Abstract
Several factors impact the immune responses such as the chemical nature of antigens, the physiologic and metabolic condition of the responsive cells, the site of antigen recognition, and neuroendocrine and pharmacological received agents. Incompatibility of host immune responses to the entrapped antigens leads to an immune pathological manner instead of an immune protection which results in the disharmony of the immune effective factors. Besides the fact that stress is one of the most common effective factors in human life, it also contributed to the protection, suppression, and pathology of the immune system. In this review article, the direct and indirect effects of the stress on the function of T cells and the contributed mechanism of action will be discussed.
Keywords: T cell, Stress, Pathology, CNS
Introduction
T cells, as the main component of cellular immunity, are highlighted for participating in defense against cancer and virally infected cells. The in vivo biological roles of T cells in immune responses and immunopathology have been largely elucidated from studies and have led to advancement of T cell-based immunotherapies in human. T cells are discussed in the context of their differentiation, function, and ontogeny. T cells primarily differentiate from hematopoietic stem cells (HSC) in the bone marrow (BM) and then migrate to the thymus for selection, maturation, and transfer to the peripheral organs. Mature naïve T cells have the capacity to the response to the specific peptide-loaded MHC (major histocompatibility complex) (Kumar et al. 2018). In the theory of two signals, naïve T cells require two distinct signals for complete activation; the first is provided from the engagement of T cell receptors by peptide-loaded MHC, and the second is delivered from the binding of costimulatory molecules on antigen-presenting cells (e.g., CD80 and CD86) to activation receptors on T cells (such as CD28) (Capece et al. 2012). After T cell activation, effector cells differentiate, proliferate, and migrate to sites of inflammation to promote efficient immune responses through direct killing (e.g., CD8+ cytotoxic T cells) or cytokine production (e.g., CD4+ T helper cells) (Kumar et al. 2018). T cells in terms of their cytokine production and cellular function are divided to several subtypes such as Th1, Th2, and Th17 (Dhabhar 2014). Cytokines are produced by Th1 (type 1 cytokines; IL-2 and IFN-γ) and Th2 (type 2 cytokines; IL-4 and IL-13) cells resulting in the cellular and humoral immunity respectively.
Different endogenous and environmental factors impact on the T cell development, activation, and function. Hormones released through the activation of the limbic-hypothalamic-pituitary-adrenal (HPA) axis in stress condition can regulate T cell function (Silverman et al. 2005). Based on a classical definition, stress physiologically is a state in which the HPA axis and sympatho-adrenomedullary system are co-activated (Jeffrey et al. 1995). Human allostatic (adaptation) systems enable us to respond to the physical state created by internal and environmental stimuli (e.g., asleep, standing, exercising, crowding, hunger, isolation, microbial and parasite infection, and danger) (McEwen 1998). Body components, including the immune system, HPA axis, metabolic response, and autonomic nervous system, are also involved in the allostasis to protect the body from the harmful effects of internal or external stresses. Stress stimulators are divided into good, tolerable, and toxic based on their effects on the body. The good stress refers to transiently increasing heart rate, blood pressure, and stress hormones which seem to be similar to the signs of acute stress. Tolerable stress is characterized by the compensative adaptive response to a time-limited stress such as homelessness or a natural disaster, the amount of responses which can return the body to the baseline condition. In the toxic condition, stress-induced alteration in the body is more than compensation response and can increase disruption of the brain architecture, cognitive impairments, and other stress-related disorders (McEwen et al. 2015; Shonkoff et al. 2009). The entire CNS is involved in the body hemostasis directly or indirectly such as HPA axis, autonomic nervous system (ANS), and particular areas in the central nervous system which are important components in cognition and the response to stress. Specific areas in the brain such as the hypothalamus and brainstem play critical roles in orchestrating the stress response (Tsigos et al. 2016).
Final effects of stress on the immune system were discussed as three distinct types: protective effects (during vaccination, wounding, and some types of infections and cancers) for the short time stress, pathologic, and immunoregulatory consequences for the chronic stress (Dhabhar 2014). In the animal models, it was demonstrated that animals received stress at the time of antigen exposure (first immunization), increasing the level of type 1 cytokines; this type of immune response leads to leukocyte infiltration in the site of antigen exposure. In addition, the short-term stress in the time of immunization enhanced the contact hypersensitivity (CHS) responses (Saint-Mezard et al. 2003). An enhancement in immune responses was observed due to short-term stress in the animal model of studies. This adjuvant effect of short-term stress is mediated by physiological concentration of glucocorticoid and epinephrine hormones (Dhabhar and McEwen 1999). Nevertheless, the studies suggested that pharmacologic concentration of endogenous hormones and their synthetic forms are immune suppressive (Dhabhar 2014).
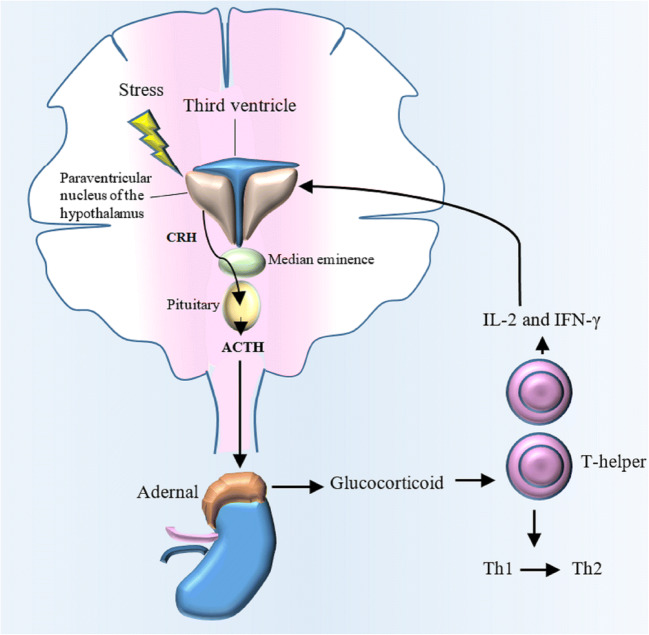
Two proposed mechanisms were found for the coordination between neural and immune components, including the delivery of messengers such as norepinephrine (NE) from the brain to the organs including immune cells and the secret of messengers such as cytokine from activated immune cells which migrate to the brain (Sanders 2012). Stress hormones can regulate the development and function of T cell-dependent cellular immunity. There is a bidirectional communication between the T cell and HPA axis. T cell cytokines such as IL-2 and IFN-γ induce HPA axis to release glucocorticoid. On the other hand, glucocorticoid (GC) plays an important role in the polarization of T cell into the type 2 immune responses (Th2/anti-inflammatory) (Fig. 1). In addition, GC induces immune system to suppress the synthesis of pro-inflammatory cytokines (Silverman et al. 2005).
Fig. 1.
The communication among stress, HPA axis, and T cell function. Stress-induced HPA axis activation leads to release of the glucocorticoid (GC) from adrenal glands. GCs can modulate T cell function by causing a shift from Th1 to Th2. On the other hand, T cell cytokines such as IL-2 and IFN-γ can stimulate HPA axis to release glucocorticoids
In this review, the effects of stress on the function of T cells were discussed from two aspects: firstly, direct effects of stress hormones on T cells and secondly, the evaluation of stress-induced systemic and metabolic changes on T cell function and differentiation. Finally, the immunopathology of T cells in stress conditions is described.
Sensing of stress by T cells; β2-adrenergic receptors on T cells
One of the central regulatory pathways between the nervous system and T cells is mediated by the expression of the β2-adrenergic receptor on T cells. Murine naïve CD4+ T cells and the effector Th1 cells express β2-adrenergic receptor but developed Th2 cells from naïve CD4+ T cells repress the expression of β2-adrenergic receptor throughout an epigenetic mechanism (Sanders 2012). Environmental cytokines, genetic variation, and epigenetic factors such as histone acetylation have an impact on the expression level of β2-adrenergic receptors on the T cell. Noradrenergic nerve fibers presented in the parenchymal of lymphoid organs release NE where resident T cells can be affected through the expression of the β2-adrenergic receptor. Engagement of the β2-adrenergic receptor (a G protein-coupled receptor) triggers a cascade signaling that increases cAMP and activates protein kinase A (Fan and Wang 2009; Sanders 2012; ThyagaRajan et al. 2012). When β2-adrenergic receptors on Th1 cells are engaged, the IFN-γ production can be affected depending on the time of Th1 cell activation. IFN-γ production will be decreased in Th1 cells which β2-adrenergic receptor engagement occurred before their cell activation. However, after cell activation, the receptor engagement leads to the increase in the level of IFN-γ production in comparison with the control cell that activated alone (Sanders 2012).
Although the role of β2-adrenergic receptors on the Th1 is more investigated, a higher density of β2-adrenergic receptors was found on the CD8+ T cells as well. Regarding the previous in vitro study, the expression of β2-adrenergic receptors on the CD8+ T cells was induced by IL-2 stimulation (Wahle et al. 2001). For determining the effect of NE on the memory CD8+ T cells, the in vitro NE-treated CD8+ T cells were stimulated by antibodies specific for CD3 and CD28. In addition, memory T cells from individuals with a low and high level of NE were assayed. It was determined that an elevated level of β2-adrenergic receptors was expressed in memory T cells in comparison with naïve T cells, and NE-dependent effects on these cells were mediated by β2-adrenergic receptors. They demonstrated that the expression of inflammatory cytokines and chemokines (such as IL-6 and TNF) was increased while growth-related cytokine production was reduced (Slota et al. 2015). The effect of stress on the CD8+ memory T cells was also shown in another study done by Maydych et al. (2017). They screened the alteration of T cell subsets throughout a period of academic examination stress. In this study, the stress factor was in correlation with an increase in memory and a decrease in naïve T cells.
Glucocorticoid receptor in T cells
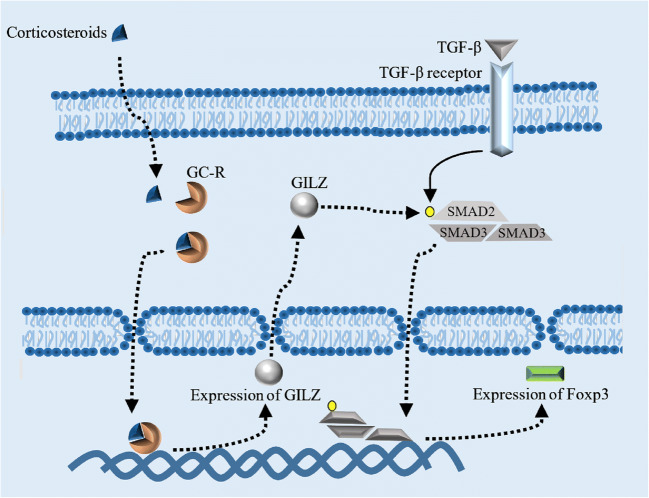
The second stress mediators which directly regulate T cell function are GCs. GC, a potent immunomodulator and immunosuppressor, is predominantly produced by adrenal cortex in response to the pituitary ACTH and ultimately established a negative feedback on HPA axis which was activated in stress condition. GC diffuses passively into the cells and binds to the GC receptor to create an active complex with different biological effects. GC receptors in the absence of GCs are found in the complex with immunophilins and heat shock proteins (HSPs). When GC binds to the complex, GC/GC receptor is released from HSPs and translocated to the nucleus (Herold et al. 2006). Monomer forms of GC/GC receptor complexes interact with several transcription factors such as AP-1, NF-κB, and STAT5 while homodimer forms are the promoter of a wide range of genes such as anti-inflammatory genes (Herold et al. 2006; Libert and Dejager 2014). GC receptor knocked out mouse revealed that in vivo thymocyte refractory to TCR-induced apoptosis was increased by GCs (Brewer et al. 2002) while when in the mouse model with over-expressing genes of GC receptor, the sensitivity to GC-induced apoptosis was increased (Pazirandeh et al. 2002). Several mechanisms involve in GC-induced apoptosis in T cells. The final effects of GCs on the T cells depend on the stage of cell development. Double-positive thymocytes (CD8+ CD4+) are more sensitive compared with single-positive thymocytes for GC-induced apoptosis. The mitochondrial integrity which is mediated by proteins such as Bcl-2 can protect cells from GC-induced apoptosis. In a theory, secondary signal in T cells (CD28/B71-2) upregulates cell survival and anti-apoptotic gene such as bcl-2 (Herold et al. 2006). Therefore, positive secondary signal in T cells may protect them from GC-induced apoptosis. This theory is supported by the study conducted by Banuelos et al. which determined bcl-2 protected human and mouse Th17 cells from GC-induced apoptosis (Banuelos et al. 2016). Despite the positive secondary signal, the inhibitory T cell signals such as PD1/PD-L1 downregulate TCR signal and consequently inhibit T cell proliferation and cytokine production. Double-positive T cells, which are unable to recognize peptide-MHC complex or recognize with high affinity, died (it is called neglect and negative selection process, respectively) in the cortex of thymus. This finding directed us to hypothesize that developing T cell death is mediated by GC/GC signal. Although in the stage of single-positive, thymocytes recognizing MHC peptides with low affinity in the presence of CD28/CD80, CD86 signal will be developed to CD4+ or CD8+ naïve T cells; the high affinity binding of thymocytes to MHC peptides in the presence of PD1/PD-Ls signal develops T cells into regulatory T cells. In a current study, the role of GC receptor in the protection from autoimmunity in pregnancy was investigated (Engler et al. 2017). Engler et al. demonstrated that GC receptor engagement in T cells increase regulatory T cells (T-reg) frequency, the cell which protects pregnant mouse from autoimmune encephalomyelitis. Bereshchenko et al. showed a mechanism for T-reg development in the presence of GC receptor engagement which supports the main role of GCs in the maintenance of T cell tolerance. They showed the expression of an anti-inflammatory gene which was induced by GC receptor engagement (so-called GC-induced leucine zipper; GILZ) can bind to SMAD2 and lead to SMAD2 phosphorylation. SMAD2 is considered an essential signal transducing factor for TGF-β initiation activation pathway in T-reg cells (Fig. 2). Activation of SMAD2 was led to an optimal induction of Foxp3 as the main transcription factor of T-reg cells (Bereshchenko et al. 2014).
Fig. 2.
A mechanism for T-reg development in the presence of GC receptor engagement. The expression of an anti-inflammatory gene which was induced by the binding of glucocorticoids to GC receptor and translocation of this heterodimer into the cell nucleus leads to the expression of GC-induced leucine zipper (GILZ). GILZ can bind to SMAD2 and lead to SMAD2 phosphorylation. The activation of SMAD2 was led to an optimal induction of Foxp3 which transcription factor requires for T-reg differentiation
Collectively, the role of GC/GR in T-reg and protection from autoimmunity was well investigated. However, T cells which are in stress condition, particularly in chronic stress, may be affected by several factors that determine whether stress suppresses or enhances T cell function and proliferation. For example, in the mouse receiving low-dose corticosterone or epinephrine, delay skin-type hypersensitivity (DTH) and T cell drainage to the adjacent lymph node have been enhanced. In contrast, high-dose corticosterone suppressed DTH (Dhabhar and McEwen 1999). At the beginning of an immune response, function and proliferation of Th cells may receive immune-enhancement effects from GCs while at end of an immune response, it may receive immune-suppressive effects (Dhabhar 2014).
Metabolic effects of stress on T cells
In this section, metabolic and other indirect effects of stress on T cells will be discussed. Although the purpose of allostasis response in stress condition is saving energy for rapid responses to life-threatening stressors, the different metabolic conditions occur in chronic and acute stress. Hypercortisolism in chronic stress can expose individuals to visceral obesity and cardiometabolic disorders. Catabolic effect of GCs in acute stress increases available energy resources for essential response against imposed stressor. Hepatic gluconeogenesis, circulating glucose, induced lipolysis, and protein degradation are increased by GC/GCR signals. In addition, GCs represent an antagonist activity for growth, thyroid hormones, insulin, and sex steroids (Chrousos 2000).
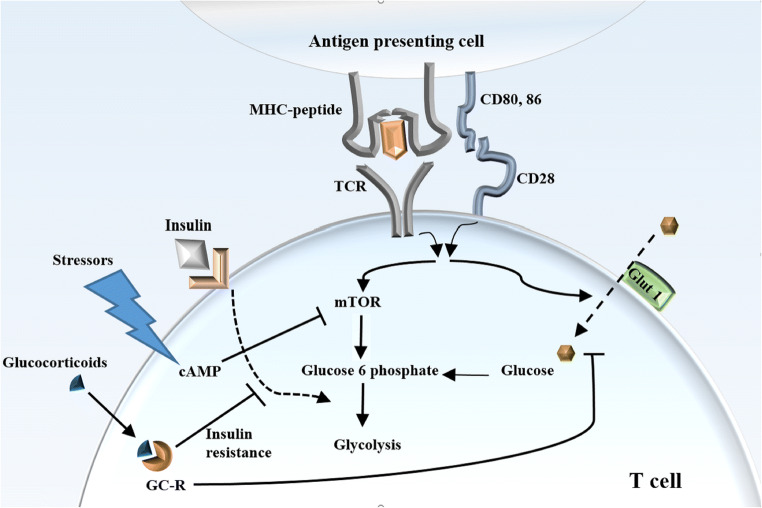
Upon T cell activation, glucose transportation 1 (Glut1) is upregulated which is mediated by TCR and CD28 signal (Jacobs et al. 2008). Effector and naïve T cells are different in consumption and generation of energy. Moreover, unique T cell subsets use different metabolic programs (Yang et al. 2015). Effector T cells mostly use glycolysis, protein phosphate pathway, and glutaminolysis (Fig. 3). In contrast, the tricarboxylic acid cycle, oxidative phosphorylation, and fatty acid oxidation are the most metabolic pathways which are used by memory and naïve T cells for providing energy (Yang et al. 2015). Glut1 enhances proliferation and growth in activated T cells and it overexpresses in Th1, Th2, and Th17 subtypes (Michalek et al. 2011). CD28 costimulatory signal increases glucose uptake in activated T cells using an Akt-dependent and an Akt-independent pathways (Jacobs et al. 2008). In contrast, PD1 co-inhibitory signal inhibits glycolysis and increases lipolysis and fatty acid oxidation in activated T cells (Patsoukis et al. 2015), which metabolic profile is similar to that in anergic or T-reg cells. CTLA-4 pathway, as the first known co-inhibitory signal, also inhibits glycolysis but without augmenting in fatty acid oxidation and lipolysis. The metabolic reprogramming which was induced by CTLA-4 signal alters the cell to the metabolic condition similar to that in naïve T cells (Patsoukis et al. 2015).
Fig. 3.
Indirect and metabolic effects of stress on T cells. After T cell activation by TCR and CD28 signal, glucose transportation 1 (Glut1) is upregulated and then mTOR pathway and glycolysis are activated. Glucocorticoids can inhibit glycolysis, decrease intercellular glucose, and may limit glucose availability in activated T cells using GC-induced insulin resistance
Previously, it was believed that suppression of immune reactions in an adaptive response to stress conserves energy for vital reactions. This theory has been challenged in the condition of acute stress where some non-vital functions of the body such as ovulation, digestion, or copulation can be delayed during the presence of a stressor. The immune response can be critical in stress situation particularly in response to wound or infection. In addition, immune suppression mechanisms are with consumption of energy, and the time course used for immune suppression usually is more than the time of acute stress (Dhabhar 2014). In contrast, chronic stresses manipulate T cell responses throughout ligand-receptor and metabolic pathways. Several mechanisms are proposed for the indirect effect of stress situation on the T cell function. GCs can inhibit glycolysis in T cells and decrease intercellular glucose. In addition, GC-induced insulin resistance may limit glucose availability in activated T cells, and the situation similar to those cells has involved by the PD1/PD-L1 signal. The function of activated T cells (Th1, Th2, and Th17) is dependent on ATPs produced in glycolysis. Therefore, these cells are more sensitive to starvation induced by stress in comparison with naïve T cells and T-reg cells where TCA cycle, oxidative phosphorylation, and fatty acid beta-oxidation pathways can be used to provide cell essential ATPs. Mammalian target of rapamycin (mTOR) signaling in activated T cells increases glycolysis and inhibits OXPHOS (van der Windt and Pearce 2012). cAMP, created in stressed cells, inhibits mTOR signaling and consequently removes OXPHOS inhibition which pathways desired in T-reg cells. Collectively, induced metabolic alterations in chronic stress much support T-reg development and interact with activated T cell function.
In addition to the metabolic changes of T cells which are induced by HPA axis and neurological stimuli, intracellular stress (e.g., endoplasmic reticulum stress; ER stress) stimuli can also influence the T cell metabolism and function. In ER stress, IRE1α excises a 26-nucleotide fragment of XBP1 mRNA and creates an active splice of XBP1MRNA that binds to the XBP1 protein, a transcription factor that has a role in the adaptation to the ER stress. In a study done by Song et al., the effect of microenvironment of ovarian cancer cells on the T cell function was evaluated (Song et al. 2018). Ascites fluid from malignant ovarian cancer patients induced some metabolic changes in T cells, including the inhibition of glucose uptake, N-linked protein glycosylation defects, and XBP1 activation which leading to the suppression of IFN-γ production and mitochondrial activity. This finding demonstrated that ovarian cancer cells employ ER stress and IRE1α–XBP1 activation in infiltrating T cells to evade antitumor responses.
In addition to the metabolic effects of stress on T cells, stress-induced adrenergic activation suppresses cholinergic control of digestive function. In the long term of chronic situation, individuals had to receive food for providing energy. Incompatibility of the adrenergic situation and food taken has led to digestive disfunction which their manifestations can be in impairing of intestinal flora. Cross talk between gut microbiota and T cells is contributed in the development of inflammatory disorders in CNS (Fung et al. 2017), activation of immune system (Miller and Raison 2016), stress-induced alteration in neural circuits (Krishnan and Nestler 2008), and depression (Fung et al. 2017). For example, epithelial-associated bacteria such as segmented filamentous bacteria induce TH17 production in mouse small intestine, the phenotype of T cell which is associated with immunopathology of CNS such as experimental autoimmune encephalomyelitis (EAE) (Fung et al. 2017). It was demonstrated that Th17 cells are increased in the animal model of learned helplessness and chronic restraint stress. The increase in Th17 cells promotes depression-like behavior in the mice, and the inhibition of TH17 function or production can reduce the vulnerability toward depression-like behavior (Beurel et al. 2013).
Pathologic and neuroprotective roles of T cells in stress condition
Although inflammation-activated HPA axis was considered a negative regulator of immune responses, inflammatory pathways were activated by stressor including nuclear factor-κB (NF-κB) activation (Bierhaus et al. 2003), and markedly enhancement in pro-inflammatory cytokines, such as IL-6 (Pace et al. 2006) which pathways can represent several dramatic pathologic manifestation in the body. T cells may play a critical role in translating allostasis to immunosuppression or immunopathologic presentations. There are shreds of evidence suggesting the neuroprotective role of T cells following CNS injury. IL-4 produced by CD4+ T cells restores neural homeostasis in the murine model of CNS injury (Walsh et al. 2015). It was determined that IL-4-producing T cells with an antigen-independent manner were induced by molecular mediators that originated from CNS injured cells (Fig. 4(b)). Affected T cells are activated throughout Myd88 pathway with MHC-II-independent signaling which leads to IL-4 production. The produced IL-4 induces Akt map kinase pathway in neurons which increases neurotrophin signaling and survival of affected neurons (Walsh et al. 2015). Previous studies demonstrated that autoreactive T cells are required for supporting neural actions including hippocampal-dependent learning, memory, and hippocampal neurotrophic factor production (Ziv et al. 2006). Accumulation results suggest the neuroprotection role of CNS-autoreactive T cells. Lewitus et al. (2008) hypothesized that stress-induced lymphocyte trafficking to the brain increases stress resilience. They determined that in a short time predator odor stress model, T cell trafficking to the brain was increased throughout corticosteroid-mediated ICAM-1 expression on the surface of choroid plexus cells (Lewitus et al. 2008). To support these findings, passively transferred CNS-specific T cells improved locomotor activity in rats which were faced with nerve injury (Yoles et al. 2001). According to the data, it can be concluded that the brain-infiltrated T cells in stress situations enhance resilience to further stress; the phenomenon is called behavioral immunization. Although behavioral immunization was determined in the short time stress, the effect of chronic stress might be different. Studies conducted by Dhabhar et al. also support the immunoprotective role of acute stress, while they determined the fact that chronic stress decreases immune cell trafficking (Dhabhar 2014; Dhabhar and Mcewen 1997) which might arrest creation of behavioral immunization during a long time stress. They proposed that chronic stress polarizes T cell to type 2 cytokines (Th2 cells) such as IL-4 and IL-10. As noted before about the neuroprotective role of IL-4 (Walsh et al. 2015), polarization of the immune response to Th2 cytokines can be considered a physiological response to protect CNS from more damages during long time stress.
Fig. 4.
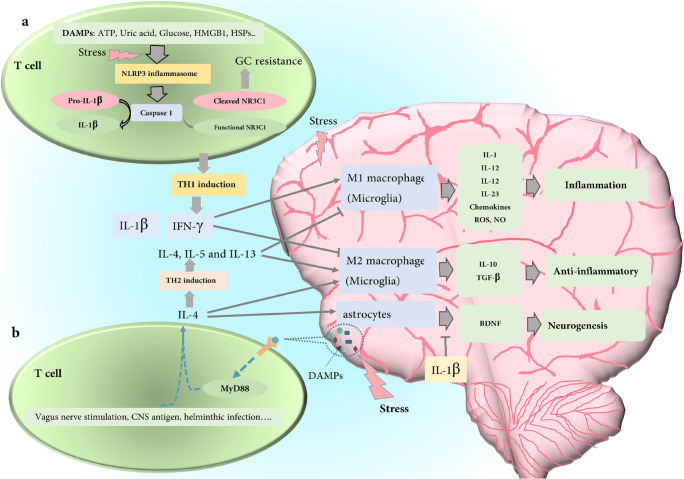
Proposed mechanism for immunopathology or immunoprotection roles of T cell in CNS: (a) stress-induced DAMPs such as ATP, uric acid, glucose HMGB1, and HSPs activate NLRP3 inflammasome and then caspase 1. This proteolytic enzyme degrades pro-IL-1 to IL-1 as well as dose degradation of the glucocorticoid receptor (NR3C1). After cleaving glucocorticoid receptor, T cells changed into a glucocorticoid resistance cells and polarized to TH1 subtype (IFN-γ producing subtype) as well. The inflammatory phenotype of microglia cell (M1) is differentiated and affected by IFN-γ and then produces inflammatory cytokines which can create an inflammation condition in the brain. This cleavage also results in GC resistance, so the phenomenon can remove the immunosuppressive effect of stress released CGs on the inflammatory function of TH1 cells. (b) Stress-induced DAMPs can also trigger CD4+ T cells throughout MyD88 signaling without dependency on MHC-II peptide, and the activation leads to IL-4 production by T cells. Other stimuli such as vagus nerve stimulation, CNS antigen, and helminthic infection also polarize T cell to the IL-4-producing T cell (TH2). IL-4 differentiates microglia cells to anti-inflammatory phenotypes and induces astrocytes to produce BDNF, as a neurogenesis factor. BDNF production can be inhibited by IL-1β
Protective or pathologic effects of T cells in stress condition can be affected by several factors such as aging and compartmentalization of T cells. Special function of T cell subsets is associated with its anatomical compartment and life stage. With age, thymic volume reduces and thymic tissue is replaced by fat. Humans are born with developed T cells, which are sufficient to fight against pathogens and regulate immune responses (Kumar et al. 2018). Although, naïve T-reg and conventional naïve T cell populations declined with age (Thome et al. 2016), the proportion of terminal effector CD8+ T cells (Temra CD8+ T cells) in blood and BM increases (Di Benedetto et al. 2015; Gordon et al. 2017). As naïve and effector T cells have different metabolic and secretome, their responses in the stress condition can differ as individual age, and more investigations are needed to explain how aging can influence allostasis responses of T cells.
According to the immunosuppressive effects of chronic stress, it may be theoretically hypothesized that chronic stress has a protective role in autoimmune disease. Most of the immunosuppressive effects of chronic stress are referred to the glucocorticoid concentration. However, several inflammatory disorder such as rheumatoid arthritis, asthma, and inflammatory bowel disease have been associated with glucocorticoid resistance (Chikanza and Kozaci 2004; De Iudicibus et al. 2011; Loke et al. 2002). It can be hypothesized that immunopathology or immunoprotective effects of chronic stress may contribute to the GC signaling.
There was some evidence that showed insufficient GC/GR signaling in stress condition impaired relevance stress-adaptive responses such as the immunosuppressive and anti-inflammatory effects of the stress (Raison and Miller 2003). Insufficient GC signaling, either as a result of inadequate secretion (hypocortisolism) or as GC unresponsiveness (GC resistance), leads to impair in restrain relevant stress-responsive system particularly for immune/inflammation responses (Raison and Miller 2003). Decreased concentration of cortisol in plasma, urinary, and salivary sources has been reported in patients suffering from posttraumatic stress disorder (PTSD) (Yehuda 2001). Studies indicated that individuals with a low level of cortisol after acute trauma belong to the high-risk individuals for developing PTSD (Delahanty et al. 2000). Although the mechanism of cortisol dysfunction still needs to be investigated, there are several mechanisms suggesting that cortisol dysfunction during chronic stress leads to insufficient anti-inflammatory effects of allostasis (Hannibal and Bishop 2014).
T cell, as one of the main targets of immune-suppressive effects of GC, can be converted to a GC resistance cell in stress condition. Arbore et al. (2016) demonstrated that reactive oxygen species (ROS) and dependent NLRP3 activation in CD4+ T cells led to IL-1β production and secretion (Arbore et al. 2016). A positive association between NLRP3 activation and TH1 response (IFN-γ production) was also discovered in this study. NLRP3-mediated caspase1 activation cleaves pro-IL-1β and pro-caspase1 to create active IL-1β and caspase1. Currently, a significant NLRP3-mediated caspase1 activation was identified in the patients with leukemia (B and T cell leukemia), and the activation leads to cleave in GC receptor (NR3C1) which results in GC resistance in leukemia patients (Paugh et al. 2015). In the innate immune response, caspase1 is activated when an external agent arrives into the macrophage and leads to the induction of IL-1β processing and secretion (Sani et al. 2014).
Studies carried out in animal models can help to understand how psychological and mixed psychological-physiological stressors trigger inflammasome using endogenous damage-associated molecular patterns (DAMPs), including high mobility group box 1 (HMGB1), ATP, heat shock proteins (HSPs), uric acid, and molecules related to ROS (Raison and Miller 2003). According to the data, in order to resolve the immunopathology of T cells in stress condition, it can be hypothesized that stress-induced DAMPs in T cells lead to NLRP3 activation and consequently proteolytic degradation of GC receptor. The created GC resistance by GC receptor degradation leads to remove immunosuppressive effects of GC (physiologic production or therapeutic administration) on T cells (Fig. 4(a)). On the other hand, NLRP3 activation induces Th1 cell polarization and IFN-γ production. IFN-γ activates numerous transcription factors such as NF-κB and AP-1. This type of transcription factors stimulates the expression of several genes which are involved in the phagocytosis and inflammatory response of macrophages such as genes corresponding to the production of inducible nitric oxide synthase (iNOS) and ROS (M1 phenotypes). Thus, it can be concluded that the migration of Th1 cells to the brain during the stress condition alters immune protective status to an inflammatory condition.
Concluding remarks
In this paper, the contribution of stress and T cell responses under the title of direct and indirect was discussed. Although the first conception of the stress condition with glucocorticoid secretion is T cell suppression, the glucocorticoid resistance in T cells can alter the equivalence to other destiny. According to the previous studies, in this review, a mechanism for immunopathology of T cell in stress condition is introduced. IL-4 and BDNF, the cytokine produced in Th2 responses, can protect CNS from stress-induced damages. Th1 inflammatory responses can be limited by HPA axis if their activated intracellular caspase does not degrade GC receptor. All discussed mechanisms were attributed to the stressors involving HPA axis, but for the stressors such as regulated starvation regimen without HPA axis involvement, the correlation between T cell function and metabolic alteration can be different. The difference between the adaptive response to psychological and physical stressors (such as starvation and inflammation) can affect T cell function separately. In addition, the difference between the animal model of the stress and human patient is another effective note. Human behavior and physiologic condition can be affected by several factors such as patient’s occupation, religion, and other mental factors. T cells, protectively or pathologically, adapt themselves to the new condition.
Acknowledgments
The author would like to thank Kashan University of Medical Sciences.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Arbore G, et al. T helper 1 immunity requires complement-driven NLRP3 inflammasome activity in CD4+ T cells. Science. 2016;352:aad1210. doi: 10.1126/science.aad1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banuelos J, Shin S, Cao Y, Bochner BS, Morales-Nebreda L, Budinger GR, Zhou L, Li S, Xin J, Lingen MW, Dong C, Schleimer RP, Lu NZ. BCL-2 protects human and mouse Th17 cells from glucocorticoid-induced apoptosis. Allergy. 2016;71:640–650. doi: 10.1111/all.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereshchenko O, Coppo M, Bruscoli S, Biagioli M, Cimino M, Frammartino T, Sorcini D, Venanzi A, di Sante M, Riccardi C. GILZ promotes production of peripherally induced Treg cells and mediates the crosstalk between glucocorticoids and TGF-β signaling. Cell Rep. 2014;7:464–475. doi: 10.1016/j.celrep.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Beurel E, Harrington LE, Jope RS. Inflammatory T helper 17 cells promote depression-like behavior in mice. Biol Psychiatry. 2013;73:622–630. doi: 10.1016/j.biopsych.2012.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bierhaus A, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci. 2003;100:1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Kanagawa O, Sleckman BP, Muglia LJ. Thymocyte apoptosis induced by T cell activation is mediated by glucocorticoids in vivo. J Immunol. 2002;169:1837–1843. doi: 10.4049/jimmunol.169.4.1837. [DOI] [PubMed] [Google Scholar]
- Capece D, Verzella D, Fischietti M, Zazzeroni F, Alesse E (2012) Targeting costimulatory molecules to improve antitumor immunity. Biomed Res Int 2012. 10.1155/2012/926321 [DOI] [PMC free article] [PubMed]
- Chikanza I, Kozaci D. Corticosteroid resistance in rheumatoid arthritis: molecular and cellular perspectives. Rheumatology. 2004;43:1337–1345. doi: 10.1093/rheumatology/keh333. [DOI] [PubMed] [Google Scholar]
- Chrousos G. The role of stress and the hypothalamic–pituitary–adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes. 2000;24:S50. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- De Iudicibus S, Franca R, Martelossi S, Ventura A, Decorti G. Molecular mechanism of glucocorticoid resistance in inflammatory bowel disease. World J Gastroenterol. 2011;17:1095–1108. doi: 10.3748/wjg.v17.i9.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty DL, Raimonde AJ, Spoonster E. Initial posttraumatic urinary cortisol levels predict subsequent PTSD symptoms in motor vehicle accident victims. Biol Psychiatry. 2000;48:940–947. doi: 10.1016/s0006-3223(00)00896-9. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS. Effects of stress on immune function: the good, the bad, and the beautiful. Immunol Res. 2014;58:193–210. doi: 10.1007/s12026-014-8517-0. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Mcewen BS. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11:286–306. doi: 10.1006/brbi.1997.0508. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, McEwen BS. Enhancing versus suppressive effects of stress hormones on skin immune function. Proc Natl Acad Sci. 1999;96:1059–1064. doi: 10.1073/pnas.96.3.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Benedetto S, Derhovanessian E, Steinhagen-Thiessen E, Goldeck D, Müller L, Pawelec G. Impact of age, sex and CMV-infection on peripheral T cell phenotypes: results from the Berlin BASE-II study. Biogerontology. 2015;16(5):631–643. doi: 10.1007/s10522-015-9563-2. [DOI] [PubMed] [Google Scholar]
- Engler JB, Kursawe N, Solano ME, Patas K, Wehrmann S, Heckmann N, Lühder F, Reichardt HM, Arck PC, Gold SM, Friese MA. Glucocorticoid receptor in T cells mediates protection from autoimmunity in pregnancy. Proc Natl Acad Sci. 2017;114:E181–E190. doi: 10.1073/pnas.1617115114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang Y. β2 adrenergic receptor on T lymphocytes and its clinical implications. Prog Nat Sci. 2009;19:17–23. [Google Scholar]
- Fung TC, Olson CA, Hsiao EY. Interactions between the microbiota, immune and nervous systems in health and disease. Nat Neurosci. 2017;20:145–155. doi: 10.1038/nn.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon CL, Miron M, Thome JJ, Matsuoka N, Weiner J, Rak MA, Igarashi S, Granot T, Lerner H, Goodrum F. Tissue reservoirs of antiviral T cell immunity in persistent human CMV infection. J Exp Med. 2017;214(3):651–667. doi: 10.1084/jem.20160758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys Ther. 2014;94:1816–1825. doi: 10.2522/ptj.20130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold M, McPherson K, Reichardt H. Glucocorticoids in T cell apoptosis and function. Cell Mol Life Sci. 2006;63:60–72. doi: 10.1007/s00018-005-5390-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs SR, Herman CE, MacIver NJ, Wofford JA, Wieman HL, Hammen JJ, Rathmell JC. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J Immunol. 2008;180:4476–4486. doi: 10.4049/jimmunol.180.7.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey SF, Lisa HU, George PC (1995) The Hypothalamic–Pituitary–Adrenal Axis and Immune-Mediated Inflammation. J Clin Med 332 (20):1351–1363 [DOI] [PubMed]
- Krishnan V, Nestler EJ. The molecular neurobiology of depression. Nature. 2008;455:894. doi: 10.1038/nature07455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout life. Immunity. 2018;48(2):202–221. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewitus GM, Cohen H, Schwartz M. Reducing post-traumatic anxiety by immunization. Brain Behav Immun. 2008;22:1108–1114. doi: 10.1016/j.bbi.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Libert C, Dejager L. How steroids steer T cells. Cell Rep. 2014;7:938–939. doi: 10.1016/j.celrep.2014.04.041. [DOI] [PubMed] [Google Scholar]
- Loke T-K, Sousa AR, Corrigan CJ, Lee TH. Glucocorticoid-resistant asthma. Curr Allergy Asthma Rep. 2002;2:144–150. doi: 10.1007/s11882-002-0009-y. [DOI] [PubMed] [Google Scholar]
- Maydych V, Claus M, Dychus N, Ebel M, Damaschke J, Diestel S, Wolf OT, Kleinsorge T, Watzl C. Impact of chronic and acute academic stress on lymphocyte subsets and monocyte function. PLoS One. 2017;12:e0188108. doi: 10.1371/journal.pone.0188108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–179. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Bowles NP, Gray JD, Hill MN, Hunter RG, Karatsoreos IN, Nasca C. Mechanisms of stress in the brain. Nat Neurosci. 2015;18:1353–1363. doi: 10.1038/nn.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD et al (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 1003613. 10.4049/jimmunol.1003613 [DOI] [PMC free article] [PubMed]
- Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. 2016;16:22–34. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Patsoukis N, Bardhan K, Chatterjee P, Sari D, Liu B, Bell LN, Karoly ED, Freeman GJ, Petkova V, Seth P, Li L, Boussiotis VA. PD-1 alters T-cell metabolic reprogramming by inhibiting glycolysis and promoting lipolysis and fatty acid oxidation. Nat Commun. 2015;6:6692. doi: 10.1038/ncomms7692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paugh SW, Bonten EJ, Savic D, Ramsey LB, Thierfelder WE, Gurung P, Malireddi RK, Actis M, Mayasundari A, Min J, Coss DR, Laudermilk LT, Panetta JC, McCorkle J, Fan Y, Crews KR, Stocco G, Wilkinson MR, Ferreira AM, Cheng C, Yang W, Karol SE, Fernandez CA, Diouf B, Smith C, Hicks JK, Zanut A, Giordanengo A, Crona D, Bianchi JJ, Holmfeldt L, Mullighan CG, den Boer M, Pieters R, Jeha S, Dunwell TL, Latif F, Bhojwani D, Carroll WL, Pui CH, Myers RM, Guy RK, Kanneganti TD, Relling MV, Evans WE. NALP3 inflammasome upregulation and CASP1 cleavage of the glucocorticoid receptor cause glucocorticoid resistance in leukemia cells. Nat Genet. 2015;47:607–614. doi: 10.1038/ng.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazirandeh A, Xue Y, Prestegaard T, Jondal M, Okret S. Effects of altered glucocorticoid sensitivity in the T cell lineage on thymocyte and T cell homeostasis. FASEB J. 2002;16:727–729. doi: 10.1096/fj.01-0891fje. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Saint-Mezard P, et al. Psychological stress exerts an adjuvant effect on skin dendritic cell functions in vivo. J Immunol. 2003;171:4073–4080. doi: 10.4049/jimmunol.171.8.4073. [DOI] [PubMed] [Google Scholar]
- Sanders VM. The beta2-adrenergic receptor on T and B lymphocytes: do we understand it yet? Brain Behav Immun. 2012;26:195–200. doi: 10.1016/j.bbi.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani MRM, Moghaddam MM, Aghamollaei H, Hassanpour K, Taheri RA, Farnoosh G. Investigation of caspase-1 activity and interleukin-1β production in murine macrophage cell lines infected with Leishmania major. Asian Pac J Trop Med. 2014;7:S70–S73. doi: 10.1016/S1995-7645(14)60205-4. [DOI] [PubMed] [Google Scholar]
- Shonkoff JP, Boyce WT, McEwen BS. Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA. 2009;301:2252–2259. doi: 10.1001/jama.2009.754. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Pearce BD, Biron CA, Miller AH. Immune modulation of the hypothalamic-pituitary-adrenal (HPA) axis during viral infection. Viral Immunol. 2005;18(1):41–78. doi: 10.1089/vim.2005.18.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slota C, Shi A, Chen G, Bevans M, N-p W. Norepinephrine preferentially modulates memory CD8 T cell function inducing inflammatory cytokine production and reducing proliferation in response to activation. Brain Behav Immun. 2015;46:168–179. doi: 10.1016/j.bbi.2015.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song M, Sandoval TA, Chae C-S, Chopra S, Tan C, Rutkowski MR, Raundhal M, Chaurio RA, Payne KK, Konrad C. IRE1α–XBP1 controls T cell function in ovarian cancer by regulating mitochondrial activity. Nature. 2018;562(7727):423–428. doi: 10.1038/s41586-018-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome JJ, Bickham KL, Ohmura Y, Kubota M, Matsuoka N, Gordon C, Granot T, Griesemer A, Lerner H, Kato T. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat Med. 2016;22(1):72–77. doi: 10.1038/nm.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ThyagaRajan S, Priyanka H, Pundir U (2012) Aging alters sympathetic noradrenergic innervation and immune reactivity in the lymphoid organs: strategies to reverse neuro-immune senescence. BrainImmune Online J
- Tsigos C, Kyrou I, Kassi E, Chrousos GP (2016) Stress, endocrine physiology and pathophysiology
- Van der Windt GJ, Pearce EL. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol Rev. 2012;249:27–42. doi: 10.1111/j.1600-065X.2012.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle M, Stachetzki U, Krause A, Pierer M, Häntzschel H, Baerwald C. Regulation of beta2-adrenergic receptors on CD4 and CD8 positive lymphocytes by cytokines in vitro. Cytokine. 2001;16:205–209. doi: 10.1006/cyto.2001.0965. [DOI] [PubMed] [Google Scholar]
- Walsh JT, Hendrix S, Boato F, Smirnov I, Zheng J, Lukens JR, Gadani S, Hechler D, Gölz G, Rosenberger K, Kammertöns T, Vogt J, Vogelaar C, Siffrin V, Radjavi A, Fernandez-Castaneda A, Gaultier A, Gold R, Kanneganti TD, Nitsch R, Zipp F, Kipnis J. MHCII-independent CD4+ T cells protect injured CNS neurons via IL-4. J Clin Invest. 2015;125:699–714. doi: 10.1172/JCI76210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Matteson EL, Goronzy JJ, Weyand CM. T-cell metabolism in autoimmune disease. Arthritis Res Ther. 2015;17:29. doi: 10.1186/s13075-015-0542-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R. Biology of posttraumatic stress disorder. J Clin Psychiatry. 2001;62:41–44. [PubMed] [Google Scholar]
- Yoles E, Hauben E, Palgi O, Agranov E, Gothilf A, Cohen A, Kuchroo V, Cohen IR, Weiner H, Schwartz M. Protective autoimmunity is a physiological response to CNS trauma. J Neurosci. 2001;21:3740–3748. doi: 10.1523/JNEUROSCI.21-11-03740.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]