Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection can give rise to different clinical manifestations that are directly related to viral tissue damage or indirectly induced by the antiviral immune response. Hyper-activation of the immune system in an attempt to eradicate the infection may trigger autoimmunity. Several immune-mediated disorders have been described in SARS-CoV-2-infected individuals. These include cutaneous rashes and vasculitis, autoimmune cytopenia, anti-phospholipid syndrome, central or peripheral neuropathy, myositis and myocarditis. On the other hand, rheumatic patients were reported to have similar coronavirus disease 2019 (COVID-19) incidence, morbidity and mortality rates compared to general population. This opinion review will summarize the crucial immunologic steps which occur during SARS-CoV-2-infection that may link autoimmunity to COVID-19 and provides an opportunity for further discussion regarding this association.
Keywords: COVID-19, SARS-CoV-2, Autoimmunity, Autoimmune diseases, Rheumatic diseases, Host-virus interaction
Core tip: The immune system plays a central role in coronavirus disease 2019 (COVID-19), being responsible for clinical manifestations and prognosis. Hyper-activation of the immune response against severe acute respiratory syndrome coronavirus 2 may result, in some cases, in development of unwanted autoimmune disorders. COVID-19 has been associated with immune-mediated systemic or organ-selective manifestations, some of which fulfill the diagnostic or classification criteria of specific autoimmune diseases. Though it is still unknown whether these medical conditions represent transitory post-infectious epiphenomena, the use of therapeutic agents targeting the immune system may perhaps prevent their chronicization which leads to development of autoimmune diseases.
INTRODUCTION
The coronavirus disease 2019 (COVID-19) pandemic, to date affecting more than 15 million people worldwide [World Health Organization (WHO) report, June 2020], has at its base the infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). SARS-CoV-2 belongs to the family of Coronaviridae, characterized by a positive single strand RNA genome embedded into a capsid and an envelope[1]. The envelope contains several structural (spike or S, membrane or M, envelope or E, nucleoprotein or N) and non-structural proteins. The S protein is crucial for virus entry into cells and confers the viral typical crown-shape as seen by electron microscopy. Several strains of CoV (229E, OC43, NL63 and HKU1) infect mammals and mostly induce mild respiratory symptoms[2]. Conversely, SARS-CoV-2 infection in humans has been associated with development of severe pneumonia and acute respiratory distress syndrome (ARDS). Since the first cases described in Wuhan in December 2019[3], the infection rapidly spread across continents and led to the WHO declaring the emergency status of this global pandemic on March 11, 2020. Distinctive symptoms of COVID-19 are cough, fever, dyspnea, myalgia and fatigue, but patients may also experience gastrointestinal manifestations, and multi-organ related complications[4]. The disease is characterized by high morbidity and mortality rates, with many infected individuals requiring hospitalization and intensive care. However, a high percentage (approximately 30%) of SARS-CoV-2 positive individuals remain asymptomatic, representing virus carriers who are unaware of their potential role in transmission in the population[5]. A Korean study showed that more than half of COVID-19 recovered patients, having a negative nasopharyngeal swab at discharge, may become SARS-CoV-2 positive at follow-up, though not complaining of any associated symptoms[5]. This underlines the possibility that in some individuals, the immune response may not confer a long-lasting protection against SARS-CoV-2 in the context of a humoral response although recent studies suggest that the cell-mediated response may be long lasting as seen with SARS for up to 17 years[6,7]. It may also be that the tests were detecting residual viral particles containing RNA genomes that may not have been completely cleared. Further, it should be clearly stated that a positive signal by real-time PCR only provides evidence of viral RNA and not of infectious virion particles.
Clinical manifestations and disease course of COVID-19 are extremely variable in different individuals, as it would depend on a delicate balance between SARS-CoV-2 virulence and host's characteristics. The viral cytopathic effects as well as intervention by the immune system response may, in fact, contribute, to some extent, to the degree of organ damage and inflammation. Importantly, the efficiency of the immune response is critical for control of SARS-CoV-2 infection; however, excessive inflammation has also been associated with severe COVID-19 outcomes. In support of this view, the use of immunomodulatory and immunosuppressive drugs, including steroids, anti-malarials and interleukin (IL)-6 inhibitors has showed some degree of efficacy and safety, especially in the management of the most aggressive cases[8].
Additionally, hyper-activation of the immune system, more pronounced in younger individuals[9], may ultimately trigger autoimmunity. In these cases, SARS-CoV-2 infected patients may have symptoms or laboratory findings that overlap with those commonly described in autoimmune diseases. It is well known that viruses may trigger or exacerbate autoimmunity in genetically predisposed individuals, through aberrant activation of immunologic pathways involving either the innate or the adaptive immune response[10,11]. Mechanisms include molecular mimicry between viral and self-epitopes, breakdown of tolerance, non-specific bystander activation, super-antigen presentation, stimulation of inflammasome platforms and release of type I interferon (IFN)[10,11]. Immune-mediated manifestations have been described in COVID-19 patients, and may occur during recovery or represent the first clue of infection in otherwise healthy individuals. Thanks to growing awareness among physicians who are treating COVID-19 patients, the number of reports describing atypical forms of COVID-19, involving skin, heart and skeletal muscles, blood cells, central and peripheral nervous system and vessels, has dramatically increased in the last months[12-18]. Similarly, an association between COVID-19 and thrombotic events, along with detection of anti-phospholipid (aPL) antibodies, has also been reported[19]. Whether these manifestations are transitory epiphenomena following viral infection or mirror the onset of a definite autoimmune disease is not understood.
Patients already affected by autoimmune rheumatic diseases have been reported to have similar COVID-19 incidence, morbidity and mortality rates to those estimated in the general population[20-22]. However, the real impact and long-term effect of SARS-CoV-2 infection on the immune system of these patients remain elusive. Rheumatic patients have an unbalanced immune response, partly due to the underlining disease and partly due to concomitant immunosuppressive therapies. Being pharmacologically immunosuppressed, they are more susceptible to external infections, which may trigger, in turn, the activation of immune pathways which results in autoimmune disease flares[10].
Given the complex immunologic scenario underlying SARS-CoV-2 infection, the aim of this opinion review is to summarize the evidence linking autoimmunity to COVID-19 and to provide an insight on the pathophysiologic mechanisms which may predispose infected people to autoimmunity.
THE IMMUNE RESPONSE AGAINST SARS-COV-2
SARS-CoV-2 and host interaction
SARS-CoV-2 spreads through aerial and, to a lesser extent, fecal-oral route[23-25]. It infects target cells[26] by the interaction of the S1 subunit of the S protein with the angiotensin-converting enzyme 2 (ACE2) expressed on plasma membrane[27]. The transmembrane serine protease 2 (TMPRSS2) is also of crucial importance in cleaving the viral S protein and facilitating the envelope fusion with the plasma membrane[28]. Animal models showed that ACE2 is broadly expressed in cells of the respiratory apparatus[29,30], including goblet and Clara cells, type I and type II pneumocytes, ciliated and non-ciliated cells, endothelial and smooth muscle cells. In humans, the expression of ACE2 follows a similar pattern, being also detectable in many other organs (gallbladder, heart muscle, kidney, epididymis, breast, ovary, prostate, pancreas)[31]. The expression of TMPRSS2 is even broader than that of ACE2 across different human tissues[32,33].
In nasal cavities, the mucosal secretory and ciliated cells are more susceptible to SARS-CoV-2 compared to other nasal cells, due to a major expression of ACE2[33]. SARS-CoV-2 may also attack olfactory cells placed at the top of the nasal cavities and reach, through this way, the central nervous system[34]. Notably, in a study conducted on 417 European patients suffering from COVID-19, anosmia and dysgeusia have been reported in more than 80% of individuals[35].
By infecting type II pneumocytes, SARS-CoV-2 may deplete the alveoli of surfactant leading to cavity collapse[36]. Furthermore, SARS-CoV-2 may invade lung stem cells, which reside at the bronco-alveolar junction and express ACE2[37]. Viral or immune-mediated cytolysis may deprive lungs of progenitors of Clara cells and pneumocytes and impair the lung regenerative properties.
In the gastrointestinal tract, ACE2 and TMPRSS2[25] are particularly abundant in the epithelial cells of colon and ileum[32]. According to one study, goblet, Paneth and enterochromaffin cells have undetectable expression of ACE2 in human gut[38]. ACE2 has instead been found in salivary glands and tongue[31,39], and SARS-CoV-2 infection of these cells may be responsible for gustatory dysfunction[35].
Both lung and intestine harbor a population of immune cells at the localized sites that represent the first-line defense against pathogens. Immune cells may aggregate to form proper lymphoid organs (Waldeyer's tonsil ring in the upper airways[40] and Peyer's patches in small bowel[41]) or be scattered in sub-epithelial connective tissue, interweaving an intricate network with epithelial cells, vessels and fibroblasts.
Mucosal immunity is of fundamental importance in counteracting the entry of pathogens into the body and acts, therefore, during the very first phase of infections. In addition, mucosal immunity may also contribute to controlling the Phyla composition of resident microbiota, which, in turn, may be influenced by external agents, including SARS-CoV-2[42], or also influence the interaction with these pathogenic agents.
The role of innate immunity in SARS-CoV-2 infection
The innate immune system is highly represented in both respiratory and intestinal mucosa and continuously scavenges and counteracts potentially dangerous stimuli through a complex interplay of cells and soluble mediators[43]. These include resident macrophages and monocytes, natural killer (NK) cells, innate lymphoid cells, polymorphonuclear and dendritic cells, cytokines, chemokines, and the complement system. Intracellular agents, like viruses, are usually cleared through direct phagocytosis and cytolysis of infected cells. In cases where pathogen clearance is impaired, this can lead to persistence of antigenic stimulation, and to a shift in the immune response from innate to adaptive immunity. The latter is characterized by higher rapidity and robustness, due to antigen selectivity and memory[44].
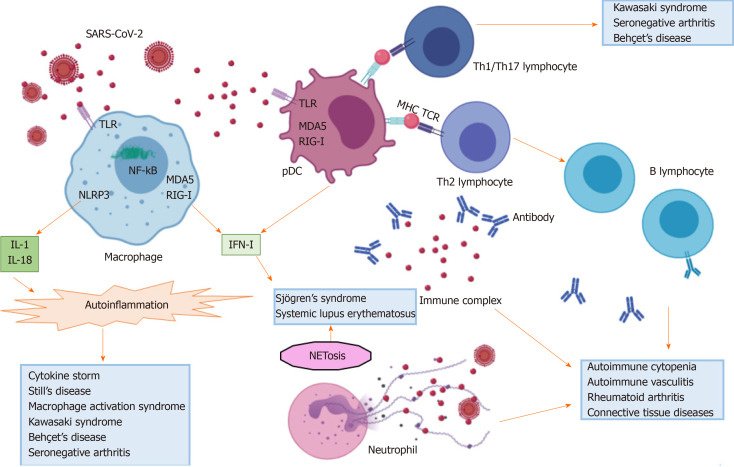
The interferon pathway: Cells belonging to the monocyte-macrophage system are able to directly recognize viral motifs through pattern recognition receptors (PRR), including the endosomal toll-like receptors (TLR), the cytosolic platforms retinoic-acid inducible gene I (RIG-I), the nucleotide oligomerization domain-like receptors (NLR) and melanoma differentiation-associated protein (MDA)5, and to set an antiviral response, mainly explicated through the production of IFN[45]. Three different types of IFN have been characterized so far: type I IFN comprises IFNα and IFNβ, type II IFNγ and type III IFNλ, all of which act via a Janus kinase-signal transducer and activator of transcription proteins (JAK-STAT) signaling pathway[46]. Plasmacytoid dendritic cells (pDC) are the main source of type I and II IFN, while type III IFN are mostly released by epithelial cells at barrier interfaces of the respiratory and gastrointestinal tracts[45]. The antiviral property of IFN is explicated through transcription of IFN-stimulated genes (ISG), able, in turn, to potentially prevent each step of viral lifecycle[47]. Viruses have evolutionary developed many escape mechanisms against the IFN antiviral pathway. In this regard, it has been shown that SARS-CoV-2 open reading frame (ORF)6 can impede the transcription of some ISG[27]. Notably, the hyper-production of IFN (mainly type I) dominates the pathogenesis of some autoimmune diseases, like systemic lupus erythematosus (SLE) and primary Sjögren's syndrome (SS)[48], and may link the etiology of these diseases to a primitive viral infection[49,50].
The inflammasome pathway: In addition to IFN response, viral proteins and nucleic acids can activate the inflammasome platforms in cells belonging to the monocyte-macrophage system with the following production of IL-1 and IL-18[51,52], having a systemic pro-inflammatory effect. Specifically, viral pathogen-associated molecular patterns (PAMPs) of RNA viruses, including SARS-CoV-2, can activate the NOD-like receptor family pyrin domain (PYD)-containing 3 (NLRP3), while their RNA fragments can sensitize RIG-I and MDA5 and activate mitochondrial antiviral signaling (MAV) platforms in the cytosol. These events culminate in the intra-nuclear translocation of nuclear factor kB (NF-kB), the transcription of genes coding for pro-IL-1β and pro-IL-18 and the final conversion of these precursors into active cytokines by means of inflammasome-activated caspases[52]. Inflammasome platforms dominate the pathogenesis of auto-inflammatory syndromes, a group of genetically induced rheumatic diseases characterized by recurrent episodes of fever and other systemic manifestations, often triggered by external infections[53]. Recent evidence links autoinflammation to other multifactorial rheumatic diseases, including Behçet's syndrome, seronegative arthritis and Still's disease[54-56].
HMGB-1 and viral clearance: Viral nucleic acids can further complex with high mobility group box 1 protein (HMGB-1) released by necrotic cells and activated immune cells, and bind the receptor for advanced glycation end-products (RAGE), TLR2, TLR4 and TLR9 in either monocyte-macrophage cells or lymphocytes. This cascade of events culminates in lysosomal membrane disruption, pyroptosis, cytokine release and activation of autoreactive T and B cells[10,57,58]. Furthermore, the release of HMGB-1 in lungs can trigger acute inflammation through the secretion of pro-inflammatory cytokines and the recruitment of neutrophils in the interstitial and alveolar space[59]. The increase in HMGB-1 inversely correlates with ACE2, and drugs targeting HMGB-1 may be promising candidates in treating pulmonary manifestations of COVID-19[58].
Neutrophils and viral clearance: A recent study enrolling 8 COVID-19 patients, 146 community-acquired pneumonia patients and 20 controls evidenced a distinct metatranscriptomic profile in the bronchoalveolar lavage fluid (BALF) of COVID-19 patients[60]. Specifically, neutrophils were the most abundant cells and the chemokines CXCL17, CXCL2, CXCL8, CCL2 and CCL7, attracting neutrophils and monocytes, and systemic cytokines (IL-1β and type I IFN) were also hyper-expressed. Remarkably, the authors described a positive correlation between the inflammatory burden and the viral load, both of which tended to attenuate in the late stages of the disease and to persist in fatal cases. Neutrophils are a source of transforming growth factor (TGF)β, whose secretion in lung has been associated with interstitial fibrosis[61]. Moreover, neutrophils are typically increased in peripheral blood samples of COVID-19 patients and may contribute to systemic inflammation and thrombosis through the process of neutrophil extracellular trap (NET)osis[62]. The process of NETosis also plays a crucial role in the pathogenesis of some autoimmune diseases, like SLE and anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis[63,64]. Noteworthy, both NETosis and inflammasome activation are related to an impaired removal of reactive oxygen species (ROS), which may arise in lung during COVID-19, following the unbalanced ACE1/ACE2 ratio[65]. It was shown that type II pneumocytes of the elderly have a noted down-regulation in expression of superoxide dismutase (SOD)3[66], and the inadequate clearance of ROS may be at the basis of the severe forms of COVID-19 pneumonia observed in older when compared to younger individuals. Recent studies showed that sera of COVID-19 individuals may trigger NETosis in vitro, and that NETs may be considered to be a marker of a more severe course of disease[67,68].
The complement system and viral clearance: The complement system takes part in the antiviral response through inactivation of virions, the recruitment of immune cells, viral opsonization, cytolysis, and the induction of B and T cell responses[69]. Its activation may follow classical, alternative and mannose-binding protein pathways[70]. The glycoproteins S of SARS-CoV can interact with mannose binding lectin and trigger the complement cascade[69], inducing a direct (cytolysis of infected cells) or indirect (immune complex-mediated) viral clearance. However, the persistent activation of the complement system may lead to detrimental consequences, like organ fibrosis and musculoskeletal inflammation[69]. In SLE, the complement system cascade, triggered by autoantibodies and immune complexes via the classical pathway, is typically hyper-activated and consumption of its components represents a hallmark of the disease[71]. Hypo-complementemia has been associated with SLE disease activity and specific organ involvements, like glomerulonephritis[72]. Some viruses, like Parvovirus B19, notoriously associated to SLE[73], may affect the function of the complement system and indirectly influence the clinical course of autoimmune diseases[73,74]. Data concerning the interplay between SARS-CoV-2 and the complement system are controversial. According to one study, SARS-CoV-2 seems not to affect the serum levels of C3 and C4[75]. However, it was recently reported that the intravenous (i.v.) administration of the C3-inhibitor AMY-101 for 14 consecutive days ameliorated the laboratory and clinical picture of a 71-year-old patient affected by COVID-19 ARDS[76].
The role of adaptive immunity in SARS-CoV-2 infection
An efficient adaptive immune response is a basic requirement for a definitive viral clearance, as well as for the prevention of re-infection. According to the antigenic nature (intracellular vs extracellular), the adaptive immune response can follow a cellular or humoral pathway, with T and B lymphocytes being protagonists of the two scenarios, respectively.
T cell immunity: Dendritic cells act as connectors between the innate and adaptive immune system, since they may directly set an unselective pro-inflammatory response after interaction with pathogens or behave as antigen presenting cells (APC) and, thus, clonally activating lymphocytes. Proteins of SARS-CoV-2 envelope, like S, E and M, contain several epitopes which, once recognized by dendritic cells, can trigger an adaptive immune response[27]. Epitopes derived from the digestion of intracellular antigens are usually presented by the major histocompatibility complex (MHC) class I to CD8+ T lymphocytes, which ultimately act by means of a direct cytotoxic mechanism. Additionally, CD4+ T helper (h) cells may also be activated following the stimulation of their T cell receptors (TCR) by epitopes presented by MHC class II. According to the cytokine milieu, Th cells further differentiate in Th1, Th2, Th17, Th22, Th9, Th follicular (f) or T regulatory (reg) lymphocytes, giving rise to different immunologic cascades[77].
Peripheral lymphopenia is typically associated with COVID-19 and represents a predictive biomarker of morbidity and mortality[4]. SARS-CoV-2 may spread into lymphocytes through the interaction with CD147 molecule[78]. In T lymphocytes, CD147 seems to preside over the reorganization of lipid rafts and inhibits TCR-mediated proliferation[79]. The engagement of the SARS-CoV-2 S protein to CD147 expressed on T lymphocytes may result either in lymphopenia or in an impairment of T cell physiologic functions. Noteworthy, CD147 seems to be hyper-expressed in CD3+ T lymphocytes of SLE individuals compared to healthy controls, predisposing these patients to SARS-CoV-2 cytopathic effect on white blood cells[80]. Although the role of SARS-CoV-2 in human secondary and tertiary lymphoid organs is unclear, it has been shown that a virulent strain of bovine CoV may induce depletion of T cells in calf Peyer's patches and mesenteric lymph nodes[81]. In SARS-CoV-2 infected people, lymphopenia especially affects T and NK lymphocyte populations and memory cells[27]. In addition, CD8+ T and NK lymphocytes display an exhausted phenotype[82]. Among CD4+ T lymphocyte subsets, an unbalance in the Th17/Treg ratio has also been described in sick patients[83]. The increase in serum IL-17 and IFNγ, as well as the detection of multinucleated giant cells at the histological examination of lung specimens of COVID-19 patients, suggest the activation of Th1 and Th17 responses[27]. The expansion of autoreactive Th1 and Th17 lymphocytes at the detriment of Treg cells also lies at the basis of many autoimmune diseases[84,85].
B cell immunity: Th2 lymphocytes cooperate with B cells in supporting the final maturation in plasma cells and the secretion of specific antiviral antibodies, which may belong to different isotypic classes (IgM, IgG, IgA). Additionally, follicular dendritic cells can also stimulate B cells in a MHC-independent manner, by inducing the cross-linking of their B cell receptors (BCR) in response to multimerized antigens[86]. Although a depletion in CD19+ cells has been reported[27,87], COVID-19 patients are usually able to mount a humoral response and synthesize serum antibodies against SARS-CoV-2 antigens[88,89].
According to a study on 173 COVID-19 patients, more than 90% of them developed anti-SARS-CoV-2 IgM and approximately 79% patients had detectable IgG 15 days from the onset of the disease[89]. Notably, humoral immunity was not always paralleled by viral clearance, suggesting that other immunologic mechanisms may be additionally needed to effectively counteract the infection. Moreover, the authors found a significant positive correlation between higher antibody titer measured after 2 weeks of disease and clinical severity. This unusual association may rely on the activation of the complement system by immune complexes, the stimulation of immune cells (especially monocytes and macrophages) through the interaction of the fragment crystallizable (Fc) of antibodies with Fc receptors (FcR)[90,91] or antibody-dependent enhancement of viral entry[92]. FcR may be blocked by treating patients with i.v. human polyclonal Ig (IVIG)[93]: Of interest, this therapeutic approach proved to be effective in several reported cases of SARS-CoV-2 pneumonia, especially when started early[94,95]. IVIG play an immunomodulatory role, being therefore indicated for the treatment of autoimmune diseases[96]. IVIG, in fact, counteract the expansion and the activation of T, B and dendritic cells, interrupt the TLR signaling cascade and rebalance cytokine milieu[97], restoring immune tolerance. Many of these effects are mediated by the interaction of polyclonal IgG with FcγRIIb expressed on the plasma membrane of cells belonging to the monocyte-macrophage line[98], which is known for imparting an inhibitory effect on either the innate or adaptive immune response[99]. A polyclonal hyper-gammaglobulinemia is often found in patients affected by autoimmune diseases sustained by an aberrant humoral response, including rheumatoid arthritis (RA) and SLE[100]. Similarly to IVIG, polyclonal antibodies may exert an immunomodulatory role in infected rheumatic patients[101]. Natural antibodies with different specificities for pathogen conserved patterns are able to rapidly neutralize microorganisms and maintain B cell memory reservoir[102]. It is however unclear whether up-regulation of the humoral response may protect rheumatic patients from complications due to SARS-CoV-2 infection.
Despite the controversial findings regarding humoral immunity, the transfusion of sick patients with anti-SARS-CoV-2 neutralizing antibodies of already recovered individuals was shown to be an effective and safe therapeutic option[103,104]. Doubts still remain concerning optimal dosages and timing, donor recruitment and Ig screening assays[105].
The cytokine storm
Both the innate and acquired immune system can contribute in generating a cytokine storm syndrome or hemophagocytic lymphohistiocytosis, a potentially fatal condition triggered by the uncontrolled release of many pro-inflammatory mediators. This clinical condition can have a genetic etiology or follow infectious, autoimmune or cancer disorders. In rheumatic patients, a cytokine storm syndrome, also known as secondary macrophage activation syndrome (MAS), is more common during the pediatric age. MAS sub-clinically occurs in 30%-40% of patients suffering from juvenile idiopathic arthritis (JIA), and may have a fatal course in 10% of cases[106]. Minor prevalence and incidence rates have been reported in SLE (0.9%-9% of cases), Kawasaki syndrome (1%-2% of cases)[107,108] and adult Still's disease (10% of cases)[106]. In rheumatic patients, MAS can be triggered in 1/3 of cases by external infections exacerbating a pre-existent inflammatory background. The subsequent generation of PAMPs or damage-associated molecular patterns (DAMPs) may stimulate macrophages, T lymphocytes and inflammasome platforms, with the final transition of macrophages into histiocytes responsible for hemophagocytosis, tissue repair and fibrosis.
MAS symptoms include high fever, lymphadenopathy, hepato-splenomegaly, hyper-coagulation, visceral hemorrhages and multiorgan impairment (especially of liver, central nervous system and kidney). This medical condition also occurs in approximately 3%-4% of septicemic individuals[109]. Polymorphic variants of the PRR, cytokine gene expression kinetics, virus characteristics and an unbalance between pro-inflammatory and anti-inflammatory mediators have been all considered risk factors[110].
In COVID-19, the development of a cytokine storm syndrome has been reported to occur after an interval of 2-3 weeks since the onset of the disease and to be associated with multiorgan failure, ARDS, disseminated intravascular coagulation (DIC)[111] and death[27]. Released cytokines include IL-1β, IL-6, IL-18, IL-33, IFNγ and tumor necrosis factor–α (TNF-α)[112], which have both a local and systemic effect and are secreted by a panel of activated immune and non-immune cells (B and T lymphocytes, NK cells, macrophages, dendritic cells, neutrophils, monocytes, epithelial and endothelial cells). COVID-19 patients with augmented level of serum ferritin, TNF-α and IL-6 and lymphopenia have been considered to be at higher risk of developing high-grade systemic inflammation and MAS[87].
In lung parenchyma, cytokine storm syndrome eventually results in interstitial fibrosis, promoted by the hyper-activation of macrophages and lymphocytes. This event occurs in the most severe cases of COVID-19 and is associated with high morbidity and mortality[113]. Of note, lung fibrosis is characterized by distinctive histological patterns found in several rheumatic diseases, including systemic sclerosis (SSc) and RA[114]. Similarly to what happens in rheumatic lung fibrotic disease[115], the aberrant secretion of TGFβ by macrophages and lymphocytes plays a central role in extracellular matrix remodeling during COVID-19[61]. Finally, hyper-activation of cells belonging to the monocyte-macrophage system is also at the basis of the aberrant release of tissue factor (TF)[116], which activates the coagulation cascade through the extrinsic pathway and may trigger DIC.
SARS-COV-2-INDUCED AUTOIMMUNE DISORDERS
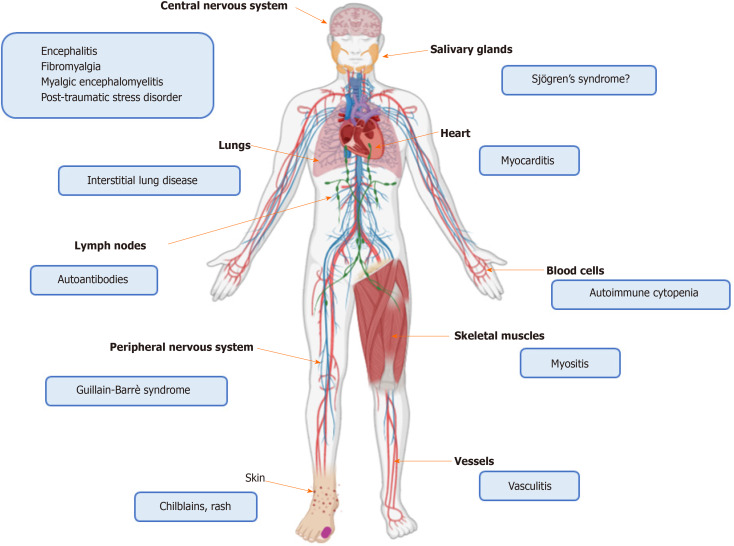
On the basis of the aforementioned evidence, SARS-CoV-2 infection seems to profoundly impact the immune system of the host. The balance seems shifted towards the potentiation of innate immune pathways at the disadvantage of the adaptive immune response. In the next subparagraphs we review this complex interplay from a clinical viewpoint, reporting data on autoimmune disorders developed during COVID-19, as well as the on the COVID-19 course described in rheumatic patients. Figures 1 and 2 depict the role of SARS-CoV-2 in triggering autoinflammation and autoimmunity, and the potentially related organ manifestations.
Figure 1.
Immunologic pathways activated by severe acute respiratory syndrome coronavirus 2. SARS-CoV2: Severe acute respiratory syndrome coronavirus 2; TLR: Toll-like receptor; IL: Interleukin; NLRP3: Nucleotide oligomerization domain-like receptor family pyrin domain (PYD)-containing 3; NF-kB: Nuclear factor-kB; RIG-I: Retinoic-acid inducible gene I; MDA5: Melanoma differentiation-associated protein 5; IFN-I: Type I interferon; pDC: Plasmacytoid dendritic cell; MHC: Major histocompatibility complex; TCR: T cell receptor; Th: T helper; NET: Neutrophil extracellular trap.
Figure 2.
Organ localization of immune-mediated disorders related to severe acute respiratory syndrome coronavirus 2 infection.
Autoantibodies
APL antibodies, including anti-cardiolipin and anti-β2-glycoprotein Ig, have been detected in the sera of COVID-19 patients, but their contribution to thrombosis is uncertain[117,118]. Although benign aPL may appear during infections as a transient epiphenomenon[119], Zhang et al[19] reported 3 cases of ischemia of upper and lower limbs and cerebral infarcts in COVID-19 patients associated with positivity of anti-cardiolipin and anti-β2-glycoprotein IgA and IgG, that may orient towards an anti-phospholipid syndrome (APS). Contrary to anti-cardiolipin antibodies, anti-β2-glycoprotein antibodies are classically regarded as thrombogenic and represent a distinctive tract of APS. These antibodies may also appear during bacterial infections[120], but in these cases they display different biomolecular properties, being not able to elicit the coagulation cascade. However, since infections may trigger APS de novo[121] and even complicate a pre-existing APS with a catastrophic syndrome[120], follow-up is important to ensure whether such immunologic response might spontaneously recover or rather result into full-blown autoimmunity. Other authors have reported the presence of anti-52 kDa and anti-60 kDa Ro-SSA antibodies, antinuclear antibodies (ANA) and anti-citrullinated protein antibodies (ACPA) in the serum of 20%, 25%, 50% and 20% of COVID-19 patients, respectively[122,123]. Nevertheless, their pathogenicity is unknown, as well as the likelihood to induce autoimmune disorders in the long term. It is well-known that autoantibodies may be detectable in the serum of patients many years before the onset of autoimmune diseases[124,125], but it should not be ignored that autoantibody seropositivity may also be found in healthy individuals[126,127].
Immune-mediated neuropathy
Neurological manifestations, involving central and peripheral nervous system have been reported in up to 36% of COVID-19 ARDS cases[128]. Brain neurons express ACE2[129] and can be infected by SARS-CoV-2 spreading via the olfactory cells and bulbs[35] and crossing the cribriform plate of the ethmoid bone[34]. Accordingly, neuronal damage may derive from direct viral cytotoxicity or from an immune-mediated attack[130]. Immune cells may recognize viral antigens expressed in infected cells, as well as cross-reactive self-antigens, like gangliosides. Neuronal inflammation may further generate DAMPs and autoantigens, thus amplifying the inflammatory cascade and predisposing to autoimmunity[131]. This pathway is likely exploited by several viral agents that contribute to the onset of autoimmune diseases, like multiple sclerosis or autoimmune peripheral neuropathy[132-135].
Pilotto et al[136] reported the case of a 60-year-old man presenting with COVID-19 neurologic symptoms. Nasopharyngeal swab was positive for SARS-CoV-2 and an increase in serum D-dimer was found along with a bilateral interstitial pneumonia. Cerebrospinal fluid was negative for SARS-CoV-2 and showed lymphocytosis and an increase in IL-6, IL-8, TNF-α and β2-microglobulin. Despite a treatment with antiviral agents, antibiotics and anti-malarials, the patient recovered only after the administration of i.v. pulses of steroids. Altogether, these findings suggest that neuronal damage might have been secondary to the immune response rather than to a viral cytopathic effect.
Guillain-Barrè[135] syndrome consists of an acute flaccid paralysis, symmetrically affecting upper and lower limbs. This medical condition develops after a respiratory or gastrointestinal infection in 2/3 of the cases and pathogenesis is immune-mediated. Specifically, the most accredited mechanism is the formation of cross-reacting anti-ganglioside antibodies that can induce a demyelinating polyneuropathy or a motor axonal neuropathy[135]. Cases of Guillain-Barrè syndrome with an unpredictable course have been reported prior or following the classical symptoms of SARS-CoV-2 infection[12,137]. Lymphopenia, thrombocytopenia and anamnesis may orient towards COVID-19 in those cases in which Guillain-Barrè syndrome is the primitive manifestation.
A recent meta-analysis, focusing on the neuropsychiatric manifestations occurring in patients undergoing severe coronavirus infections, showed that post-traumatic stress disorder, depression or anxiety, memory impairment, fatigue, insomnia and sleep disturbances were frequent in the post-illness stage (follow-up 60 days to 12 years) of SARS and Middle East respiratory syndrome (MERS)[138]. These symptoms also characterize two rheumatic dysfunctional diseases, namely fibromyalgia (FM) and myalgic encephalomyelitis[139]. These two clinical conditions share many similarities and may coexist; however, contrary to FM, myalgic encephalomyelitis has often been associated with previous infections and may be the result of an autoimmune process[140]. Although the development of an algodysfunctional syndrome may be plausible in COVID-19 recovered patients, epidemiologic data are still lacking.
Immune-mediated blood disorders
A number of papers described the occurrence of immune-mediated blood cytopenia in COVID-19 individuals. Lazarian et al[13] reported 7 cases of autoimmune hemolytic anemia sustained by both warm and cold IgG. This condition occurred after a mean of 9 days in middle-aged patients affected by COVID-19 pneumonia. Of note, the majority of patients had coexistent lympho-proliferative disorders. Another two cases of hemolytic anemia sustained by cold agglutinins during COVID-19 have been described in the literature. The first patient was a 46-year-old woman, with a past history of immune thrombocytopenic purpura and splenectomy and testing negative for autoantibodies, who developed a rapidly fatal course of the disease[141]. The second patient was a 62-year-old oncologic man, who was hospitalized due to SARS-CoV-2-related fever, asthenia, respiratory symptoms and a mild hemolytic anemia precipitating in the following two weeks of follow-up[142].
Other authors reported a case of thrombocytopenic purpura occurring in a 65-year-old woman eight days after the onset of COVID-19 symptoms[14]. Anti-platelet antibodies were not detected and the bone marrow aspiration showed a normal cellularity.
Evans syndrome, consisting of the combination of autoimmune hemolytic anemia and thrombocytopenia and often following viral infections, has also been reported in a patient affected by COVID-19[143]. In this case, thrombocytopenia preceded hemolytic anemia and was already present at admission along with fever and respiratory symptoms. Given the current recommendations discouraging the use of steroids in COVID-19 ill patients[144,145], these hematologic complications were successfully managed with IVIG.
Immune-mediated myositis and myocarditis
The contribution of viral agents to the pathogenesis of autoimmune myositis has been so far postulated and supported by the hyper-expression of MHC class I and the presence of CD8+ T lymphocyte foci at the histological examination of muscle biopsies of patients affected by polymyositis and necrotizing myopathy[146]. An interesting study identified three SARS-CoV-2 highly specific immunogenic epitopes in the antibody epitope repertoire of 20 adult individuals affected by dermatomyositis[147]. Epitopes mapped to SARS-CoV-2 2'-O-ribose methyltransferase, 3'-to-5' exonuclease proteins and RNA-dependent RNA polymerase, the latter being associated with HLA-A*01:01-restricted CD8+ T cell expansion.
To date, only one case of COVID-19-related myositis has been reported[15]. Myositis appeared as the first clinical manifestation and was followed after 4 days by fever and respiratory distress. The patient complained of hyposthenia of the muscles of the pelvic girdle, with clinical, laboratory and imaging findings being undistinguishable from those commonly found in autoimmune myositis. SARS-CoV-2 was detected in BAL but not in nasopharyngeal swab. Additionally, the patient was lymphopenic while autoantibodies were absent. Notably, respiratory symptoms and bilateral lower lobe ground-glass opacities at lung computer tomography (CT) scan are present in up to 40% of autoimmune myositis cases[148], making differential diagnosis with COVID-19 challenging in case of negative nasopharyngeal swab.
A few cases of COVID-19-associated myocarditis have also been reported. Myocarditis, consisting of the inflammation of myocardium, is a severe and potentially fatal complication of many autoimmune diseases, including acute rheumatic fever, RA, sarcoidosis, vasculitis and connective tissue diseases[149-151]. Additionally, myocarditis may be the result of viral infections, mostly sustained by Coxsackievirus B and adenovirus[150]. Myocarditis may manifest as acute coronary syndrome, arrhythmia, sudden death or heart failure. Viruses proliferating inside myocardiocytes can induce a direct tissue injury or trigger an immune response and inflammation, followed by myocardium remodeling and fibrosis. These events subsequently lead to systolic and diastolic dysfunction[149]. As endothelial and myocardial cells express ACE2, SARS-CoV-2 may directly invade these cells and hamper the beneficial effect played by ACE2 in counteracting heart failure[152]. In addition, the balance between IL-17 and IFNγ is crucial in dictating the final course (acute vs chronic damage) of myocarditis[153].
An acute COVID-19-associated eosinophilic myocarditis was described in a 17-year-old male patient without any prior risk factors[16]. Myocarditis appeared 2 days after the onset of a clinical picture characterized by gastrointestinal symptoms, headache and dizziness. SARS-CoV-2 was isolated in the nasopharyngeal swab. Eosinophilic myocarditis has been associated with drug and allergen hypersensitivity, ANCA vasculitis[149,151], and less frequently with viral infections[153].
Another case of COVID-19 myocarditis was described in a 57-year-old male subject, affected by arterial hypertension[154]. The patient developed ARDS and acute myocardial failure, characterized by an increase in serum troponin I and N-terminal pro-B-type natriuretic peptide (NT-proBNP), tachycardia at electrocardiogram, diffuse hypo-kinesis without pericardial effusion at transthoracic echocardiogram, and myocardial edema at cardiac magnetic resonance imaging (MRI). A combinatory therapeutic strategy based on the use of corticosteroids, tocilizumab and the experimental compound aldose reductase inhibitor was effective in inducing myocardial recovery, underlining that heart damage was mostly immune-mediated.
Immune-mediated dermatologic manifestations
Patients with suspected or confirmed COVID-19 have been reported to suffer from polyhedral cutaneous manifestations[155]. Among them, chilblains have been described in several case series[17,156]. Chilblains are localized red or purple cutaneous lesions due to an inflammatory reaction triggered by the exposure to cold temperatures[157]. These manifestations can be found in SLE and other connective tissue diseases or vasculitis[158,159], and be related to an excessive production of type I IFN[160]. Autoimmune chilblains mostly occur in fertile or middle-aged woman, are associated with typical autoantibody patterns and tend to chronically persist, being less influenced by variations in external temperature[161]. On the contrary, COVID-19 chilblains were mostly reported in pediatric male patients approximately 2 weeks from disease onset. The lesions were mainly localized in the lower extremities of the toes or feet and rapidly disappeared following the recovery of the underlining infectious disease. Tests for SARS-CoV-2 at the oropharyngeal swab were positive in a few cases[17,156]. Given the chronologic interval, it may be argued that skin lesions following COVID-19 might be the result of the antiviral response characterized by the hyper-production of IFN.
Vasculitis and pediatric inflammatory multisystem syndrome
Kawasaki syndrome is an autoimmune necrotizing vasculitis of medium- and small-sized vessels, affecting coronary arteries in more than 20% of cases[162]. The disease occurs in pediatric patients and classically starts with fever, mucocutaneous manifestations and cervical lymphadenopathy. Thus, external infections of the upper and lower respiratory tract, especially sustained by RNA viruses[163], have been suspected as triggering factors. This hypothesis is supported by the detection of CD8+ T aggregates and oligoclonal IgA in coronaries, along with the hyper-expression of ISG, and the presence of cytosolic inclusion bodies in ciliated bronchial epithelium[163].
An increased incidence of Kawasaki vasculitis-like syndrome has been reported in Bergamo province, which has been one of the most affected territories by SARS-CoV-2 infection in Italy. Here, Verdoni et al[164] described 10 cases of Kawasaki-like disease developing in children (mean age 7.5 years) between February 2020 and April 2020. Five of them had a classical syndrome with mucocutaneous manifestations and lymphadenopathy, while the others had incomplete forms. Compared to Kawasaki cases attending their medical Institute prior to the COVID-19 pandemic, the authors found a higher incidence of cardiac involvement, MAS and shock syndrome requiring adjunctive steroidal treatment. Additional cases of Kawasaki vasculitis, mainly occurring as incomplete forms, have been reported worldwide in young COVID-19 patients[18,165-167]. It is still debated whether this clinical condition represents a true Kawasaki vasculitis or rather reflects nonspecific post-infectious immune-mediated manifestations. Notably, in these patients, classical symptoms of COVID-19, including cough and dyspnea, appeared less frequent, while other manifestations like gastrointestinal symptoms[18,168] or cardiac hypotension were prevalent[165,166]. Heart involvement mostly consisted of pericardial effusion and myocarditis rather than coronary vasculitis[168]. Besides the detection of SARS-CoV-2 in nasopharyngeal swab, some children were found positive for group A Streptococcus[165,166] at the nasopha-ryngeal rapid test. Group A Streptococcus is responsible for rheumatic fever and pancarditis through a molecular mimicry mechanism[169] due to structural homologies between epitopes of the streptococcal N-acetyl-beta-D-glucosamine and M protein and heart valve endothelium, basement membrane and cardiac myosin, respectively[170]. Thus, a synergistic effect played by streptococci and SARS-CoV-2 in setting an immune response eventually responsible for this incomplete Kawasaki-like syndrome may be hypothesized.
So far, in United Kingdom, the term "pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2" (PIMS-TS) was coined in order to distinguish this clinical condition from other apparently overlapping diseases[171]. PIMS, also known as multisystem inflammatory syndrome in children (MIS-C), is characterized by a variegate spectrum of clinical manifestations that may include incomplete forms of Kawasaki vasculitis, MAS, myocarditis and toxic shock. Epidemiological data showed that PIMS-TS usually follows COVID-19 symptoms after a delay of at least two weeks; is more common among Afro-Caribbean descendants; manifests with a more frequent cardiac, gastrointestinal and hematological involvement compared to Kawasaki syndrome; and, finally, appears more refractory to IVIG treatment than classical vasculitis, often requiring the addition of glucocorticoids or IL-6 inhibitors[172]. Belhadjer et al[173] recently reported a case series of 35 pediatric patients admitted to French and Swiss hospitals with fever, cardiogenic shock or signs of left ventricular dysfunction. SARS-CoV-2 infection was confirmed in 88.5% of them, prevalently by anti-SARS-CoV-2 IgG serodiagnosis. Interestingly, COVID-19 manifested with atypical symptoms, like gastrointestinal disorders and meningeal irritation, and, despite the clinical severity at admission, most of patients rapidly recovered with i.v. inotropic agents, steroids, anticoagulants and IVIG, whilst 3 patients needed a treatment with anakinra. From March 1 to May 17 2020, a total of 156 PIMS diagnoses were notified to the French National surveillance[174]. Among them, 79 were clearly associated with SARS-CoV-2 infection and were characterized by a high prevalence of incomplete forms of Kawasaki disease, myocarditis and MAS, requiring critical care intervention in 67% of cases. Similarly, a retrospective study in United Kingdom collected clinical data of 58 cases of PIMS-TS, following an atypical presentation of COVID-19[175]. Noteworthy, only 13 cases fulfilled the classification criteria of the American Heart Association for Kawasaki disease[176], whilst a hyper-inflammatory status leading to cardiogenic shock in 29 patients was reported. These results are in line with those reported by larger retrospective United States cohort analyses including 186 and 191 juvenile/pediatric cases of MIS-C, respectively[177,178]. Gastrointestinal symptoms were the most commonly reported followed by cardiovascular, hematological, mucocutaneous, ocular and pulmonary manifestations.
THE IMPACT OF COVID-19 ON PRE-EXISTING AUTOIMMUNE DISEASES
Surprisingly, patients already affected by rheumatic diseases and pharmacologically immunosuppressed were reported to have similar incidence and prevalence rates of COVID-19 compared to general population[179,180]. A similar trend has also been observed concerning COVID-19 morbidity and mortality rates, even in those subjects affected by autoimmune diseases, like RA and SSc, having an intrinsic risk of lung interstitial disease and fibrosis[181]. Additionally, the worsening of pre-existing rheumatic symptoms has not been described. This may be due, on the one hand, to the hyper-activation of the anti-viral pathways in subjects with autoimmune diseases, and, on the other hand, to the concomitant therapeutic background able to counteract the excessive activation of the immune system.
Even though nasopharyngeal swabs were not offered to all the rheumatic patients, a very low incidence of COVID-19 was reported in two independent Italian cohorts undergoing treatment with biologic or synthetic disease modifying anti-rheumatic drugs (DMARDs)[21,22]. Preliminary data from the COVID-19 Global Rheumatology Alliance provider registries, gathered from a cohort of 110 infected individuals suffering from rheumatic diseases, showed that 35% of them were admitted to hospital due to SARS-CoV-2 infection and 5% died[182]. Patients were mostly affected by RA and treated with conventional, biologic or synthetic DMARDs with or without glucocorticoids. Twenty per cent of them suffered from lung disease. Symptoms reported did not differ from those described in the general population.
An observational analysis on 123 connective tissue disease patients treated with either conventional or biologic drugs showed only one fatal case of COVID-19[20]. The patient was a 32-year-old SSc female receiving hydroxychloroquine and rituximab for a pre-existent lung involvement and developing a severe interstitial pneumonia following SARS-CoV-2 infection. Other 14 patients reported mild symptoms consistent with COVID-19 but they had no access to nasopharyngeal swab and did not require the discontinuation of the anti-rheumatic treatment. Overall prevalence of confirmed COVID-19 in this cohort was in line with that reported in general population from this geographic area.
Risk factors for a more severe disease in rheumatic patients appear to be the same as those reported for the general population and include male gender, older age, cardiovascular disease, diabetes mellitus, obesity, lymphopenia, and increased serum level of IL-6, C-reactive protein (CRP) and lactate dehydrogenase (LDH)[183].
Although real-life data do not show evidence of an increased risk of COVID-19 severe complications in rheumatic cohorts and the use of immunosuppressive drugs may even protect from SARS-CoV-2-induced hyper-inflammation, caution should be used in the therapeutic management of these patients. In this regard, the American College of Rheumatology (ACR) recently proposed a panel of conditional therapeutic recommendations in order to guide physicians' decisions in treating rheumatic patients with ascertained COVID-19. In these cases, the discontinuation of conventional, synthetic, and biologic DMARDs and immunosuppressive drugs is advised, while non-steroidal anti-inflammatory drugs (NSAIDs), low doses of glucocorticoids, anti-malarials and anti-IL-6 inhibitors may be maintained[183]. Anti-malarials prevent the disruption of the lysosomal membrane induced by the internalization of viral RNA and HMGB-1 (45), and appeared useful in limiting both the viral and immune-mediated organ damage. Similarly, IL-6- and IL-1β-inhibitors, IVIG and glucocorticoids[94,136,184,185] have been successfully used in controlling symptoms related to the hyper-activation of the immune response against SARS-CoV-2 infection and may perhaps prevent the precipitation of COVID-19 towards MAS and organ failure.
DISCUSSION
Since the description of the first COVID-19 cases, classically dealing with fever, cough and dyspnea, other manifestations spilling over the rheumatologic field have been reported, Table 1. These include cutaneous rashes and vasculitis[17,156,164], hematologic disorders[13,14], neuropathy[12,136,137], myositis and myocarditis[15,16,154], along with the positivity of autoantibodies. Immune-mediated manifestations seem more frequent in younger patients, while virus-related organ failure is more common among adults. This may be due to several reasons, including a reduced expression of ACE2 and the attenuation of the immune response in elderly people[9]. However, it is also noticed that COVID-19 prognosis is worse in patients developing an extremely high inflammatory response. While a male gender predilection was observed in the most severe COVID-19 cases[83], the influence of gender in SARS-CoV-2 related immune-mediated disorders appears less evident.
Table 1.
Studies describing autoimmune disorders during coronavirus disease 2019
| Ref. | Year | Number of subjects | Type of study | Comments |
| Harzallah et al[117] | 2020 | 56 | Retrospective cohort analysis | Positivity of LAC and anti-cardiolipin/anti-β2-glycoprotein IgG or IgM in 45% and 10% of COVID-19 patients, respectively |
| Zhou et al[123] | 2020 | 21 | Case series | Positivity of anti-52 kDa Ro-SSA, anti-60 kDa Ro-SSA antibodies and ANA in 20%, 25% and 50% of critically or severely ill COVID-19 patients, respectively. Anti-SCL70, ACA, anti-U1-RNP, anti-Jo1, anti-Sm-D1, anti-dsDNA antibodies and RF not detected |
| Gao et al[122] | 2020 | 10 | Case series | Positivity of ACPA in 20% of COVID-19 patients with ANA and RF negative |
| Helms et al[118] | 2020 | 150 | Retrospective cohort analysis | Thromboembolic events reported in 64 COVID-19 cases; positivity of LAC in 87.7% of the tested patient cohort (57 individuals) |
| Zhang et al[19] | 2020 | 3 | Case series | Ischemia of upper and lower limbs and cerebral infarcts in 3 COVID-19 patients with positivity of anti-cardiolipin and anti-β2-glycoprotein IgA and IgG |
| Pilotto et al[136] | 2020 | 1 | Case report | Immune-mediated encephalitis in a 60-year-old man recovering with i.v. pulses of methylprednisolone (1 g/d for five days) |
| Ottaviani et al[137] | 2020 | 1 | Case report | Guillain-Barré syndrome occurring in a 66-year-old woman 10 days after the recovery from cough, rash and fever; neurological symptoms worsening with the development of transient episodes of confusion and psychomotor agitation. Progressive worsening of gas exchanges ending in a multi-organ failure |
| Zhao et al[12] | 2020 | 1 | Case report | Guillain-Barré syndrome as the first COVID-19 manifestation in a 61-year-old woman; SARS occurring 8 days later. Neurologic symptoms disappearing at COVID-19 recovery |
| Lazarian et al[13] | 2020 | 7 | Case series | Autoimmune hemolytic anemia developing after a mean delay of 9 days since the first COVID-19 symptoms and sustained by either warm or cold IgG; association with pre-existent lympho-proliferative disorders, partial response to steroid and rituximab |
| Zagorski et al[141] | 2020 | 1 | Case report | Hemolytic anemia sustained by cold agglutinins in a 46-year-old woman, admitted to hospital with fever, jaundice and dyspnea; SARS-CoV-2 RT-PCR and direct Coombs test positive and detection of cold IgG and complement. Rapidly fatal course of COVID-19 due to cardiogenic shock |
| Capes et al[142] | 2020 | 1 | Case report | Hemolytic anemia sustained by cold agglutinins in a 62-year-old oncologic male patient with COVID-19 (asthenia, fever, dyspnea with bilateral lung infiltrates); SARS-CoV-2 RT-PCR, direct Coombs test and ANA positive; anti-ENA and APL antibodies negative |
| Zulfiqar et al[14] | 2020 | 1 | Case report | Immune thrombocytopenic purpura developing 8 days after the first COVID-19 symptoms managed with platelet transfusion, i.v. prednisolone, eltrombopag and IVIG; anti-platelet antibodies not detected |
| Li et al[143] | 2020 | 1 | Case report | Evans syndrome developing in a 39-year-old man complaining of classical symptoms of COVID-19; chronological distance between thrombocytopenia, present at admission, and hemolytic anemia, occurring after discharge |
| Beydon et al[15] | 2020 | 1 | Case report | Myositis of the proximal lower limb muscles as the exordium manifestation of COVID-19, confirmed by CK serum increase and MRI findings and followed by fever and respiratory symptoms. SARS-CoV-2 detected in bronchoalveolar lavage fluid but not in nasopharyngeal swab |
| Craver et al[16] | 2020 | 1 | Case report | Fatal eosinophilic myocarditis in a 17-year-old male patient |
| Coyle et al[154] | 2020 | 1 | Case report | Myocarditis and ARDS recovering with steroids, anti-IL-6R and aldose reductase inhibitor |
| Andina et al[17] | 2020 | 22 | Retrospective case series | Chilblains of the feet and fingers in a pediatric cohort (median age 12 years) following respiratory and gastrointestinal symptoms (median time delay 1-28 days); oropharyngeal swab positive for SARS-CoV-2 only in one case. Rapid recovery with symptomatic oral or topical drugs |
| Landa et al[156] | 2020 | 6 | Case series | Chilblain-like lesions of toe, fingers and heels in an asymptomatic 31-year median aged cohort of patients; respiratory symptoms and fever reported in few of them 3-4 weeks before; oropharyngeal swab positive for SARS-CoV-2 in 2 cases |
| Verdoni et al[164] | 2020 | 29 | Observational study | 30-fold increase in Kawasaki vasculitis and MAS in SARS-CoV-2-infected compared to non-infected children (mean age 7.5 years); frequent cases of shock syndrome and cardiac involvement requiring high dose steroidal therapy in addition to IVIG |
| Toubiana et al[168] | 2020 | 21 | Prospective observational study | Kawasaki syndrome reported in 21 subjects (median age 7.9 years, 12 females, 12 of African descent, 90% being SARS-CoV-2 positive at nasopharyngeal swab or at blood test for anti-SARS-CoV-2 IgG); myocarditis found in 76% of patients; gastrointestinal involvement present in 100% of patients; coronary artery dilatation found in 24% of patients. Rapid recovery in all the cases with IVIG and steroids |
| Licciardi et al[18] | 2020 | 2 | Case series | Kawasaki-like syndrome developing in two SARS-CoV-2-infected male children (7-year-old and 12-year-old), characterized by fever, mucocutaneous manifestations, blood cytopenia, complement consumption, hypoalbuminemia, increased serum ferritin and inflammatory markers and cardiac injury recovering with i.v. methylprednisolone and IVIG. Nasopharyngeal swab negative for SARS-CoV-2; anti-SARS-CoV-2 antibodies present |
| Deza Leon et al[165] | 2020 | 1 | Case report | Kawasaki-like syndrome developing in a 6-year-old female, along with respiratory distress, hypotension and myocarditis, treated with antibiotics, IVIG, aspirin and extra corporeal membrane oxygenation. Nasopharyngeal swab for SARS-CoV-2 and Group A Streptococcus nasopharyngeal rapid test positive |
| Rivera-Figueroa et al[166] | 2020 | 1 | Case report | Incomplete Kawasaki disease in a 5-year-old male patient, characterized by mucocutaneous manifestations, cervical lymphadenopathy, pericardial effusion and hypotension. Resolution after supportive therapy, IVIG, i.v. steroids and aspirin. Nasopharyngeal swab for SARS-CoV-2 and Group A Streptococcus nasopharyngeal rapid test positive |
| Pouletty et al[167] | 2020 | 16 | Multicentre retrospective case-control analysis | Kawasaki and Kawasaki-like syndromes reported in a cohort of 16 patients (median age 10 years); complete form described in 10 cases and cardiogenic shock developing in 7 patients; SARS-CoV-2 detected in nasopharyngeal secretions and stool in 9 and 2 patients, respectively; serology positive in 7 out of 8 patients. Limited response to single IVIG infusion; additional lines of treatment (multiple infusions of IVIG, steroids, IL-1 or IL-6 inhibitors and ASA) required |
| Belhadjer et al[173] | 2020 | 35 | Case series | MIS-C (fever, cardiogenic shock, increased CRP serum value and acute left ventricle failure) described in pediatric patients (median age 10 years) admitted to French and Swiss hospitals from March to April 2020. Gastrointestinal symptoms reported in 80% of cases and SARS-CoV-2 positivity detected in 88.5% of cases (positive nasopharyngeal swab in 34% of patients and presence of anti-SARS-CoV-2 antibodies in 86% of individuals). Cardiogenic shock present in 80% of subjects at admission; Takotsubo syndrome, pericardial effusion and coronary artery dilatation without aneurysms also described. Rapid resolution with i.v. supportive therapy, IVIG, steroids. Anakinra required in 3 cases |
| Feldstein et al[177] | 2020 | 186 | Retrospective cohort analysis | MIS-C reported in 186 United States patients (median age 8.3 years, 115 males), 70% of whom testing positive for SARS-CoV-2 (RT-PCR and/or antibodies). Gastrointestinal symptoms most commonly reported (92% of cases), followed by cardiovascular (80% of cases), hematological (76% of cases), mucocutaneous (74% of cases) and respiratory (70% of cases) manifestations. Detection of coronary artery aneurysms in 8% of cases and death occurring in 4 patients. Treatment based on the administration of IVIG, steroids, IL-6- and IL-1-inhibitors beyond supportive therapy |
| Dufort et al[178] | 2020 | 191 | Retrospective cohort analysis | SARS-CoV-2-related MIS-C identified in 99 out of the 191 reported cases to the NYSDOH (53 males mostly aged 6-12 years; 40% black and 36% Hispanic); fever and increased systemic inflammatory markers described in all the patients; gastrointestinal symptoms occurring in 80% of patients, followed by cutaneous (60% of cases), ocular (56% of cases), and mucosal (27% of cases) manifestations; myocarditis detected in 53% of patients; 2 deaths recorded |
| Whittaker et al[175] | 2020 | 58 | Case series | MIS-C described in a United Kingdom cohort of pediatric/juvenile patients (median age 9 years; 33 females); COVID-19 symptoms reported in 78% of patients, with the gastrointestinal tract being the most commonly involved; anti-SARS-CoV-2 IgG found in 87% of patients. Complete Kawasaki syndrome ascertained in 13 patients and coronary artery dilatation found in 14% of cases. Cardiogenic shock developing in 29 subjects and requiring resuscitation procedures |
| Belot et al[174] | 2020 | 156 | Nationwide surveillance analysis | Confirmed association with SARS-CoV-2 infection in 79 out of 156 PIMS reported cases; Kawasaki-like syndrome, myocarditis and MAS described in 61%, 70%, and 23% of patients (median age 8 years), respectively, and requiring critical care intervention in 67% of cases |
ACPA: Anti-citrullinated protein antibodies; ANA: Antinuclear antibodies; anti-dsDNA: Anti-double stranded DNA; anti-ENA: Anti-extractable nuclear antigen; APL: Anti-phospholipid; ARDS: Acute respiratory distress syndrome; ASA: Acetylsalicylic acid; CK: Creatine phosphokinase; CRP: C-reactive protein; COVID-19: Coronavirus disease 2019; i.v.: Intravenous; Ig: Immunoglobulin; IL-6R: Interleukin-6 receptor; IVIG: Intravenous immunoglobulins; LAC: Lupus anticoagulant; MAS: Macrophage activation syndrome; MIS-C: Multisystem inflammatory syndrome in children; MRI: Magnetic resonance imaging; NYSDOH: New York State Department of Health; PIMS: Pediatric inflammatory multisystem syndrome; RF: Rheumatoid factor; RNP: Ribonucleoprotein; RT-PCR: Real-time reverse transcription polymerase chain reaction; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
It is known that the majority of autoimmune diseases affect more women than men, and this may depend on the unbalanced expression of genes placed on the X chromosome, which code for proteins involved in the immune response[186].
Some genes of crucial importance in the SARS-CoV-2 infection, like those coding for ACE2 and TLR7, map in the X chromosome. A hyper-expression of ACE2 has been shown in CD4+ T lymphocytes of SLE patients, having both favorable (immunomodulatory and anti-oxidative function played by ACE2) and unfavorable (viral entry and lymphopenia) outcomes[187]. On the other hand, the hyper-expression of TLR7 in women may confer protection to SARS-CoV-2 through a more efficient viral clearance[188]. Still, clinical implications of these findings are unclear: For instance, SARS-CoV-2 infection in SLE patients was reported to give rise to clinical pictures not diverging from those observed in general population[20].
COVID-19 indeed has at its base a complex immunological scenario, in which the immune system acts as a double-edged sword, being responsible for either viral clearance or excessive inflammation and autoimmunity. Once recognized by PRR of monocyte-macrophage cells[45], SARS-CoV-2 may activate several immunologic pathways. The infection may in fact generate a type I IFN response[27], associated with COVID-19 cutaneous manifestations and Kawasaki syndrome[160,163]; induce autoinflammation through the assembly of the inflammasome platform[52] or the interaction with HMGB-1[59], both of which are in turn associated with systemic COVID-19 complications, like cytokine storm and PIMS; trigger NETosis[62], lung oxidative stress[65], and complement and coagulation cascades[70], which lead to ARDS, lung fibrosis and DIC; phenotypically and functionally reset the lymphocyte subsets in favor of CD8+ and CD4+ Th1/Th17 responses, which, however, often display exhaustion[27,82] and are associated with COVID-19 myositis, myocarditis, encephalitis and Kawasaki vasculitis[136,147,150,163]; and finally give rise to the production of autoantibodies, which may cross-react with self-antigens, inducing the development of hematologic disorders or Guillain-Barrè syndrome[12,13,137,141-143]. The immunologic cascade underneath COVID-19 seems to be unbalanced and shifted towards the innate response in spite of the hyper-activation of T and B cells. Accordingly, cytokine storm syndrome and MAS have been reported in either old or pediatric SARS-CoV-2-infected patients as a complication of ARDS or Kawasaki-like syndromes[18,111,164,166,171]. Since an excessive stimulation of monocyte-macrophage cells may eventually result in autoimmunity, in these cases the early use of colchicine, IL-1β, TNF-α and IL-6 inhibitors or recombinant human IL-37 and IL-38 constraining macrophage activation and systemic cytokine release[111,145,184,185,189-191], may prevent in the long term the evolution towards a definite autoimmune disease. Tocilizumab is an IL-6R-targeting monoclonal antibody approved for moderate to severe RA and systemic and polyarticular JIA, and also usable for giant cell arteritis and adult and pediatric cytokine storm syndrome. The drug has shown to reduce the risk of death and invasive mechanical ventilation in critical COVID-19 patients[192], without increasing the risk of opportunistic infections compared to conventional treatments[193].
However, systemic hyper-inflammatory syndromes, like PIMS, have shown to mostly occur during or after seroconversion, and may be mediated by anti-SARS-CoV-2 antibodies reacting with FcR expressed on phagocytic cells[172], hence explaining the effectiveness of IVIG in these cases.
Other reports described autoimmune phenomena occurring during COVID-19, based on T or B cell-mediated responses[12,13,137,147]. Contrary to PIMS, autoimmune disorders reported in adults may be the first clinical expression of COVID-19 and may be due to type I IFN response or to a molecular mimicry mechanism[172]. Several questions still remain concerning the chronicization of these manifestations, the future onset of rheumatic diseases in recovered SARS-CoV-2 infected individuals and the role of the immunosuppressive therapy in preventing such consequences. Though often being lymphopenic, SARS-CoV-2-infected patients have a hyper-activation of the CD8+ and CD4+ Th1/Th17 lymphocyte axes at the detriment of the Treg compartment[27]. Therefore, lymphopenia may be interpreted as the result of lymphocyte margination into inflamed organs[136,194]. Some immunosuppressive drugs like cyclosporin A (CyA), used to manage autoimmune diseases, may also hamper the calcineurin-dependent SARS-CoV-2 replication and thus protect treated patients from COVID-19[145]. The real effectiveness of immunotherapies aiming at the expansion of the pool of Treg lymphocytes, like IL-2- or cytotoxic T-lymphocyte-associated protein 4 (CTLA-4)-based agents, is instead unclear, as the efficiency of Treg cells in contrasting the immune response may be impaired by the hyper-secretion of pro-inflammatory cytokines. JAK-inhibitors, currently licensed for RA and other immune-mediated diseases, may interrupt cytokine signaling pathways but may meantime weaken the IFN-mediated antiviral response[144].
Although no meaningful differences were noticed in the clinical course of COVID-19 nor in its incidence and prevalence rates between rheumatic and non-rheumatic patients[21,22], it cannot be ruled out that SARS-CoV-2 may eventually influence the immune response. Concomitant immunosuppressive therapies prescribed to rheumatic patients may constrain the activation of the innate and adaptive immune system and protect from the development of a highly inflammatory cascade associated to worse COVID-19 outcomes[144].
CONCLUSION
In conclusion, it is not yet time for considering autoimmunity as the comet tail of COVID-19, and many of the reported immune-mediated disorders in SARS-CoV-2 may represent transitory epiphenomena accompanying a viral infection. Anti-inflammatory and immunosuppressive therapies may have a protective role in preventing the development of definite autoimmune diseases, but current evidence in support is still developing and may take a few more years to have any definitive answers.
Footnotes
Conflict-of-interest statement: The author declares no conflict of interests.
Manuscript source: Invited manuscript
Peer-review started: July 4, 2020
First decision: July 24, 2020
Article in press: August 26, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: Italy
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bayry J S-Editor: Gong ZM L-Editor: A P-Editor: Xing YX
Contributor Information
Rossella Talotta, Department of Clinical and Experimental Medicine, Rheumatology Unit, AOU “Gaetano Martino”, University of Messina, Messina 98100, Italy. talotta1@virgilio.it.
Erle Robertson, Department of Otorhinolaryngology-Head and Neck Surgery, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA 19014, United States.
References
- 1.Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2020 doi: 10.1016/j.jmii.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Y, Peng F, Wang R, Yange M, Guan K, Jiang T, Xu G, Sun J, Chang C. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434. doi: 10.1016/j.jaut.2020.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workman J. The proportion of COVID-19 cases that are asymptomatic in South Korea: Comment on Nishiura et al. Int J Infect Dis. 2020;96:398. doi: 10.1016/j.ijid.2020.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vabret N, Britton GJ, Gruber C, Hegde S, Kim J, Kuksin M, Levantovsky R, Malle L, Moreira A, Park MD, Pia L, Risson E, Saffern M, Salomé B, Esai Selvan M, Spindler MP, Tan J, van der Heide V, Gregory JK, Alexandropoulos K, Bhardwaj N, Brown BD, Greenbaum B, Gümüş ZH, Homann D, Horowitz A, Kamphorst AO, Curotto de Lafaille MA, Mehandru S, Merad M, Samstein RM Sinai Immunology Review Project. Immunology of COVID-19: Current State of the Science. Immunity. 2020;52:910–941. doi: 10.1016/j.immuni.2020.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Channappanavar R, Zhao J, Perlman S. T cell-mediated immune response to respiratory coronaviruses. Immunol Res. 2014;59:118–128. doi: 10.1007/s12026-014-8534-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang W, Zhao Y, Zhang F, Wang Q, Li T, Liu Z, Wang J, Qin Y, Zhang X, Yan X, Zeng X, Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dhochak N, Singhal T, Kabra SK, Lodha R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr. 2020;87:537–546. doi: 10.1007/s12098-020-03322-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim B, Kaistha SD, Rouse BT. Viruses and autoimmunity. Autoimmunity. 2006;39:71–77. doi: 10.1080/08916930500484708. [DOI] [PubMed] [Google Scholar]
- 11.Mackay IR. The etiopathogenesis of autoimmunity. Semin Liver Dis. 2005;25:239–250. doi: 10.1055/s-2005-916330. [DOI] [PubMed] [Google Scholar]
- 12.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–384. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, Mekinian A, Braun T, Damaj G, Delmer A, Cymbalista F. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190:29–31. doi: 10.1111/bjh.16794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zulfiqar AA, Lorenzo-Villalba N, Hassler P, Andrès E. Immune Thrombocytopenic Purpura in a Patient with Covid-19. N Engl J Med. 2020;382:e43. doi: 10.1056/NEJMc2010472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beydon M, Chevalier K, Al Tabaa O, Hamroun S, Delettre AS, Thomas M, Herrou J, Riviere E, Mariette X. Myositis as a manifestation of SARS-CoV-2. Ann Rheum Dis. 2020:Online ahead of print. doi: 10.1136/annrheumdis-2020-217573. [DOI] [PubMed] [Google Scholar]
- 16.Craver R, Huber S, Sandomirsky M, McKenna D, Schieffelin J, Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c) Fetal Pediatr Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andina D, Noguera-Morel L, Bascuas-Arribas M, Gaitero-Tristán J, Alonso-Cadenas JA, Escalada-Pellitero S, Hernández-Martín Á, de la Torre-Espi M, Colmenero I, Torrelo A. Chilblains in children in the setting of COVID-19 pandemic. Pediatr Dermatol. 2020;37:406–411. doi: 10.1111/pde.14215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Licciardi F, Pruccoli G, Denina M, Parodi E, Taglietto M, Rosati S, Montin D. SARS-CoV-2-Induced Kawasaki-Like Hyperinflammatory Syndrome: A Novel COVID Phenotype in Children. Pediatrics. 2020;146:e20201711. doi: 10.1542/peds.2020-1711. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, Chen H, Ding X, Zhao H, Zhang H, Wang C, Zhao J, Sun X, Tian R, Wu W, Wu D, Ma J, Chen Y, Zhang D, Xie J, Yan X, Zhou X, Liu Z, Wang J, Du B, Qin Y, Gao P, Qin X, Xu Y, Zhang W, Li T, Zhang F, Zhao Y, Li Y, Zhang S. Coagulopathy and Antiphospholipid Antibodies in Patients with Covid-19. N Engl J Med. 2020;382:e38. doi: 10.1056/NEJMc2007575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Favalli EG, Agape E, Caporali R. Incidence and Clinical Course of COVID-19 in Patients with Connective Tissue Diseases: A Descriptive Observational Analysis. J Rheumatol. 2020;47:1296. doi: 10.3899/jrheum.200507. [DOI] [PubMed] [Google Scholar]
- 21.Favalli EG, Ingegnoli F, Cimaz R, Caporali R. What is the true incidence of COVID-19 in patients with rheumatic diseases? Ann Rheum Dis. 2020:Online ahead of print. doi: 10.1136/annrheumdis-2020-217615. [DOI] [PubMed] [Google Scholar]
- 22.Monti S, Balduzzi S, Delvino P, Bellis E, Quadrelli VS, Montecucco C. Clinical course of COVID-19 in a series of patients with chronic arthritis treated with immunosuppressive targeted therapies. Ann Rheum Dis. 2020;79:667–668. doi: 10.1136/annrheumdis-2020-217424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cascella M, Rajnik M, Cuomo A, Dulebohn SC, Di Napoli R. Features, Evaluation and Treatment Coronavirus (COVID-19). In: StatPearls [Internet] Treasure Island (FL): StatPearls Publishing; 2020. [PubMed] [Google Scholar]
- 24.Nikhat S, Fazil M. Overview of Covid-19; its prevention and management in the light of Unani medicine. Sci Total Environ. 2020;728:138859. doi: 10.1016/j.scitotenv.2020.138859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zang R, Gomez Castro MF, McCune BT, Zeng Q, Rothlauf PW, Sonnek NM, Liu Z, Brulois KF, Wang X, Greenberg HB, Diamond MS, Ciorba MA, Whelan SPJ, Ding S. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci Immunol. 2020;5:eabc3582. doi: 10.1126/sciimmunol.abc3582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Brüggen MC, O'Mahony L, Gao Y, Nadeau K, Akdis CA. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. 2020;75:1564–1581. doi: 10.1111/all.14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tay MZ, Poh CM, Rénia L, MacAry PA, Ng LFP. The trinity of COVID-19: immunity, inflammation and intervention. Nat Rev Immunol. 2020;20:363–374. doi: 10.1038/s41577-020-0311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van den Brand JM, Haagmans BL, Leijten L, van Riel D, Martina BE, Osterhaus AD, Kuiken T. Pathology of experimental SARS coronavirus infection in cats and ferrets. Vet Pathol. 2008;45:551–562. doi: 10.1354/vp.45-4-551. [DOI] [PubMed] [Google Scholar]
- 30.Wiener RS, Cao YX, Hinds A, Ramirez MI, Williams MC. Angiotensin converting enzyme 2 is primarily epithelial and is developmentally regulated in the mouse lung. J Cell Biochem. 2007;101:1278–1291. doi: 10.1002/jcb.21248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Zhong L, Deng J, Peng J, Dan H, Zeng X, Li T, Chen Q. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burgueño JF, Reich A, Hazime H, Quintero MA, Fernandez I, Fritsch J, Santander AM, Brito N, Damas OM, Deshpande A, Kerman DH, Zhang L, Gao Z, Ban Y, Wang L, Pignac-Kobinger J, Abreu MT. Expression of SARS-CoV-2 Entry Molecules ACE2 and TMPRSS2 in the Gut of Patients With IBD. Inflamm Bowel Dis. 2020;26:797–808. doi: 10.1093/ibd/izaa085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera-López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baig AM, Khaleeq A, Ali U, Syeda H. Evidence of the COVID-19 Virus Targeting the CNS: Tissue Distribution, Host-Virus Interaction, and Proposed Neurotropic Mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 35.Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A, Dequanter D, Blecic S, El Afia F, Distinguin L, Chekkoury-Idrissi Y, Hans S, Delgado IL, Calvo-Henriquez C, Lavigne P, Falanga C, Barillari MR, Cammaroto G, Khalife M, Leich P, Souchay C, Rossi C, Journe F, Hsieh J, Edjlali M, Carlier R, Ris L, Lovato A, De Filippis C, Coppee F, Fakhry N, Ayad T, Saussez S. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol. 2020;277:2251–2261. doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bracco L. Covid-19, Type II Alveolar Cells and Surfactant. J Med-Clin Res Rev [Internet] 2020;4:1–3. Available from: https://www.isinnova.it/easy-covid19/ [Google Scholar]
- 37.Ling TY, Kuo MD, Li CL, Yu AL, Huang YH, Wu TJ, Lin YC, Chen SH, Yu J. Identification of pulmonary Oct-4+ stem/progenitor cells and demonstration of their susceptibility to SARS coronavirus (SARS-CoV) infection in vitro. Proc Natl Acad Sci USA. 2006;103:9530–9535. doi: 10.1073/pnas.0510232103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Li HB, Lyu JR, Lei XM, Li W, Wu G, Lyu J, Dai ZM. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int J Infect Dis. 2020;96:19–24. doi: 10.1016/j.ijid.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:11. doi: 10.1038/s41368-020-0080-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hellings P, Jorissen M, Ceuppens JL. The Waldeyer's ring. Acta Otorhinolaryngol Belg. 2000;54:237–241. [PubMed] [Google Scholar]
- 41.Jung C, Hugot JP, Barreau F. Peyer's Patches: The Immune Sensors of the Intestine. Int J Inflam. 2010;2010:823710. doi: 10.4061/2010/823710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhar D, Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Res. 2020;285:198018. doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 44.Eisen HN, Chakraborty AK. Evolving concepts of specificity in immune reactions. Proc Natl Acad Sci USA. 2010;107:22373–22380. doi: 10.1073/pnas.1012051108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rönnblom L, Leonard D. Interferon pathway in SLE: one key to unlocking the mystery of the disease. Lupus Sci Med. 2019;6:e000270. doi: 10.1136/lupus-2018-000270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Katze MG, He Y, Gale M., Jr Viruses and interferon: a fight for supremacy. Nat Rev Immunol. 2002;2:675–687. doi: 10.1038/nri888. [DOI] [PubMed] [Google Scholar]
- 48.Rönnblom L, Eloranta ML. The interferon signature in autoimmune diseases. Curr Opin Rheumatol. 2013;25:248–253. doi: 10.1097/BOR.0b013e32835c7e32. [DOI] [PubMed] [Google Scholar]
- 49.Nelson P, Rylance P, Roden D, Trela M, Tugnet N. Viruses as potential pathogenic agents in systemic lupus erythematosus. Lupus. 2014;23:596–605. doi: 10.1177/0961203314531637. [DOI] [PubMed] [Google Scholar]
- 50.Mariette X, Gottenberg JE. Pathogenesis of Sjögren's syndrome and therapeutic consequences. Curr Opin Rheumatol. 2010;22:471–477. doi: 10.1097/BOR.0b013e32833c36c5. [DOI] [PubMed] [Google Scholar]
- 51.Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, Kritas SK. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. 2020;34:327–331. doi: 10.23812/CONTI-E. [DOI] [PubMed] [Google Scholar]
- 52.Chen IY, Ichinohe T. Response of host inflammasomes to viral infection. Trends Microbiol. 2015;23:55–63. doi: 10.1016/j.tim.2014.09.007. [DOI] [PubMed] [Google Scholar]
- 53.Broderick L, De Nardo D, Franklin BS, Hoffman HM, Latz E. The inflammasomes and autoinflammatory syndromes. Annu Rev Pathol. 2015;10:395–424. doi: 10.1146/annurev-pathol-012414-040431. [DOI] [PubMed] [Google Scholar]
- 54.Lopalco G, Cantarini L, Vitale A, Iannone F, Anelli MG, Andreozzi L, Lapadula G, Galeazzi M, Rigante D. Interleukin-1 as a common denominator from autoinflammatory to autoimmune disorders: premises, perils, and perspectives. Mediators Inflamm. 2015;2015:194864. doi: 10.1155/2015/194864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.del Río-Martínez PS. Spondyloarthritis: pathogenesis, clinical manifestations, diagnosis, and management. Eur Med J. 2016;1:96–102. [Google Scholar]
- 56.Caso F, Costa L, Nucera V, Barilaro G, Masala IF, Talotta R, Caso P, Scarpa R, Sarzi-Puttini P, Atzeni F. From autoinflammation to autoimmunity: old and recent findings. Clin Rheumatol. 2018;37:2305–2321. doi: 10.1007/s10067-018-4209-9. [DOI] [PubMed] [Google Scholar]
- 57.Andersson U, Ottestad W, Tracey KJ. Extracellular HMGB1: a therapeutic target in severe pulmonary inflammation including COVID-19? Mol Med. 2020;26:42. doi: 10.1186/s10020-020-00172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Street ME. HMGB1: A Possible Crucial Therapeutic Target for COVID-19? Horm Res Paediatr. 2020:1–3. doi: 10.1159/000508291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chen G, Chen DZ, Li J, Czura CJ, Tracey KJ, Sama AE, Wang H. Pathogenic role of HMGB1 in SARS? Med Hypotheses. 2004;63:691–695. doi: 10.1016/j.mehy.2004.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhou Z, Ren L, Zhang L, Zhong J, Xiao Y, Jia Z, Guo L, Yang J, Wang C, Jiang S, Yang D, Zhang G, Li H, Chen F, Xu Y, Chen M, Gao Z, Yang J, Dong J, Liu B, Zhang X, Wang W, He K, Jin Q, Li M, Wang J. Heightened Innate Immune Responses in the Respiratory Tract of COVID-19 Patients. Cell Host Microbe. 2020;27:883–890.e2. doi: 10.1016/j.chom.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen W. A potential treatment of COVID-19 with TGF-β blockade. Int J Biol Sci. 2020;16:1954–1955. doi: 10.7150/ijbs.46891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mozzini C, Girelli D. The role of Neutrophil Extracellular Traps in Covid-19: Only an hypothesis or a potential new field of research? Thromb Res. 2020;191:26–27. doi: 10.1016/j.thromres.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bouts YM, Wolthuis DF, Dirkx MF, Pieterse E, Simons EM, van Boekel AM, Dieker JW, van der Vlag J. Apoptosis and NET formation in the pathogenesis of SLE. Autoimmunity. 2012;45:597–601. doi: 10.3109/08916934.2012.719953. [DOI] [PubMed] [Google Scholar]
- 64.Söderberg D, Segelmark M. Neutrophil Extracellular Traps in ANCA-Associated Vasculitis. Front Immunol. 2016;7:256. doi: 10.3389/fimmu.2016.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meng Y, Li T, Zhou GS, Chen Y, Yu CH, Pang MX, Li W, Li Y, Zhang WY, Li X. The angiotensin-converting enzyme 2/angiotensin (1-7)/Mas axis protects against lung fibroblast migration and lung fibrosis by inhibiting the NOX4-derived ROS-mediated RhoA/Rho kinase pathway. Antioxid Redox Signal. 2015;22:241–258. doi: 10.1089/ars.2013.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abouhashem AS, Singh K, Azzazy HME, Sen CK. Is Low Alveolar Type II Cell SOD3 in the Lungs of Elderly Linked to the Observed Severity of COVID-19? Antioxid Redox Signal. 2020;33:59–65. doi: 10.1089/ars.2020.8111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps in COVID-19. JCI Insight. 2020;5 doi: 10.1172/jci.insight.138999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zuo Y, Yalavarthi S, Shi H, Gockman K, Zuo M, Madison JA, Blair C, Weber A, Barnes BJ, Egeblad M, Woods RJ, Kanthi Y, Knight JS. Neutrophil extracellular traps (NETs) as markers of disease severity in COVID-19. medRxiv. 2020:2020.04.09.20059626. [Google Scholar]
- 69.Stoermer KA, Morrison TE. Complement and viral pathogenesis. Virology. 2011;411:362–373. doi: 10.1016/j.virol.2010.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sarma JV, Ward PA. The complement system. Cell Tissue Res. 2011;343:227–235. doi: 10.1007/s00441-010-1034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4 Suppl 3:S279–S293. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramos-Casals M, Campoamor MT, Chamorro A, Salvador G, Segura S, Botero JC, Yagüe J, Cervera R, Ingelmo M, Font J. Hypocomplementemia in systemic lupus erythematosus and primary antiphospholipid syndrome: prevalence and clinical significance in 667 patients. Lupus. 2004;13:777–783. doi: 10.1191/0961203304lu1080oa. [DOI] [PubMed] [Google Scholar]
- 73.Aslanidis S, Pyrpasopoulou A, Kontotasios K, Doumas S, Zamboulis C. Parvovirus B19 infection and systemic lupus erythematosus: Activation of an aberrant pathway? Eur J Intern Med. 2008;19:314–318. doi: 10.1016/j.ejim.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Hashizume H, Kageyama R. Hypocomplementemia is a diagnostic clue for parvovirus B19 infection in adults. J Dermatol. 2017;44:e27. doi: 10.1111/1346-8138.13469. [DOI] [PubMed] [Google Scholar]
- 75.Dheir H, Sipahi S, Yaylaci S, Köroğlu M, Erdem AF, Karabay O. Is there relationship between SARS-CoV 2 and the complement C3 and C4? Turk J Med Sci. 2020;50:687–688. doi: 10.3906/sag-2004-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mastaglio S, Ruggeri A, Risitano AM, Angelillo P, Yancopoulou D, Mastellos DC, Huber-Lang M, Piemontese S, Assanelli A, Garlanda C, Lambris JD, Ciceri F. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin Immunol. 2020;215:108450. doi: 10.1016/j.clim.2020.108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Luckheeram RV, Zhou R, Verma AD, Xia B. CD4+T cells: differentiation and functions. Clin Dev Immunol. 2012;2012:925135. doi: 10.1155/2012/925135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang K, Chen W, Zhou Y-S, Lian J-Q, Zhang Z, Du P, Gong L, Zhang Y, Cui H-Y, Geng J-J, Wang B, Sun X-X, Wang C-F, Yang X, Lin P, Deng Y-Q, Wei D, Yang X-M, Zhu Y-M, Zhang K, Zheng Z-H, Miao J-L, Guo T, Shi Y, Zhang J, Fu L, Wang Q-Y, Bian H, Zhu P, Chen Z-N. SARS-CoV-2 invades host cells via a novel route: CD147-spike protein. bioRxiv [Internet] 2020:2020.03.14.988345. [Google Scholar]
- 79.Staffler G, Szekeres A, Schütz GJ, Säemann MD, Prager E, Zeyda M, Drbal K, Zlabinger GJ, Stulnig TM, Stockinger H. Selective inhibition of T cell activation via CD147 through novel modulation of lipid rafts. J Immunol. 2003;171:1707–1714. doi: 10.4049/jimmunol.171.4.1707. [DOI] [PubMed] [Google Scholar]
- 80.Pistol G, Matache C, Calugaru A, Stavaru C, Tanaseanu S, Ionescu R, Dumitrache S, Stefanescu M. Roles of CD147 on T lymphocytes activation and MMP-9 secretion in systemic lupus erythematosus. J Cell Mol Med. 2007;11:339–348. doi: 10.1111/j.1582-4934.2007.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kapil S, Goyal SM, Trent AM. Cellular immune status of coronavirus-infected neonatal calves. Comp Immunol Microbiol Infect Dis. 1994;17:133–138. doi: 10.1016/0147-9571(94)90038-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diao B, Wang C, Tan Y, Chen X, Liu Y, Ning L, Chen L, Li M, Liu Y, Wang G, Yuan Z, Feng Z, Zhang Y, Wu Y, Chen Y. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19) Front Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin C, Zhou L, Hu Z, Zhang S, Yang S, Tao Y, Xie C, Ma K, Shang K, Wang W, Tian DS. Dysregulation of Immune Response in Patients With Coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jäger A, Kuchroo VK. Effector and regulatory T-cell subsets in autoimmunity and tissue inflammation. Scand J Immunol. 2010;72:173–184. doi: 10.1111/j.1365-3083.2010.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Mai J, Wang H, Yang XF. Th 17 cells interplay with Foxp3+ Tregs in regulation of inflammation and autoimmunity. Front Biosci (Landmark Ed) 2010;15:986–1006. doi: 10.2741/3657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.El Shikh ME, El Sayed RM, Sukumar S, Szakal AK, Tew JG. Activation of B cells by antigens on follicular dendritic cells. Trends Immunol. 2010;31:205–211. doi: 10.1016/j.it.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, Ntaganou M, Kyriakopoulou M, Dimopoulos G, Koutsodimitropoulos I, Velissaris D, Koufargyris P, Karageorgos A, Katrini K, Lekakis V, Lupse M, Kotsaki A, Renieris G, Theodoulou D, Panou V, Koukaki E, Koulouris N, Gogos C, Koutsoukou A. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020;27:992–1000.e3. doi: 10.1016/j.chom.2020.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee YL, Liao CH, Liu PY, Cheng CY, Chung MY, Liu CE, Chang SY, Hsueh PR. Dynamics of anti-SARS-Cov-2 IgM and IgG antibodies among COVID-19 patients. J Infect. 2020;81:e55–e58. doi: 10.1016/j.jinf.2020.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020:Online ahead of print. doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoepel W, Newling M, Vogelpoel LTC, Sritharan L, Hansen IS, Kapsenberg ML, Baeten DLP, Everts B, den Dunnen J. FcγR-TLR Cross-Talk Enhances TNF Production by Human Monocyte-Derived DCs via IRF5-Dependent Gene Transcription and Glycolytic Reprogramming. Front Immunol. 2019;10:739. doi: 10.3389/fimmu.2019.00739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Acharya D, Li XRL, Heineman RE, Harrison RE. Complement Receptor-Mediated Phagocytosis Induces Proinflammatory Cytokine Production in Murine Macrophages. Front Immunol. 2019;10:3049. doi: 10.3389/fimmu.2019.03049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wan Y, Shang J, Sun S, Tai W, Chen J, Geng Q, He L, Chen Y, Wu J, Shi Z, Zhou Y, Du L, Li F. Molecular Mechanism for Antibody-Dependent Enhancement of Coronavirus Entry. J Virol. 2020;94 doi: 10.1128/JVI.02015-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fu Y, Cheng Y, Wu Y. Understanding SARS-CoV-2-Mediated Inflammatory Responses: From Mechanisms to Potential Therapeutic Tools. Virol Sin. 2020;35:266–271. doi: 10.1007/s12250-020-00207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cao W, Liu X, Bai T, Fan H, Hong K, Song H, Han Y, Lin L, Ruan L, Li T. High-Dose Intravenous Immunoglobulin as a Therapeutic Option for Deteriorating Patients With Coronavirus Disease 2019. Open Forum Infect Dis. 2020;7:ofaa102. doi: 10.1093/ofid/ofaa102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Xie Y, Cao S, Dong H, Li Q, Chen E, Zhang W, Yang L, Fu S, Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mulhearn B, Bruce IN. Indications for IVIG in rheumatic diseases. Rheumatology (Oxford) 2015;54:383–391. doi: 10.1093/rheumatology/keu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hartung HP. Advances in the understanding of the mechanism of action of IVIg. J Neurol. 2008;255 Suppl 3:3–6. doi: 10.1007/s00415-008-3002-0. [DOI] [PubMed] [Google Scholar]
- 98.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–486. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 99.Espéli M, Smith KG, Clatworthy MR. FcγRIIB and autoimmunity. Immunol Rev. 2016;269:194–211. doi: 10.1111/imr.12368. [DOI] [PubMed] [Google Scholar]
- 100.Zhao EJ, Carruthers MN, Li CH, Mattman A, Chen LYC. Conditions associated with polyclonal hypergammaglobulinemia in the IgG4-related disease era: a retrospective study from a hematology tertiary care center. Haematologica. 2020;105:e121–e123. doi: 10.3324/haematol.2019.219725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schluter SF, Adelman MK, Taneja V, David C, Yocum DE, Marchalonis JJ. Natural autoantibodies to TCR public idiotopes: potential roles in immunomodulation. Cell Mol Biol (Noisy-le-grand) 2003;49:193–207. [PubMed] [Google Scholar]
- 102.Montes CL, Acosta-Rodríguez EV, Merino MC, Bermejo DA, Gruppi A. Polyclonal B cell activation in infections: infectious agents' devilry or defense mechanism of the host? J Leukoc Biol. 2007;82:1027–1032. doi: 10.1189/jlb.0407214. [DOI] [PubMed] [Google Scholar]
- 103.Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X, Zou Y, Zhang S. Treatment With Convalescent Plasma for Critically Ill Patients With Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, van Buskirk C, Grossman BJ, Joyner M, Henderson JP, Pekosz A, Lau B, Wesolowski A, Katz L, Shan H, Auwaerter PG, Thomas D, Sullivan DJ, Paneth N, Gehrie E, Spitalnik S, Hod EA, Pollack L, Nicholson WT, Pirofski LA, Bailey JA, Tobian AA. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. 2020;130:2757–2765. doi: 10.1172/JCI138745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mehta P, Cron RQ, Hartwell J, Manson JJ, Tattersall RS. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. Lancet Rheumatol. 2020;2:e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bracaglia C, Prencipe G, De Benedetti F. Macrophage Activation Syndrome: different mechanisms leading to a one clinical syndrome. Pediatr Rheumatol Online J. 2017;15:5. doi: 10.1186/s12969-016-0130-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Latino GA, Manlhiot C, Yeung RS, Chahal N, McCrindle BW. Macrophage activation syndrome in the acute phase of Kawasaki disease. J Pediatr Hematol Oncol. 2010;32:527–531. doi: 10.1097/MPH.0b013e3181dccbf4. [DOI] [PubMed] [Google Scholar]
- 109.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16–32. doi: 10.1128/MMBR.05015-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Coperchini F, Chiovato L, Croce L, Magri F, Rotondi M. The cytokine storm in COVID-19: An overview of the involvement of the chemokine/chemokine-receptor system. Cytokine Growth Factor Rev. 2020;53:25–32. doi: 10.1016/j.cytogfr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ding M, Zhang Q, Li Q, Wu T, Huang YZ. Correlation analysis of the severity and clinical prognosis of 32 cases of patients with COVID-19. Respir Med. 2020;167:105981. doi: 10.1016/j.rmed.2020.105981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wells AU, Denton CP. Interstitial lung disease in connective tissue disease--mechanisms and management. Nat Rev Rheumatol. 2014;10:728–739. doi: 10.1038/nrrheum.2014.149. [DOI] [PubMed] [Google Scholar]
- 115.Rice LM, Padilla CM, McLaughlin SR, Mathes A, Ziemek J, Goummih S, Nakerakanti S, York M, Farina G, Whitfield ML, Spiera RF, Christmann RB, Gordon JK, Weinberg J, Simms RW, Lafyatis R. Fresolimumab treatment decreases biomarkers and improves clinical symptoms in systemic sclerosis patients. J Clin Invest. 2015;125:2795–2807. doi: 10.1172/JCI77958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Harzallah I, Debliquis A, Drénou B. Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. 2020;18:2064–2065. doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Helms J, Tacquard C, Severac F, Leonard-Lorant I, Ohana M, Delabranche X, Merdji H, Clere-Jehl R, Schenck M, Fagot Gandet F, Fafi-Kremer S, Castelain V, Schneider F, Grunebaum L, Anglés-Cano E, Sattler L, Mertes PM, Meziani F CRICS TRIGGERSEP Group (Clinical Research in Intensive Care and Sepsis Trial Group for Global Evaluation and Research in Sepsis) High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46:1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Uthman IW, Gharavi AE. Viral infections and antiphospholipid antibodies. Semin Arthritis Rheum. 2002;31:256–263. doi: 10.1053/sarh.2002.28303. [DOI] [PubMed] [Google Scholar]
- 120.Sène D, Piette JC, Cacoub P. Antiphospholipid antibodies, antiphospholipid syndrome and infections. Autoimmun Rev. 2008;7:272–277. doi: 10.1016/j.autrev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 121.Mendoza-Pinto C, García-Carrasco M, Cervera R. Role of Infectious Diseases in the Antiphospholipid Syndrome (Including Its Catastrophic Variant) Curr Rheumatol Rep. 2018;20:62. doi: 10.1007/s11926-018-0773-x. [DOI] [PubMed] [Google Scholar]
- 122.Gao ZW, Wang X, Lin F, Dong K. The correlation between SARS-CoV-2 infection and rheumatic disease. Autoimmun Rev. 2020;19:102557. doi: 10.1016/j.autrev.2020.102557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhou Y, Han T, Chen J, Hou C, Hua L, He S, Guo Y, Zhang S, Wang Y, Yuan J, Zhao C, Zhang J, Jia Q, Zuo X, Li J, Wang L, Cao Q, Jia E. Clinical and Autoimmune Characteristics of Severe and Critical Cases of COVID-19. Clin Transl Sci. 2020:Online ahead of print. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Dellavance A, Coelho Andrade LE. Immunologic derangement preceding clinical autoimmunity. Lupus. 2014;23:1305–1308. doi: 10.1177/0961203314531346. [DOI] [PubMed] [Google Scholar]
- 125.Sokolove J, Bromberg R, Deane KD, Lahey LJ, Derber LA, Chandra PE, Edison JD, Gilliland WR, Tibshirani RJ, Norris JM, Holers VM, Robinson WH. Autoantibody epitope spreading in the pre-clinical phase predicts progression to rheumatoid arthritis. PLoS One. 2012;7:e35296. doi: 10.1371/journal.pone.0035296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Shapira Y, Poratkatz BS, Gilburd B, Barzilai O, Ram M, Blank M, Lindeberg S, Frostegård J, Anaya JM, Bizzaro N, Jara LJ, Damoiseaux J, Shoenfeld Y, Levin NA. Geographical differences in autoantibodies and anti-infectious agents antibodies among healthy adults. Clin Rev Allergy Immunol. 2012;42:154–163. doi: 10.1007/s12016-010-8241-z. [DOI] [PubMed] [Google Scholar]
- 127.Dancygier H. Autoantibodies. Clin Hepatol Princ Pract Hepatobiliary Dis. 2010:345–55. [Google Scholar]
- 128.Conde Cardona G, Quintana Pájaro LD, Quintero Marzola ID, Ramos Villegas Y, Moscote Salazar LR. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J Neurol Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen R, Wang K, Yu J, Chen Z, Wen C, Xu Z. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in human and mouse brain. bioRxiv. 2020:2020.04.07.030650. doi: 10.3389/fneur.2020.573095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Berger JR. COVID-19 and the nervous system. J Neurovirol. 2020;26:143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Zindler E, Zipp F. Neuronal injury in chronic CNS inflammation. Best Pract Res Clin Anaesthesiol. 2010;24:551–562. doi: 10.1016/j.bpa.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 132.Levin MC, Lee SM, Kalume F, Morcos Y, Dohan FC, Jr, Hasty KA, Callaway JC, Zunt J, Desiderio D, Stuart JM. Autoimmunity due to molecular mimicry as a cause of neurological disease. Nat Med. 2002;8:509–513. doi: 10.1038/nm0502-509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ascherio A, Munger KL, Lünemann JD. The initiation and prevention of multiple sclerosis. Nat Rev Neurol. 2012;8:602–612. doi: 10.1038/nrneurol.2012.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yadav SK, Mindur JE, Ito K, Dhib-Jalbut S. Advances in the immunopathogenesis of multiple sclerosis. Curr Opin Neurol. 2015;28:206–219. doi: 10.1097/WCO.0000000000000205. [DOI] [PubMed] [Google Scholar]
- 135.van den Berg B, Walgaard C, Drenthen J, Fokke C, Jacobs BC, van Doorn PA. Guillain-Barré syndrome: pathogenesis, diagnosis, treatment and prognosis. Nat Rev Neurol. 2014;10:469–482. doi: 10.1038/nrneurol.2014.121. [DOI] [PubMed] [Google Scholar]
- 136.Pilotto A, Odolini Si, Masciocchi S, Comelli A, Volonghi I, Gazzina S, Nocivelli S, Pezzini A, Foca, Emanuele, Caruso A, Leonardi M, Pasolini MP, Gasparotti R, Castelli F, Padovani A. Steroid-responsive severe encephalopathy in SARS-CoV-2 infection. medRxiv [Internet] 2020:2020.04.12.20062646. [Google Scholar]
- 137.Ottaviani D, Boso F, Tranquillini E, Gapeni I, Pedrotti G, Cozzio S, Guarrera GM, Giometto B. Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci. 2020;41:1351–1354. doi: 10.1007/s10072-020-04449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rogers JP, Chesney E, Oliver D, Pollak TA, McGuire P, Fusar-Poli P, Zandi MS, Lewis G, David AS. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Natelson BH. Myalgic Encephalomyelitis/Chronic Fatigue Syndrome and Fibromyalgia: Definitions, Similarities, and Differences. Clin Ther. 2019;41:612–618. doi: 10.1016/j.clinthera.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Morris G, Berk M, Galecki P, Maes M. The emerging role of autoimmunity in myalgic encephalomyelitis/chronic fatigue syndrome (ME/cfs) Mol Neurobiol. 2014;49:741–756. doi: 10.1007/s12035-013-8553-0. [DOI] [PubMed] [Google Scholar]
- 141.Zagorski E, Pawar T, Rahimian S, Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19) Br J Haematol. 2020:Online ahead of print. doi: 10.1111/bjh.16892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99:1679–1680. doi: 10.1007/s00277-020-04137-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li M, Nguyen CB, Yeung Z, Sanchez K, Rosen D, Bushan S. Evans syndrome in a patient with COVID-19. Br J Haematol. 2020;190:e59–e61. doi: 10.1111/bjh.16846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bonam SR, Kaveri SV, Sakuntabhai A, Gilardin L, Bayry J. Adjunct Immunotherapies for the Management of Severely Ill COVID-19 Patients. Cell Rep Med. 2020;1:100016. doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, Mekinian A, Anunciacion-Llunell A, Miró-Mur F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun Rev. 2020;19:102569. doi: 10.1016/j.autrev.2020.102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Dalakas MC. Inflammatory muscle diseases: a critical review on pathogenesis and therapies. Curr Opin Pharmacol. 2010;10:346–352. doi: 10.1016/j.coph.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 147.Megremis S, Walker TDJ, He X, Ollier WER, Chinoy H, Hampson L, Hampson I, Lamb JA. Antibodies against immunogenic epitopes with high sequence identity to SARS-CoV-2 in patients with autoimmune dermatomyositis. Ann Rheum Dis. 2020:Online ahead of print. doi: 10.1136/annrheumdis-2020-217522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fujisawa T, Hozumi H, Kono M, Enomoto N, Hashimoto D, Nakamura Y, Inui N, Yokomura K, Koshimizu N, Toyoshima M, Shirai T, Yasuda K, Hayakawa H, Suda T. Prognostic factors for myositis-associated interstitial lung disease. PLoS One. 2014;9:e98824. doi: 10.1371/journal.pone.0098824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sagar S, Liu PP, Cooper LT., Jr Myocarditis. Lancet. 2012;379:738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bracamonte-Baran W, Čiháková D. Cardiac Autoimmunity: Myocarditis. Adv Exp Med Biol. 2017;1003:187–221. doi: 10.1007/978-3-319-57613-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E. Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes. J Am Coll Cardiol. 2017;70:2363–2375. doi: 10.1016/j.jacc.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 152.Patel VB, Zhong JC, Grant MB, Oudit GY. Role of the ACE2/Angiotensin 1-7 Axis of the Renin-Angiotensin System in Heart Failure. Circ Res. 2016;118:1313–1326. doi: 10.1161/CIRCRESAHA.116.307708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rose NR. Viral myocarditis. Curr Opin Rheumatol. 2016;28:383–389. doi: 10.1097/BOR.0000000000000303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Coyle J, Igbinomwanhia E, Sanchez-Nadales A, Danciu S, Chu C, Shah N. A Recovered Case of COVID-19 Myocarditis and ARDS Treated with Corticosteroids, Tocilizumab, and Experimental AT-001. JACC Case Rep. 2020;2:1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sachdeva M, Gianotti R, Shah M, Bradanini L, Tosi D, Veraldi S, Ziv M, Leshem E, Dodiuk-Gad RP. Cutaneous manifestations of COVID-19: Report of three cases and a review of literature. J Dermatol Sci. 2020;98:75–81. doi: 10.1016/j.jdermsci.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Landa N, Mendieta-Eckert M, Fonda-Pascual P, Aguirre T. Chilblain-like lesions on feet and hands during the COVID-19 Pandemic. Int J Dermatol. 2020;59:739–743. doi: 10.1111/ijd.14937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Vano-Galvan S, Martorell A. Chilblains. CMAJ. 2012;184:67. doi: 10.1503/cmaj.110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yang X, Perez OA, English JC., 3rd Adult perniosis and cryoglobulinemia: a retrospective study and review of the literature. J Am Acad Dermatol. 2010;62:e21–e22. doi: 10.1016/j.jaad.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 159.de Argila Fernández-Aurán D, Revenga Arranz F, Iglesias Díez L. [Perniosis and lupus anticoagulant] Rev Clin Esp. 1996;196:24–27. [PubMed] [Google Scholar]
- 160.Volpi S, Picco P, Caorsi R, Candotti F, Gattorno M. Type I interferonopathies in pediatric rheumatology. Pediatr Rheumatol Online J. 2016;14:35. doi: 10.1186/s12969-016-0094-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Viguier M, Pinquier L, Cavelier-Balloy B, de la Salmonière P, Cordoliani F, Flageul B, Morel P, Dubertret L, Bachelez H. Clinical and histopathologic features and immunologic variables in patients with severe chilblains. A study of the relationship to lupus erythematosus. Medicine (Baltimore) 2001;80:180–188. doi: 10.1097/00005792-200105000-00004. [DOI] [PubMed] [Google Scholar]
- 162.Newburger JW, Takahashi M, Burns JC. Kawasaki Disease. J Am Coll Cardiol. 2016;67:1738–1749. doi: 10.1016/j.jacc.2015.12.073. [DOI] [PubMed] [Google Scholar]
- 163.Rowley AH, Shulman ST. The Epidemiology and Pathogenesis of Kawasaki Disease. Front Pediatr. 2018;6:374. doi: 10.3389/fped.2018.00374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Verdoni L, Mazza A, Gervasoni A, Martelli L, Ruggeri M, Ciuffreda M, Bonanomi E, D'Antiga L. An outbreak of severe Kawasaki-like disease at the Italian epicentre of the SARS-CoV-2 epidemic: an observational cohort study. Lancet. 2020;395:1771–1778. doi: 10.1016/S0140-6736(20)31103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Deza Leon MP, Redzepi A, McGrath E, Abdel-Haq N, Shawaqfeh A, Sethuraman U, Tilford B, Chopra T, Arora H, Ang J, Asmar B. COVID-19-Associated Pediatric Multisystem Inflammatory Syndrome. J Pediatric Infect Dis Soc. 2020;9:407–408. doi: 10.1093/jpids/piaa061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Rivera-Figueroa EI, Santos R, Simpson S, Garg P. Incomplete Kawasaki Disease in a Child with Covid-19. Indian Pediatr. 2020;57:680–681. doi: 10.1007/s13312-020-1900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pouletty M, Borocco C, Ouldali N, Caseris M, Basmaci R, Lachaume N, Bensaid P, Pichard S, Kouider H, Morelle G, Craiu I, Pondarre C, Deho A, Maroni A, Oualha M, Amoura Z, Haroche J, Chommeloux J, Bajolle F, Beyler C, Bonacorsi S, Carcelain G, Koné-Paut I, Bader-Meunier B, Faye A, Meinzer U, Galeotti C, Melki I. Paediatric multisystem inflammatory syndrome temporally associated with SARS-CoV-2 mimicking Kawasaki disease (Kawa-COVID-19): a multicentre cohort. Ann Rheum Dis. 2020;79:999–1006. doi: 10.1136/annrheumdis-2020-217960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Toubiana J, Poirault C, Corsia A, Bajolle F, Fourgeaud J, Angoulvant F, Debray A, Basmaci R, Salvador E, Biscardi S, Frange P, Chalumeau M, Casanova JL, Cohen JF, Allali S. Kawasaki-like multisystem inflammatory syndrome in children during the covid-19 pandemic in Paris, France: prospective observational study. BMJ. 2020;369:m2094. doi: 10.1136/bmj.m2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Goyal P, Vijayvergiya R. Rheumatic Fever and Rheumatic Heart Disease. Int Encycl Public Heal. 2016:357–362. [Google Scholar]
- 170.Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24:408–416. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Viner RM, Whittaker E. Kawasaki-like disease: emerging complication during the COVID-19 pandemic. Lancet. 2020;395:1741–1743. doi: 10.1016/S0140-6736(20)31129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Galeotti C, Bayry J. Autoimmune and inflammatory diseases following COVID-19. Nat Rev Rheumatol. 2020;16:413–414. doi: 10.1038/s41584-020-0448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Belhadjer Z, Méot M, Bajolle F, Khraiche D, Legendre A, Abakka S, Auriau J, Grimaud M, Oualha M, Beghetti M, Wacker J, Ovaert C, Hascoet S, Selegny M, Malekzadeh-Milani S, Maltret A, Bosser G, Giroux N, Bonnemains L, Bordet J, Di Filippo S, Mauran P, Falcon-Eicher S, Thambo JB, Lefort B, Moceri P, Houyel L, Renolleau S, Bonnet D. Acute heart failure in multisystem inflammatory syndrome in children (MIS-C) in the context of global SARS-CoV-2 pandemic. Circulation. 2020:Online ahead of print. doi: 10.1161/CIRCULATIONAHA.120.048360. [DOI] [PubMed] [Google Scholar]
- 174.Belot A, Antona D, Renolleau S, Javouhey E, Hentgen V, Angoulvant F, Delacourt C, Iriart X, Ovaert C, Bader-Meunier B, Kone-Paut I, Levy-Bruhl D. SARS-CoV-2-related paediatric inflammatory multisystem syndrome, an epidemiological study, France, 1 March to 17 May 2020. Euro Surveill. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.22.2001010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, Ramnarayan P, Fraisse A, Miller O, Davies P, Kucera F, Brierley J, McDougall M, Carter M, Tremoulet A, Shimizu C, Herberg J, Burns JC, Lyall H, Levin M PIMS-TS Study Group and EUCLIDS and PERFORM Consortia. Clinical Characteristics of 58 Children With a Pediatric Inflammatory Multisystem Syndrome Temporally Associated With SARS-CoV-2. JAMA. 2020:e2010369. doi: 10.1001/jama.2020.10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, Baker AL, Jackson MA, Takahashi M, Shah PB, Kobayashi T, Wu MH, Saji TT, Pahl E American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Cardiovascular Surgery and Anesthesia; and Council on Epidemiology and Prevention. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation. 2017;135:e927–e999. doi: 10.1161/CIR.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 177.Feldstein LR, Rose EB, Horwitz SM, Collins JP, Newhams MM, Son MBF, Newburger JW, Kleinman LC, Heidemann SM, Martin AA, Singh AR, Li S, Tarquinio KM, Jaggi P, Oster ME, Zackai SP, Gillen J, Ratner AJ, Walsh RF, Fitzgerald JC, Keenaghan MA, Alharash H, Doymaz S, Clouser KN, Giuliano JS, Jr, Gupta A, Parker RM, Maddux AB, Havalad V, Ramsingh S, Bukulmez H, Bradford TT, Smith LS, Tenforde MW, Carroll CL, Riggs BJ, Gertz SJ, Daube A, Lansell A, Coronado Munoz A, Hobbs CV, Marohn KL, Halasa NB, Patel MM, Randolph AG Overcoming COVID-19 Investigators and the CDC COVID-19 Response Team. Multisystem Inflammatory Syndrome in U.S. Children and Adolescents. N Engl J Med. 2020;383:334–346. doi: 10.1056/NEJMoa2021680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Dufort EM, Koumans EH, Chow EJ, Rosenthal EM, Muse A, Rowlands J, Barranco MA, Maxted AM, Rosenberg ES, Easton D, Udo T, Kumar J, Pulver W, Smith L, Hutton B, Blog D, Zucker H New York State and Centers for Disease Control and Prevention Multisystem Inflammatory Syndrome in Children Investigation Team. Multisystem Inflammatory Syndrome in Children in New York State. N Engl J Med. 2020;383:347–358. doi: 10.1056/NEJMoa2021756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Michelena X, Borrell H, López-Corbeto M, López-Lasanta M, Moreno E, Pascual-Pastor M, Erra A, Serrat M, Espartal E, Antón S, Añez GA, Caparrós-Ruiz R, Pluma A, Trallero-Araguás E, Barceló-Bru M, Almirall M, De Agustín JJ, Lladós J, Julià A, Marsal S. Incidence of COVID-19 in a cohort of adult and paediatric patients with rheumatic diseases treated with targeted biologic and synthetic disease-modifying anti-rheumatic drugs. Semin Arthritis Rheum. 2020;50:564–570. doi: 10.1016/j.semarthrit.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Filocamo G, Minoia F, Carbogno S, Costi S, Romano M, Cimaz R Pediatric Rheumatology Group of the Milan Area. Absence of severe complications from SARS-CoV-2 infection in children with rheumatic diseases treated with biologic drugs. J Rheumatol. 2020:Online ahead of print. doi: 10.3899/jrheum.200483. [DOI] [PubMed] [Google Scholar]
- 181.Cheng C, Li C, Zhao T, Yue J, Yang F, Yan Y, Liu X. COVID-19 with rheumatic diseases: a report of 5 cases. Clin Rheumatol. 2020;39:2025–2029. doi: 10.1007/s10067-020-05160-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Gianfrancesco MA, Hyrich KL, Gossec L, Strangfeld A, Carmona L, Mateus EF, Sufka P, Grainger R, Wallace Z, Bhana S, Sirotich E, Liew J, Hausmann JS, Costello W, Robinson P, Machado PM, Yazdany J COVID-19 Global Rheumatology Alliance Steering Committee. Rheumatic disease and COVID-19: initial data from the COVID-19 Global Rheumatology Alliance provider registries. Lancet Rheumatol. 2020;2:e250–e253. doi: 10.1016/S2665-9913(20)30095-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, Chatham W, Cohen S, Costenbader K, Gravallese EM, Kalil AC, Weinblatt ME, Winthrop K, Mudano AS, Turner A, Saag KG. American College of Rheumatology Guidance for the Management of Rheumatic Disease in Adult Patients During the COVID-19 Pandemic: Version 1. Arthritis Rheumatol. 2020:Online ahead of print. doi: 10.1002/art.41301. [DOI] [PubMed] [Google Scholar]
- 184.McGonagle D, Sharif K, O'Regan A, Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19:102537. doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Monteagudo LA, Boothby A, Gertner E. Continuous Intravenous Anakinra Infusion to Calm the Cytokine Storm in Macrophage Activation Syndrome. ACR Open Rheumatol. 2020;2:276–282. doi: 10.1002/acr2.11135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Bianchi I, Lleo A, Gershwin ME, Invernizzi P. The X chromosome and immune associated genes. J Autoimmun. 2012;38:J187–J192. doi: 10.1016/j.jaut.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 187.Sawalha AH, Zhao M, Coit P, Lu Q. Epigenetic dysregulation of ACE2 and interferon-regulated genes might suggest increased COVID-19 susceptibility and severity in lupus patients. Clin Immunol. 2020;215:108410. doi: 10.1016/j.clim.2020.108410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Conti P, Younes A. Coronavirus COV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J Biol Regul Homeost Agents. 2020;34:339–343. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 189.Deftereos SG, Siasos G, Giannopoulos G, Vrachatis DA, Angelidis C, Giotaki SG, Gargalianos P, Giamarellou H, Gogos C, Daikos G, Lazanas M, Lagiou P, Saroglou G, Sipsas N, Tsiodras S, Chatzigeorgiou D, Moussas N, Kotanidou A, Koulouris N, Oikonomou E, Kaoukis A, Kossyvakis C, Raisakis K, Fountoulaki K, Comis M, Tsiachris D, Sarri E, Theodorakis A, Martinez-Dolz L, Sanz-Sánchez J, Reimers B, Stefanini GG, Cleman M, Filippou D, Olympios CD, Pyrgakis VN, Goudevenos J, Hahalis G, Kolettis TM, Iliodromitis E, Tousoulis D, Stefanadis C. The Greek study in the effects of colchicine in COvid-19 complications prevention (GRECCO-19 study): Rationale and study design. Hellenic J Cardiol. 2020;61:42–45. doi: 10.1016/j.hjc.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Khan F, Fabbri L, Stewart I, Robinson K, Smyth AR, Jenkins G. A systematic review of Anakinra, Tocilizumab, Sarilumab and Siltuximab for coronavirus-related infections. medRxiv [Internet] 2020:2020.04.23.20076612. [Google Scholar]
- 191.Duret PM, Sebbag E, Mallick A, Gravier S, Spielmann L, Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann Rheum Dis. 2020:Online ahead of print. doi: 10.1136/annrheumdis-2020-217362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guaraldi G, Meschiari M, Cozzi-Lepri A, Milic J, Tonelli R, Menozzi M, Franceschini E, Cuomo G, Orlando G, Borghi V, Santoro A, Di Gaetano M, Puzzolante C, Carli F, Bedini A, Corradi L, Fantini R, Castaniere I, Tabbì L, Girardis M, Tedeschi S, Giannella M, Bartoletti M, Pascale R, Dolci G, Brugioni L, Pietrangelo A, Cossarizza A, Pea F, Clini E, Salvarani C, Massari M, Viale PL, Mussini C. Tocilizumab in patients with severe COVID-19: a retrospective cohort study. Lancet Rheumatol. 2020:1–11. doi: 10.1016/S2665-9913(20)30173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Campochiaro C, Della-Torre E, Cavalli G, De Luca G, Ripa M, Boffini N, Tomelleri A, Baldissera E, Rovere-Querini P, Ruggeri A, Monti G, De Cobelli F, Zangrillo A, Tresoldi M, Castagna A, Dagna L TOCI-RAF Study Group. Efficacy and safety of tocilizumab in severe COVID-19 patients: a single-centre retrospective cohort study. Eur J Intern Med. 2020;76:43–49. doi: 10.1016/j.ejim.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cardone M, Yano M, Rosenberg AS, Puig M. Lessons Learned to Date on COVID-19 Hyperinflammatory Syndrome: Considerations for Interventions to Mitigate SARS-CoV-2 Viral Infection and Detrimental Hyperinflammation. Front Immunol. 2020;11:1131. doi: 10.3389/fimmu.2020.01131. [DOI] [PMC free article] [PubMed] [Google Scholar]