Abstract
Objective
The present study evaluates and compares the effectiveness of Simvastatin (SIM), Hydroxyapatite (HA), and platelet-rich fibrin (PRF) in bone regeneration of periapical defects.
Material& method
Thirty-nine patients were selected and randomized into three groups, Group 1: HA (n = 13), Group 2: PRF (n = 13), Group 3: SIM (n = 13). After completion of RCT and apicoectomy, the grafts were placed locally in the defect and sutured.
Results
At the end of twelve months, postoperative symptoms and radiographic analysis assessed the outcome of the treatment
Conclusion
Intragroup analysis of CBCT- Periapical Index (PAI) scores at 6th and 12th month revealed a significant change in the SIM group (p = 0.018 and 0.001 respectively), compared to PRF (p = 0.026 and 0.001 respectively) and HA (p = 0.053 and 0.039 respectively). Intergroup analysis of change in the level of CBCT-PAI score was highly significant (p = 0.003).
SIM caused a more considerable change in the level of CBCT-PAI score compared to other groups, thereby indicating a faster rate of bone regeneration.
Keywords: CBCT-PAI, Hydroxyapatite, Bone regeneration, Platelet rich fibrin, Simvastatin
1. Introduction
The success of an endodontic therapy depends on the complete elimination of microbial infection from the root canal and over-all repair and regeneration of periapical tissue. Most of the time periapical lesion heals spontaneously by proper non-surgical endodontic therapy but sometimes it fails and needs a surgical intervention along with regenerative therapy. Various specialties of dentistry have used different autogenous graft and autologous bone graft to enhance tissue formation in the defects created by lesion and surgical excavation.Recently, the introduction of various anabolic drugs like bisphosphonates, calcitonin, and statins also have shown promising results in bone regeneration in medicine.
Statins [3-hydroxy-3-methylglutaryl coenzyme A (HMG CoA) reductase inhibitors] have been used in the field of medicine as a cholesterol-lowering agents to reduce cardiovascular diseases and mortality. It was Mundy et al.1 in 1991 who discovered bone anabolic properties of statin which led its application in diseases like osteoporosis.Since then, many researchers have shown pertinent evidence related to statin-induced osteogenesis by reducing farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP) levels,2 statin-inhibited osteoblast apoptosis,3 and statin-suppressed osteoclastogenesis.4
Many researchers have confirmedthat oral administration of Statin enhances the rate of bone formation by increasing the expression of bone morphogenic proteins-2 (BMP-2) gene5 and by reducing bone resorption markers.6 Its pleiotropic actions like vasodilative, antithrombotic, antioxidant, anti-inflammatory, and immune-modulatory effects7,8 have proven to be beneficial in other bone catabolic diseases associated with oral and maxillofacial bone tissues. Several clinical studies on humans showed a positive response to oral,9 and local administration10 of Statin on the periodontal health including a greater decrease in tooth mobility, greater improvement in clinical attachment levels and greater bone fill in type II diabetes and chronic periodontitis cases.11
As a regenerative medicine, Simvastatin potentially has shown to induce odontogenic differentiation of Human Dental Pulp Stem Cells, thereby promoting pulp regeneration in cases of pulpitis.12, 13, 14 Studies have shown its positive effect in the early healing of extracted third molar sockets in humans15,16 and in attenuating the rat periapical lesions by suppressing the expression of Cysteine-rich 61(Cyr61) in osteoblasts and eventually, CD-68 positive macrophage migration into the lesions.17
Such favorable results in different streams of medicine and dentistry have open new fronts for the use of Statins to promote bone formation. One such aspect is its use in periapical surgery. Apical surgery is seen as a last resort to save a tooth before extraction, and with recent advancements, in the techniques and armamentarium, the success rates have approached or exceeded 90%.18 Bone regeneration following periapical surgery is an important event for long-term success. Hydroxyapatite (HA)19 and platelet-rich fibrin (PRF)20 are some of the bone regenerative materials of predictable results. To the best of our knowledge, there are no studies regarding the use of Statins in endodontics. Therefore the present trial was conducted to evaluate the effect of Simvastatin (SIM) on the rate of bone regeneration of peri-apical defects of endodontic origin. It was hypothesized that SIM, PRF, HA had no significant difference in the amount of bone fill in periapical defect post periapical surgery.
2. Materials and methods
Institutional Ethical Clearance was obtained from Secretariat, Research cell, “XXX,” India, to conduct this randomized clinical trial. The study was also submitted to the Clinical Trials Registry-India (CTRI), under the reference no- REF/2017/05/014445 and registration number CTRI/2017/06/008881 before commencement.
2.1. Eligibility criteria
Systemically healthy, well-motivated persons between the age group of 20yrs–40yrs requiring periapical surgery in anterior teeth were selected. Failed Re-RCT cases with recurrent sinus and pus discharge or those with periapical radiolucency more than 5 mm or CBCT-periapical score 4 & 5 were included.
While patients requiring re-apicoectomy, smokers, alcoholics, pregnant or lactating females, periodontally compromised or unrestorable or fractured teeth were excluded. Subjects having allergies or hypersensitivity to drugs, or on any systemic medication which can affect the periapical healing, e.g., bis-phosphonates and anti-resorptive treatment, hormone replacement therapy, immune-suppressants, steroids, selective serotonin reuptake inhibitors or statins were also excluded.
Depending upon the inclusion and exclusion criteria, a total of 39 patients were selected and randomized by the allocation concealment method. For this purpose, sequentially numbered, opaque, sealed envelopes (SNOSE) were prepared. Thirty-nine group information notes depending upon the regenerative therapy to be given (13 for each group) were packed randomly into 39 envelopes. Each envelope was numbered sequentially and handed over to the patient at the time of treatment. The three groups were as follows.
Group 1: Hydroxyapatite bone graft (n = 13).
Group 2: Autologous Platelet Rich Fibrin (n = 13).
Group 3: Simvastatin (n = 13).
All the steps of non-surgical and surgical endodontic treatment were performed by a single operator to remove inter-operator bias. However, the assessment and documentation of baseline and follow up parameters were performed by three different endodontists blind to the intervention given to that particular patient. A preoperative CBCT and CBCT-PAI scoring done as per Estrela et al. (2008).21 The lesions were measured by the working tools of CS 3D Imaging software (Kodak Dental Systems, Carestream Health, Rochester, NY, EUA) in three different dimensions: buccopalatal, mesiodistal, and diagonal. The final CBCT-PAI score was based on the largest measurement observed in a given lesion in one of the planes selected. A 6-point (0–5) scoring system was defined as follows:Score 0 = intact periapical structures; Score 1 = periapical radiolucency with the major diameter of 0.5 mm–1.0 mm; Score 2 = periapical radiolucency with the major diameter of 1 mm–2 mm; Score 3 = periapical radiolucency with the major diameter of 2 mm–4 mm; Score 4 = periapical radiolucency with the major diameter of 4 mm–8 mm; Score 5 = major diameter >8 mm. Two more variables were added to the system: E−expansion of cortical bone and D-destruction of cortical bone.21
2.2. Treatment procedures
2.2.1. Non-surgical treatment
Under rubber dam isolation, a straight-line access cavity prepared with a sterile Endo access bur after giving local anesthesia containing 2% lignocaine with 1:100,000 adrenaline. No. 15 k file maintained the patency of the canal. The working length measured radiographically. Removal of necrotic tissue from the root canal was accomplished by gently irrigating the root canal with normal saline dispensed through a syringe with a 27-gauge needle. In cases with frank purulent discharge, the tooth was left open for drainage with a cotton pellet in the pulp chamber, and then the patient was called after 24 h. Biomechanical preparation (BMP) accomplished with rotary instruments to an apical size of ProTaper F3. BMP was followed by irrigation with 10 ml of 2% chlorhexidine (CHX) as a final rinse. Finally, the canals were dried using sterile paper points and obturated with corresponding gutta-percha points and sealer by cold lateral condensation. The access cavity was then closed by composite resin.
3. Surgical technique
Under local anesthesia, a full-thickness mucoperiosteal flap was raised involving one tooth on either side of the diseased tooth. A window was prepared on the bony surface by the help of carbide burs in a slow speed handpiece along with copious saline irrigation. Once accessibility to the osseous defect was made, periradicular curettage was done to remove the granulation tissue from the osseous cavity. Apical 3 mm of the root end was resected, and root end preparation was done with the help of the ultrasonic system, and retrocavity was filled with MTA under hemostatic conditions.
3.1. Regenerative approach
Group 1: Synthetic HA (BioGraft HTR, IFGL Bio Ceramics) was used to fill the bony cavity before suturing the flap. (n = 13); Group 2: Autologous PRF was filled in the bony cavity before suturing the flap. (n = 13); Group 3: SIM10 mg was used with a gelatin sponge (surgispon) as a bone fill material prior to suturing the flap. (n = 13).
3.1.1. Preparation of PRF
10 ml of whole venous blood was obtained from the patient's anticubital vein. It was then transferred to a test tube without anticoagulant and centrifuged at 3000 rpm for 10 min. After centrifugation, three layers were naturally formed in the tube: platelet-poor plasma (PPP) at the surface, platelet-rich fibrin (PRF) clot in the middle, and RBC's at the bottom. With the help of a sterile tweezer, the fibrin clot was gently grabbed and removed out of the test tube and placed into the bone cavity without disturbing the root-end filling. Finally, the flap was sutured.
3.2. Preparation of the simvastatin graft
SIM10mg (Zosta-10 mg, UsvPvt Ltd) tablets were used. The outer coating was scraped off, and the tablets were then crushed into powder form.15,16,22,23 The pre-weighed SIM powder along with surgispon gelatin sponge as carrier moistened with 2 ml normal saline solution in a dappen dish was prepared and carefully grafted in the bone-defect taking care not to disturb the MTA retro-filling, and the flap was sutured.We selected 10 mg as a safe dose to place in the bone defect with a carrier gelatin sponge engulfing it.Direct placement of simvastatin into the bone tissue may lead to severe inflammatory reaction. Thus to prevent this untoward reaction the SIM was engulfed in gelatin sponge. The idea was to protect the statin by gelatin sponge and as the gel foam resorbed slowly, the powder would have a slow sustained release effect. We never got any adverse reaction to the drug in this way.
Finally, the postoperative instructions were given. Antibiotics (amoxicillin & clavulanic acid) and NSAIDs were prescribed for a period of 5 days. The patient was recalled after 24 h and one week. Sutures were removed on the 7th day. Follow-up was planned at 6th and 12th month for clinical and CBCT evaluation.
Clinical evaluation at follow-up: the patient was assessed for edema, postoperative pain, signs of infection, sinus, and pus discharge after 24 h, one week, 6th, and 12th month. Postoperative pain intensity was assessed according to the criteria established by Yoldas et al. on a 4-point descriptive scale (1 no pain; 2 mild; 3 moderate; 4 severe pain).24,25
3.3. Radiographic evaluation at the follow-up
Radiographically, CBCT scans were taken at 6th month and 12th month to determine the bone fill with time. The measurements were compared with baseline scans to assess the reduction in the PAI score of the lesion. The data were collected at the end of the 12th month and statistically analyzed.
3.4. Evaluation of the outcome
At the 12-month re-evaluation visit, teeth were classified as healed, healing, or diseased. Healed: clinical normalcy with CBCT-PAI score of 0, 1, or 2; Healing: clinical normalcy with a reduction in the size of the periradicular lesion and a reduction in the PAI score; Diseased: clinical signs and symptoms with PAI score of 3 or higher or an increase in the size of the periradicular lesion or an increase in the PAI score. The overall outcome was further dichotomized; teeth classified as healed or healing at 12th month were considered successful, and diseased teeth were considered unsuccessful. The data obtained were further analyzed.
4. Statistical analysis
The results were analyzed using descriptive statistics and making comparisons among various groups. Categorical data were summarized as proportions and percentages (%). The software used for the analysis was IBM-SPSS version 18 and MS- excel worksheet. Kappa test is used to evaluate inter-rater agreement between different observers. Fisher Exact test/chi square (χ2) tests were used to determine whether there are any statistically significant differences between the proportions of groups. A two-sided (α = 2) p < 0.05 was considered statistically significant.
5. Results
The inter-observer reliability of clinical and radiographical parameters was analyzed by the Kappa-Cohen test (k value) and was found to be k = 0.82 at all periods indicating high reliability of observed outcomes.
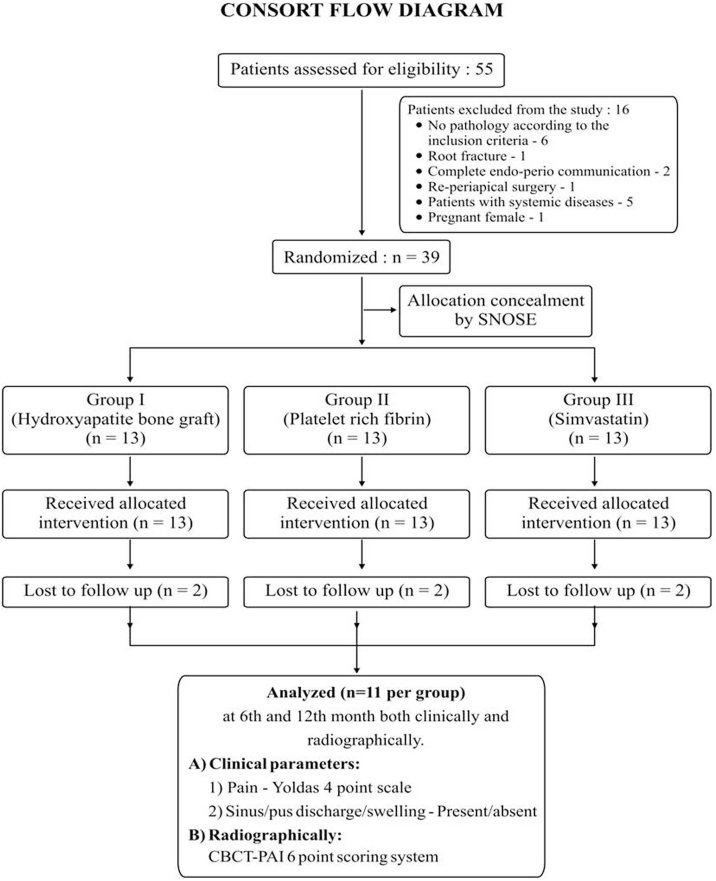
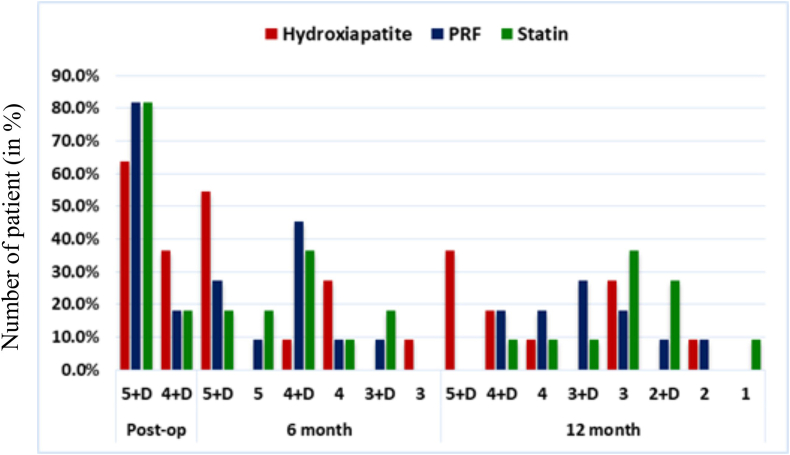
Initially, 55 patients were assessed, out of which only 39 were randomized, as the remaining didn't fulfill the inclusion criteria. However, only 11 per group were analyzed, while 2 per group were lost to follow up. Fig. 1 shows the consort flowchart. Intragroup analysis of CBCT-PAI scores at 6th and 12th month revealed a significant change in Simvastatin group (χ2 = 10.10 & 18.00 respectively; p = 0.018 and < 0.001 respectively), compared to PRF (χ2 = 7.29 & 18.00 respectively; p = 0.026 and < 0.001 respectively) and Hydroxyapatite (χ2 = 5.88 & 6.48 respectively; p = 0.053 and 0.039 respectively). This is represented in the form of graph in Fig. 2, showing greater percentage of patients with improved CBCT-PAI scores at 6th and 12th month.
Figure-1.
Consort flowchart.
Figure-2.
Bar graph showing Intergroup distribution of CBCT-PAIscores among HA, PRF and SIM groups.
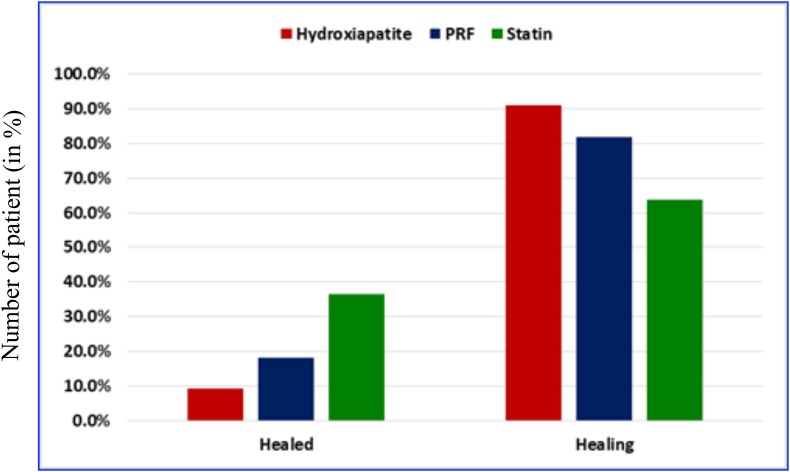
For intergroup analysis, a change in PAI score of one was taken as one level change, and the data were tabulated. A significant difference was found in the proportion of various Score changes at 12 months in group I-II (χ2 = 7.66, p = 0.022) and group I-III (χ2 = 12.4, p = 0.002) (Table 1, Table 2, Table 3). Similarly, it was seen that the Intergroup analysis of change in the level of CBCT-PAI score among the three groups was highly significant (χ2 = 16.1; p = 0.003) (Table 4).Clinically no untoward findings were noted at 6th and 12th month. Graph in Fig. 3 shows that one (9.1%) case of group-I, two (18.2%) cases of group-II, and four (36.4%) cases of group-III were healed while remaining were improving.
Table 1.
Intergroup comparison of Score Change of bone regeneration between Hydroxyapatite and PRF.
| follow-up interval | Change in Level | Hydroxyapatite N = 11) Grp-I |
PRF (N = 11) Grp-II |
Comparison |
|||
|---|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | chisq/F value | p-value | ||
| 6 month | Unchanged | 6 | 54.5% | 4 | 36.4% | 0.80 | 0.670 |
| level 1–2 | 4 | 36.4% | 6 | 54.5% | |||
| level 3–4 | 1 | 9.1% | 1 | 9.1% | |||
| 12 month | Unchanged | 4 | 36.4% | 0 | 0.0% | 7.66 | 0.022 |
| level 1–3 | 6 | 54.5% | 5 | 45.5% | |||
| level 4 & above | 1 | 9.1% | 6 | 54.5% | |||
Table 2.
Intergroup comparison of Score Change of bone regeneration between Hydroxyapatite and Statin.
| follow-up interval | Change in Level | Hydroxyapatite N = 11) Grp-I |
Statin (N = 11) Grp-III |
Comparison |
|||
|---|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | chisq/F value | p-value | ||
| 6 month | Unchanged | 6 | 54.5% | 2 | 18.2% | 3.33 | 0.189 |
| level 1–2 | 4 | 36.4% | 8 | 72.7% | |||
| level 3–4 | 1 | 9.1% | 1 | 9.1% | |||
| 12 month | Unchanged | 4 | 36.4% | 0 | 0.0% | 12.4 | 0.002 |
| level 1–3 | 6 | 54.5% | 2 | 18.2% | |||
| level 4 & above | 1 | 9.1% | 9 | 81.8% | |||
Table 3.
Intergroup comparison of ScoreChange of bone regeneration between PRF and Statin.
| Follow-up interval | Change in Level | PRF (N = 11) Grp-II |
Statin (N = 11) Grp-III |
Comparison |
|||
|---|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | chisq/F value | p-value | ||
| 6 month | Unchanged | 4 | 36.4% | 2 | 18.2% | 0.95 | 0.621 |
| level 1–2 | 6 | 54.5% | 8 | 72.7% | |||
| level 3–4 | 1 | 9.1% | 1 | 9.1% | |||
| 12 month | Unchanged | 0 | 0.0% | 0 | 0.0% | 1.89 | 0.170 |
| level 1–3 | 5 | 45.5% | 2 | 18.2% | |||
| level 4 & above | 6 | 54.5% | 9 | 81.8% | |||
Table 4.
Intergroup comparison of score Change of bone regeneration among Hydroxyapatite, PRF and Statin.
| Follow-up interval | Change in Level | Hydroxyapatite N = 11) |
PRF (N = 11) |
Statin (N = 11) |
Total |
Comparison |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of cases | % | No. of cases | % | No. of cases | % | No. of cases | % | Chisq/F value | p-value | ||
| 6 month | Unchanged | 6 | 54.5 | 4 | 36.4 | 2 | 18.2 | 12 | 36.4 | 3.33 | 0.504 |
| level 1–2 | 4 | 36.4 | 6 | 54.5 | 8 | 72.7 | 18 | 54.5 | |||
| level 3–4 | 1 | 9.1 | 1 | 9.1 | 1 | 9.1 | 3 | 9.1 | |||
| 12 month | Unchanged | 4 | 36.4 | 0 | 0.0 | 0 | 0.0 | 4 | 12.1 | 16.1 | 0.003 |
| level 1–3 | 6 | 54.5 | 5 | 45.5 | 2 | 18.2 | 13 | 39.4 | |||
| level 4 & above | 1 | 9.1 | 6 | 54.5 | 9 | 81.8 | 16 | 48.5 | |||
Figure-3.
Bar graph showing Intergroup comparison of final outcome among HA, PRF and SIM groups.
6. Discussion
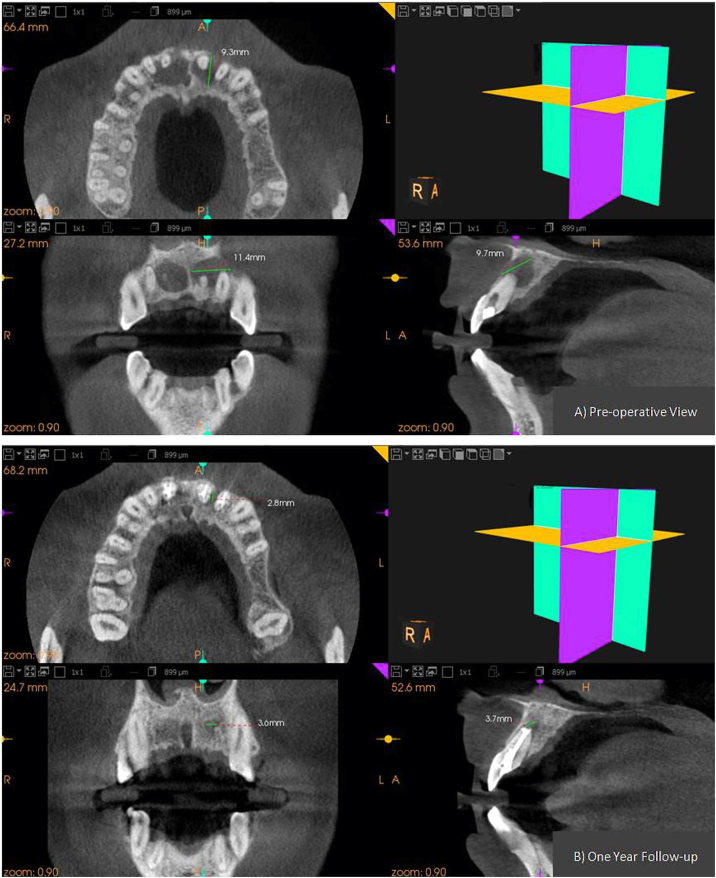
Formation of new bone involves production of new bone matrix by osteoblast, and its mineralization. Various growth factors like bone morphogenic protein plays an important role in the proliferation of osteoblast. Various researchers have found in their study that statin can enhance new bone formation effectively by stimulating BMP-2 genes.5,12,15 In our study Statins, PRF & HA was used to observe the bone formation in endodontic bone defect. The result ofthe study showed that the local delivery of the drug into the defect led to an enhanced rate of bone formation at these sites in comparison to the PRF and HA (Figure-4, Figure-5, Figure-6). Clinically there were no untoward reactions against the graft postoperatively, and there was no statistically significant difference in the clinical parameters at the follow-up intervals. The bone anabolic and pleiotropic properties of Statins owe the credit for the increased bone formation rate and mineral apposition rate of hard tissue.8 This accelerated rate of healing can be well appreciated by Table 1 where healing of SIM-group III lesions occurred at a significantly faster rate when compared to HA-group I and PRF-group II which is evident by the intra-group comparison where the p-value at six months was 0.053, 0.026, 0.018 for the group I, II, and III respectively.Rate of healing: Simvastatin > PRF > hydroxyapatite.
Figure-4.
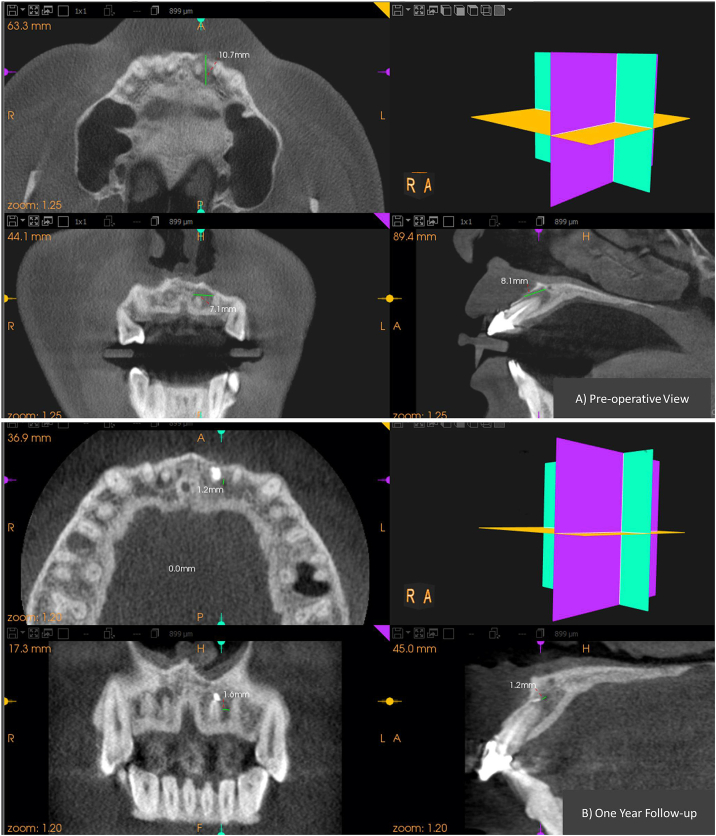
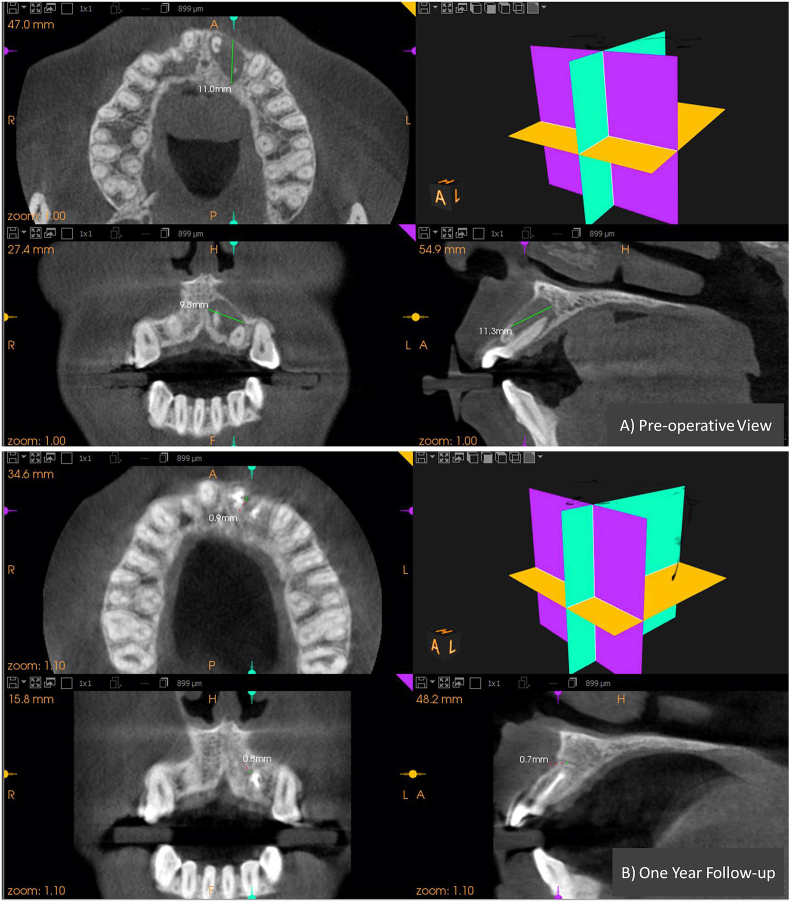
CBCT images of group-I (HA) showing pre and post-operative dimension of the periapical lesion in all the three planes. A) Pre-operative view. B) at 12month follow-up.
Figure-5.
CBCT images of group-II (PRF) showing pre and post-operative dimension of the periapical lesion in all the three planes. A) Pre-operative view.B) at 12month follow-up.
Figure-6.
CBCT images of group-III (SIM) showing pre and post-operative dimension of the periapical lesion in all the three planes. A) pre-operative view. B) at 12month follow-up.
Table 4 showed that no case showed failure at the end of the 12th month. But when healed cases are taken into consideration, 4 cases of SIM, 2 cases of PRF, and 1 case of HA were improved. Healed: Simvastatin > PRF > hydroxyapatite
Apart from the material aspect, this higher success rate was attributed to the strict inclusion criteria that affected the prognosis following surgery in the present trial. Similarly, the use of regenerative therapy in apical surgery helped to accelerate periradicular healing and to allow healing in a compromised clinical situation.26This helps in the prevention of osteogenesis due to connective tissue migration into the osseous defect, leading to healing by connective tissue formation rather than by hard tissue. HA is neither osteogenic nor osteoinductive but instead is osteophilic and osteoconductive, unlike PRF and SIM. Lack of angiogenic factors within the bone graft hampers the periapical wound healing and decreases the rate of bone regeneration in group-I.19
On the contrary, PRF, a second-generation platelet concentrate, contains numerous growth factors such as platelet-derived growth factor (PDGF), vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF beta1) and helps in graft stabilization and improved handling properties and its anti-inflammatory action.20
Fig. 2 showed that in group II, a decrease in the PAI score was significant at 6 and 12 months (χ2 = 7.29,p = 0.026; χ2 = 18.0,p=<0.001 respectively). The observation for the group II (PRF) is in agreement with the study conducted by Vaishnavi et al. (2011) which suggested that the group using Platelet concentrate as a regenerative material, showed evidence of bone formation at three month and six month due to the presence of biological modulators within its composition. PRF is osteoinductive and results in an enhanced rate of woven bone formation.27
In the present study, SIM 10 mg tablets, along with absorbable gelatin sponge was used with 2 ml of sterile saline as a regenerative approach. Simvastatin possesses topical and systemic anti-inflammatory properties, but this property alters at high-dose local applications. Stein et al. applied Simvastatin at different doses and found the signs of clinical inflammation can be reduced by lowering the simvastatin dose. The study of Chauhan AS. et al. supported these parameters where 10 mg SIM tablets with gel foam as a carrier were used in the 3rd molar extraction sockets. They concluded that the experimental site, when compared to the control sites, showed accelerated bone formation with no difference in clinical parameters indicating no adverse reaction to the drug.15 Another similar study by Sezavar M et al. showed the same results.16
The Gelatin sponge used in the present study induced platelet adhesion and degranulation of the α-granules.28 It is a biocompatible material and acted as a carrier matrix for SIM. Many studies have supported its use in the treatment of extraction sockets, calvarial defects, and in the procurement of iliac bone to test bony healing.29 Studies have further supported its use as a scaffold for the slow release of growth factors in the healing of the extraction sockets.30 Statins are small molecular weight compounds that quickly get incorporated into the carrier matrixes, which lead to their slow-release locally over a period of time. Statins combined with different graft have shown significantly greater new bone formation.31
Table 4 showed that when all the three regenerative modalities were compared and analyzed, there was a highly significant difference of p = 0.003, and thus SIM performed better than the two materials in the present trial. CBCT scans used for evaluation of healing outcomes after surgery can detect the periapical bone changes, much earlier time as compared to conventional periapical radiograph (Figure-4, Figure-5, Figure-6). This is in agreement with the literature, which states that CBCT is significantly better in terms of sensitivity, positive or negative predictive values, and diagnostic accuracy than digital or conventional radiographs. However, CBCT scans are always associated with the risks of radiation. As per a publication in Report No. 116 (NCRP, 1993), the cumulative effective dose received due to exposure of three CBCT scans in a patient was much lower than the annual dose limit.32
To the best of the authors' knowledge, this is the first study to investigate the outcome of local application of SIM in the endodontically associated osseous defect post periapical surgery. It was concluded that the use of SIM as a newer bone regenerative material should be promoted in the periapical defects. Though the effect of SIM and PRF are comparable, the overall beneficial effects of the Statin may surpass the useful role of PRF and bone grafts in their role of bone formation because of its lesser price, easy handling properties, reduced risks of cross-infection and no additional trauma to the patient as in the case of autologous PRF.
Since bone regeneration progresses slowly and may take four years and more, so studies in large sample sizes and longer follow up are required for more definitive and conclusive results both clinically and radiographically. The limitations of this study need to be considered in the design of future research in this area of study, but this research study helped to provide insight into the direction and considerations that future researchers must consider in their studies.
Ethics committee approval with the reference number
Approved By Institutional Ethics Committee.
Reference number
ECM IIB-Thesis/P9.
Source of funding
None
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
No other relationships/conditions/circumstances that present a potential conflict of interest
Acknowledgments
My sincere thanks to all the co-authors for their contribution to the study. The authors deny any conflicts of interest related to this study.
Contributor Information
Sweety Gupta, Email: sweetygupta.2608@gmail.com.
Promila Verma, Email: promilarajesh@yahoo.co.in.
Aseem Prakash Tikku, Email: crown_tikku@yahoo.com.
Anil Chandra, Email: ahanachandra@yahoo.com.
Rakesh Kumar Yadav, Email: rakeshanita10@yahoo.in.
Ramesh Bharti, Email: r_bharti14@yahoo.com.
Rhythm Bains, Email: docrhythm77@gmail.com.
References
- 1.Mundy G., Garrett R., Harris S., Chan J. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286:1946–1949. doi: 10.1126/science.286.5446.1946. [DOI] [PubMed] [Google Scholar]
- 2.Ruan F., Zheng Q., Wang J. Mechanisms of bone anabolism regulated by statins. Biosci Rep. 2012;32:511–519. doi: 10.1042/BSR20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borton A.J., Frederick J.P., Datto M.B., Wang X.F., Weinstein R.S. The loss of Smad3 results in a lower rate of bone formation and osteopenia through dysregulation of osteoblast differentiation and apoptosis. J Bone Miner Res. 2001;16:1754–1764. doi: 10.1359/jbmr.2001.16.10.1754. [DOI] [PubMed] [Google Scholar]
- 4.Kaji H., Kanatani M., Sugimoto T., Chihara K. Statins modulate the levels of osteoprotegerin/receptor activator of NF kappaB ligand mRNA in mouse bone-cell cultures. Horm Metab Res. 2005;37:589–592. doi: 10.1055/s-2005-870538. [DOI] [PubMed] [Google Scholar]
- 5.Garrett I.R., Gutierrez G., Mundy G.R. Statins and bone formation. CurrPharm Des. 2001;7(8):715–736. doi: 10.2174/1381612013397762. [DOI] [PubMed] [Google Scholar]
- 6.Gradosova I., Zivna H., Palicka V., Hubena S., Svejkovska K., Zivny P. Protective effect of atorvastatin on bone tissue in orchidectomised male albino Wistar rats. Eur J Pharmacol. 2012;15:144–150. doi: 10.1016/j.ejphar.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 7.Mundy G.R. Statins and their potential for osteoporosis. Bone. 2001;29:495–497. doi: 10.1016/s8756-3282(01)00606-8. [DOI] [PubMed] [Google Scholar]
- 8.Estanislau I.M., Terceiro I.R., Lisboa M.R. Pleiotropic effects of statins on the treatment of chronic periodontitis–a systematic review. Br J Clin Pharmacol. 2015;79(6):877–885. doi: 10.1111/bcp.12564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Subramanian S., Emami H., Vucic E. High-dose atorvastatin reduces periodontal inflammation: a novel pleiotropic effect of statins. J Am Coll Cardiol. 2013;62:2382–2391. doi: 10.1016/j.jacc.2013.08.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pradeep A.R., Thorat M.S. Clinical effect of subgingivally delivered simvastatin in the treatment of patients with chronic periodontitis: a randomized clinical trial. J Periodontol. 2010;81:214–222. doi: 10.1902/jop.2009.090429. [DOI] [PubMed] [Google Scholar]
- 11.Pradeep A.R., Rao N.S., Bajaj P., Kumari M. Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol. 2013;84:24–31. doi: 10.1902/jop.2012.110721. [DOI] [PubMed] [Google Scholar]
- 12.Karanxha L., Park S.J., Son W.J., Nor J.E., Min K.S. Combined effects of simvastatin and enamel matrix derivative on odontoblastic differentiation of human dental pulp cells. J Endod. 2013;39(1):76–82. doi: 10.1016/j.joen.2012.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okamoto Y., Sonoyama W., Ono M. Simvastatin induces the odontogenic differentiation of human dental pulp stem cells in vitro and in vivo. J Endod. 2009;35(3):367–372. doi: 10.1016/j.joen.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Jia W., Zhao Y., Yang J. Simvastatin promotes dental pulp stem cell-induced coronal pulp regeneration in pulpotomized teeth. J Endod. 2016;42(7):1049–1054. doi: 10.1016/j.joen.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Chauhan A.S., Maria A., Managutti A. Efficacy of simvastatin in bone regeneration after surgical removal of mandibular third molars: a clinical pilot study. J Oral Maxillofac Surg. 2015;14(3):578–585. doi: 10.1007/s12663-014-0697-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sezavar M., Bohlouli B., Farhadi S., Tabatabaee S., Latifi R. Simvastatin effects on dental socket quality: a comparative study. Contemp Clin Dent. 2018;9(1):55–59. doi: 10.4103/ccd.ccd_719_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin S.K., Kok S.H., Lee Y.L. Simvastatin as a novel strategy to alleviate periapical lesions. J Endod. 2009;35(5):657–662. doi: 10.1016/j.joen.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 18.vonArx T. Failed root canals: the case for apicoectomy (periradicular surgery) J Oral Maxillofac Surg. 2005;63:832–837. doi: 10.1016/j.joms.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 19.Aichelmann-Reidy M.E., Yukna R.A. Bone replacement grafts. The bone substitutes. Dent Clin. 1998;42:491–503. [PubMed] [Google Scholar]
- 20.DohanEhrenfest D.M., de Peppo G.M., Doglioli P., Sammartino G. Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies. Growth Factors. 2009;27(1):63–69. doi: 10.1080/08977190802636713. [DOI] [PubMed] [Google Scholar]
- 21.Estrela C., Bueno M.R., Azevedo B.C., Azevedo J.R., Pécora J.D. A new periapical index based on cone beam computed tomography. J Endod. 2008;34(11):1325–1331. doi: 10.1016/j.joen.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 22.Saifi A.M., Giraddi G.B., Ahmed N. Healing of extraction socket following local application of simvastatin: a split mouth prospective study. J Oral BiolCraniofac Res. 2017;7(2):106–112. doi: 10.1016/j.jobcr.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park J.B. The use of simvastatin in bone regeneration. Med Oral Patol Oral Cir Bucal. 2009;14(9):e485–e488. [PubMed] [Google Scholar]
- 24.García B., Penarrocha M., Martí E., Gay-Escodad C., von Arx T. Pain and swelling after periapical surgery related to oral hygiene and smoking. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104(2):271–276. doi: 10.1016/j.tripleo.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Yoldas O., Topuz A., Isçi A.S., Oztunc H. Postoperative pain after endodontic retreatment: single-versus two-visit treatment. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004;98(4):483–487. doi: 10.1016/j.tripleo.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 26.Von Arx T. Apical surgery: a review of current techniques and outcome. Saudi Dent J. 2011;23(1):9–15. doi: 10.1016/j.sdentj.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaishnavi C., Mohan B., Narayanan L.L. Treatment of endodontically induced periapical lesions using hydroxyapatite, platelet-rich plasma, and a combination of both: an in vivo study. J Conserv Dent. 2011;14:140–146. doi: 10.4103/0972-0707.82614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cenni E., Ciapetti G., Stea S., Corradini A., Carozzi F. Biocompatibility and performance in vitro of a hemostatic gelatin sponge. J Biomater Sci Polym Ed. 2000;11:685–699. doi: 10.1163/156856200743959. [DOI] [PubMed] [Google Scholar]
- 29.Liu Y., Han J.X., Xiao S.Q., Wang S.L., Wang M. Study of recombinant human osteogenic protein-1 expressed in prokaryocyte on the repair of extracted socket in rabbits. J Biomed Mater Res. 2006;77:324–330. doi: 10.1002/jbm.a.30565. [DOI] [PubMed] [Google Scholar]
- 30.Anders J.O., Mollenhauer J., Beberhold A., Kinne R.W., Venbrocks R.A. Gelatin-based haemostypticSpongostan as a possible three-dimensional scaffold for a chondrocyte matrix?: an experimental study with bovine chondrocytes. J Bone Joint Surg Br. 2009;91:409–416. doi: 10.1302/0301-620X.91B3.20869. [DOI] [PubMed] [Google Scholar]
- 31.Sousa D.N., Roriz V.M., Oliveira G.J. Local effect of simvastatin combined with different osteoconductive biomaterials and collagen sponge on new bone formation in critical defects in rat calvaria. Acta Cir Bras. 2020;35(1) doi: 10.1590/s0102-865020200010000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Council on Radiation Protection and Measurements . 2004. Recent Applications of the NCRP Public Dose Limit Recommendation for Ionizing Radiation. Statement No. 10. [Google Scholar]