Summary
Carbon monoxide (CO) plays an important role in the regulation of a variety of physiological processes and thus is regarded as a promising pharmaceutical agent. Nevertheless, therapeutic applications of CO are severely hampered by the difficulty of the delivery of controlled amounts of CO to biological targets. To address this deficiency, we present a spatiotemporally controllable CO-releasing platform (designated as Neu-MnO2/Fla) for synergistic anti-inflammation. With the assistance of neutrophil membrane coating, Neu-MnO2/Fla can target to inflammatory sites. Subsequently, excess H2O2 at the inflamed tissues can be decomposed into oxygen because of MnO2 as nanozymes possessing catalase (CAT) activity, which not only relieves oxidative stress but also achieves in situ rapid photo-induced CO release. The in vitro and in vivo results indicate our CO-releasing platform exhibits a strong synergistic anti-inflammatory effect. Our work will shed light on targeted CO release to avoid side effects of therapeutic applications of CO.
Subject Areas: Chemistry, Biochemistry, Nanotechnology
Graphical Abstract
Highlights
-
•
The Neu-MnO2/Fla was successfully constructed
-
•
Neu-MnO2/Fla can deliver CO to inflammatory sites in a safe and effective way
-
•
Synergistic anti-inflammatory effects were achieved by combining MnO2 and CO
-
•
The Neu-MnO2/Fla reduced the level of ROS and pro-inflammatory cytokines
Chemistry; Biochemistry; Nanotechnology
Introduction
The emerging researches of carbon monoxide (CO) gas therapy have attracted widespread attention, including therapy of neurodegenerative diseases (Queiroga et al., 2015) and as an antibacterial (Wareham et al., 2015), anti-cancer (Wegiel et al., 2013; Wu et al., 2018a), and especially anti-inflammatory agent (He et al., 2015; Ji et al., 2016b; Popova et al., 2018; Zheng et al., 2018; Wang et al., 2019). Moreover, studies have revealed that CO serves as an endogenous signaling molecule and shows significant anti-inflammatory effects at low doses in many inflammation-related diseases (Otterbein et al., 2000). However, the clinical application of inhaled CO presents several disadvantages, including lack of tissue specificity, difficulty in controlling precise amounts, and the need for complex hospital devices (Ji et al., 2016a). To overcome these limitations, many CO-releasing molecules (CORMs) have been developed as non-gaseous forms of CO delivery (Mann, 2012; Heinemann et al., 2014; Garcia-Gallego and Bernardes, 2014; Chakraborty et al., 2014; Chaves-Ferreira et al., 2015). Yet, most of these CORMs are transition metal carbonyl complexes, which often show potential toxicity and background CO release (Kautz et al., 2016). Fortunately, emerging organic CORMs offer an alternative way for safer and more effective CO delivery (Popova et al., 2018; Zheng et al., 2018; Li et al., 2018b; Ji and Wang, 2018). Recently, Berreau's group reported a flavonol-based organic CORM, 3-hydroxybenzo [g]flavone (Fla), featuring the capacities of fluorescence traceability in cells, low toxicity, and CO release in the presence of oxygen and the light (Popova et al., 2018; Anderson et al., 2015; Soboleva et al., 2017).
However, these small molecules like CORMs usually exhibit unsatisfactory biological effects because of their random biodistribution, fast renal excretion, poor tissue permeability, and low retention at lesion areas (Ji et al., 2016a; Garcia-Gallego and Bernardes, 2014; Li et al., 2018a). To address the aforementioned challenges, various nanocarriers have been constructed for drug delivery (He et al., 2015; Hasegawa et al., 2010; Yao et al., 2018a; Yang et al., 2017). In particular, hollow manganese dioxide (MnO2) nanoparticles are promising drug carriers owing to high drug-loading capacity, intelligent biodegradability (Fan et al., 2016; Yang et al., 2018), and inherent catalase (CAT) mimic activity (Wan et al., 2012; Li et al., 2017). On the one hand, MnO2 nanocarriers can deliver these drugs safely and effectively, avoiding rapid pervasion within the body after administration; on the other hand, MnO2 as nanozymes possessing catalase (CAT) activity can efficiently decompose the endogenous H2O2 to evolve ample oxygen to boost in situ CO release in inflamed tissues. Moreover, MnO2 nanoparticles can be decomposed to biocompatible Mn2+ ions rapidly discharged by kidneys. Hence, there should be no long-term toxicity issues for MnO2 nanoparticles as a therapeutic agent in biological systems (Yang et al., 2017; Zhu et al., 2016; Chen et al., 2016). Taking these advantages into consideration, we aim to develop a CO delivery platform based on hollow mesoporous MnO2 nanozymes.
Neutrophils, a type of white blood cells, can autonomously move along the chemotactic gradients toward the inflammatory sites (behavior known as chemotaxis) (Xue et al., 2017; Shao et al., 2017; Wu et al., 2018b; Zhang et al., 2019). Thus, neutrophil cell membrane-derived nanoparticles have been reported as a promising targeted drug delivery platform for many autoimmune diseases and inflammatory disorders (Yurkin and Wang, 2017; Chu et al., 2018; Zhang et al., 2018).
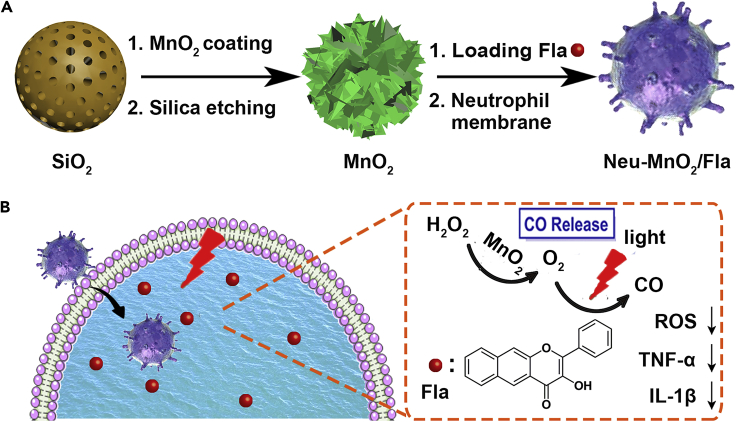
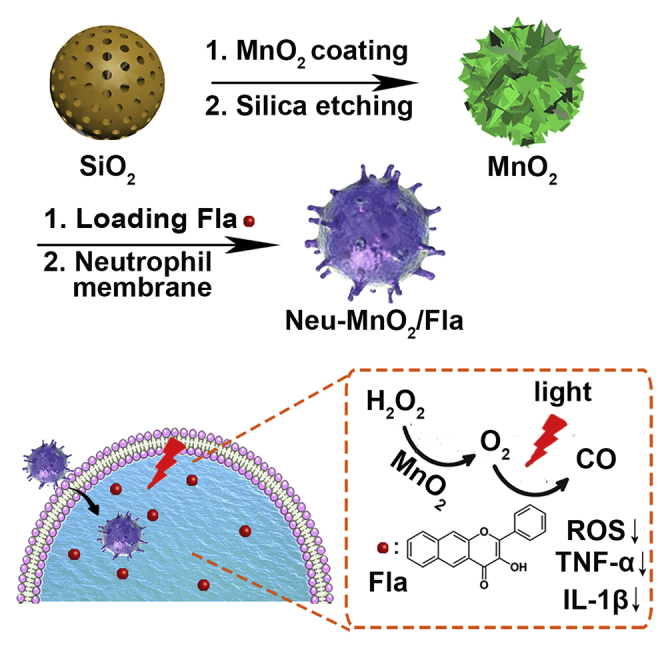
By the integration of Fla, MnO2, and neutrophil membrane, we presented a CO-releasing nano-platform for in vivo synergistic anti-inflammation, which could achieve targeted CORMs delivery and spatiotemporally controllable in situ CO release. As illustrated in Figure 1, the hollow mesoporous MnO2 nanoparticles were chosen as the carriers and used for encapsulating Fla, a small molecule prodrug for CO release (Anderson et al., 2015). Then the MnO2 nanoparticles were further coated with neutrophil cell membrane to obtain Neu-MnO2/Fla. In a lipopolysaccharide (LPS)-induced inflammation model, the subsequently administrated Neu-MnO2/Fla were primed by the chemoattractants and migrated to the inflammatory sites. Afterward, Neu-MnO2/Fla achieved in situ rapid photo-induced CO release in the presence of ample oxygen and the light and thus produced a significant synergistic anti-inflammatory effect.
Figure 1.
Schematic Representation of Synergistic Anti-inflammation
Schematic illustration of (A) the preparation of Neu-MnO2/Fla and (B) neutrophil membrane-targeted in situ CO release for synergistic anti-inflammatory effects promoted by MnO2 nanozymes.
Results and Discussion
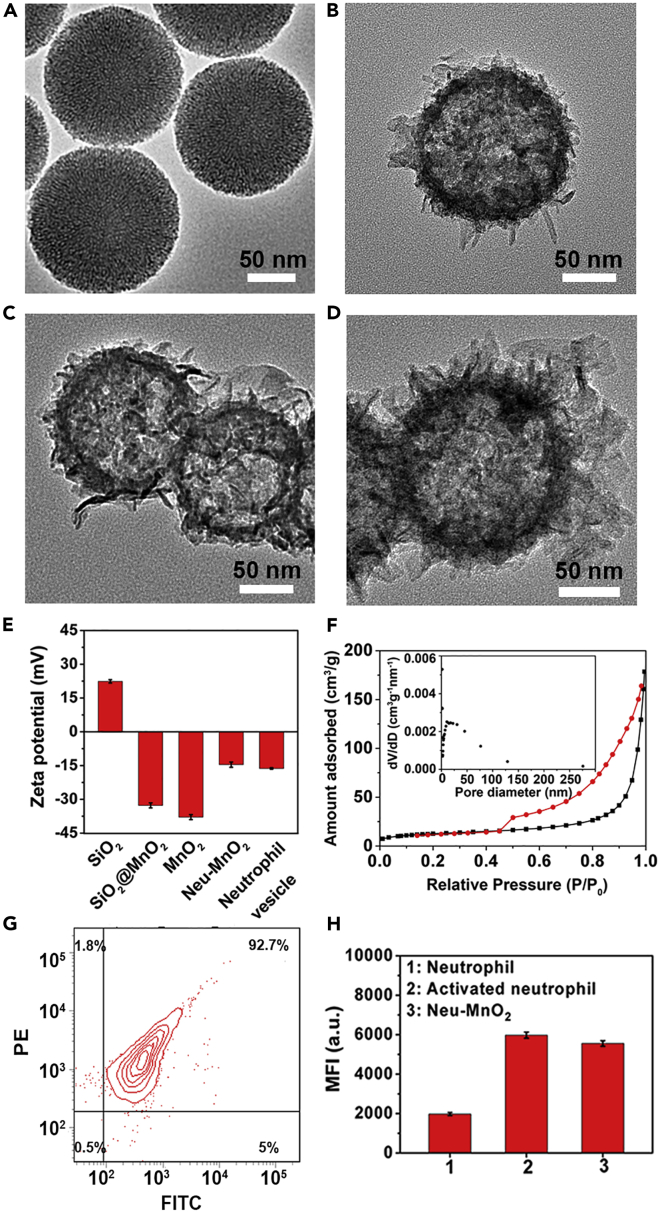
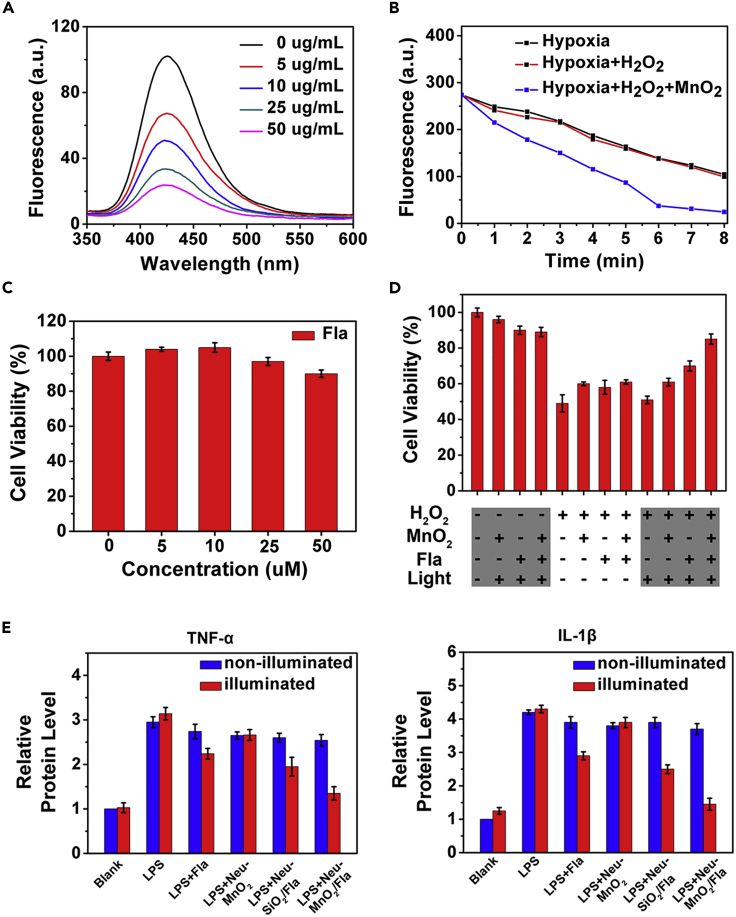
In order to verify our hypothesis, the Neu-MnO2/Fla were synthesized by embedding Fla into the hollow mesoporous MnO2 nanoparticles and further coating them with neutrophil membrane. The mesoporous silica nanoparticles (SiO2) with uniform spherical morphology were synthesized as the templates (Figures 2A and S1). Uniform mesoporous MnO2 layers were deposited on the surface of SiO2 (SiO2@MnO2) through a hydrothermal process (Figure 2B). After etching silica, uniform hollow mesoporous MnO2 nanoparticles were obtained (Figure 2C). Elemental mapping further confirmed the hollow structure of MnO2 nanoparticles (Figure S2). The surface zeta potential transformed from positive to negative after MnO2 coating and more negative after silica etching treatment (Figure 2E). Dynamic light scattering (DLS) demonstrated the hydrodynamic diameters of MnO2 increased after neutrophil membrane coating (Figure S3). Besides, the Fourier transform infrared (FTIR) spectrum and X-ray photoelectron spectroscopy (XPS) analysis additionally indicated the preparation of MnO2 (Figures S4 and S5). X-ray diffraction (XRD) patterns showed that the diffraction peaks could match well with the crystal phase of δ-MnO2 (JCPDS No. 80-1098, Figure S6). Nitrogen adsorption-desorption curves of the MnO2 were measured. Figure 2F showed a pronounced hysteresis at higher p/p0 as a result of the creation of mesoporosity. The created mesopores showed a broad distribution centered around approximately 10.80 nm. The hollow and mesoporous structures made them ideal for efficient drug loading. To employ the MnO2 nanoparticles for safe and effective CO delivery, a photo-induced CORM Fla was synthesized and characterized (Figures S7–S9) and was then loaded into the hollow mesoporous MnO2 (MnO2/Fla). The Fla-loading capacities reached a rather high value of 26% determined by ultraviolet-visible (UV-vis) spectra (Figure S10). The fluorescence spectra also suggested the successful embedment of Fla into MnO2 (Figures S11 and S12). These results demonstrated that Fla was stably embedded into the hollow mesoporous MnO2.
Figure 2.
Synthesis and Characterization of Neu-MnO2/Fla
(A–D) TEM images of (A) SiO2, (B) SiO2@MnO2, (C) MnO2, and (D) Neu-MnO2. The ethanol solution of the samples was dripped onto the Formvar Stabilized with Carbon Support Films for the test.
(E) Zeta potential of SiO2, SiO2@MnO2, MnO2, Neu-MnO2, and Neutrophil vesicle. The samples were dispersed in an aqueous solution.
(F) Nitrogen adsorption-desorption curves of the MnO2. Inset figure: pore size distributions derived from the adsorption branch according to the BJH model.
(G) Flow cytometry analysis of the purity of neutrophils co-stained with anti-Ly6g antibody (FITC) and PE-anti rat CD11b/c antibody (OX-42). The lower-left, lower-right, upper-left, and upper-right quadrants represent the populations of FITC-/PE-, FITC+/PE-, FITC-/PE+, and FITC+/PE + cells, respectively.
(H) Mean fluorescence intensity (MFI) measured in neutrophils, activated neutrophils, and Neu-MnO2 stained with PE-anti rat CD11b/c antibody (OX-42). The results are presented as means ± standard deviation from three independent experiments.
Then the mature neutrophils were isolated from the rat bone marrow and the purity was measured to be higher than 90% (Figure 2G). LPS was chosen to activate neutrophils in response to inflammatory cues. CD11b/c, neutrophil-specific surface proteins, are upregulated on the occurrence of inflammation and facilitates neutrophil aggregation, migration, and adhesion to substrates by opsonization, chemotaxis (Orden et al., 2014; Diamond and Springer, 1993). As expected, the CD11b/c expression level of neutrophils dramatically increased after treatment with LPS, which confirmed the activation of neutrophils (Figure 2H). Then membrane derived from the purified and activated rat bone marrow neutrophils was cloaked on MnO2/Fla (Neu-MnO2/Fla) by previous methods (Zhang et al., 2018). The Neu-MnO2/Fla exhibited obvious neutrophil membrane coating by transmission electron microscopy (TEM) (Figure 2D). In addition, the surface zeta potential of Neu-MnO2/Fla was less negative than that of MnO2 and matched with neutrophil membrane-derived vesicles (Figure 2E). Furthermore, flow cytometry measurements confirmed the presence of neutrophil-specific surface protein CD11b/c on Neu-MnO2/Fla (Figure 2H). These results further demonstrated the successful coating of neutrophil membrane. Besides, Neu-MnO2/Fla showed <10% of Fla release in different solutions for the whole experiment period (Figure S13A) and could stably disperse in different solutions (Figures S13B and S14). These results indicated that Neu-MnO2/Fla was highly stable in different solutions under our experimental conditions. In addition, as shown in Figure S15, Fla was almost completely decomposed under white light within 7 days. Thus, the Fla and Neu-MnO2/Fla should be protected from prolonged exposure to white light.
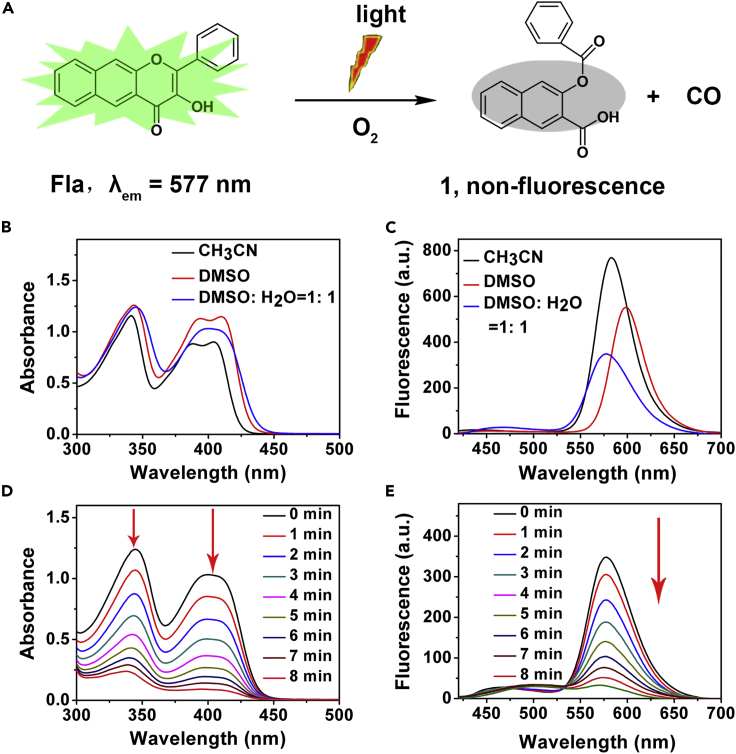
As shown in Figure 3A, Fla could undergo a photo-induced CO release reaction in aerobic environment accompanied with generation of non-emissive 3-(benzoyloxy)-2-naphthoic acid (marked as 1, Figures S16 and 17). The absorption and emission features of Fla in different solutions were distinct indicating that the CO release was identifiable by either absorption or emission spectroscopy (Figures 3B and 3C). The absorption and emission features of Fla at concentration of biological experiments (1% DMSO, v/v) were also observed (Figure S18). The conversion of Fla to compound 1 would lead to the release of CO and the disappearance of absorption and emission features (Soboleva et al., 2017), so it was feasible to achieve “real-time” monitoring of CO release by spectral changes. Subsequently, the capability of photo-induced CO release of Fla in DMSO: H2O (1: 1, v/v) was performed during light illumination (λ = 410 nm; power = 15 mW/cm2). Gradually decreasing of the absorbance and fluorescence was observed upon increasing of the illumination time within 8 min (Figures 3D and 3E). In view of the photolysis products 1 possessing no corresponding absorption and emission features, these results suggested the effective occurrence of CO release reaction under our experimental conditions. Moreover, we also measured the changes in the absorption and emission spectra of Neu-MnO2/Fla upon illumination (Figure S19). Subsequently, the CO release of Neu-MnO2/Fla in a mixed solution (DMSO/PBS, pH 5.5) was investigated by gas chromatography-mass spectrometry (GC-MS). Figure S20 indicated that our design had effective and reliable CO-producing ability. Based on the analysis of GC-MS, 14.7 ppm of CO was released from the Neu-MnO2/Fla. By calculating, approximately 58% of Fla incorporated into the MnO2 converted to CO. The quantum yield was measured to be 0.006 by the absolute quantum yield measurement system.
Figure 3.
Photo-Induced CO Release Reaction of Fla
(A) Schematic illustration of photo-induced CO release reaction of Fla.
(B and C) (B) Absorption and (C) Emission spectra of Fla (0.1 mM) in various solvents (λex = 409 nm).
(D and E) (D) Absorption and (E) Emission spectra of Fla (0.1 mM) with the light illumination (λ = 410 nm; power = 15 mW/cm2) under air for 8 min at 37°C in DMSO: H2O (1:1, v/v).
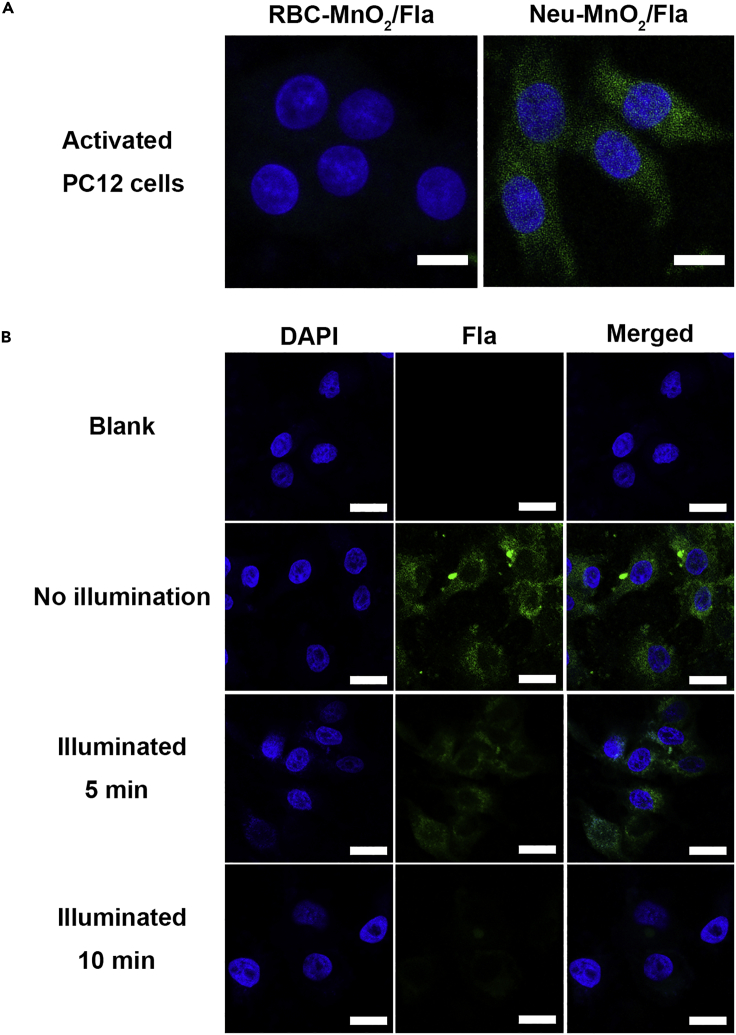
Thereafter, the inflammation targeting ability and intracellular CO delivery of Neu-MnO2/Fla were investigated. Before cell-based studies, we first tested the cytotoxicity of Neu-MnO2/Fla in the presence/absence of illumination. MTT assays showed low toxicity of Neu-MnO2/Fla toward PC12 cells under non-illuminated or illuminated conditions at our experimental concentration (Figures S21 and S22). We next comparatively examined the inflammation targeting ability of Neu-MnO2/Fla. Neutrophil membrane coating endowed the Neu-MnO2/Fla with the specific inflammation targeting ability (Zhang et al., 2019). Herein, Neu-MnO2/Fla was added to PC12 cells activated with LPS. Red blood cell membrane-coated MnO2/Fla (RBC-MnO2/Fla) was used as the control because RBC-MnO2/Fla had analogous structures as Neu-MnO2/Fla but lacked the inflammation targeting ability. In contrast to RBC-MnO2/Fla, the fluorescence of Fla was much stronger for cells treated with Neu-MnO2/Fla (Figures 4A and S23). These results demonstrated that the Neu-MnO2/Fla successfully targeted to inflamed cells by the neutrophil membrane coating. The protein-receptor interactions mediated by CD11b/c on the neutrophil membrane may play a key role (Orden et al., 2014; Diamond and Springer, 1993; Weaver et al., 2000). The drug release behaviors of Fla from Neu-MnO2/Fla was then measured in PBS at different pH values (Figure S24). Compared with the release curve in acidic solution (pH = 5.5), the release amount of Fla was much higher than that in the physiological solution (pH = 7.4) after 24 h, owing to the acidic triggered decomposition of MnO2 nanoshells. We further examined the intracellular CO release of Neu-MnO2/Fla by monitoring the green fluorescence of Fla. PC12 cells were incubated with Neu-MnO2/Fla for 8 h. After washing with PBS, cells were illuminated (λ = 410 nm; power = 15 mW/cm2) for different periods of time. Importantly, the green fluorescence was gradually attenuated over illumination time (Figures 4B and S25). These results demonstrated that Neu-MnO2/Fla could responsively release CO in cells.
Figure 4.
The Inflammation Targeting Ability and Intracellular CO Delivery of Neu-MnO2/Fla
(A) Fluorescent images of PC12 cells after incubation with RBC-MnO2/Fla (25 μg/mL) and Neu-MnO2/Fla (25 μg/mL), respectively. Cells were activated with LPS before being treated with nanoparticles. Scale bars: 10 μM.
(B) Fluorescent images of PC12 cells exposed to Neu-MnO2/Fla (25 μg/mL) with subsequent illumination. Blue and green represented DAPI and Fla fluorescence, respectively. Scale bars: 25 μM.
Having demonstrated the inflammation targeting ability and intracellular CO release of Neu-MnO2/Fla, we further studied the synergistic anti-inflammatory effects of Neu-MnO2/Fla. The catalase (CAT)-like activity of MnO2 was first investigated via a terephthalic acid (TA) reaction (Figure 5A) (Li et al., 2017). In addition, the concentration of H2O2 was directly evaluated via UV-vis spectroscopy according to the absorbance at 240 nm (Figure S26). Coinciding with the results of TA reaction assay, H2O2 could be decomposed with the assistance of MnO2 in a dose-dependent manner (Figure S27). The concentration of H2O2 sharply decreased along with the reaction time (Figure S28). Then, we examined the photo-induced CO release under hypoxic conditions with MnO2 boost. Illumination of Fla (0.1 mM) in DMSO:H2O (1: 1, v/v) under hypoxic conditions resulted in 49% conversion to CO within 6 min. Addition of H2O2 (1 mM) and MnO2 (5 µg/mL) to the solution boosted this reaction, which reached >90% completion within 6 min (Figures 5B and S29). These results indicated that the oxygen from MnO2-catalyzed H2O2 decomposition remarkably boosted the CO release reactivity of Fla. Given the fluctuations of H2O2 concentrations within inflammatory cells, we performed an additional experiment with 25 μM Fla and 25 μM H2O2 under hypoxic conditions. Figure S30 showed that CO release was also almost completed within 6 min. This result indicated that, under low concentration of H2O2 (25 μM), the O2 produced from the nanozymes catalysis was sufficient to promote the CO release in physiological conditions. As reported, aberrant reactive oxygen species (ROS) generation was a key mediator during the inflammatory process (Yao et al., 2018b; Yang et al., 2019; Zhang and Kaufman, 2008; Huang et al., 2016). Thus, we further examined the synergistic antioxidative effects of MnO2 nanozymes and Fla-generated CO in PC12 cells. First, MTT assays showed low toxicity of MnO2 and Fla toward PC12 cells at our experimental concentration (Figures 5C and S31). As shown in Figure 5D, H2O2 (500 μM) was employed as an inducer of oxidative stress in PC12 cells. Compared with the control without H2O2 treatment, cytotoxic H2O2 obviously caused cell death; however, the individual MnO2 (25 μg/mL) and Fla-generated CO both improved the cell viability, respectively. As expected, even better effect was observed for cells when treated together with MnO2 (25 μg/mL) and Fla (25 μM), verifying the synergistic antioxidative effect of Neu-MnO2/Fla.
Figure 5.
The In Vitro Synergistic Anti-inflammatory Effects of Neu-MnO2/Fla
(A) CAT-like activity of MnO2 with different concentrations.
(B) Linear curves of photo-induced emission changes of Fla under hypoxic conditions with MnO2 boost (λ = 410 nm; power = 15 mW/cm2).
(C) PC12 cell viability after incubation with different concentrations of Fla for 24 h.
(D) MTT assays of the synergistic antioxidative effects in PC12 cells.
(E) Anti-inflammatory effects of the Neu-MnO2/Fla in PC12 cells under illuminated and non-illuminated conditions (λ = 410 nm; power = 15 mW/cm2).
Afterward, we examined the anti-inflammatory effects of Neu-MnO2/Fla against LPS-induced inflammation in PC12 cells. TNF-α and IL-1β, typical pro-inflammatory cytokines, were selected to verify the inflammatory response of PC12 cells. As shown in Figure 5E, PC12 cells displayed a strong inflammatory response after stimulation with LPS as the levels of TNF-α and I`L-1β were significantly increased. In the absence of illumination, all the treatments had no obvious effects on these pro-inflammatory cytokines (blue bars). Illumination with the treatment of Neu-MnO2 (without Fla) also did not lead to obvious influence of these pro-inflammatory cytokines. When PC12 cells were treated with Neu-MnO2/Fla and light illumination (λ = 410 nm; power = 15 mW/cm2), this caused significant inhibition of the expression of TNF-α and IL-1β. The anti-inflammatory effect of Neu-MnO2/Fla was better than that of Neu-SiO2/Fla. This result indicated that the nanozyme activity of MnO2 did help to improve the synergistic effect of Neu-MnO2/Fla.
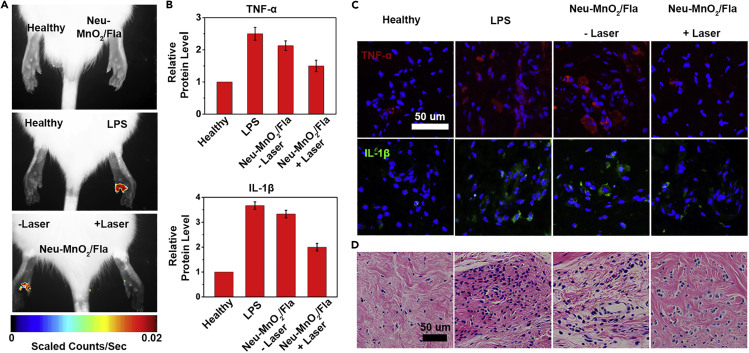
Then, the in vivo anti-inflammatory effects of Neu-MnO2/Fla were investigated by using an LPS-induced paw inflammation model following the published study with minor modification (Wan et al., 2017). Owing to the low phototoxicity and deep tissue penetration of two-photon technology, a two-photon approach was used to trigger CO release from Neu-MnO2/Fla (Li et al., 2018b). First, the in vivo ROS scavenging capability of Neu-MnO2/Fla was studied. Levels of ROS in inflamed paws were measured using the in vivo imaging system. As demonstrated in Figure 6A, there was no obvious fluorescence in the healthy paw or that treated with the Neu-MnO2/Fla alone. In contrast, a strong fluorescence was observed in the LPS-treated paw, confirming the production of excess ROS in the LPS-induced inflamed tissues. When treated with Neu-MnO2/Fla and the radiation (marked as Neu-MnO2/Fla + Laser), the fluorescence intensity of the inflamed paw was significantly weaker than that of the inflamed paw without laser illumination (marked as Neu-MnO2/Fla - Laser), indicating laser illumination decreases the ROS level in the inflamed tissues. Treatment with Neu-MnO2/Fla + Laser also significantly reduced the TNF-α and IL-1β levels in the inflamed tissues (Figures 6B and 6C). Hematoxylin and eosin (H&E)-stained images showed that treatment with Neu-MnO2/Fla + Laser reduced infiltration of the inflammatory cells (Figure 6D). These results indicated that Neu-MnO2/Fla produced a high enough local therapeutic concentration of CO and exhibited a significant anti-inflammatory effect.
Figure 6.
The In Vivo Synergistic Anti-inflammatory Effects of Neu-MnO2/Fla
(A) In vivo imaging of ROS in LPS-induced inflamed paws following treatment with Neu-MnO2/Fla (25 μg/mL) without/with two-photon laser irradiation (λ = 820 nm, using confocal laser source, 4,100 mW/cm2 at 4% laser power).
(B–D) (B) Levels of inflammatory cytokines TNF-α and IL-1β and (C) corresponding fluorescence images and (D) H&E-stained images of inflamed paw.
Conclusions
In summary, using a photo-induced CORM and neutrophil membrane coated hollow mesoporous MnO2 nanoparticles as carriers and nanozymes, we successfully constructed a spatiotemporally controllable CO-releasing platform for synergistic anti-inflammation in biological system. This approach could deliver CO to the desired location in a safe and effective way. Importantly, remarkable synergistic anti-inflammatory effects were achieved through combining MnO2 nanozymes and CO gas therapy. The Neu-MnO2/Fla reduced the level of ROS and pro-inflammatory cytokines both in vitro and in vivo. Histological examinations of tissue sections confirmed the ability of Neu-MnO2/Fla to mitigate tissue inflammation. Our work may promote controllable CO-based targeting gas therapy for in vivo synergistic anti-inflammation.
Limitations of the Study
We constructed a spatiotemporally controllable CO-releasing platform for CO gas therapy, and a photo-induced CORM, Fla, was the CO donor. Fla was known to undergo CO release via direct illumination using visible light, but the visible light limited a lot of applications of CO gas therapy. Although Fla could be effectively excited by two-photon laser, two photon experiment had certain requirements for equipment and operation. Moreover, the low fluorescence quantum yield of Fla may affect the amount of CO released in biological tissues. Therefore, it is necessary to develop near-infrared light-induced CORM with high fluorescence quantum yield to broaden the applications of CO gas therapy.
Resource Availability
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Xiaogang Qu (xqu@ciac.ac.cn).
Materials Availability
This study did not generate new unique reagents.
Data and Code Availability
The published article includes all datasets generated or analyzed during this study.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
The authors are grateful for support from National Natural Science Foundation of China (21533008, 91856205, 21871249, and 21820102009), Key Research Program of Frontier Sciences of CAS (QYZDY-SSW-SLH052).
Author Contributions
C.L. and Z.D. contributed equally to this work.
Declaration of Interests
The authors declare no competing interests.
Published: September 25, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101483.
Supplemental Information
References
- Anderson S.N., Richards J.M., Esquer H.J., Benninghoff A.D., Arif A.M., Berreau L.M. A structurally-tunable 3-hydroxyflavone motif for visible light-induced carbon monoxide-releasing molecules (CORMs) ChemistryOpen. 2015;4:590–594. doi: 10.1002/open.201500167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty I., Carrington S.J., Mascharak P.K. Design strategies to improve the sensitivity of photoactive metal carbonyl complexes (photoCORMs) to visible light and their potential as CO-donors to biological targets. Acc. Chem. Res. 2014;47:2603–2611. doi: 10.1021/ar500172f. [DOI] [PubMed] [Google Scholar]
- Chaves-Ferreira M., Albuquerque I.S., Matak-Vinkovic D., Coelho A.C., Carvalho S.M., Saraiva L.M., Romao C.C., Bernardes G.J. Spontaneous CO release from Ru(II)(CO)2-protein complexes in aqueous solution, cells, and mice. Angew. Chem. Int. Ed. 2015;54:1172–1175. doi: 10.1002/anie.201409344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Q., Feng L., Liu J., Zhu W., Dong Z., Wu Y., Liu Z. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016;28:7129–7136. doi: 10.1002/adma.201601902. [DOI] [PubMed] [Google Scholar]
- Chu D., Dong X., Shi X., Zhang C., Wang Z. Neutrophil-based drug delivery systems. Adv. Mater. 2018;30:e1706245. doi: 10.1002/adma.201706245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond M.S., Springer T.A. A subpopulation of Mac-1 (CD11b/CD18) molecules mediates neutrophil adhesion to ICAM-1 and fibrinogen. J. Cell Biol. 1993;120:545–556. doi: 10.1083/jcb.120.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H., Yan G., Zhao Z., Hu X., Zhang W., Liu H., Fu X., Fu T., Zhang X.B., Tan W. A smart photosensitizer-manganese dioxide nanosystem for enhanced photodynamic therapy by reducing glutathione levels in cancer cells. Angew. Chem. Int. Ed. 2016;55:5477–5482. doi: 10.1002/anie.201510748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gallego S., Bernardes G.J. Carbon-monoxide-releasing molecules for the delivery of therapeutic CO in vivo. Angew. Chem. Int. Ed. 2014;53:9712–9721. doi: 10.1002/anie.201311225. [DOI] [PubMed] [Google Scholar]
- Hasegawa U., van der Vlies A.J., Simeoni E., Wandrey C., Hubbell J.A. Carbon monoxide-releasing micelles for immunotherapy. J. Am. Chem. Soc. 2010;132:18273–18280. doi: 10.1021/ja1075025. [DOI] [PubMed] [Google Scholar]
- He Q., Kiesewetter D.O., Qu Y., Fu X., Fan J., Huang P., Liu Y., Zhu G., Liu Y., Qian Z., Chen X. NIR-responsive on-demand release of CO from metal carbonyl-caged graphene oxide nanomedicine. Adv. Mater. 2015;27:6741–6746. doi: 10.1002/adma.201502762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S.H., Hoshi T., Westerhausen M., Schiller A. Carbon monoxide--physiology, detection and controlled release. Chem. Commun. 2014;50:3644–3660. doi: 10.1039/c3cc49196j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Liu Z., Liu C., Ju E., Zhang Y., Ren J., Qu X. Self-assembly of multi-nanozymes to mimic an intracellular antioxidant defense system. Angew. Chem. Int. Ed. 2016;55:6646–6650. doi: 10.1002/anie.201600868. [DOI] [PubMed] [Google Scholar]
- Ji X., Wang B. Strategies toward organic carbon monoxide prodrugs. Acc. Chem. Res. 2018;51:1377–1385. doi: 10.1021/acs.accounts.8b00019. [DOI] [PubMed] [Google Scholar]
- Ji X., Damera K., Zheng Y., Yu B., Otterbein L.E., Wang B. Toward carbon monoxide-based therapeutics: critical drug delivery and developability issues. J. Pharm. Sci. 2016;105:406–416. doi: 10.1016/j.xphs.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji X., Zhou C., Ji K., Aghoghovbia R.E., Pan Z., Chittavong V., Ke B., Wang B. Click and release: a chemical strategy toward developing gasotransmitter prodrugs by using an intramolecular Diels-Alder reaction. Angew. Chem. Int. Ed. 2016;55:15846–15851. doi: 10.1002/anie.201608732. [DOI] [PubMed] [Google Scholar]
- Kautz A.C., Kunz P.C., Janiak C. CO-releasing molecule (CORM) conjugate systems. Dalton Trans. 2016;45:18045–18063. doi: 10.1039/c6dt03515a. [DOI] [PubMed] [Google Scholar]
- Li W., Liu Z., Liu C., Guan Y., Ren J., Qu X. Manganese dioxide nanozymes as responsive cytoprotective shells for individual living cell encapsulation. Angew. Chem. Int. Ed. 2017;56:13661–13665. doi: 10.1002/anie.201706910. [DOI] [PubMed] [Google Scholar]
- Li L.L., Guo J.W., Wang Y.Q., Xiong X.X., Tao H., Li J., Jia Y., Hu H.Y., Zhang J.X. A broad-spectrum ROS-eliminating material for prevention of inflammation and drug-induced organ toxicity. Adv. Sci. 2018;5:1800781. doi: 10.1002/advs.201800781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Shu Y., Liang M., Xie X., Jiao X., Wang X., Tang B. A two-photon H2O2-activated CO photoreleaser. Angew. Chem. Int. Ed. 2018;57:12415–12419. doi: 10.1002/anie.201805806. [DOI] [PubMed] [Google Scholar]
- Mann B.E. CO-releasing molecules: a personal view. Organometallics. 2012;31:5728–5735. [Google Scholar]
- Orden S., de Pablo C., Rios-Navarro C., Martinez-Cuesta M.A., Peris J.E., Barrachina M.D., Esplugues J.V., Alvarez A. Efavirenz induces interactions between leucocytes and endothelium through the activation of Mac-1 and gp150,95. J. Antimicrob. Chemother. 2014;69:995–1004. doi: 10.1093/jac/dkt468. [DOI] [PubMed] [Google Scholar]
- Otterbein L.E., Bach F.H., Alam J., Soares M., Lu H.T., Wysk M., Davis R.J., Flavell R.A., Choi A.M.K. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- Popova M., Soboleva T., Ayad S., Benninghoff A.D., Berreau L.M. Visible-light-activated quinolone carbon-monoxide-releasing molecule: prodrug and albumin-assisted delivery enables anticancer and potent anti-inflammatory effects. J. Am. Chem. Soc. 2018;140:9721–9729. doi: 10.1021/jacs.8b06011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queiroga C.S., Vercelli A., Vieira H.L. Carbon monoxide and the CNS: challenges and achievements. Br. J. Pharmacol. 2015;172:1533–1545. doi: 10.1111/bph.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao J., Xuan M., Zhang H., Lin X., Wu Z., He Q. Chemotaxis-guided hybrid neutrophil micromotors for targeted drug transport. Angew. Chem. Int. Ed. 2017;56:12935–12939. doi: 10.1002/anie.201706570. [DOI] [PubMed] [Google Scholar]
- Soboleva T., Esquer H.J., Benninghoff A.D., Berreau L.M. Sense and release: a thiol-responsive flavonol-based photonically driven carbon monoxide-releasing molecule that operates via a multiple-input AND logic gate. J. Am. Chem. Soc. 2017;139:9435–9438. doi: 10.1021/jacs.7b04077. [DOI] [PubMed] [Google Scholar]
- Wan W.L., Lin Y.J., Chen H.L., Huang C.C., Shih P.C., Bow Y.R., Chia W.T., Sung H.W. In situ nanoreactor for photosynthesizing H2 gas to mitigate oxidative stress in tissue inflammation. J. Am. Chem. Soc. 2017;139:12923–12926. doi: 10.1021/jacs.7b07492. [DOI] [PubMed] [Google Scholar]
- Wan Y., Qi P., Zhang D., Wu J., Wang Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012;33:69–74. doi: 10.1016/j.bios.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Wang S.B., Zhang C., Chen Z.X., Ye J.J., Peng S.Y., Rong L., Liu C.J., Zhang X.Z. A versatile carbon monoxide nanogenerator for enhanced tumor therapy and anti-inflammation. ACS. Nano. 2019;13:5523–5532. doi: 10.1021/acsnano.9b00345. [DOI] [PubMed] [Google Scholar]
- Wareham L.K., Poole R.K., Tinajero-Trejo M. CO-releasing metal carbonyl compounds as antimicrobial agents in the post-antibiotic era. J. Biol. Chem. 2015;290:18999–19007. doi: 10.1074/jbc.R115.642926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver K.D., Branch C.A., Hernandez L., Miller C.H., Quattrocchi K.B. Effect of leukocyte-endothelial adhesion antagonism on neutrophil migration and neurologic outcome after cortical trauma. J. Trauma. 2000;48:1081–1090. doi: 10.1097/00005373-200006000-00014. [DOI] [PubMed] [Google Scholar]
- Wegiel B., Gallo D., Csizmadia E., Harris C., Belcher J., Vercellotti G.M., Penacho N., Seth P., Sukhatme V., Ahmed A. Carbon monoxide expedites metabolic exhaustion to inhibit tumor growth. Cancer Res. 2013;73:7009–7021. doi: 10.1158/0008-5472.CAN-13-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L.H., Cai X.J., Zhu H.F., Li J.H., Shi D.X., Su D.F., Yue D., Gu Z.W. PDT-driven highly efficient intracellular delivery and controlled release of CO in combination with sufficient singlet oxygen production for synergistic anticancer therapy. Adv. Funct. Mater. 2018;28:1804324. [Google Scholar]
- Wu M., Zhang H., Tie C., Yan C., Deng Z., Wan Q., Liu X., Yan F., Zheng H. MR imaging tracking of inflammation-activatable engineered neutrophils for targeted therapy of surgically treated glioma. Nat. Commun. 2018;9:4777. doi: 10.1038/s41467-018-07250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J., Zhao Z., Zhang L., Xue L., Shen S., Wen Y., Wei Z., Wang L., Kong L., Sun H. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017;12:692–700. doi: 10.1038/nnano.2017.54. [DOI] [PubMed] [Google Scholar]
- Yang B., Chen Y., Shi J. Reactive oxygen species (ROS)-based nanomedicine. Chem. Rev. 2019;119:4881–4985. doi: 10.1021/acs.chemrev.8b00626. [DOI] [PubMed] [Google Scholar]
- Yang G., Xu L., Chao Y., Xu J., Sun X., Wu Y., Peng R., Liu Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017;8:902. doi: 10.1038/s41467-017-01050-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Chueng S.D., Li Y., Patel M., Rathnam C., Dey G., Wang L., Cai L., Lee K.B. A biodegradable hybrid inorganic nanoscaffold for advanced stem cell therapy. Nat. Commun. 2018;9:3147. doi: 10.1038/s41467-018-05599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Cheng Y., Zhou M., Zhao S., Lin S., Wang X., Wu J., Li S., Wei H. ROS scavenging Mn3O4 nanozymes for in vivo anti-inflammation. Chem. Sci. 2018;9:2927–2933. doi: 10.1039/c7sc05476a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C., Wang W., Wang P., Zhao M., Li X., Zhang F. Near-infrared upconversion mesoporous cerium oxide hollow biophotocatalyst for concurrent pH-/H2O2-responsive O2-evolving synergetic cancer therapy. Adv. Mater. 2018;30:1704833. doi: 10.1002/adma.201704833. [DOI] [PubMed] [Google Scholar]
- Yurkin S.T., Wang Z. Cell membrane-derived nanoparticles: emerging clinical opportunities for targeted drug delivery. Nanomedicine. 2017;12:2007–2019. doi: 10.2217/nnm-2017-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhang L., Wu W., Gao F., Li R.Q., Song W., Zhuang Z.N., Liu C.J., Zhang X.Z. Artificial super neutrophils for inflammation targeting and HClO generation against tumors and infections. Adv. Mater. 2019:e1901179. doi: 10.1002/adma.201901179. [DOI] [PubMed] [Google Scholar]
- Zhang K., Kaufman R.J. From endoplasmic-reticulum stress to the inflammatory response. Nature. 2008;454:455–462. doi: 10.1038/nature07203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Dehaini D., Zhang Y., Zhou J., Chen X., Zhang L., Fang R.H., Gao W., Zhang L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018;13:1182–1190. doi: 10.1038/s41565-018-0254-4. [DOI] [PubMed] [Google Scholar]
- Zheng Y., Ji X., Yu B., Ji K., Gallo D., Csizmadia E., Zhu M., Choudhury M.R., de La Cruz L.K.C., Chittavong V. Enrichment-triggered prodrug activation demonstrated through mitochondria-targeted delivery of doxorubicin and carbon monoxide. Nat. Chem. 2018;10:787–794. doi: 10.1038/s41557-018-0055-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W.W., Dong Z.L., Fu T.T., Liu J.J., Chen Q., Li Y.G., Zhu R., Xu L.G., Liu Z. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv. Funct. Mater. 2016;26:5490–5498. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The published article includes all datasets generated or analyzed during this study.