talbert DR, Allred CD, Zaytseva YY, Kilgore MW (2008) Transactivation of ERalpha by Rosiglitazone induces proliferation in breast cancer cells. Breast Cancer Res Treat. 108, 23–33. [DOI] [PubMed] [Google Scholar]
- [34].Zabirnyk O, Liu W, Khalil S, Sharma A, Phang JM (2010) Oxidized low-density lipoproteins upregulate proline oxidase to initiate ROS-dependent autophagy. Carcinogenesis. 31, 446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Govindarajan R, Ratnasinghe L, Simmons DL, Siegel ER, Midathada MV, Kim L, Kim PJ, Owens RJ, Lang NP (2007) Thiazolidinediones and the risk of lung, prostate, and colon cancer in patients with diabetes. J Clin Oncol. 25, 1476–1481. [DOI] [PubMed] [Google Scholar]
- [36].Kim KY, Ahn JH, Cheon HG (2007) Apoptotic action of peroxisome proliferator-activated receptor-gamma activation in human non small-cell lung cancer is mediated via proline oxidase-induced reactive oxygen species formation. Mol Pharmacol. 72, 674–685. [DOI] [PubMed] [Google Scholar]
- [37].Wang J, Lv X, Shi J, Hu X, Du Y. (2011) Troglitazone induced apoptosis via PPARgamma activated POX-induced ROS formation in HT29 cells. Biomed Environ Sci. 24, 391–399. [DOI] [PubMed] [Google Scholar]
- [38].Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM (1998) Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 93, 229–240. [DOI] [PubMed] [Google Scholar]
- [39].Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116, 281–297. [DOI] [PubMed] [Google Scholar]
- [40].Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM (2012) Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci U S A. 109, 8983–8988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Xu HJ, Quinlan DC, Davidson AG, Hu SX, Summers CL, Li J, Benedict WF (1994) Altered retinoblastoma protein expression and prognosis in early-stage non-small-cell lung carcinoma. J Natl Cancer Inst. 86, 695–699. [DOI] [PubMed] [Google Scholar]
- [42].Zarse K, Schmeisser S, Groth M, Priebe S, Beuster G, Kuhlow D, Guthke R, Platzer M, Kahn CR, Ristow M. (2012) Impaired insulin/IGF1 signaling extends life span by promoting mitochondrial L-proline catabolism to induce a transient ROS signal. Cell Metab. 15, 451–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Catchpole G, Platzer A, Weikert C, Kempkensteffen C, Johannsen M, Krause H, Jung K, Miller K, Willmitzer L, Selbig J, Weikert S. (2009) Metabolic profiling reveals key metabolic features of renal cell carcinoma. J Cell Mol Med. 15, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hirayama A, Kami K, Sugimoto M, Sugawara M, Toki N, Onozuka H, Kinoshita T, Saito N, Ochiai A, Tomita M, Esumi H, Soga T. (2009) Quantitative metabolome profiling of colon and stomach cancer microenvironment by capillary electrophoresis time-of-flight mass spectrometry. Cancer Res. 69, 4918–4925. [DOI] [PubMed] [Google Scholar]
- [45].Deryugina EI, Quigley JP (2006) Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 25, 9–34. [DOI] [PubMed] [Google Scholar]
- [46].Kakkad SM, Solaiyappan M, O’rourke B, Stasinopoulos I, Ackerstaff E, Raman V, Bhujwalla ZM, Glunde K. (2010) Hypoxic tumor microenvironments reduce collagen I fiber density. Neoplasia. 12, 608–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Phang JM, Downing SJ, Yeh GC (1980) Linkage of the HMP pathway to ATP generation by the proline cycle. Biochem Biophys Res Commun. 93, 462–470. [DOI] [PubMed] [Google Scholar]
- [48].Gordon G, Mackow MC, Levy HR (1995) On the mechanism of interaction of steroids with human glucose 6-phosphate dehydrogenase. Arch Biochem Biophys. 318, 25–29. [DOI] [PubMed] [Google Scholar]
- [49].Phang JM, Donald SP, Pandhare J, Liu Y. (2008) The metabolism of proline, a stress substrate, modulates carcinogenic pathways. Amino Acids. 35, 681–690. [DOI] [PubMed] [Google Scholar]
- [50].Reiling JH, Sabatini DM (2006) Stress and mTORture signaling. Oncogene. 25, 6373–6383. [DOI] [PubMed] [Google Scholar]
- [51].Sengupta S, Peterson TR, Sabatini DM (2010) Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 40, 310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Liu W, Glunde K, Bhujwalla ZM, Raman V, Sharma A, Phang JM (2012) Proline oxidase promotes tumor cell survival in hypoxic tumor microenvironments. Cancer Res. doi: 10.1158/0008-5472.CAN-12-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Tontonoz P, Nagy L, Alvarez JG, Thomazy VA, Evans RM (1998) PPARgamma promotes monocyte/macrophage differentiation and uptake of oxidized LDL. Cell. 93, 241–252. [DOI] [PubMed] [Google Scholar]
- [54].Suzuki K, Ito Y, Wakai K, Kawado M, Hashimoto S, Toyoshima H, Kojima M, Tokudome S, Hayakawa N, Watanabe Y, Tamakoshi K, Suzuki S, Ozasa K, Tamakoshi A. (2004) Serum oxidized low-density lipoprotein levels and risk of colorectal cancer: a case-control study nested in the Japan Collaborative Cohort Study. Cancer Epidemiol Biomarkers Prev. 13, 1781–1787. [PubMed] [Google Scholar]
- [55].Delimaris I, Faviou E, Antonakos G, Stathopoulou E, Zachari A, Dionyssiou-Asteriou A. (2007) Oxidized LDL, serum oxidizability and serum lipid levels in patients with breast or ovarian cancer. Clin Biochem. 40, 1129–1134. [DOI] [PubMed] [Google Scholar]
- [56].Cao Y, Klionsky DJ (2007) Physiological functions of Atg6/Beclin 1: a unique autophagy-related protein. Cell Res. 17, 839–849. [DOI] [PubMed] [Google Scholar]