Dear Editor,
Chronic neutrophilic leukemia (CNL) is a rare myeloproliferative neoplasm (MPN) that includes only about 200 patients described to date meeting the World Health Organization (WHO) criteria and the recently reported CSF3R T618I mutation. Diagnosis of CNL using the World Health Organization 2008 criteria[1] requires a WBC count of ≥25 × 109/L, ≥80 % segmented/band neutrophils, absence of dysgranulopoiesis, exclusion of genetic drivers that are known to occur in others MPNs (such as BCR-ABL1, PDGFRA/B, or FGFR1 rearrangements), and <10 % immature myeloid cells [2]. Previously, CNL was often confused with chronic myeloid leukemia (CML), atypical CML (aCML), or chronic myelomonocytic leukemia (CMML); however, this changed with the identification of oncogenic mutations in the granulocyte colony-stimulating 3 factor receptor (CSF3R) gene in approximately 83 % of WHO-defined CNL patients [3]. CSF3R mutations in CNL are either nonsense or frameshift mutations truncating the cytoplasmic tail (truncation mutations) or point mutations in the extracellular domain (membrane proximal mutations) [3, 4]. Truncation mutations are sensitive to dasatinib in vitro, while membrane proximal mutations show ligand-independent signaling through the JAKSTAT pathway and cells harboring this mutation are sensitive to Jak1/2 inhibitors in vitro. Additional observations from Mayo Clinic showed that concomitant SETBP1 and ASXL1 mutations in CNL patients with a CSF3R mutation help to further distinguish CNL from aCML and are associated with poorer outcomes [5]. There is as yet limited clinical experience with ruxolitinib in patients with CNL, given the low prevalence of the disease [6, 7]. After the initial case reported by Maxson et al., Dao et al. presented the case of another patient with CNL and a CSF3RT618I mutation who experienced reduction in hepatosplenomegaly and improvement in blood counts and constitutional symptoms with ruxolitinib, but there was no reduction in CSF3R T618I allele frequency or overall bone marrow cellularity after 3 months of treatment [6]. This argues for the inability of ruxolitinib to cytoreduce the malignant clone in the short-term. Dao et al. described a double-mutated CNL patient (CSF3RT618I and SETBP1 D868N) who was refractory to the treatment with both ruxolitinib and hydroxyurea both in vitro and in vivo [7]. The authors speculated that the co-expression of SETBP1 in these CSF3R T618I mutations in a patient with CNL might have contributed to the ineffectiveness of JAK inhibitor therapy in CNL. We report the case of a patient with a CSF3R and SETBP1 mutation, who responded to ruxolitinib, at least for a brief period of time.
Our patient is a 76-year-old man, who presented with a new sharp intermittent left upper quadrant (LUQ) abdominal pain and fatigue without other constitutional symptoms. Complete blood count (CBC) showed a white blood cell count (WBC) of 147 × 109/L, hemoglobin (Hb) 9.7 g/dL, MCV 104, and platelet count 235 × 109/L. The differential count included 73 % neutrophils, 4 % lymphocytes, 1 % monocytes, 1 % myelocytes, 5 % promyelocytes, and 2 % blasts. Blood smear showed dysgranulopoiesis (Fig. 1a). A CBC done 7 months earlier was normal. Examination demonstrated mild LUQ tenderness without organomegaly or lymphadenopathy. Uric acid and LDH were elevated while liver enzymes and kidney function were normal. Computed tomography (CT) scan of the abdomen and pelvis showed hepatomegaly (18.4 cm) and splenomegaly 15.5 cm), which was absent on prior CT in 2012, as well as a wedge-shaped splenic hypodensity possibly representing a splenic infarct (Fig. 2a, b, c). Marrow biopsy was 100 % cellular with profound erythropoietic hypoplasia, mild myeloid dysplasia, and diffused interstitial reticulin fibrosis (Fig. 1b). No aspirate could be obtained. Flow cytometric studies of blood confirmed the majority of cells being mature neutrophils and less than 1 % of cells being CD34+ CD117+ myeloblasts. There was no increase in monocytes and no evidence for monoclonal lymphocytosis.
Fig. 1.
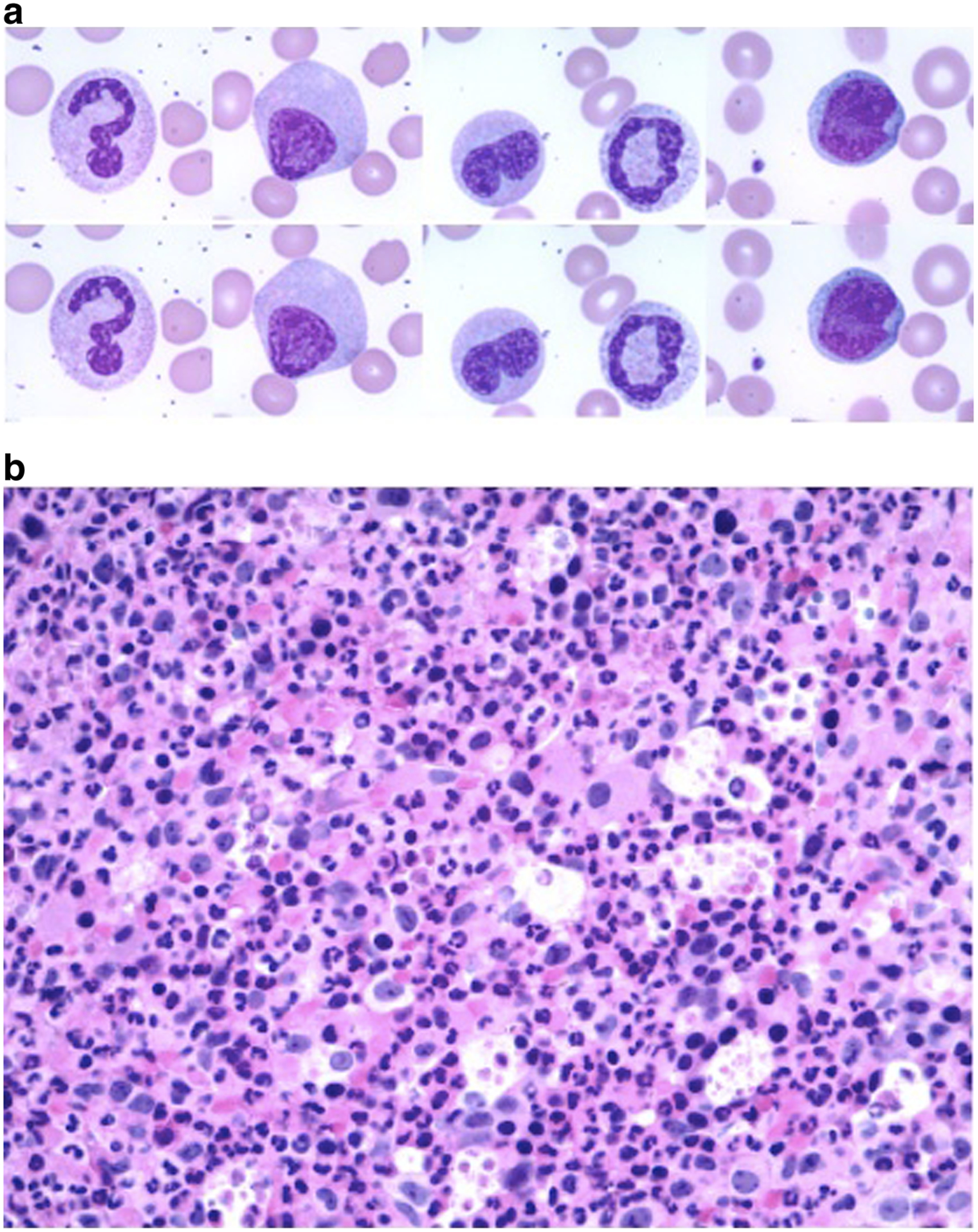
a Wright-Giemsa-stained blood smear (x400) demonstrating dysgranulopoiesis. b Bone marrow biopsy with myeloid hyperplasia and multilineage dysplasia (x100)
Fig. 2.
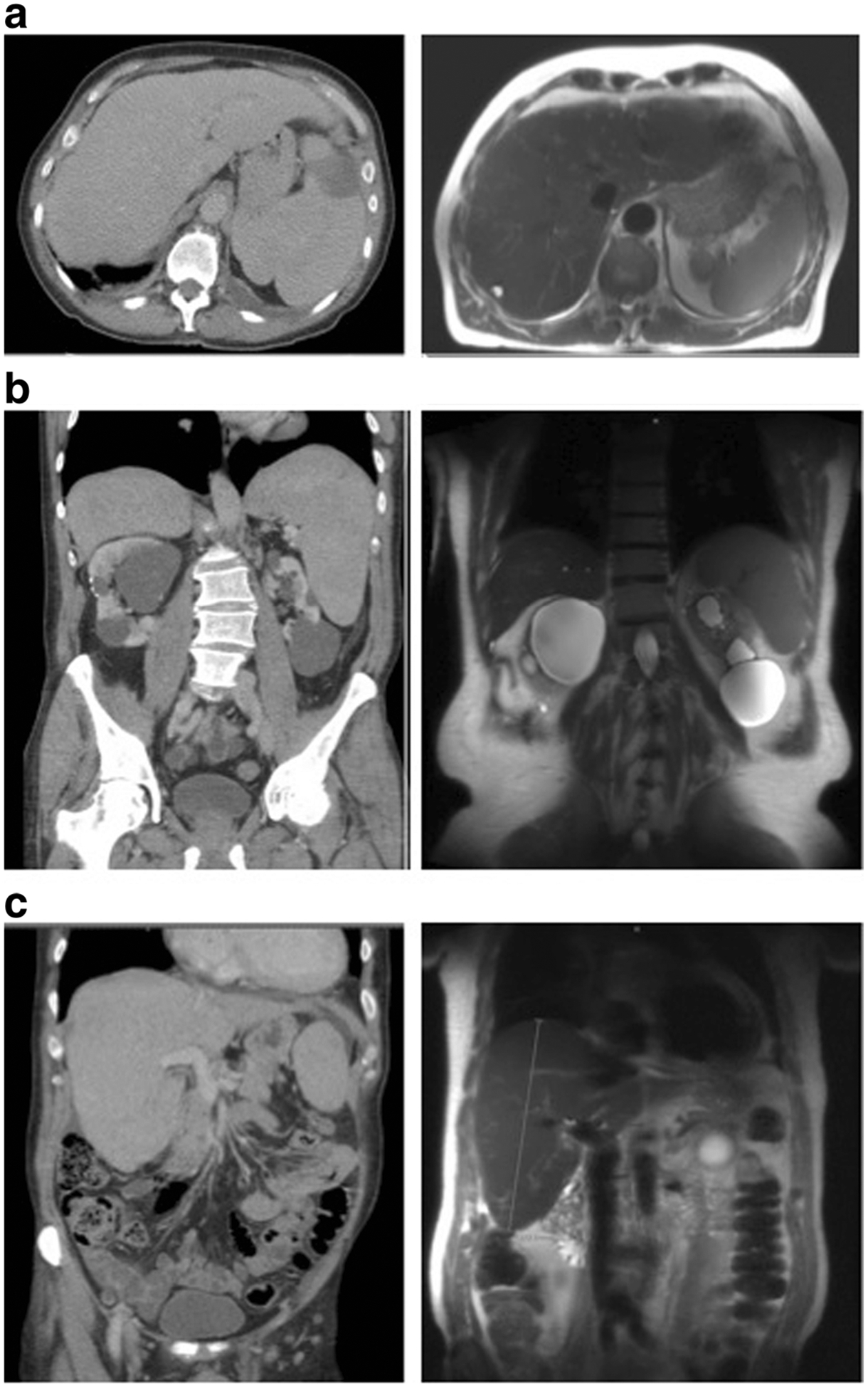
Pre- and post-ruxolitinib therapy contrast-enhanced CT scans. a Axial view pre- and post-treatment. b Coronal view splenomegaly preand post-treatment. c Coronal view hepatomegaly pre- and post-treatment
The patient was treated with hydroxyurea while awaiting results of florescence in situ hybridization (FISH) and polymerase chain reaction (PCR) studies for t(9; 22) and BCR-ABL fusion, respectively, which were negative. Supportive care with rasburicase, allopurinol, and fluids was administered. FISH showed three copies of chromosome 8q in 10 % of the bone marrow cells. Molecular profiling demonstrated no mutations of CEBPA, NPM1, FLT3, KIT, JAK2, PDFGR, FGR1, or CALR. CSF3R T618I mutation was present, suggestive of a diagnosis of chronic neutrophilic leukemia (CNL)[5]; SETBP1 (SETBP1 G870S) and ASXL1 (ASXL1 R404 and Y700) mutations, both of which have been reported to be associated with worse outcomes and resistance to JAK2 inhibitor therapy in patients with CNL, were also present [7–9]. The patient declined allogeneic stem cell transplant. Ruxolitinib was started at 5 mg twice daily and the dose increased in 5-mg increments to 20 mg twice daily (Fig. 3). On follow-up 3 months after initiation of ruxolitinib therapy, symptoms resolved, the patient was able to resume strenuous physical activity, and the CBC markedly improved (Fig. 3). Additionally, he achieved a dramatic reduction in spleen size (11.6 cm in length from previously 15.5 cm) and moderate reduction in liver size (17.6 cm in length from previously 18.4 cm) (Fig. 2 a, b, c). Five months after initiating ruxolitinib, he lost response with increasing WBC and worsening platelet count and Hb, so hydoxyurea was added with stabilization of his WBC count (Fig. 3).
Fig. 3.
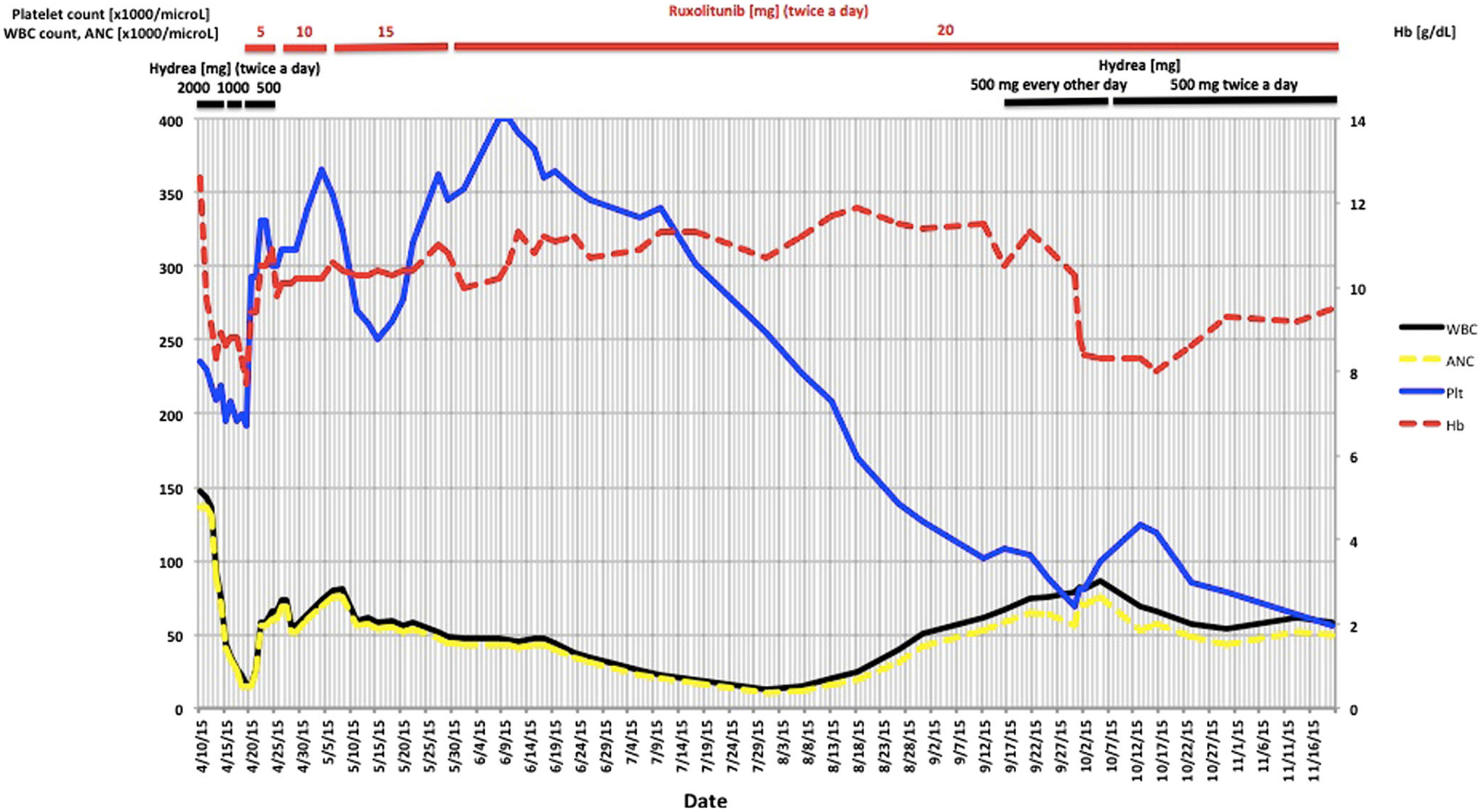
WBC, ANC, Hb, and platelet count upon presentation and during treatment with hydroxyurea and ruxolitinib
The response in our case suggests that patients with SETBP1 mutations may respond to ruxolitinib, at least for a brief period of time. An ongoing small clinical trial is evaluating the impact of ruxolitinib on natural history of CNL (Clinicaltrial.gov NCT02092324). As ruxolitinib therapy has not been shown to change the natural history of CNL, ASCT should be considered for transplant-eligible patients with CNL.
Footnotes
Conflict of interest Dr. Steensma and Dr. Rampal consulted for Incyte and received honoraria. All other authors have no relevant conflicts.
References
- 1.Tefferi A, Vardiman JW (2008) Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 22(1): 14–22. doi: 10.1038/sj.leu.2404955 [DOI] [PubMed] [Google Scholar]
- 2.Tefferi A, Elliott M, Pardanani A (2015) Chronic neutrophilic leukemia: novel mutations and their impact on clinical practice. Curr Opin Hematol 22(2):171–176. doi: 10.1097/MOH.0000000000000114 [DOI] [PubMed] [Google Scholar]
- 3.Gotlib J, Maxson JE, George TI, Tyner JW (2013) The new genetics of chronic neutrophilic leukemia and atypical CML: implications for diagnosis and treatment. Blood 122(10):1707–1711. doi: 10.1182/blood-2013-05-500959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, Bottomly D, Wilmot B, McWeeney SK, Tognon CE, Pond JB, Collins RH, Goueli B, Oh ST, Deininger MW, Chang BH, Loriaux MM, Druker BJ, Tyner JW (2013) Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med 368(19):1781–1790. doi: 10.1056/NEJMoa1214514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pardanani A, Lasho TL, Laborde RR, Elliott M, Hanson CA, Knudson RA, Ketterling RP, Maxson JE, Tyner JW, Tefferi A (2013) CSF3R T618I is a highly prevalent and specific mutation in chronic neutrophilic leukemia. Leukemia 27(9):1870–1873. doi: 10.1038/leu.2013.122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dao KH, Solti MB, Maxson JE, Winton EF, Press RD, Druker BJ, Tyner JW (2014) Significant clinical response to JAK1/2 inhibition in a patient with CSF3R-T618I-positive atypical chronic myeloid leukemia. Leukemia Res Rep 3(2):67–69. doi: 10.1016/j.lrr.2014.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasho TL, Mims A, Elliott MA, Finke C, Pardanani A, Tefferi A (2014) Chronic neutrophilic leukemia with concurrent CSF3R and SETBP1 mutations: single colony clonality studies, in vitro sensitivity to JAK inhibitors and lack of treatment response to ruxolitinib. Leukemia 28(6):1363–1365. doi: 10.1038/leu.2014.39 [DOI] [PubMed] [Google Scholar]
- 8.Makishima H, Yoshida K, Nguyen N, Przychodzen B, Sanada M, Okuno Y, Ng KP, Gudmundsson KO, Vishwakarma BA, Jerez A, Gomez-Segui I, Takahashi M, Shiraishi Y, Nagata Y, Guinta K, Mori H, Sekeres MA, Chiba K, Tanaka H, Muramatsu H, Sakaguchi H, Paquette RL, McDevitt MA, Kojima S, Saunthararajah Y, Miyano S, Shih LY, Du Y, Ogawa S, Maciejewski JP (2013) Somatic SETBP1 mutations in myeloid malignancies. Nat Genet 45(8):942–946. doi: 10.1038/ng.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elliott MA, Pardanani A, Hanson CA, Lasho TL, Finke CM, Belachew AA, Tefferi A (2015) ASXL1 mutations are frequent and prognostically detrimental in CSF3R-mutated chronic neutrophilic leukemia. Am J Hematol 90(7):653–656. doi: 10.1002/ajh.24031 [DOI] [PubMed] [Google Scholar]


