Abstract
The application of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) has revolutionized the treatment of acute promyelocytic leukemia (APL). More than 80–90% of patients are expected to be cured with a combination of ATRA, ATO and/or chemotherapy. In this review, we focus on the remaining obstacles to a cure for all patients with APL. We review the issue of early death and coagulopathy and discuss the particular challenges in the care of patients with high-risk APL and patients with relapsed APL. We also give recommendations and highlight ongoing efforts to improve the persistently high early death rate and the outcomes of high risk and relapsed APL patients.
Keywords: APL, ATRA, cure
Introduction
Acute promyelocytic leukemia (APL) is a unique subtype of acute myeloid leukemia (AML) defined by the fusion of promyelocytic leukemia (PML) gene with the retinoic acid receptor-α gene (RARα) as a result of a balanced translocation between chromosome 15 and 17 creating PML-RARα [1]. This oncogenic fusion protein leads to a block of myeloid differentiation at the promyelocytic stage of myelopoiesis [1]. Over the last decades the development of treatment targeting PML-RARα with all-trans retinoic acid and (ATRA) and arsenic trioxide (ATO) has transformed APL from a disease with a high mortality and long-term survival of less than 50% to the most curable subtype of adult acute leukemia in adults [2–4].
Although with ATRA and ATO long-term survival has improved to 80–90% or higher [3,4], challenges remain in the treatment of APL. In this commentary, we focus on these remaining challenges in the field of APL. We discuss the issues of early death and coagulopathy and review the management of challenging patient populations including those with high-risk disease and patients with relapsed APL. Lastly, we offer advice on how to overcome the remaining obstacles to cure for all APL patients (Figure 1).
Figure 1.
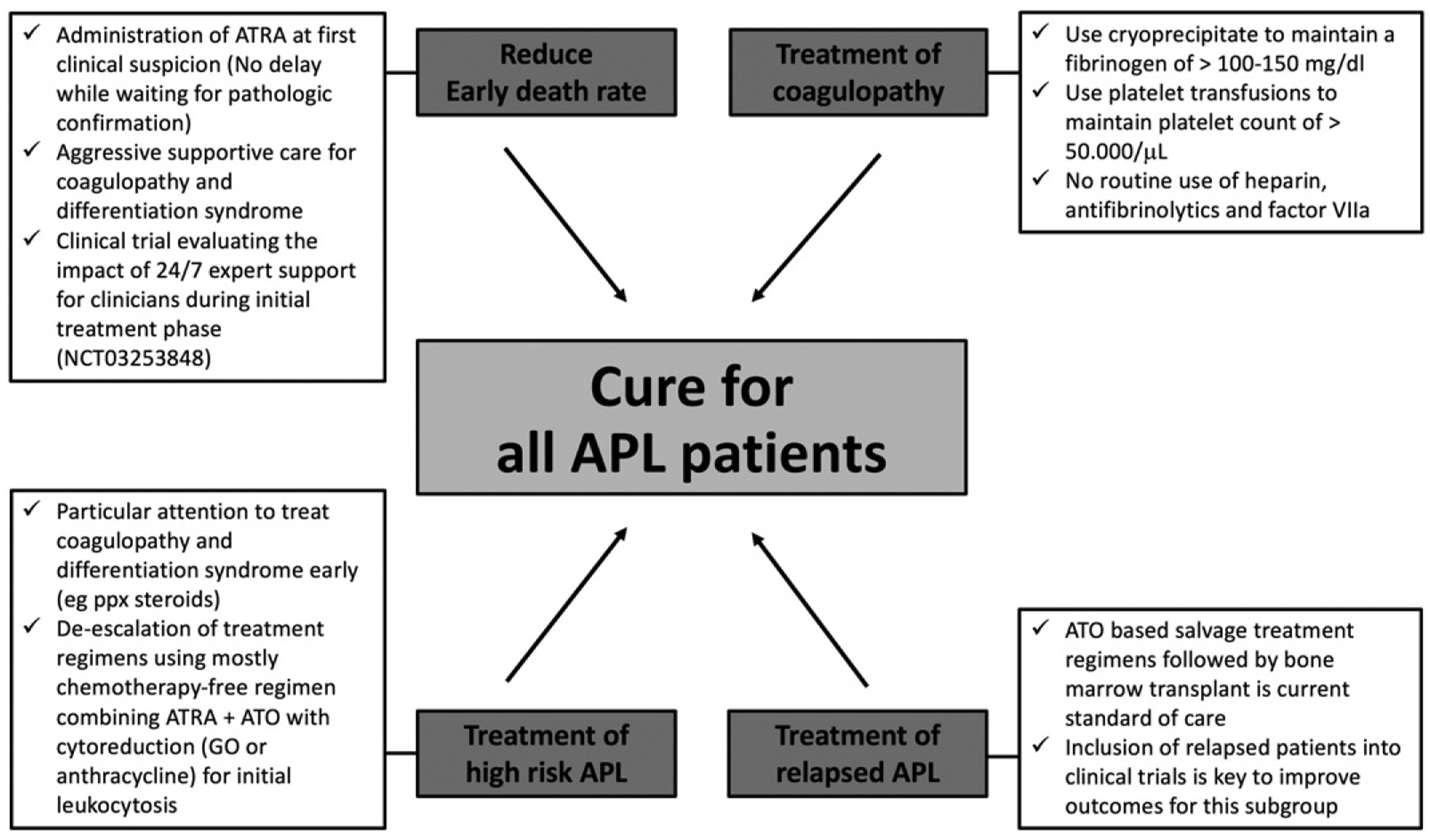
Remaining obstacles to cure for all APL patients and approaches to overcome them.
Early death
Early death, most commonly defined as death within the first 30 days of presentation to medical care, is the major cause of treatment failure in APL [5] (Figure 1). Causes for early death are mainly coagulopathy leading to intracerebral, gastrointestinal and pulmonary hemorrhage and less commonly infection and differentiation syndrome [6]. In an analysis of two large PETHEMA group studies of APL patients treated with ATRA and idarubicin, 91% of patients achieved a complete remission whereas 9% died during induction therapy with hemorrhage being the most common cause of death (5%), followed by infection (2.3%) and differentiation syndrome (1.4%) [7]. Most of the early deaths in the PETHEMA studies (57%) occurred during the first week of induction therapy and the type of lethal hemorrhage was most commonly intracranial hemorrhage (65%) followed by pulmonary (32%) and gastrointestinal bleeding (3%). Similarly, other large cooperative group studies have reported a relatively low early death rate (EDR) of about 5–10% [8–11] (Table 1). However, clinical trials frequently exclude patients with severe coagulopathy and/or hemorrhage at the time of presentation who may die before treatment is initiated and therefore likely underestimate the EDR. For instance, in the PETHEMA LPA 96 and LPA 99 trials half of the patients excluded from the study had life-threatening hemorrhages [12].
Table 1.
Early death rate (EDR) in a selection of cooperative group studies vs population-based studies.
| Study | N | Regimen | EDR | Reported time to first ATRA |
|---|---|---|---|---|
| Cooperative group studies | ||||
| PETHEMA [12] | 732 | ATRA + Idarubicin | 7% | Day 1 of trial, exact time to ATRA not reported |
| Published: 2008 | ||||
| Study years: 1999–2005 | ||||
| JALSG [13] | 283 | ATRA/Idarubicin/ara-C | 5% | |
| Published: 2007 | ||||
| Study years: not reported | ||||
| GAMLCG [14] | 142 | ATRA/high dose ara-C | 8% | |
| Published: 2009 | ||||
| Study years: 1994–2005 | ||||
| GIMEMA [15] | 807 | ATRA + Idarubicin | 6% | |
| Published: 2011 | ||||
| Study years: 1993–2010 | ||||
| AML17 [16] | 119 | ATRA + ATP or ATRA + Idarubicin | 6% | |
| Published: 2015 | ||||
| Study years: 2009–2013 | ||||
| APL 0406 [4] | 77 | ATRA + ATO or ATRA + Idarubicin | 0% | |
| Published: 2013 | ||||
| Study years: 2007–2010 | ||||
| Population-based studies | ||||
| Jeddi et al [17] | 41 | ATRA/Daunorubicin/ara-C or ATRA + Idarubicin | 16% | time to ATRA not reported |
| Published: 2008 | ||||
| Study years: 1998–2006 | ||||
| Lehmann et al [18] | 105 | ATRA based or no treatment in 21% of patients with ED | 29% | Patients with ED: |
| Published: 2011 | First health-care contact to first contact with hematologist: 1 day (R, 0–17 days) | |||
| Study years: 1997–2006 | ||||
| First contact with hematologist to diagnosis: 1 day (R, −1 to 8 days) | ||||
| Diagnosis to start of ATRA treatment: 0 days (R, −2 to 5 days) | ||||
| McClellan et al. [19] | 70 | ATRA based or no treatment in 6% of patients | 26% | Time from presentation to the hospital to ATRA: |
| Published: 2012 | Patients with ED: 1 day | |||
| Study years: 1997–2009 | Patients without ED: 1.25 days (p > .1). | |||
| Park et al. [20] | 1400 | No information about treatment (SEER study) | 17.3% | time to ATRA not reported |
| Published: 2011 | ||||
| Study years: 1992–2011 | ||||
| Altman et al. [21] | 204 | 11% | Initial presentation to suspicion of APL: 1 day (R, 0–37 days; 95% CI, 0–9 days) | |
| Published: 2013 | ||||
| Study years: 1992–2009 | Suspicion of APL to ordering ATRA: 1 day (R, 0–99 days; 95%CI, 0–6.5 days | |||
| Ordering ATRA to its administration: 0 days (R, 0–3 days; 95%CI, 0–1 day) | ||||
| Presentation to ATRA administration: 2 days (R, 0–100 days, 95%CI, 0–16 days) | ||||
ED: early death; EDR: early death rate; R: range; 95%CI: 95% confidence interval; PETHEMA: Progra]ma para el Estudio de la Terapéutica en Hemopatía Maligna; JALSG: Japan Adult Leukemia Study Group; GAMLCG: The German Acute Myeloid Leukemia Cooperative Group; GIMEMA: Gruppo Italiano Malattie EMatologiche dell’Adulto; ATRA: all-trans retinoic acid; ara-C: cytarabine.
Not surprisingly, the analysis of ‘real world’ registries and large population-based cancer database data tell a much different story regarding the EDR in APL (Table 1). In a Swedish registry study of 105 APL patients, the EDR was reported as 29% with hemorrhage being the most common cause of death (41% of patients with early death); 35% of patients with early death never received ATRA [18]. Similarly, in an analysis of 1400 newly diagnosed APL patients using the Surveillance, Epidemiology and End Results (SEER) database, an EDR of 17.3% was reported, which was even higher for patients older than 55 years reaching 24.2% in that patient population [20]. Importantly, the EDR did change, but only modestly over time after ATRA was approved and rapidly incorporated into routine practice with survival improving from the period of 1992–1995 (22.1%) to the period 1996–2011 (14.7%), but no further improvements after 2001 (17.5%). This brings up the concern that ATRA may be frequently administered too late into the presentation of patients with APL contributing to a persistently high EDR.
In fact, in a retrospective analysis of 204 consecutive newly diagnosed APL patients, who presented between 1992 and 2009 to four major academic centers with experience caring for patients with APL, showed that ATRA was only ordered on the day APL was suspected in 31% of patients [21]. The study showed an EDR of 11% with hemorrhage being the cause for early death in 61% of cases. The median time from presentation until suspicion of an APL diagnosis was 1 day and on average it took an additional 1 day until ATRA was administered. In 39% of patients, ATRA was only administered once a suspected diagnosis of APL was pathologically confirmed either by bone marrow biopsy review or by demonstrating the PML-RARα translocation by cytogenetics, fluorescence in situ hybridization (FISH) or polymerase chain reaction (PCR).
Given that APL is a rare disease expert management is essential in the early stages of the disease in order to improve survival. For instance, utilizing the National Cancer Database, it has been shown that compared to APL patients treated at nonacademic centers (community cancer program, comprehensive community cancer program and others), APL patients treated at academic medical centers had a lower 30-day mortality (22% vs. 25%, p = .03) and improved 1-year OS (83% vs. 78%; p < .001) [22]. Innovative approaches are clearly needed to deliver expert care rapidly to patients both in the academic and community hospital setting. One such approach is to establish a support system for emergency room physicians and other providers by having a group of oncologists with expertise in the treatment of APL available for advice over phone, text or email 24 hours a day 7 days a week. This approach has demonstrated some promising results in a pilot study in Georgia and South Carolina [23] and is currently tested in a unique NCI funded clinical trial led by the ECOG-ACRIN clinical trials group (NCT03253848) (Figure 1).
Coagulopathy
Coagulopathy is present in almost all patients with APL at the time of presentation and, if left untreated, can develop into disseminated intravascular coagulation (DIC) [24] (Figure 1). DIC is characterized by coagulopathy, fibrinolysis and proteolysis and prolonged prothrombin (PT), partial thromboplastin (PTT) time and a deceased platelet count and fibrinogen are seen on laboratory evaluation. Although the true incidence of DIC in APL is not known, a recent epidemiological survey from Taiwan showed that about 78% of APL patients developed DIC [25].
The coagulopathy in APL is a complex process driven by expression of the tumor-associated procoagulants including tissue factor (TF) and cancer procoagulant (CP) [26–28] as well as hyperfibrinolysis induced by elevated levels of urokinase-type plasminogen activator (u-PA) and tissue-type plasminogen activator (tPA) and reduced levels of plasminogen and a2-antiplasmin [29–31]. Additionally, the release of cytokines from promyelocytes including IL-1β and TNFα have been shown to induce cell death of endothelial cells with subsequent increase in TF expression [32]. More recently, the podoplanin gene is found to be highly-expressed on APL promyelocytes leading to aberrant platelet binding, activation, and aggregation [33]. Given its complex pathophysiology, it is not surprising that APL can present with both bleeding and thrombosis [24,32].
In a retrospective study of 995 APL patients treated within 5 large clinical trials with ATRA-containing treatment regimens, the two independent predictors for increased rates of hemorrhagic death during the first 30 days following induction therapy were an ECOG performance status of 3–4 (vs. 0–2; HR 2.17, 95% CI 0.84–5.62) and a white blood cell count (WBC) of ≥20 000/mL (vs. <20 000/L; HR 5.2, 95%CI 2.7–10.02) [34]. In a retrospective analysis of 124 APL patients treated with the AIDA regimen (ATRA plus Idarubicin) of the GIMEMA group, 8.9% of patients experienced a thrombotic event [35]. Patients with thrombotic events had a higher median WBC and a higher prevalence of the bcr3 transcript type (72 vs 48%, p = .01), expression of FLT3-ITD (64 vs 28%, p = .02) as well as a CD2 (54 vs 20%, p = .0001) and CD15- positive immunophenotyped (36 vs 8%, p = .01) compared to patients without a thrombotic event [35]. In contrast, a larger retrospective study of 733 APL patients treated with the AIDA regimen in a PETHEMA group study, 4.5% of patients developed a thrombotic event and risk factors for thrombosis were the M3-variant subtype (11% vs 4%, p = .02) and fibrinogen <170 mg/dL (7% vs 3%, p =.02), but not the expression of FLT3-ITD or a CD2 or CD15-positive immunophenotype [36]. Additionally, severe APL differentiation syndrome has been shown to be a risk factor for developing both hemorrhage and thrombosis [37].
Early initiation of ATRA and or ATO and aggressive supportive care utilizing cryoprecipitate and platelet transfusions remain the mainstay of therapy for APL-associated coagulopathy [24]. An expert panel of the European Leukemia Network has published guidelines for the management of patients with suspected APL [38]. These guidelines emphasize the immediate initiation of therapy with ATRA without waiting for genetic confirmation of the PML-RARα fusion gene and utilizing aggressive supportive care measures to reverse any existing and prevent the development of coagulopathy. Recommended supportive care measures include cryoprecipitate and platelet transfusions to maintain a fibrinogen of >100–150 mg/dl and a platelet count of >50.000/μL, with the additional use of fresh frozen plasma in the case of prolonged activated partial thromboplastin time and/or prothrombin time [25,38]. These guidelines also recommend sending diagnostic samples to a reference laboratory for rapid and reliable diagnostic confirmation of the suspected APL diagnosis. Importantly all three actions should be taken simultaneously without any delay in treatment with ATRA or initiation of aggressive supportive measures. Additionally, aggressive treatment of differentiation syndrome by both stopping the offending agent (ATRA or ATO) and treating the patient with high dose steroids (dexamethasone 10 mg/m2 every 12 h) is essential [39].
The role of heparin, antifibrinolytics and recombinant factor VII in the treatment of APL-related coagulopathy is more controversial [24]. Heparin was initially thought to be beneficial in reducing hemorrhage in APL by inhibiting fibrin formation and thereby reducing the consumption of clotting factors and platelets [24,40,41]. Conversely, antifibrinolytic agents (aminocaproic acid or tranexamic acid) have been found to be beneficial in small studies [42,43]. However, the studies showing a potential beneficial effect of heparin and antifibrinolytics were carried out prior to the advent of ATRA-based therapy, were not randomized and enrolled only a small number of patients. In fact, a large retrospective study of 268 patients treated for APL by the GIMEMA before the advent of ATRA showed no difference in response, survival and early hemorrhagic death between heparin, antifibrinolytics (tranexamic acid, epsilon-aminocaproic acid, or aprotinin) and supportive therapy alone [44]. In addition, the use of tranexamic acid for patients treated with the AIDA regimen in the LPA 99 trial led by the PETHEMA group did not reduce the rate of hemorrhagic deaths and was associated with a higher rate of thrombotic events [7,36]. Lastly, the role of recombinant factor VIIa in APL-associated hemorrhage remains unclear. Several case studies reported successful reversal of life-threatening hemorrhage in APL with recombinant factor VIIa [45–47]; however, no clinical trials examining the use of recombinant factor VIIa in APL have been undertaken.
In summary, in order to improve outcomes in APL, particular attention needs to be paid to the persistently high EDR in patients presenting with newly diagnosed APL, which is mainly due to life-threatening coagulopathy and fatal hemorrhage. This can frequently be reversed by the early administration of ATRA and utilizing aggressive transfusion support with blood product. Heparin and antifibrinolytic agents are not recommended anymore for the routine use in APL (Figure 1).
High-Risk APL
The APL 0406 study demonstrated that low- and intermediate-risk APL patients can be effectively treated with a chemotherapy-free regimen utilizing only ATRA and ATO [4,48]. A small pilot study in China took this a step further by showing that 65% and 100% of low- and intermediate-risk APL patients treated with an outpatient regimen using oral ATO and ATRA achieve a complete molecular remission at 3 months and at 6 months, respectively [49]. High-risk APL patients, defined as patients with a WBC > 10 × 109/L, present a challenge to the treating physician. With initiation of treatment, patients are at risk for developing a rapidly rising WBC and potentially life-threatening differentiation syndrome, hemorrhage, and DIC. Therefore, the NCCN guidelines recommend prophylactic administration of corticosteroids in all APL patients with a WBC > 10 × 109/L [50] and particular attention needs to be paid to correcting any existing or worsening coagulopathy as discussed above.
The standard of care recommended by the NCCN guidelines for high-risk patients is induction therapy utilizing ATRA in combination with daunorubicin plus cytarabine or ATRA in combination with ATO and idarubicin. This is followed by consolidation therapy with ATRA plus daunorubicin or ATRA plus ATO followed by maintenance therapy [2,50]. If administered consistently, these treatment regimens lead to excellent clinical outcomes [2,50]. The PETHEMA LPA99 trial showed that adding ATRA to anthracycline chemotherapy during consolidation therapy was superior to anthracycline monotherapy as used in the PETHEMA LPA96 trial [51]. The LPA2005 trial demonstrated that the addition of cytarabine to ATRA plus idarubicin for induction therapy led to a 3-year relapse rate of 11%, which was significantly lower than in the LPA99 trial (26%; p = .03) [52]. In the APML4 trial the combination of ATRA, ATO, and idarubicin followed by ATRA and ATO consolidation and ATRA, 6-mercaptopurine, and methotrexate maintenance therapy led to excellent outcomes with a 5-year disease-free survival (DFS) of 95% and OS of 87% [53].
Significant progress has been made toward using chemotherapy-free regimens similar to what has been achieved for patients with low-risk APL. The MD Anderson group attempted to reduce exposure to traditional chemotherapy by adding gemtuzumab ozogamicin (GO), an anti-CD33 monoclonal antibody conjugated to the anthracycline antibiotic calicheamicin, at a dose of 9 mg/m2 on day 1 of induction therapy with ATRA and ATO for high-risk APL patients, which led to 5-year DFS and OS of 89% and 86%, respectively [54]. In the AML17 trial by the National Cancer Research Institute in the UK, APL patients were randomized to receive either chemotherapy-free treatment with ATRA and ATO or ATRA and idarubicin [16]. While prior trials had excluded high-risk APL patients [4,48], the AML17 trial included 57 high-risk APL patients with a WBC > 10 × 109/L, who were given a single dose of GO at 6 mg/m2 to control the elevated WBC and prevent complications induced by differentiation treatment. Both ATRA plus ATO and ATRA plus idarubicin resulted in high rates of molecular remission (91% vs 88%, p = .48) and 4-year survival (93% vs 89%, p = .25) with a significantly lower 4-year cumulative incidence of morphological relapse in the ATRA plus ATO arm (1% vs. 18%, p = .0007) [16]. Of note, the APL17 trial also administered ATO on a novel twice weekly treatment schedule instead of a daily treatment schedule after completion of daily ATO administration for the first 5 days of each treatment course.
In a thought-provoking small pilot study in China, 20 high-risk APL patients were treated with a chemotherapy-free outpatient based induction regimen using oral ATRA and ATO with the addition hydroxyurea until a WBC < 10 × 109/L was achieved [55]. Long-term outcomes were excellent with a complete molecular response rate of 85% and 100% at 3 and 6 months, respectively, and an estimated 3-year OS and EFS of 100% and 89.4%, respectively. Two patients experienced a molecular relapse at 12 and 15 months but achieved a complete molecular response again using the same treatment protocol. However, 16 patients out of 20 patients required the simultaneous administration of cytarabine for a median of 6 days and the total time in the hospital was a median of 25 days per patient.
In summary, these studies show that even high-risk APL patients can be effectively treated with mostly chemotherapy-free regimens utilizing only GO or anthracyclines for initial cytoreduction (Figure 1).
Relapsed APL
The vast majority of patients with APL achieve a complete remission after induction therapy and molecular remission after completion of consolidation therapy. However, about 10% of patients with high-risk APL experience a relapse of their disease [2]. ATO based regimens have also become the standard of care for patients with relapsed APL as ATO has been shown to result in CR rates of 80–90% and 3-year survival of 50–70% [56–58] (Figure 1). In fact, the risk of relapse has been significantly reduced with using ATO during induction and/or consolidation therapy, including in patients with high-risk APL; however, it is unclear how this will affect the efficacy of ATO in the relapsed disease setting [2].
The general treatment strategy for relapsed disease consists of induction therapy with the goal of achieving a molecular remission followed by consolidation therapy with either autologous or allogeneic stem cell transplantation (SCT) depending on the response. The specific induction treatment approach depends on whether the patient received ATO and anthracycline-containing regimens during induction and/or consolidation therapy and whether the relapse occurred early (<6 months) or late (≥6 months) after completion of an ATO containing treatment regimen [50]. Patients without prior exposure to ATO or with late relapse after treatment with an ATO containing regimen or early relapse after ATRA plus anthracycline-containing regimens are generally treated with ATO until count recovery with marrow confirmation of remission [50]. Patients, who experience an early relapse after ATRA and ATO based treatment without receiving anthracyclines, are treated with ATO and idarubicin instead [50]. For all of these regimens, ATRA can be added but, the degree of benefit if any is unclear in the relapsed disease setting. Missense mutations affecting the ligand-binding domain of the RARα region of the PML-RARα fusion protein conferring resistance to ATRA have been described [59,60]. Furthermore, in a small randomized trial, the addition of ATRA to ATO in relapsed APL patients was not associated with any added benefit [57].
Once patients achieve a second molecular remission, high-dose chemotherapy followed by autologous SCT is most commonly used as consolidation therapy whereas for patients, who do not achieve a molecular remission, allogeneic transplant is preferred if a donor is available [2,61]. In a retrospective analysis of relapsed APL patients, who achieved a molecular remission with salvage therapy, autologous SCT was superior compared to ATO based consolidation therapy with a 5-year event-free survival of 83% in the transplanted group and 34% in the ATO based therapy group, respectively [61]. The benefit of autologous SCT is seen in the subgroup of patients, who have no evidence of minimal residual disease (MRD) as evidenced by PML-RARα negativity by PCR prior to undergoing autologous stem cell transplant. In a prospective study by the GIMEMA group, all 7 APL patients with evidence of MRD by positive PCR for PML-RARα prior to autologous SCT, still had evidence of MRD after receiving an autologous SCT and relapsed after just a median of 5 months after transplant [62]. On the contrary, all 8 patients without evidence of MRD by PCR prior to autologous SCT, remained PCR negative during initial follow up controls after SCT; one patient relapsed at 10 months after transplant, one died of a secondary (PML/RARα−) leukemia, and six remained in molecular remission at a median time of 28 months. In patients with presence of MRD by PCR, allogeneic transplant is recommended as consolidation therapy if a donor is available and the patient is deemed fit for transplant [63].
Patients who do not achieve a remission with initial salvage therapy represent a particular challenge to the treating physician. For these patients, GO can be considered as a salvage treatment option and inclusion of patients into clinical trials should be strongly considered [64,65] (Figure 1).
Conclusions
Targeting PML-RARα with ATRA and ATO has transformed APL from a frequently lethal disease to a curable disease in most patients. The major remaining obstacles to a cure for all patients with APL are a relatively high EDR due to coagulopathy and hemorrhage, as well as, the care for high-risk patients and patients with relapsed disease. Reducing the EDR in patients with APL likely will not depend on the approval of new drugs, but will require a rigorous implementation of existing guidelines including the start of ATRA at first suspicion of APL and aggressive reversal of coagulopathy.
High-risk APL patients with a WBC > 10 × 109/L are at a particular risk for developing coagulopathy and differentiation syndrome once induction treatment is started and should be treated with prophylactic steroids and blood products to counteract any coagulopathy. De-escalation of treatment in patients with high-risk APL unitizing mostly chemotherapy -free regimen combining ATRA with ATO and using cytoreductive treatment with chemotherapy or GO for initial leukocytosis does not result in inferior results compared to ATRA plus idarubicin.
ATO based salvage therapy has improved outcomes of relapsed APL. Patients who do not achieve a remission can be treated with GO but are best included in clinical trials.
Footnotes
Potential conflict of interest: Disclosure forms provided by the authors are available with the full text of this article online at https://doi.org/10.1080/10428194.2019.1613540.
References
- [1].Grignani F, Ferrucci PF, Testa U, et al. The acute promyelocytic leukemia-specific PML-RAR alpha fusion protein inhibits differentiation and promotes survival of myeloid precursor cells. Cell. 1993;74:423–431. [DOI] [PubMed] [Google Scholar]
- [2].Watts JM, Tallman MS. Acute promyelocytic leukemia: what is the new standard of care? Blood Rev. 2014; 28:205–212. [DOI] [PubMed] [Google Scholar]
- [3].Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120:1570–1580. [DOI] [PubMed] [Google Scholar]
- [4].Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369:111–121. [DOI] [PubMed] [Google Scholar]
- [5].Tallman MS, Lo-Coco F, Kwaan HC, et al. Early death in patients with acute promyelocytic leukemia. Proceedings from a live roundtable at the 2010 American Society of Hematology Annual Meeting, December 4–7, 2010, Orlando, Florida. Clin Adv Hematol Oncol. 2011;9:1–16. [PubMed] [Google Scholar]
- [6].Di Bona E, Avvisati G, Castaman G, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. 2000; 108:689–695. [DOI] [PubMed] [Google Scholar]
- [7].de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111:3395–3402. [DOI] [PubMed] [Google Scholar]
- [8].Tallman MS, Andersen JW, Schiffer CA, et al. All-transretinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337:1021–1028. [DOI] [PubMed] [Google Scholar]
- [9].Fenaux P, Chastang C, Chevret S, et al. A randomized comparison of all transretinoic acid (ATRA) followed by chemotherapy and ATRA plus chemotherapy and the role of maintenance therapy in newly diagnosed acute promyelocytic leukemia. The European APL Group. Blood. 1999;94:1192–1200. [PubMed] [Google Scholar]
- [10].Sanz MA, Martin G, Rayon C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94:3015–3021. [PubMed] [Google Scholar]
- [11].Asou N, Adachi K, Tamura J, et al. Analysis of prognostic factors in newly diagnosed acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Japan Adult Leukemia Study Group. Jco. 1998;16:78–85. [DOI] [PubMed] [Google Scholar]
- [12].Sanz MA, Montesinos P, Vellenga E, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA Group. Blood. 2008;112:3130–3134. [DOI] [PubMed] [Google Scholar]
- [13].Asou N, Kishimoto Y, Kiyoi H, et al. A randomized study with or without intensified maintenance chemotherapy in patients with acute promyelocytic leukemia who have become negative for PML-RARalpha transcript after consolidation therapy: the Japan Adult Leukemia Study Group (JALSG) APL97 study. Blood. 2007;110:59–66. [DOI] [PubMed] [Google Scholar]
- [14].Lengfelder E, Haferlach C, Saussele S, et al. High dose ara-C in the treatment of newly diagnosed acute promyelocytic leukemia: long-term results of the German AMLCG. Leukemia. 2009;23:2248–2258. [DOI] [PubMed] [Google Scholar]
- [15].Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117:4716–4725. [DOI] [PubMed] [Google Scholar]
- [16].Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16:1295–1305. [DOI] [PubMed] [Google Scholar]
- [17].Jeddi R, Kacem K, Ben Neji H, et al. Predictive factors of all-trans-retinoic acid related complications during induction therapy for acute promyelocytic leukemia. Hematology. 2008;13:142–146. [DOI] [PubMed] [Google Scholar]
- [18].Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia. 2011;25: 1128–1134. [DOI] [PubMed] [Google Scholar]
- [19].McClellan JS, Kohrt HE, Coutre S, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012; 97:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood. 2011;118:1248–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Altman JK, Rademaker A, Cull E, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res. 2013;37:1004–1009. [DOI] [PubMed] [Google Scholar]
- [22].Smith Giri KM, Vijaya Raj B. Overall survival (OS) of acute promyelocytic leukemia (APL) treated in academic (AC) versus non academic (NAC) centers. American Society of Clinical Oncology Meeting 2016, Abstract e18142; 2017. [Google Scholar]
- [23].Anand Jillella MA. Asad Bashey et al Decreasing Early Deaths in Acute Promyelocytic Leukemia (APL) By Using a Simplified Treatment Algorithm and Establishing a Network with Academic and Community Centers in USA. Presented at the American Society of Hematology Meeting 2015. [Google Scholar]
- [24].Choudhry A, DeLoughery TG. Bleeding and thrombosis in acute promyelocytic leukemia. Am J Hematol. 2012;87:596–603. [DOI] [PubMed] [Google Scholar]
- [25].Chang H, Kuo MC, Shih LY, et al. Clinical bleeding events and laboratory coagulation profiles in acute promyelocytic leukemia. Eur J Haematol. 2012;88: 321–328. [DOI] [PubMed] [Google Scholar]
- [26].Falanga A, Alessio MG, Donati MB, et al. A new procoagulant in acute leukemia. Blood. 1988;71:870–875. [PubMed] [Google Scholar]
- [27].Koyama T, Hirosawa S, Kawamata N, et al. All-trans retinoic acid upregulates thrombomodulin and down-regulates tissue-factor expression in acute promyelocytic leukemia cells: distinct expression of thrombomodulin and tissue factor in human leukemic cells. Blood. 1994;84:3001. [PubMed] [Google Scholar]
- [28].Falanga A, Iacoviello L, Evangelista V, et al. Loss of blast cell procoagulant activity and improvement of hemostatic variables in patients with acute promyelocytic leukemia administered all-trans-retinoic acid. Blood. 1995;86:1072–1081. [PubMed] [Google Scholar]
- [29].Bennett B, Booth NA, Croll A, et al. The bleeding disorder in acute promyelocytic leukaemia: fibrinolysis due to u-PA rather than defibrination. Br J Haematol. 1989;71:511–517. [DOI] [PubMed] [Google Scholar]
- [30].Sakata Y, Murakami T, Noro A, et al. The specific activity of plasminogen activator inhibitor-1 in disseminated intravascular coagulation with acute promyelocytic leukemia. Blood. 1991;77:1949–1957. [PubMed] [Google Scholar]
- [31].Dombret H, Scrobohaci ML, Daniel MT, et al. In vivo thrombin and plasmin activities in patients with acute promyelocytic leukemia (APL): effect of all-trans retinoic acid (ATRA) therapy. Leukemia. 1995;9:19–24. [PubMed] [Google Scholar]
- [32].Stein E, McMahon B, Kwaan H, et al. The coagulopathy of acute promyelocytic leukaemia revisited. Best Pract Res Clin Haematol. 2009;22:153–163. [DOI] [PubMed] [Google Scholar]
- [33].Lavallee VP, Chagraoui J, MacRae T, et al. Transcriptomic landscape of acute promyelocytic leukemia reveals aberrant surface expression of the platelet aggregation agonist Podoplanin. Leukemia. 2018;32:1349–1357. [DOI] [PubMed] [Google Scholar]
- [34].Mantha S, Goldman DA, Devlin SM, et al. Determinants of fatal bleeding during induction therapy for acute promyelocytic leukemia in the ATRA era. Blood. 2017;129:1763–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Breccia M, Avvisati G, Latagliata R, et al. Occurrence of thrombotic events in acute promyelocytic leukemia correlates with consistent immunophenotypic and molecular features. Leukemia. 2007;21:79–83. [DOI] [PubMed] [Google Scholar]
- [36].Montesinos P, de la Serna J, Vellenga E, et al. Incidence and risk factors for thrombosis in patients with acute promyelocytic leukemia. experience of the PETHEMA LPA96 and LPA99 protocols. Blood. 2006; 108:1503. [Google Scholar]
- [37].Montesinos P, Bergua JM, Vellenga E, et al. Differentiation syndrome in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and anthracycline chemotherapy: characteristics, outcome, and prognostic factors. Blood. 2009;113: 775–783. [DOI] [PubMed] [Google Scholar]
- [38].Sanz MA, Grimwade D, Tallman MS, et al. Management of acute promyelocytic leukemia: recommendations from an expert panel on behalf of the European Leukemia Net. Blood. 2009;113:1875–1891. [DOI] [PubMed] [Google Scholar]
- [39].Sanz MA, Montesinos P. How we prevent and treat differentiation syndrome in patients with acute promyelocytic leukemia. Blood. 2014;123:2777–2782. [DOI] [PubMed] [Google Scholar]
- [40].Gralnick HR, Bagley J, Abrell E. Heparin treatment for the hemorrhagic diathesis of acute promyelocytic leukemia. Am J Med. 1972;52:167–174. [DOI] [PubMed] [Google Scholar]
- [41].Hoyle CF, Swirsky DM, Freedman L, et al. Beneficial effect of heparin in the management of patients with APL. Br J Haematol. 1988;68:283–289. [DOI] [PubMed] [Google Scholar]
- [42].Avvisati G, ten Cate JW, Buller HR, et al. Tranexamic acid for control of haemorrhage in acute promyelocytic leukaemia. Lancet. 1989;2:122–124. [DOI] [PubMed] [Google Scholar]
- [43].Keane TJ, Gorman AM, O’Connell LG, et al. epsilon-Amino-caproic acid in the management of acute promyelocytic leukaemia. Acta Haematol. 1976;56: 202–204. [DOI] [PubMed] [Google Scholar]
- [44].Rodeghiero F, Avvisati G, Castaman G, et al. Early deaths and anti-hemorrhagic treatments in acute promyelocytic leukemia. A GIMEMA retrospective study in 268 consecutive patients. Blood. 1990;75: 2112–2117. [PubMed] [Google Scholar]
- [45].Pemmaraju N, Sasaki K, Johnson D, et al. Successful treatment of intracranial hemorrhage with recombinant activated factor VII in a patient with newly diagnosed acute myeloid leukemia: a case report and review of the literature. Front Oncol. 2015;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nosari A, Caimi TM, Zilioli V, et al. Cerebral hemorrhage treated with NovoSeven in acute promyelocytic leukemia. Leuk Lymphoma. 2012;53:160. [DOI] [PubMed] [Google Scholar]
- [47].Zver S, Andoljsek D, Cernelc P. Effective treatment of life-threatening bleeding with recombinant activated factor VII in a patient with acute promyelocytic leukaemia. Eur J Haematol. 2004;72:455–456. [DOI] [PubMed] [Google Scholar]
- [48].Platzbecker U, Avvisati G, Cicconi L, et al. Improved outcomes with retinoic acid and arsenic trioxide compared with retinoic acid and chemotherapy in non-high-risk acute promyelocytic leukemia: final results of the randomized italian-german APL0406 trial. JCO. 2017;35:605–612. [DOI] [PubMed] [Google Scholar]
- [49].Zhu HH, Huang XJ. Oral arsenic and retinoic acid for non-high-risk acute promyelocytic leukemia. N Engl J Med. 2014;371:2239–2241. [DOI] [PubMed] [Google Scholar]
- [50].O’Donnell MR, Tallman MS, Abboud CN, et al. Acute Myeloid Leukemia, Version 3.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2017. [DOI] [PubMed] [Google Scholar]
- [51].Sanz MA, Martin G, Gonzalez M, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans-retinoic acid and anthracycline monochemotherapy: a multicenter study by the PETHEMA group. Blood. 2004;103:1237–1243. [DOI] [PubMed] [Google Scholar]
- [52].Sanz MA, Montesinos P, Rayon C, et al. Risk-adapted treatment of acute promyelocytic leukemia based on all-trans retinoic acid and anthracycline with addition of cytarabine in consolidation therapy for high-risk patients: further improvements in treatment outcome. Blood. 2010;115:5137–5146. [DOI] [PubMed] [Google Scholar]
- [53].Iland HJ, Collins M, Bradstock K, et al. Use of arsenic trioxide in remission induction and consolidation therapy for acute promyelocytic leukaemia in the Australasian Leukaemia and Lymphoma Group (ALLG) APML4 study: a non-randomised phase 2 trial. Lancet Haematol. 2015;2:e357–e366. [DOI] [PubMed] [Google Scholar]
- [54].Estey E, Garcia-Manero G, Ferrajoli A, et al. Use of all-trans retinoic acid plus arsenic trioxide as an alternative to chemotherapy in untreated acute promyelocytic leukemia. Blood. 2006;107:3469–3473. [DOI] [PubMed] [Google Scholar]
- [55].Zhu HH, Liu YR, Jia JS, et al. Oral arsenic and all-trans retinoic acid for high-risk acute promyelocytic leukemia. Blood. 2018;131:2987–2989. [DOI] [PubMed] [Google Scholar]
- [56].Au WY, Lie AK, Chim CS, et al. Arsenic trioxide in comparison with chemotherapy and bone marrow transplantation for the treatment of relapsed acute promyelocytic leukaemia. Ann Oncol. 2003;14: 752–757. [DOI] [PubMed] [Google Scholar]
- [57].Raffoux E, Rousselot P, Poupon J, et al. Combined treatment with arsenic trioxide and all-trans-retinoic acid in patients with relapsed acute promyelocytic leukemia. J Clin Oncol. 2003;21:2326–2334. [DOI] [PubMed] [Google Scholar]
- [58].Shigeno K, Naito K, Sahara N, et al. Arsenic trioxide therapy in relapsed or refractory Japanese patients with acute promyelocytic leukemia: updated outcomes of the phase II study and postremission therapies. Int J Hematol. 2005;82:224–229. [DOI] [PubMed] [Google Scholar]
- [59].Ding W, Li YP, Nobile LM, et al. Leukemic cellular retinoic acid resistance and missense mutations in the PML-RARalpha fusion gene after relapse of acute promyelocytic leukemia from treatment with all-trans retinoic acid and intensive chemotherapy. Blood. 1998; 92:1172–1183. [PubMed] [Google Scholar]
- [60].Fasan A, Haferlach C, Perglerova K, et al. Molecular landscape of acute promyelocytic leukemia at diagnosis and relapse. Haematologica. 2017;102:e222–e224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Thirugnanam R, George B, Chendamarai E, et al. Comparison of clinical outcomes of patients with relapsed acute promyelocytic leukemia induced with arsenic trioxide and consolidated with either an autologous stem cell transplant or an arsenic trioxide-based regimen. Biol Blood Marrow Transplant. 2009; 15:1479–1484. [DOI] [PubMed] [Google Scholar]
- [62].Meloni G, Diverio D, Vignetti M, et al. Autologous bone marrow transplantation for acute promyelocytic leukemia in second remission: prognostic relevance of pre-transplant minimal residual disease assessment by reverse-transcription polymerase chain reaction of the PML/RAR alpha fusion gene. Blood. 1997;90:1321–1325. [PubMed] [Google Scholar]
- [63].Ramadan SM, Di Veroli A, Camboni A, et al. Allogeneic stem cell transplantation for advanced acute promyelocytic leukemia in the ATRA and ATO era. Haematologica. 2012;97:1731–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Petti MC, Pinazzi MB, Diverio D, et al. Prolonged molecular remission in advanced acute promyelocytic leukaemia after treatment with gemtuzumab ozogamicin (Mylotarg CMA-676). Br J Haematol. 2001;115: 63–65. [DOI] [PubMed] [Google Scholar]
- [65].Takeshita Akihiro, Ono Takaaki, Kojima Yumi, et al. Efficacy of Gemtuzumab Ozogamicin (GO) Monotherapy on Relapsed/Refractory Acute Promyelocytic Leukemia (APL). Presented at American Society of Hematology (ASH) Annual meeting 2011; 2011. [Google Scholar]


