Abstract
As the only stable binary compound formed between an alkali metal and nitrogen, lithium nitride possesses remarkable properties and is a model material for energy applications involving the transport of lithium ions. Following a materials design principle drawn from broad structural analogies to hexagonal graphene and boron nitride, we demonstrate that such low dimensional structures can also be formed from an s-block element and nitrogen. Both one- and two-dimensional nanostructures of lithium nitride, Li3N, can be grown despite the absence of an equivalent van der Waals gap. Lithium-ion diffusion is enhanced compared to the bulk compound, yielding materials with exceptional ionic mobility. Li3N demonstrates the conceptual assembly of ionic inorganic nanostructures from monolayers without the requirement of a van der Waals gap. Computational studies reveal an electronic structure mediated by the number of Li-N layers, with a transition from a bulk narrow-bandgap semiconductor to a metal at the nanoscale.
Subject terms: Chemistry, Materials science
Lithium nitride is the only stable binary alkali metal-nitrogen compound and shows promise for energy applications involving the transport of lithium ions. Here, the authors demonstrate that lithium nitride nanostructures can be grown as fibres and sheets despite the absence of a van der Waals gap.
Introduction
Lithium nitride, Li3N, was originally proposed for use as an electrolyte in all solid-state Li+ ion batteries given its exceptional ionic conductivity at room temperature (ca. 10−3 S cm−1)1. Indeed, for several decades it remained the highest conducting crystalline Li+ ion conductor at ambient conditions hampered chiefly by its low decomposition potential despite many ongoing attempts to stabilise it. Doping with late transition metals, however, triggers electronic conductivity that can be exploited in anodes with more than twice the charging capacity of graphite2. Li3N has also been proposed for a myriad of other applications, e.g. as a means of converting CO2 into useful products3, as the electron injection layer in organic light-emitting diodes4 and as an unusual reducing agent in preparative organic and organometallic chemistry5. Further, in 2002 Li3N was revealed as a potential candidate for solid-state hydrogen storage given its capability to accommodate up to 10.4 wt.% H26. Slow kinetics for H2 sorption and high (de)hydrogenation temperatures are the primary hurdles to overcome before the Li–N–H system can be exploited commercially, however. By combining experiment and calculation, we demonstrate how the changes in electronic structure and reduction of diffusion lengths brought about by chemically nanostructuring Li3N can lead to dramatic changes in electronic properties and ionic transport behaviour.
Results
Synthesis and characterisation
Li3N nanocrystals grow following the heating and cooling of the bulk nitride powder under a reduced pressure of nitrogen (see “Methods”). Powder X-ray diffraction (PXD) data for the fine powder product matches to hexagonal α-Li3N (P6/mmm; a = 3.656(2) Å; c = 3.868(4) Å) (Supplementary Fig. 1). In the nanofibre syntheses, long structures with diameters ranging from 200 nm to 2 μm and lengths in excess of 10 μm were produced (Fig. 1). Atomic force microscopic (AFM) measurements confirmed these ranges of thicknesses in the different fibres observed (Supplementary Fig. 2). The morphology of the nanostructured material can be tailored by controlling the preparative conditions to yield different types of nanofibres (see “Methods”).
Fig. 1. SEM micrographs of typical Li3N nanofibres (type I) at different magnifications.
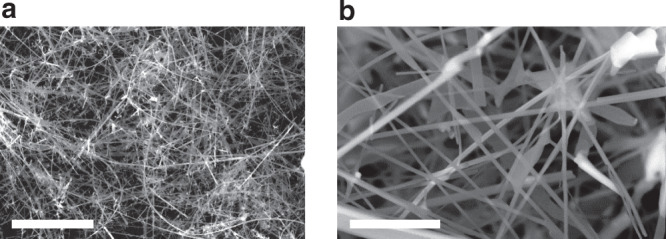
Images are taken at: a ×500 and b ×5000. Scale bars correspond to 50 μm (a) and 5 μm (b).
The growth of the one-dimensional (1D) nanostructures can be rationalised in terms of a self-assisted vapour–liquid–solid (VLS) mechanism. Under vacuum, Li3N will decompose at a temperature below the ambient pressure decomposition temperature (815 °C; ΔH(298K) = −171.3 +/− 7.7 kJ mol−1)7,8 (Eq. 1), forming droplets of liquid Li, since the melting point of Li (T = 180.5 °C) lies well below the applied reaction temperatures. These Li seeds would act as nucleation sites for the N2(g) species, which supersaturate the Li droplets and eventually lead to anisotropic growth and Li3N fibre formation. Energy-dispersive X-ray spectroscopy (EDX) data of the fibrous material did not show any traces of other metals such as Fe (from the wire used to support the reaction vessel), ruling out the possibility of Fe acting as a catalyst or seed for the formation of the fibres described here (Supplementary Fig. 3). Self-catalytic growth of GaN nanowires from Ga droplets and gallium/nitrogen vapour species has been observed previously9. The comparison to GaN is apposite in that both binary nitrides are formed from low melting point metals.
| 1 |
Two types of single-crystalline straight fibres with different growth orientations (I and II) form under varying reaction conditions (see Supplementary Tables 1 and 2): type I nanofibres (Fig. 2a, b) exhibit a growth direction of <>, whereas, conversely, type II nanofibres exhibit a growth direction of <0001> (Fig. 2c, d). Typically, type II nanofibres were found together with a few fibres and nanosheets with zig-zag morphology (Supplementary Figs. 4 and 5). Thus Li3N nanofibres exist in which the hexagonal [Li2N] layers are stacked either parallel or perpendicular to the principal fibre axis. Carbon nanofibres can assemble in an analogous fashion, where graphene layers can be oriented either perpendicular or parallel to the principal fibre axis10,11. Our experimental evidence shows that the pressure within the reaction vessel is likely to be the crucial parameter for the preferential growth of type I and II nanofibres, i.e. under identical reaction times and temperature, type II fibres will be favoured over type I fibres at higher reaction pressures (Supplementary Tables 1 and 2). There are several examples in the literature that describe how the total pressure and/or precursor partial pressure can control the growth direction of fibres; these preferences can be both thermodynamically and kinetically driven12. Indeed, in this respect, our density functional theory (DFT) calculations suggest that type I nanofibres (−325.44 eV Li3N unit−1 see “Methods”) are more thermodynamically stable than the equivalent type II fibres (−324.59 eV Li3N unit−1). The dependence of growth direction on seed concentration in layered BN and GaN fibres has been previously reported13,14. Moreover, it has been well documented that nanowire growth direction is directly impacted by pressure across diverse systems of varying complexity (e.g. in semiconductors such as Si, In2O3 and InN)15–18. One might expect an increased Li seed concentration at elevated reaction pressure. An increase in N2 partial pressure (concentration) and the N:Li ratio at the growth interface should lead to the formation of Li-N planes and type II fibre propagation. Local variation in pressure and N:Li stoichiometry might, therefore, account for the formation of zig-zag (kinked) nanofibres and sheets observed in type II samples (Supplementary Figs. 4 and 5); analogous behaviour has been noted for Si and InN nanowires, for example16,17.
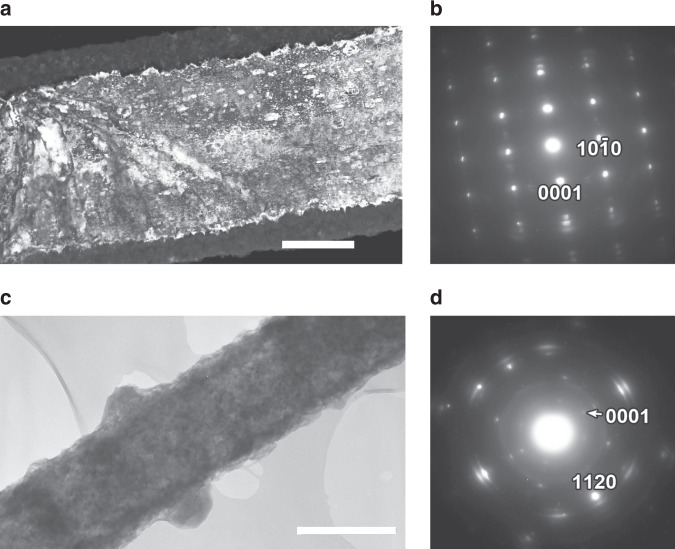
Fig. 2. Transmission electron microscopic (TEM) images and selected area electron diffraction (SAED) patterns of type I and type II fibres.
a High-magnification dark-field TEM image of a type I fibre (Li3N core and an LiOH outer layer) and b corresponding SAED pattern in the <20> projection demonstrating the <100> nanofibre growth direction; c bright-field TEM image of a type II fibre and d corresponding SAED pattern in the <010> projection (with arcs due to an LiOH surface layer) showing the <0001> growth direction. Scale bars in a and c correspond to 500 nm.
Raman spectra from nanostructured Li3N (fibres type I and type II) and bulk Li3N showed one prominent peak at ca. 580 cm−1, attributable to the only Raman active mode of Li3N, i.e. E2g (Supplementary Fig. 6). This phonon mode corresponds to the displacement of the Li(2) and N atoms along the ab plane in Li3N19 and is consistent with group theory predictions and the spectra of bulk Li3N20. We found that the E2g mode band was slightly blue-shifted (by ca. 5 cm−1) with respect to the bulk for both type I and type II fibres. Raman spectra of microcrystals of structurally similar graphite and BN21,22 and of BN nanotubes23 also revealed that the E2g band broadens and shifts to a higher frequency as particle size decreases. Ab-initio calculations on BN single-walled nanotubes attribute the blue shift in the E2g mode (of 5 cm−1) to a shortening of the sp2 bonds with respect to the bulk23. The additional bands observed at ca. 500 cm−1 and 650 cm−1 in both bulk and nanoscale Li3N samples were assigned to the acoustic (A) and optical phonon (O) modes 2 TA (z, K), 2 TO(A) and 2 LA(A) (T = transverse, L = longitudinal) by direct comparison with the second-order Raman spectra of Li3N19,24. Bands corresponding to the second-order phonon mode (2 LA (M, K)) were also observed in the 250–460 cm−1 region. The presence of these modes is attributed to the resonant conditions established when using laser irradiation, i.e. at 2.33 eV25.
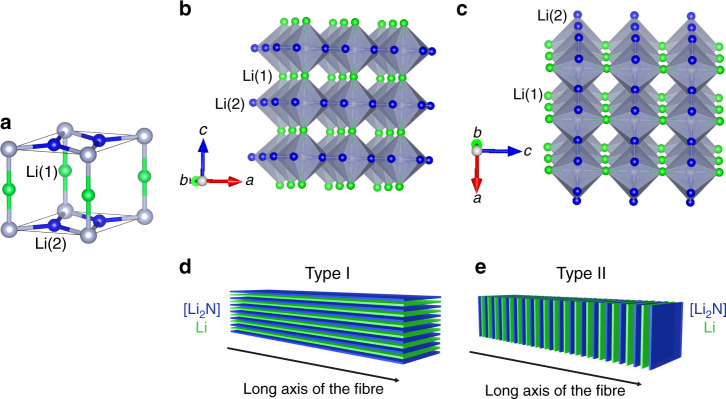
The layered crystal structure of Li3N (hexagonal P6/mmm) shares salient symmetry features with those of hexagonal graphite and BN. [Li2N] layers contain Li atoms in trigonal planar coordination to N, ostensibly by analogy to sp2 hybridised carbon in the graphene layers of graphite or to boron and nitrogen in BN (Fig. 3)1. Given the formation of anisotropic fibres (types I and II; Fig. 3) like layered chalcogenides, MX2 (M = early transition metal; X = S, Se), it may be tempting to classify Li3N as an extension of this latter class of materials26. Importantly, however, what distinguishes Li3N from the above examples is the absence of a van der Waals gap; although anisotropic, Li3N is connected in the third dimension by Li atoms (forming infinite [-Li-N-Li-] chains).
Fig. 3. Schematic diagrams illustrating the Li3N crystal structure and type I and type II nanofibres.
a Li3N unit cell, showing Li(1) atoms (green spheres), Li(2) atoms (blue spheres) and N (grey spheres); polyhedral representations of Li3N showing Li(1) atoms and Li(2) atoms: b aligned in the ab direction and c perpendicular to the ab direction; corresponding depictions of d type I and e type II Li3N nanofibres, based on the orientation of the Li (green) and [Li2N] layers (blue) with respect to the long axis of the fibre.
Li+ and H+ transport properties
Given the exceptional Li+ fast ionic conducting properties of bulk Li3N, we wished to understand how the ionic transport might vary as a consequence of nanostructuring. Variable-temperature wideline 7Li solid-state nuclear magnetic resonance (NMR) measurements between 133 and 373 K enabled the local structure of the type I and type II nanofibres to be probed and allowed the Li+ ion transport properties of the Li3N nanofibres to be determined (Fig. 4, Supplementary Note 2 and Supplementary Figs. 7 and 8). The line narrowing observed particularly for the Li(2) satellite lines between 133 and 293 K can be interpreted in terms of intra-layer Li+ diffusion, by analogy with the corresponding behaviour for bulk Li3N27. Similarly, the broadening that occurs >293 K and eventually causes both sets of satellites to disappear into the baseline results from inter-layer diffusion via an exchange of Li+ between the Li(1) and Li(2) sites. Assuming simple Arrhenius behaviour, the activation energy for the former intra-layer process can be measured from the temperature variation of the linewidth and was found to be 0.075 and 0.053 eV for type I and II Li3N nanofibres, respectively, which should be compared with the value of 0.121 eV, previously obtained for bulk Li3N28 (Supplementary Table 3). Hence, Li+ ion hopping becomes more facile within the Li-N planes as a result of nanofibre formation. Electrochemical impedance spectroscopic measurements were attempted on these samples to corroborate the conductivity results obtained by NMR. However, the high temperatures required to sinter pellets pressed from the Li3N nanofibres (i.e. to reduce grain boundary resistances) compromised their nanostructured morphology, so although high values of conductivity could be obtained (ca. 1 × 10−3 S cm−1), we could not treat the data obtained as representative of the fibres.
Fig. 4. 7Li NMR characterisation at variable temperatures of type I and II fibres.
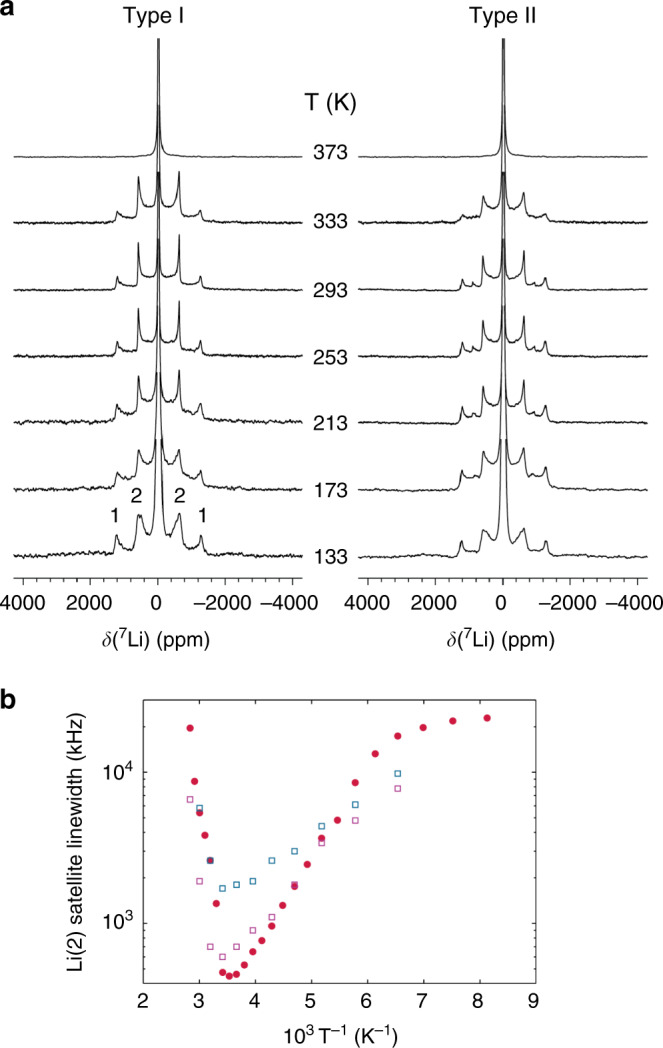
a Variable-temperature (VT) wideline 7Li NMR spectra recorded between 133 and 373 K for type I (left) and type II (right) Li3N nanofibres. 1 and 2 denote satellite bands for the two-coordinate Li(1) site and three-coordinate Li(2) site, respectively; b temperature variation of the linewidth of the Li(2) satellites for type I (magenta) and II (blue) Li3N nanofibres and bulk Li3N (red)28 extracted from the VT NMR by fitting a simulated powder lineshape.
High Li+ ion conductivity is a fundamental requirement for solid electrolytes to compete against organic electrolytes in lithium-ion batteries. To date, only a few sulfide-type electrolytes have shown comparable ionic conductivities to commercial organic electrolytes29. Thus nanostructuring of solid electrolytes might be a plausible approach to increase ionic conductivity. This field is only at its infancy, with most of the work conducted on the Li7La3Zr2O12 garnet solid electrolyte (as a sintered ceramic or as an inorganic filler component in solid composite polymer electrolytes)30. Nevertheless, given the low decomposition potential of Li3N (ca. 0.45 V vs. Li+/Li)31 and the enhanced electronic conductivity expected in these nanostructured materials when compared to the bulk (see “DFT calculations” section), the use of Li3N as a solid electrolyte seems implausible from a practical point of view. On the other hand, in lithium-containing hydrogen storage materials, e.g. the lithium amide–lithium imide system, proton mobility has been intrinsically linked to Li+ ion diffusion32. Hence, we expected that the high Li+ ion mobility observed in these Li3N nanofibres may lead to improved hydrogen absorption properties.
Preliminary volumetric experiments to determine the hydrogen uptake properties of the nanostructured Li3N material were conducted by differential pressure analysis (DPA). As compared to bulk samples of highly crystalline, single-phase Li3N, the initial H2 uptake for the nano-Li3N material was found to be lower (8.9 vs. 10.5 wt%). The latter uptake is typical for pristine bulk material and matches the theoretical capacity from a two-step reaction mechanism (Eq. 2)6:
| 2 |
One might postulate that the reduction in initial gravimetric capacity in the nanofibres is a result of surface hydrolysis (passivation) on handling in air (as observed from transmission electron microscopy (TEM)/selected area electron diffraction experiments; Supplementary Note 1 and Supplementary Fig. 9). Nevertheless, there is a greater than threefold increase in the rate of hydrogen sorption for nanocrystalline Li3N with respect to the bulk material in the first cycle, which dramatically improves further in subsequent uptake cycles (Supplementary Fig. 10). The microstructure of the dehydrogenated nitride is retained through cycling (from imide to amide and vice versa) and hence the (de)hydrogenation process is pseudomorphic and reversible (Supplementary Fig. 11). The enhanced hydrogen uptake behaviour of the nanofibres is broadly analogous to the uptake kinetics observed when Li3N is impregnated in mesoporous carbon or used in carbon nanocomposites (also at a reduced sorption temperature of 200 °C and which exhibit a hydrogen desorption enthalpy that is half that of the bulk material)33. Unlike these composites, however, the nanofibres do not suffer from the gravimetric capacity penalty imposed by an inactive component. Computational studies have demonstrated that pseudo-molecular (Li3N)n (n = 1–7) clusters would bind H2 via coordinatively unsaturated Li atoms with an adsorption energy approximately an order of magnitude smaller than that of the bulk material34. By extension, earlier DFT calculations show that the most favourable adsorption sites for both H2 and dissociated H atoms are to N positions on the (001) surface of Li3N35. Semi-quantitatively at least, both prior studies would indicate that fabrication of narrow nanofibres or thin nanosheets of Li3N should produce storage materials with improved sorption kinetics. Thus the design and optimisation of anisotropic Li-N(-H) nanomaterials could prove a useful strategy towards attaining a storage solution that meets the challenging criteria required for implementing hydrogen as a fuel for transport. Further studies should establish whether this is indeed the case.
Calculations on nanostructured α-Li3N electronic properties
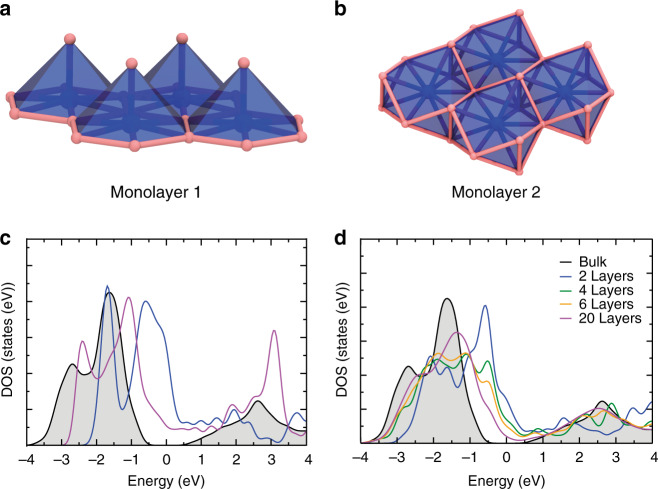
Electronic properties were computed on α-Li3N using DFT to compare the behaviour of a monolayer of Li3N with respect to the bulk material and to develop an understanding of the evolution of electronic structure with the number of layers as compared to graphene and other van der Waals nanostructures (vdWNs). Our calculations confirm that bulk α-Li3N is a semiconductor with an indirect bandgap of Eg = 1.3 eV, in agreement with several previous first-principles calculations of the α-Li3N electronic properties36–39. The electronic bandgap predicted by DFT is notably smaller than the experimental optical bandgap of 2.18 eV for Li3N single crystals40. This discrepancy is due to the well-known DFT problem in underestimating conduction band state energies41,42. Unlike layers in vdWNs, a single [Li2N] layer is unstable and computational optimisation produces two monolayer structures from the combination of [Li2N] and [Li] planes, forms 1 and 2 (Fig. 5a, b), of which the latter is more stable by −0.574 eV.
Fig. 5. Li3N monolayer configurations and electronic structures determined from density functional theory calculations.
Calculated Li3N monolayer structures of: a form 1; b form 2; c comparison of the total DOS for bulk Li3N (black) with form 1 (blue) and 2 (magenta) monolayers, respectively; d evolution of the total DOS for increasing numbers of Li3N layers (2 (blue), 4 (green), 6 (yellow) and 20 (magenta)) as compared to that of the bulk material (black).
Although the profile of the single-layer density of states (DOS) more closely resembles that of the bulk material for the form 2 monolayer, both single-layer forms are predicted to be metallic (Fig. 5c). In each case, the DOS below and approaching the Fermi level is attributable to N states, whereas the higher states of the conduction band are composed predominantly of Li states. As the number of layers in a Li3N slab is increased, the total DOS at the Fermi level decreases and a clear tendency towards an opening of a bandgap is observed (Fig. 5d). Li3N nanosheets composed of up to 20 layers (75.6 Å thick), for example, remain metallic, although using accurate quantum chemical approaches it is difficult to predict a critical thickness at which the metal–semiconductor transition occurs. The evolution of the electronic structure contrasts markedly with graphene, where there is a relatively abrupt change from zero bandgap semiconducting to semi-metallic graphite-like behaviour as the number of stacked monolayers increases43. Experimental data suggest that the bandgap in BN 5-layer nanosheets (5.56 eV) is smaller than that calculated for a BN monolayer (6 eV), but both values are larger than that of the bulk material (5.2–5.4 eV) and the nitride remains an insulator44–46.
Discussion
In summary, anisotropic 1D Li3N nanostructured materials can be formed by broad analogy to sp2-like layered solids such as graphite and boron nitride, despite the absence of a van der Waals gap in Li3N. Two distinct types of Li3N fibres have been identified, here denoted as type I and type II, which differ in the orientation of the [Li2N] layers (parallel or perpendicular) with respect to the long axis of the fibre. The formation of 1D structural variants of Li3N has important consequences for the transport of Li+ (and H+) ions, which lead to demonstrable differences in diffusion behaviour compared to the bulk material. DFT calculations have shown that the electronic properties of these Li3N materials appear scalable with size and shape.
Given their predicted metallic-like behaviour and high Li+ ion mobility, we anticipate that these nano-based Li3N materials could play an important role as additives in high-power Li-based batteries, which demand rapid ionic and electronic kinetics. Currently, bulk and surface-passivated Li3N has been used as a pre-lithiation additive in various cathode materials (e.g. LCO, NMC)47,48 to offset the first-cycle lithium loss and thus to improve the overall energy density of the material. Furthermore, bulk Li3N has been used as a protective coating for Li metal anodes in Li-ion and Li-S batteries to avoid dendrite growth and parasitic reactions between polysulfides and Li metal49,50. Importantly, the nanostructured materials allow for the rapid growth of an insulating surface passivation layer (e.g. formed from Li2CO3) conferring higher chemical stability that could eliminate reactions at the electrolyte interface. From a different perspective, the superior Li+ ion diffusion in the Li3N nanofibers leads to improved hydrogen absorption/desorption properties in the Li–N–H system given the intrinsic relationship between proton and lithium-ion conduction in this system. Thus, by analogy, it may be conceivable to revisit other materials related to the Li–N–H family of compounds to explore the impact of nanoscaling on hydrogen storage and generation more widely.
Our studies clearly demonstrate that combining an s-block element with nitrogen under the appropriate synthetic conditions can lead to the formation of anisotropic nanomaterials. Nanostructuring has a palpable influence on many chemical and physical properties in the Li–N system and there are likely to be other exciting phenomena and behaviours to be discovered. We expect that this work will stimulate further research on this system and those containing other s-block elements.
Methods
Synthesis of type I Li3N nanofibres
α-Li3N (ca. 0.1 g) prepared from the nitridation of a sodium/lithium alloy28 was contained within an iron foil liner that was placed inside a stainless-steel crucible. The crucible was suspended by an iron wire inside a long, 3 cm internal diameter stainless-steel reaction vessel that was water-cooled at the upper end. The vessel was evacuated to a pressure of ca. 5 Pa (a range of 4–6.7 Pa), then sealed and heated to 1023–1073 K in a vertical furnace for 6 days. Finally, the furnace was cooled to room temperature. Supplementary Table 1 provides detailed reaction conditions for each of the syntheses performed that yielded type I Li3N nanofibres.
Synthesis of type II Li3N nanofibres
α-Li3N (ca. 0.1 g) prepared from the nitridation of a sodium/lithium alloy28 was contained within an iron foil liner that was placed inside a stainless-steel crucible. The crucible was suspended by an iron wire inside a long, 1.5 cm internal diameter stainless steel reaction vessel that was water-cooled at the upper end. The vessel was evacuated to a pressure of ca. 10 Pa (a range of 9.3–10.7 Pa), then sealed and heated to 1023–1043 K in a vertical furnace for 6 days. Finally, the furnace was cooled to room temperature. Supplementary Table 2 shows detailed reaction conditions for each of the syntheses performed that yielded type II Li3N nanofibres.
The handling of the reactants and products took place in a nitrogen-filled glovebox (Saffron Scientific Ltd.; O2 < 5 ppm; H2O < 10 ppm) during the syntheses of both types of nanofibres. In both cases, on cooling, red/dark purple fibrous material (10–40 mg) was found deposited on the Fe wire above the reaction crucible. EDX spectra of all nanomaterials reveal peaks only from nitrogen (and oxygen from brief air exposure). Given that Li is not detectable by EDX and that no peaks from lithium metal (or other Li-containing phases) are observed in solid-state NMR spectra, the results are consistent with the presence of single-phase Li3N. The absence of metal impurity peaks corroborates a self-assisted growth mechanism.
Powder X-ray diffraction
PXD data were collected using a Philips X’Pert θ–2θ diffractometer with a PW3710 control unit using Cu Kα radiation (λ = 1.5418 Å), operating at 40 kV and 40 mA. Samples were run within a bespoke air-tight aluminium holder with Mylar windows51. Data were collected in the range from 5 to 80° 2θ in steps of 0.025° s−1. Sample preparation for analysis took place in an N2-filled glovebox. Lattice parameters were refined by least-squares fitting using the CELL software package52.
Scanning electron microscopy (SEM)
A Philips XL30 ESEM-FEG instrument was used for SEM and EDX characterisation. Experiments were performed in high vacuum mode with an applied accelerating voltage of 15 kV. Samples were loaded onto aluminium stubs using adhesive carbon tabs and the transfer was performed under a stream of flowing N2 gas.
Atomic force microscopy
AFM sample preparation consisted of dispersing the samples in n-propyl acetate in an Ar-filled glove box. Then a droplet of the concentrated Li3N solution was placed onto a silicon wafer for measurement. AFM analyses were carried out using a Keysight 5500 instrument in tapping mode to produce topographical information of the samples.
Transmission electron microscopy
TEM analyses were performed using different instruments under varying operating conditions. Measurements performed at 80 kV were conducted using a JEOL JEM-2200MCO FEGTE. Samples were loaded in an N2-filled glovebox onto a custom-made air-tight holder to minimise air exposure during transfer. Analyses performed at 200 kV were made using either a JEOL JEM-2000FX II TEM or an FEI Tecnai G2 20 TEM. TEM samples were prepared by depositing the Li3N dry onto a 3-mm holey carbon film copper grid in an N2-filled glovebox. Each grid was placed within a sealed container and transferred to the instrument under a stream of N2. In both cases, a small condenser aperture was used to reduce beam damage (the result of which is evident in Supplementary Fig. 12) and evaporation due to the instability of nanoscale Li3N under the beam.
7Li NMR spectroscopy
Wideline 7Li NMR spectra of Li3N nanomaterials were recorded from 133 to 453 K at a Larmor frequency of 116.6 MHz on a Varian InfinityPlus Spectrometer equipped with a single-resonance broadband probe. Spectra were obtained using an EXORCYCLED solid-echo sequence with π/2 pulses of 1.3 µs and an echo delay of 16.0 µs. Relaxation delays between 0.5 and 10 s were used depending on the temperature. Chemical shifts are referenced externally to aqueous LiCl.
Raman spectroscopy
Raman spectra were collected using a Horiba-Yvon LabRam HR spectrometer with a confocal microscope at room temperature using a 532-nm laser with a 600 g mm−1 grating and a Synapse CCD detector. Samples were mounted in sealed glass capillaries in an N2-filled glovebox to avoid air exposure. A ten times reduced incident laser power (15 mW) was used together with a 100-nm aperture to avoid damaging the material under the beam during analysis.
Hydrogen storage measurements
Volumetric hydrogen uptake measurements were performed by loading ca. 0.2 g sample into a Swagelock tube in an N2-filled glovebox and sealed using parafilm. The tube was connected to the DPA apparatus53 and the parafilm was seal-broken using a flow of helium gas. The sample was evacuated before exposure to hydrogen and heated to the reaction temperature. H2(g) and He(g) were dried using a liquid nitrogen trap before use. Hydrogen uptake was performed at 10 bar at 200 °C. After reaching equilibrium, the sample was desorbed at 200 °C before performing the next absorption cycle.
DFT calculations
Electronic properties of bulk and nano-sheets of α-Li3N were calculated at the gradient-corrected DFT level by applying the Perdew–Burke–Ernzerhof54 exchange-correlation functional as implemented in the SIESTA program package55. Electronic states were expanded by a double-ζ plus polarisation basis set with norm-conserving Troullier–Martins pseudopotentials56 for the description of core levels and a plane-wave representation of the charge density with a cut-off of 240 Ry. The reciprocal space was sampled by a Monkhorst–Pack grid57 of 16 × 16 × 32 k-points in the Brillouin zone (16 × 16 × 1 k-points in the case of nanosheets). Models of nanosheets are composed of an increasing number of unit cell replicas constructed in the direction perpendicular to the nano-sheet plane and a 15-Å-thick vacuum region. Geometries of all systems were relaxed until a maximum gradient of 0.02 eV Å−1 on forces was reached. In the calculations of the total energies of type I and type II nanofibres, the model structures were built with an increasing number of replicas of the unit cell in the non-periodic directions to achieve the convergence in terms of energy per number of replicas (14 replicas were used for type I nanofibre and 8 × 8 for type II nanofibre). An MP k-points sampling with 16 k-points in the periodic directions has been applied (two directions for type I nanofibre and one direction for type II nanofibre).
Supplementary information
Acknowledgements
D.H.G. acknowledges the EPSRC for financial support under grants GR/S26965 and EP/E040071, the University of Nottingham for studentships to A.G.G. and H.K.E., the University of Glasgow for a studentship to C.W.D. and ScotCHEM for a studentship to N.T.-R. E.B. acknowledges a New Directions for EPSRC Research Leaders Award (EP/G005060), the European Research Council under the European Union’s Seventh Framework Programme (FP7)/ERC grant agreement No. 307755-FIN and a Royal Society Wolfson Fellowship for financial support. M.B. and E.B. acknowledge the use of Athena at HPC Midlands+, which was funded by the EPSRC on grant EP/P020232/1 as part of the HPC Midlands+ consortium.
Author contributions
N.T.-R., A.G.G., C.M.J. and C.W.D. developed the synthesis methods and characterised materials by X-ray diffraction, spectroscopy and electron microscopy; H.K.E., P.D.B. and I.M. performed transmission electron microscopy and electron diffraction experiments and analysed and interpreted these data; J.M.B., C.P.S., C.M.J. and N.T.-R. performed hydrogen uptake experiments, analysed and interpreted these data; M.B. and E.B. performed computational calculations, analysed, interpreted the data and prepared these for publication; J.J.T. designed the solid-state NMR experiments and collected and interpreted the NMR data; D.H.G. initiated and supervised the project and analysed and interpreted the data. N.T.-R., J.J.T., E.B. and D.H.G. wrote the manuscript. All authors discussed the results and commented on the paper.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.
Competing interests
The authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks Albert Davydov, Moneesh Upmanyu and the other anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Nuria Tapia-Ruiz, Alexandra G. Gordon.
Supplementary information
Supplementary information is available for this paper at 10.1038/s41467-020-17951-6.
References
- 1.Boukamp BA, Huggins RA. Fast ionic conductivity in lithium nitride. Mater. Res. Bull. 1978;13:23–32. [Google Scholar]
- 2.Shodai T, Okada S, Tobishima S, Yamaki J. Study of Li3-xMxN (M: Co, Ni or Cu) system for use as anode material in lithium rechargeable cells. Solid State Ionics. 1996;86–88:785–789. [Google Scholar]
- 3.Hu YH, Huo Y. Fast and exothermic reaction of CO2 and Li3N into C-N-containing solid materials. J. Phys. Chem. A. 2011;115:11678–11681. doi: 10.1021/jp205499e. [DOI] [PubMed] [Google Scholar]
- 4.Duan L, et al. Thermally decomposable lithium nitride as an electron injection material for highly efficient and stable OLEDs. J. Phys. Chem. C. 2009;113:13386–13390. [Google Scholar]
- 5.Baldwin FP, Blanchard EJ, Koenig PE. Metal nitrides in organic reactions. I. Reactions of lithium nitride with acid chlorides. Preparation of N,N-diacylamides. J. Org. Chem. 1965;30:671–673. [Google Scholar]
- 6.Chen P, Xiong Z, Luo J, Lin J, Tan KL. Interaction of hydrogen with metal nitrides and imides. Nature. 2002;420:302–304. doi: 10.1038/nature01210. [DOI] [PubMed] [Google Scholar]
- 7.Adams PF, Hubberstey, P. Pulham RJ. Review of the solubility of nonmetals in liquid lithium. J. Less-Common Met. 1975;42:1–11. [Google Scholar]
- 8.Kimura H, Asano M, Kubo K. Vaporization of solid lithium nitride. J. Nucl. Mater. 1980;91:200–204. [Google Scholar]
- 9.Stach EA, et al. Watching GaN nanowires grow. Nano Lett. 2003;3:867–869. [Google Scholar]
- 10.Rodriguez NM, Chambers A, Baker RTK. Catalytic engineering of carbon nanostructures. Langmuir. 1995;11:3862–3866. [Google Scholar]
- 11.Che G, Lakshmi BB, Martin CR, Fisher ER. Chemical vapor deposition based synthesis of carbon nanotubes and nanofibres using a template method. Chem. Mater. 1998;10:260–267. [Google Scholar]
- 12.Fortuna SA, Li X. Metal-catalysed semiconductor nanowires: a review on the control of growth directions. Semicond. Sci. Technol. 2010;25:024005. [Google Scholar]
- 13.Tang D-M, Liu C, Chen H-M. Controlled synthesis of quasi-one-dimensional boron nitride nanostructures. J. Mater. Res. 2007;22:2809–2816. [Google Scholar]
- 14.Kuykendall TR, Altoe MVP, Ogletree F, Aloni S. Catalyst-directed crystallographic orientation control of GaN nanowire growth. Nano Lett. 2014;14:6767–6773. doi: 10.1021/nl502079v. [DOI] [PubMed] [Google Scholar]
- 15.Lugstein A, et al. Pressure-induced orientation control of the growth of epitaxial silicon nanowires. Nano Lett. 2008;8:2310–2314. doi: 10.1021/nl8011006. [DOI] [PubMed] [Google Scholar]
- 16.Madras P, Dailey E, Drucker J. Kinetically induced kinking of vapor-liquid-solid grown epitaxial Si nanowires. Nano Lett. 2009;9:3826–3830. doi: 10.1021/nl902013g. [DOI] [PubMed] [Google Scholar]
- 17.Liu H, et al. Reactant-governing growth direction of indium nitride nanowires. Nanotechnology. 2010;21:245601. doi: 10.1088/0957-4484/21/24/245601. [DOI] [PubMed] [Google Scholar]
- 18.Shen Y, et al. Size-induced switching of nanowire growth direction: a new approach toward kinked nanostructures. Adv. Funct. Mater. 2016;26:3687–3695. [Google Scholar]
- 19.Chandrasekhar HR, Bhattacharya G, Migoni, R., Bilz H. Infrared and Raman spectra and lattice dynamics of the superionic conductor lithium nitride (Li3N) Phys. Rev. B. 1978;17:884–893. [Google Scholar]
- 20.Chandrasekhar HR, Bhattacharya G, Migoni R, Bilz H. Phonon spectra and lattice dynamics of lithium nitride. Solid State Commun. 1977;22:681–684. [Google Scholar]
- 21.Nemanich RJ, Solin SA. First- and second-order Raman scattering from finite-size crystals of graphite. Phys. Rev. B. 1979;20:392–401. [Google Scholar]
- 22.Nemanich RJ, Solin SA, Martin RM. Light scattering study of boron nitride microcrystals. Phys. Rev. B. 1981;23:6348–6356. [Google Scholar]
- 23.Arenal R, et al. Raman spectroscopy of single-wall boron nitride nanotubes. Nano Lett. 2006;6:1812–1816. doi: 10.1021/nl0602544. [DOI] [PubMed] [Google Scholar]
- 24.Kress W, Grimm H, Press W, Lefebvre J. Lattice vibrations in lithium nitride, Li3N. Phys. Rev. B. 1980;22:4620–4625. [Google Scholar]
- 25.Nesheva D. Raman scattering from semiconductor nanoparticles and superlattices. J. Optoelectron. Adv. Mater. 2005;7:185–192. [Google Scholar]
- 26.Tenne R. Inorganic nanotubes and fullerene-like materials. Chem. Eur. J. 2002;8:5296–5304. doi: 10.1002/1521-3765(20021202)8:23<5296::AID-CHEM5296>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 27.Messer R, Birli H, Differt K. NMR study of diffusion in Li3N. J. Phys. C Solid State. 1981;14:2731–2746. [Google Scholar]
- 28.Stoeva, Z., Gomez, R., Gregory, D. H., Hix, G. B. & Titman, J. J. Evolution of structure, transport properties and magnetism in ternary lithium nitridometalates Li3-x-yMxN, M = Co, Ni, Cu. Dalton Trans. 3093–3097 (2004). [DOI] [PubMed]
- 29.Jiang C, Li H, Wang C. Recent progress in solid-state electrolytes for alkali-ion batteries. Sci. Bull. 2017;62:1473–1490. doi: 10.1016/j.scib.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 30.Chan CK, Yang TJ, Weller JM. Nanostructured garnet-type Li7La3Zr2O12: synthesis, properties and opportunities as electrolytes for Li-ion batteries. Electrochim. Acta. 2017;253:268–280. [Google Scholar]
- 31.Rabenau A. Lithium nitride and related materials case study of the use of modern solid state research techniques. Solid State Ionics. 1982;6:277–293. [Google Scholar]
- 32.David WIF, et al. A mechanism for non-stoichiometry in the lithium amide/lithium imide hydrogen storage reaction. J. Am. Chem. Soc. 2007;129:1594–1601. doi: 10.1021/ja066016s. [DOI] [PubMed] [Google Scholar]
- 33.Demir-Cakan R, Tang WS, Darwiche, Janot AR. Modification of the hydrogen storage properties of Li3N by confinement into mesoporous carbons. Energy Environ. Sci. 2011;4:3625–3631. [Google Scholar]
- 34.Jiang ZP, Zhou X, Sun Q, Wang Q, Jena P. Geometry, electronic properties, and hydrogen adsorption properties of Li3N-based nanostructures. J. Phys. Chem. C. 2010;14:19202–19205. [Google Scholar]
- 35.Jin HM, Luo JZ, Wu P. Adsorption and dissociation of hydrogen on Li3N surface: a first principles study. Appl. Phys. Lett. 2007;90:084101. [Google Scholar]
- 36.Wu S, Neo SS, Dong Z, Boey F, Wu P. Tunable ionic and electronic conduction of lithium nitride via phosphorus and arsenic substitution: a first-principles study. J. Phys. Chem. C. 2010;114:16706–16709. [Google Scholar]
- 37.Zhao Y, Tian X, Xue W, Gao T. The structure, dynamical and thermodynamic properties of α-Li3N: a first-principles study. Solid State Commun. 2009;149:2130–2134. [Google Scholar]
- 38.Baumeier B, Kruger P, Pollmann J, Vajenine GV. Electronic structure of alkali-metal fluorides, oxides, and nitrides: density-functional calculations including self-interaction corrections. Phys. Rev. B. 2008;78:125111. [Google Scholar]
- 39.Dovesi R, Pisani C, Ricca F, Roetti C, Suanders VR. Hartree-Fock study of crystalline lithium nitride. Phys. Rev. B. 1984;30:972–979. [Google Scholar]
- 40.Brendecke H, Bludau W. Photoluminescence properties of lithium nitride. Phys. Rev. B. 1980;21:805–815. [Google Scholar]
- 41.Sham LJ, Schlüter M. Density-functional theory of the band gap. Phys. Rev. B. 1985;32:3883–3889. doi: 10.1103/physrevb.32.3883. [DOI] [PubMed] [Google Scholar]
- 42.Sham LJ, Schlüter M. Density-functional theory of the energy gap. Phys. Rev. Lett. 1983;51:1888–1891. [Google Scholar]
- 43.Partoens B, Peeters FM. From graphene to graphite: electronic structure around the K point. Phys. Rev. B. 2006;74:075404. [Google Scholar]
- 44.Song L, et al. Large scale growth and characterization of atomic hexagonal boron nitride layers. Nano Lett. 2010;10:3209–3215. doi: 10.1021/nl1022139. [DOI] [PubMed] [Google Scholar]
- 45.Blase X, et al. Quasiparticle band structure of bulk hexagonal boron nitride and related systems. Phys. Rev. B. 1995;51:6868–6875. doi: 10.1103/physrevb.51.6868. [DOI] [PubMed] [Google Scholar]
- 46.Hoffman DM, Doll GL, Eklund PC. Optical properties of pyrolytic boron nitride in the energy range 0.05—10 eV. Phys. Rev. B. 1984;30:6051–6056. [Google Scholar]
- 47.Sun Y, et al. Stabilised Li3N for efficient battery cathode prelithiation. Energy Storage Mater. 2017;6:119–124. [Google Scholar]
- 48.Bian Z, et al. Dual roles of Li3N as an electrode additive for Li-excess layered cathode materials: a Li-ion sacrificial salt and electrode-stabilizing agent. Chem. Eur. J. 2018;24:13815–13820. doi: 10.1002/chem.201801809. [DOI] [PubMed] [Google Scholar]
- 49.Park K, Goodenough JB. Dendrite-suppressed lithium plating from a liquid electrolyte via wetting of Li3N. Adv. Energy Mater. 2017;7:1700732. [Google Scholar]
- 50.Baloch M, et al. Variations on Li3N protective coating using ex-situ and in-situ techniques for Li° in sulphur batteries. Energy Storage Mater. 2017;9:141–149. [Google Scholar]
- 51.Barker, M. G., Begley, M. J., Edwards, P. P., Gregory, D. H. & Smith, S. E. Synthesis and crystal structures of the new ternary nitrides Sr3CrN3 and Ba3CrN3. J. Chem. Soc. Dalton Trans. 1–5 (1996).
- 52.Holland TJB, Redfern SAT. Unit cell refinement from powder diffraction data: the use of regression diagnostics. Mineral. Mag. 1997;61:65–77. [Google Scholar]
- 53.Blackman JM, Patrick JW, Snape CP. An accurate volumetric differential pressure method for the determination of hydrogen storage capacity at high pressures in carbon materials. Carbon. 2006;44:918–927. [Google Scholar]
- 54.Perdew J, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 55.Soler JM, et al. The SIESTA method for ab initio order-N materials simulation. J. Phys. Condens. Matter. 2002;14:2745. [Google Scholar]
- 56.Troullier N, Martins JL. Efficient pseudopotentials for plane-wave calculations. Phys. Rev. B. 1991;43:1993. doi: 10.1103/physrevb.43.1993. [DOI] [PubMed] [Google Scholar]
- 57.Monkhorst HJ, Pack JD. Special points for Brillouin-zone integrations. Phys. Rev. B. 1976;13:5188–5192. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study are available within the paper and its supplementary information files.