Abstract
Background
The coronavirus disease 2019 (COVID-19) pandemic would have particularly affected acute stroke care. However, its impact is clearly inherent to the local stroke network conditions. We aimed to assess the impact of COVID-19 pandemic on acute stroke care in the Lyon comprehensive stroke center during this period.
Methods
We conducted a prospective data collection of patients with acute ischemic stroke (AIS) treated with intravenous thrombolysis (IVT) and/or mechanical thrombectomy (MT) during the COVID-19 period (from 29/02/2020 to 10/05/2020) and a control period (from 29/02/2019 to 10/05/2019). The volume of reperfusion therapies and pre and intra-hospital delays were compared during both periods.
Results
A total of 208 patients were included. The volume of IVT significantly decreased during the COVID-period [55 (54.5%) vs 74 (69.2%); p = 0.03]. The volume of MT remains stable over the two periods [72 (71.3%) vs 65 (60.8%); p = 0.14], but the door-to-groin puncture time increased in patients transferred for MT (237 [187–339] vs 210 [163–260]; p < 0.01). The daily number of Emergency Medical Dispatch calls considerably increased (1502 [1133–2238] vs 1023 [960–1410]; p < 0.01).
Conclusions
Our study showed a decrease in the volume of IVT, whereas the volume of MT remained stable although intra-hospital delays increased for transferred patients during the COVID-19 pandemic. These results contrast in part with the national surveys and suggest that the impact of the pandemic may depend on local stroke care networks.
Keywords: Stroke, Thrombolysis, Thrombectomy, COVID-19
Introduction
The healthcare system has been disrupted during the coronavirus disease 2019 (COVID-19) pandemic outbreak, leading to a massive redistribution of health care resources. The saturation of the Emergency Medical Dispatch (EMD) with COVID 19-related calls may have jeopardized the recognition and management of other emergencies [1]. In addition, this pandemic has imposed containment and social distancing measures, with potential subsequent social isolation that may have contributed to a drop in stroke admissions. Patients’ fear of contracting the infection in hospitals may have delayed or limited their demand for care, especially for transient or minor symptoms. Furthermore, the unprecedented media concentration on the pandemic may have precipitated the extinction of calls for other emergencies and insidiously replaced other healthcare needs in the collective mind [2].
The effect of the COVID-19 pandemic on stroke care is still debated. Although some studies have reported an impact of the pandemic on acute ischemic stroke (AIS) care in terms of admissions and reperfusion therapy volumes along with longer treatment times and a decrease in the use of stroke imaging compared with control periods in 2019, other reports have not detected significant effects on revascularization procedures [3–10].
Aims and/or hypothesis
The objective of our study was to assess the impact of the COVID-19 pandemic on the volume of AIS patients treated with intravenous thrombolysis (IVT) and/or mechanical thrombectomy (MT), as well as pre and intra-hospital delays (Fig. 1).
Fig. 1.
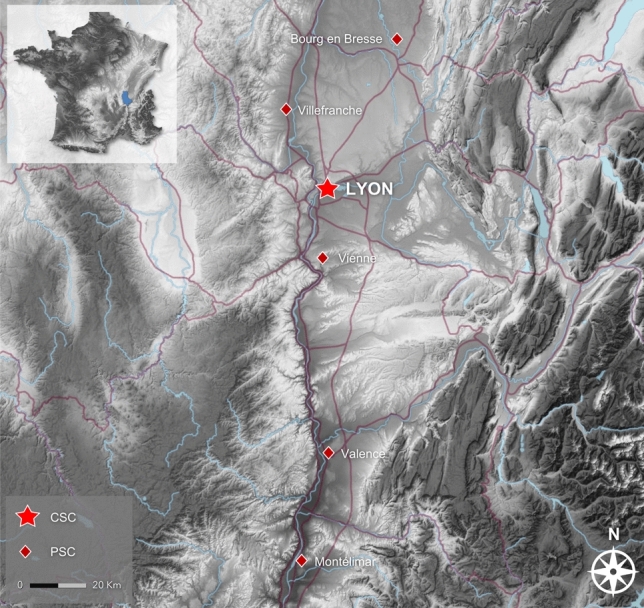
Stroke care network in the northern Rhône valley [inset, location within France; CSC Comprehensive Stroke Center, PSC Primary Stroke Center. Sourvdfce: Institut national de l’information géographique et forestière (IGN)]
Methods
Study design and data collection
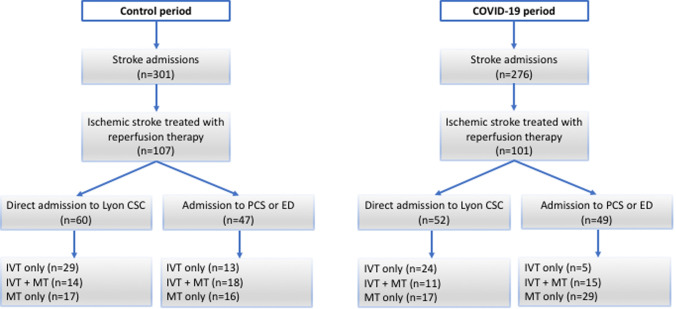
Data from these patients were collected within a regional emergency stroke network registry (RESUVAL), approved by the local ethics committee (Comité de Protection des Personnes Sud-Est II, registration E-2012-069). This observational study was carried out in accordance with the ethical standards of the Declaration of Helsinki. No patient expressed opposition to the research. The Lyon Stroke Center (tertiary university hospital), serving the greater Lyon metropolitan area (population: 2.3 million), treats ∼1700 ischemic stroke patients each year and is the only comprehensive stroke center (CSC, i.e. thrombectomy-capable) within our regional stroke network (5 primary stroke centers, population: 3.4 million) (Fig. 2).
Fig. 2.
Patient pathways and procedures during the control and the COVID-19 periods (CSC comprehensive stroke center, PCS primary stroke center, ED emergency department, IVT intravenous thrombolysis, MT mechanical thrombectomy)
The analyzed period ranged from the entry into level-2 of the pandemic in France (February 29th) and the lifting of lockdown on May 10th. The same period in 2019 served as control. To take into account the local trend (i.e. yearly increase in case volumes), we also provided data about the total number of reperfusion procedures from January 1st to May 10th of the previous 5 years.
Study population
All consecutive patients with AIS treated with IVT and/or MT in the Lyon Stroke Center, France, were included during the COVID-19 period and the control periods.
Baseline data on demographic characteristics, risk factors, and medical history were systematically collected at admission as well as times from stroke onset to hospital admission (to our CSC or to primary stroke center or to emergency department as appropriate), door to imaging, door to needle, and door to groin puncture. When the time of symptoms onset was unknown, the time when patients woke up or were identified was considered as the time of symptom onset. Neurological status was assessed by board certified neurologists using National Institute of Health Stroke Scale (NIHSS) score at admission.
Data about the volume of stroke admissions and the daily number of calls to EMD during the COVID-19 and the control periods were obtained from the hospital administrative database.
Imaging
The first-line imaging method in our CSC is magnetic resonance imaging (MRI), including diffusion-weighted imaging (DWI), T2∗-weighted imaging, Fluid-Attenuated-Inversion-Recovery (FLAIR), 3D-Time-of-Flight MR-angiography (MRA), perfusion-weighted imaging and cervical-vessels angiography were optional. If MRI was unavailable or contra-indicated, non-enhanced computed tomography (CT) followed by CT-angiography were performed; CT-perfusion was optional. Lesion side and baseline ischemic core size were assessed on DWI or CT using the Alberta Stroke Program Early CT Score (ASPECTS) for patients with stroke in the middle cerebral artery territory [11]. Baseline arterial occlusion site was evaluated with MRA or CT-angiography. A follow-up CT performed at 24 h classified any hemorrhagic transformation according to the European Co-operative Acute Stroke Study (ECASS II) classification [12].
Statistical analysis
Continuous variables are expressed as means (standard deviation [SD]) or medians (interquartile range [IQR]), and categorical variables as percentages. The Mann–Whitney U test and Fisher’s exact test were used to compare continuous and categorical variables, respectively. A p value < 0.05 was considered significant. Data were analyzed with Stata Version 15™ (STATACORP, College Station, Texas 77845 USA).
Results
A total of 301 and 276 patients were admitted for stroke during the control and the COVID-19 periods, respectively. Of them, 101 and 107 patients were treated with reperfusion therapy during the control and the COVID-19 periods, respectively, and were included in the study. Patient pathways and procedures as well as main characteristics are detailed in Fig. 2 and Table 1, respectively. Current smoking [18 (17.8%) vs 33 (30.8%); p = 0.04] and posterior circulation stroke [3 (3.0) vs 15 (14.0); p < 0.01] were less frequent during the COVID-19 period. Seven patients (6.5%) had typical chest CT findings (n = 3) or PCR confirmed (n = 4) COVID-19 infection. The clinical and radiological characteristics of COVID + patients did not differ significantly from other COVID-19 period patients. Median time from stroke onset to detection of COVID-19 infection was 0 day (range − 10 to 9 days).
Table 1.
Main characteristics of the study population
| Control period (n = 107) | COVID-19 period (n = 101) | p value | |
|---|---|---|---|
| Age, years | 72.4 ± 14.8 | 70.2 ± 14.7 | 0.24 |
| Male | 49 (45.8) | 52 (51.5) | 0.49 |
| Prestroke mRS > 2 | 11 (10.8) | 5 (5.2) | 0.20 |
| Hypertension | 60 (5.1) | 57 (56.5) | 1 |
| Hyperlipemia | 27 (25.2) | 29 (28.7) | 0.64 |
| Diabetes | 18 (16.8) | 9 (8.9) | 0.10 |
| Current smoking | 33 (30.8) | 18 (17.8) | 0.04 |
| Previous stroke/TIA | 17 (15.9) | 13 (12.9) | 0.56 |
| Ischemic heart disease | 16 (15.0) | 11 (11.0) | 0.42 |
| Atrial fibrillation | 24 (22.4) | 13 (12.9) | 0.10 |
| Antithrombotic drug | 42 (39.3) | 37 (36.6) | 0.78 |
| Etiology | 0.57 | ||
| Cardioembolism | 49 (46.7) | 36 (37.1) | |
| Large-artery atherosclerosis | 23 (21.9) | 21 (21.7) | |
| Microangiopathy | 3 (2.9) | 5 (5.2) | |
| Others | 5 (4.8) | 5 (5.2) | |
| Undetermined | 24 (22.9) | 30 (30.9) | |
| Baseline NIHSS score | 15 [5–19] | 13 [6–18] | 0.56 |
| Wake-up stroke | 27 (25.2) | 32 (31.7) | 0.36 |
| Off-hour | 52 (48.6) | 55 (56.7) | 0.26 |
| CT | 33 (30.8) | 33 (32.7) | 0.88 |
| MRI | 83 (77.6) | 82 (81.2) | 0.61 |
| Thrombus location | |||
| M1 MCA segment | 56 (52.3) | 53 (52.5) | 1 |
| M2 MCA segment | 10 (9.4) | 11 (9.9) | 1 |
| Intracranial ICA | 24 (22.4) | 16 (15.8) | 0.29 |
| Tandem occlusion | 21 (19.6) | 14 (15.6) | 0.58 |
| Posterior circulation | 15 (14.0) | 3 (3.0) | < 0.01 |
| ASPECT score | 8 [7–9] | 8 [7–9] | 0.47 |
| Right hemisphere | 56 (52.3) | 42 (41.6) | 0.20 |
| IVT | 74 (69.2) | 55 (54.5) | 0.03 |
| Telethrombolysis | 17 (15.9) | 10 (9.9) | 0.22 |
| MT | 65 (60.8) | 72 (71.3) | 0.14 |
| Secondary transfers | 34 (52.3) | 44 (61.1) | 0.31 |
| Reperfusion TICI 2b/3 | 51 (79.7) | 48 (67.6) | 0.12 |
| Hemorrhagic transformation | 0.55 | ||
| HI1 | 7 (7.2) | 13 (13.8) | |
| HI2 | 10 (10.3) | 11 (11.7) | |
| PH1 | 4 (4.1) | 4 (4.3) | |
| PH2 | 3 (3.1) | 1 (1.1) | |
| SAH | 1 (1.0) | 0 | |
| Emergency Medical Dispatch | |||
| Number of calls/day | 1023 [960 − 1410] | 1502 [1133–2238] | < 0.01 |
Significant results appear in bold
mRS modified Rankin Scale, TIA transient ischemic accident, NIHSS National Institute of Health Stroke Score, CT computed tomography, MRI magnetic resonance imaging, MCA middle cerebral artery, ICA internal carotid artery, ASPECT Alberta Stroke Program Early CT, IVT intravenous thrombolysis, MT mechanical thrombectomy, TICI thrombolysis in cerebral Infarction, HI hemorrhagic infarction, PH parenchymal hematoma, SAH subarachnoid hemorrhage
The volume of IVT was significantly lower during the COVID-19 period compared to the control period [55 (54.5%) vs 74 (69.2%); p = 0.03]. In contrast, the volume of patients treated with MT remained stable over these two periods [72 (71.3%) during the COVID-19 period vs 65 (60.8%) during the control period; p = 0.14]. The number of revascularization procedures in Lyon CSC steeply and yearly increased since 2015 (Fig. 3). The curve of cumulative cases in early 2020 was superior to that of 2019, but leveled off at the end of February 2020 and thereafter remained inferior to 2019 levels.
Fig. 3.
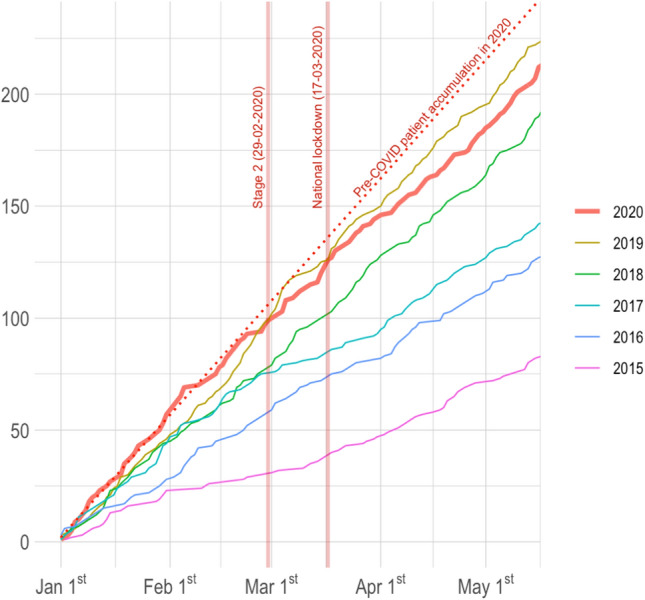
Cumulative number of reperfusion procedures (intravenous thrombolysis and thrombectomy) from January 1st to May 10th from 2015 to 2020 (the dashed line shows the slope of case numbers in 2020 before the COVID-19 period)
Onset-to-door, door-to-imaging and door-to-needle times did not differ between the two periods. In contrast, door-to-groin puncture time was increased in patients transferred for MT (237 [187–339] vs 210 [163–260]; p < 0.01) (Table 2). Note that the volume of direct admissions to CSC declined without reaching the statistical significance threshold.
Table 2.
Time intervals
| Control period | COVID-19 period | p value | |
|---|---|---|---|
| IVT-only group (n = 71) | n = 42 | n = 29 | |
| Onset-to-door time, min | 71 [61–102] | 89 [58–106] | 0.61 |
| Door-to-imaging time, min | 23 [13–52] | 16 [14–34] | 0.63 |
| Door-to-needle time, min | 53 [42–95] | 54 [43–69] | 0.77 |
| MT ± IVT group (n = 137) | n = 65 | nn = 72 | |
| Onset-to-door time, min | 84 [59–118] | 91 [60–139] | 0.34 |
| Door-to-imaging time, min | 21 [16–38] | 25 [16–54] | 0.30 |
| Door-to-needle time, min | 55 [44–80] | 60 [46–80] | 0.88 |
| Door-to-groin puncture time, min | 121 [80–210] | 185 [103–270] | 0.01 |
| Direct admission to Lyon CSC | 84 [67–94] | 81 [65–111] | 0.68 |
| Transfer from PCS or ED | 210 [163–260] | 237 [187–339] | 0.04 |
Significant results appear in bold
IVT intravenous thrombolysis, MT mechanical thrombectomy, CSC comprehensive stroke center, PCS primary stroke center, ED emergency department
The emergency call center faced a significant increase in activity during the COVID-19 period. The total daily number of calls increased considerably (1023 [960–1410] vs 1502 [1133–2238] in the control and COVID-19 periods, respectively; p < 0.01).
Discussion
We evaluated the impact of COVID-19 outbreak on a regional stroke care network. We observed a decrease in the volume of IVT, whereas the volume of MT remained stable although we observed a prolonged door-to-groin puncture time for transferred patients.
In line with previous studies, the volume of IVT markedly decreased during the COVID-19 period [3–5, 7–10]. The main factor for limiting the use of IVT among patients treated with MT was time delay. The volume of MT remained stable between the two periods while previous studies have reported conflicting results [3–5, 8–10]. The regional variability of the impact of COVID-19 on acute stroke care is illustrated by a German study which found a relevant effect on MT in a only one out of four centers [10]. Our result must be interpreted in relation to the resources available for stroke care delivery in our catchment area, which is likely undersized with regard to the large population base. This discrepancy between supply and demand may have propped up the number of revascularization procedures, despite a likely COVID-related reduction in healthcare resources, including stroke care. Nevertheless, we failed to observe our expected yearly growth in the number of reperfusion procedures. A similar observation was made by Hsiao et al. [8]. The magnitude of the COVID-19 pandemics was also lower in our region compared to other French regions as the Grand-Est and could have modified its impact of the COVID-19 on stroke care as reported in Germany [10].
An overload of EMD calls was reported in Catalonia during the COVID-19 outbreak [6]. Similarly, we observed an increase of about 50% in the total daily number of EMD calls during this period.
Another interesting finding is the significant decrease in patients managed for posterior circulation stroke during the pandemic period. Outside the pandemic period, posterior circulation strokes are more likely misdiagnosed in part because of nonspecific clinical presentation [13]. This phenomenon may have been exacerbated during the COVID-19 period. In contrast to other studies, age, NIHSS and ASPECT scores did not differ between the two periods, suggesting that criteria for treatment eligibility remained unchanged [5, 6].
Our methodological strengths come from a prospective data collection concerning all consecutive patients treated with MT in our geographical area as our stroke center is the only one to have thrombectomy facilities within our stroke regional network.
Our study has some limitations. First, the sample size is limited as a result of the short study period. Second, our registry was restricted to patients treated with reperfusion therapy while data about untreated patients were not collected. Thus we cannot draw a clear relationship between the decrease in the volume of IVT during the COVID-19 period and pre or intra-hospital delays. Still, we prospectively collected data concerning all consecutive patients treated with MT; as our CSC is the only thrombectomy-capable hospital within our stroke regional network, the count of MT cases was exhaustive. Last, the generalizability of these results found in our stroke regional network to other regions or countries with a different stroke care organization is uncertain.
The growing reports of the COVID-19 pandemic impact on acute stroke care call for implementing strategies to guarantee safe and hig-quality stroke care during the pandemic. The strategies adopted up to now varied depending on COVID-19 pandemic magnitude and preexisting regional organization of stroke care pathway as reported in Italy [14]. Future strategies should guarantee stroke pathway (beds, personnel) and reorganize it through specific stroke-COVID pathways.
Acknowledgements
We acknowledge Magali Bischoff, Julie Freyssenge and Carlos El Khoury from the RESCUe-RESUVAL network (Lucien Hussel Hospital, Vienne, France), André Lecoanet from the department of Medical Information (Hospices Civils de Lyon, France), Serkan Cakmak, Karine Blanc-Lasserre, Anne-Evelyne Vallet, Frédéric Philippeau and Chérif Heroum from primary stroke centers.
Funding
Hospices Civils de Lyon Funding.
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standards
This research was conducted according to the ethical standards issued by the Declaration of Helsinki.
References
- 1.Perlini S, Canevari F, Cortesi S, et al. Emergency Department and Out-of-Hospital Emergency System (112—AREU 118) integrated response to Coronavirus Disease 2019 in a Northern Italy centre. Intern Emerg Med. 2020 doi: 10.1007/s11739-020-02390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Coninck D, d’Haenens L, Matthijs K. Forgotten key players in public health: news media as agents of information and persuasion during the COVID-19 pandemic. Public Health. 2020;183:65–66. doi: 10.1016/j.puhe.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhao J, Li H, Kung D, et al. Impact of the COVID-19 epidemic on stroke care and potential solutions. Stroke. 2020;51:1996–2001. doi: 10.1161/STROKEAHA.120.030225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baracchini C, Pieroni A, Viaro F, et al. Acute stroke management pathway during Coronavirus-19 pandemic. Neurol Sci. 2020;41:1003–1005. doi: 10.1007/s10072-020-04375-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerleroux B, Fabacher T, Bricout N, et al. Mechanical thrombectomy for acute ischemic stroke amid the COVID-19 outbreak: decreased activity, and increased care delays. Stroke. 2020;51:2012–2017. doi: 10.1161/STROKEAHA.120.030373. [DOI] [PubMed] [Google Scholar]
- 6.Rudilosso S, Laredo C, Vera V, et al. Acute stroke care is at risk in the Era of COVID-19: experience at a Comprehensive Stroke Center in Barcelona. Stroke. 2020;51:1991–1995. doi: 10.1161/STROKEAHA.120.030329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pop R, Quenardelle V, Hasiu A, et al. Impact of the COVID-19 outbreak on acute stroke pathways—insights from the Alsace region in France. Eur J Neurol. 2020 doi: 10.1111/ene.14316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsiao J, Sayles E, Antzoulatos E, et al. Effect of COVID-19 on emergent stroke care: a regional experience. Stroke. 2020 doi: 10.1161/STROKEAHA.120.030499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frisullo G, Brunetti V, Di Iorio R, et al. Effect of lockdown on the management of ischemic stroke: an Italian experience from a COVID hospital. Neurol Sci. 2020 doi: 10.1007/s10072-020-04545-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoyer C, Ebert A, Huttner HB, et al. Acute stroke in times of the COVID-19 pandemic: a multicenter Study. Stroke. 2020;51:2224–2227. doi: 10.1161/STROKEAHA.120.030395. [DOI] [PubMed] [Google Scholar]
- 11.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/S0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 12.Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II) Lancet. 1998;352:1245–1251. doi: 10.1016/S0140-6736(98)08020-9. [DOI] [PubMed] [Google Scholar]
- 13.Gurley KL, Edlow JA. Avoiding misdiagnosis in patients with posterior circulation ischemia: a narrative review. Acad Emerg Med. 2019;26:1273–1284. doi: 10.1111/acem.13830. [DOI] [PubMed] [Google Scholar]
- 14.Zedde M, Pezzella FR, Paciaroni M, et al. Stroke care in Italy: an overview of strategies to manage acute stroke in COVID-19 time. Eur Stroke J. 2020 doi: 10.1177/2396987320942622. [DOI] [PMC free article] [PubMed] [Google Scholar]