Abstract
PURPOSE
Diffuse large B-cell lymphoma (DLBCL) presents as a limited-stage disease in 25% to 30% of patients, with better overall survival (OS) than that for advanced-stage disease but with continuous relapse regardless of treatment approach. The preferred treatment is abbreviated rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) and radiation therapy. On the basis of promising results of positron emission tomography (PET)–directed treatment approaches, we designed a National Clinical Trials Network (NCTN) study to improve outcomes and decrease toxicity.
METHODS
Patients with nonbulky (< 10 cm) stage I/II untreated DLBCL received 3 cycles of standard R-CHOP therapy and underwent a centrally reviewed interim PET/computed tomography scan (iPET). Those with a negative iPET proceeded with 1 additional cycle of R-CHOP, whereas those with a positive iPET received involved field radiation therapy followed by ibritumomab tiuxetan radioimmunotherapy.
RESULTS
Of 158 patients enrolled, 132 were eligible and 128 underwent iPET, which was positive in 14 (11%) of the patients. With a median follow-up of 4.92 years (range, 1.1-7.7 years), only 6 patients progressed and 3 died as a result of lymphoma. Eleven patients died as a result of nonlymphoma causes at a median age of 80 years. The 5-year progression-free survival estimate was 87% (95% CI, 79% to 92%) and the OS estimate was 89% (95% CI, 82% to 94%), with iPET-positive and iPET-negative patients having similar outcomes.
CONCLUSION
To our knowledge, S1001 is the largest prospective study in the United States of limited-stage DLBCL in the rituximab era, with the best NCTN results in this disease subset. With PET-directed therapy, 89% of the patients with a negative iPET received R-CHOP × 4, and only 11% had a positive iPET and required radiation, with both groups having excellent outcomes. The trial establishes R-CHOP × 4 alone as the new standard approach to limited-stage disease for the absolute majority of patients.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common non-Hodgkin lymphoma diagnosed in the United States, representing 30% to 40% of all cases.1 DLBCL presents as limited stage approximately 25% to 30% of the time. Our study in the pre-rituximab era, SWOG S8736, established that 3 courses of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) followed by radiation therapy (RT; CHOP ×3 + RT) had superior progression-free and overall survival (PFS and OS) compared with 8 courses of CHOP, but the differences disappeared by year 9 because of late relapses, without a plateau in the survival curve.2,3 S8736 also established the stage-modified (Miller) international prognostic index (smIPI) by demonstrating that patients without adverse risk factors had an excellent 10-year OS of at least 90%, whereas patients with risk factors had excess mortality because of relapse and death, with a 5-year OS of approximately 70%.2,4,5
Context
Key Objectives
To use an interim positron emission tomography (PET) scan after 3 cycles of rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) to stratify therapy for patients with limited-stage diffuse large B-cell lymphoma.
Knowledge Generated
With a median follow-up of almost 5 years, only 6 of 132 eligible patients progressed, and 3 died as a result of lymphoma, for a 5-year progression-free survival estimate of 87% and an OS estimate of 89%. Eighty-nine percent of the patients with a negative interim PET/computed tomography scan (iPET) received R-CHOP × 4, whereas only 11% had a positive iPET and required radiation-based therapy, with both groups having excellent outcomes.
Relevance
The trial establishes R-CHOP × 4 alone as the new standard approach to limited-stage disease for the absolute majority of patients
In follow-up studies, SWOG S0014 demonstrated that adding rituximab to CHOP × 3 + RT (R-CHOP × 3 + RT) had a positive effect on the high-risk group,6 and radioimmunotherapy consolidation with ibritumomab tiuxetan after CHOP × 3 + RT seemed to decrease long-term relapses in S0313.7 On the basis of S0014 and extrapolation from other DLBCL studies,8 R-CHOP × 3 + RT has remained a standard therapy approach in this setting and holds a category 1 recommendation from the National Comprehensive Cancer Network.
Midtreatment interim positron emission tomography (PET)/computed tomography (CT) scan (iPET) is prognostic in DLBCL.9,10 Preliminary results of a retrospective experience from the BC Cancer Lymphoid Cancer Database demonstrated that 80% of patients were PET negative after 3 cycles of R-CHOP (defined as Deauville 1-211), and only 8% of them relapsed after receiving 1 more cycle of R-CHOP without RT.12
We designed S1001, a prospective National Clinical Trials Network (NCTN) PET-directed study, to tailor therapy in limited-stage DLBCL after 3 cycles of R-CHOP. Our goal was to eliminate the short- and long-term toxicities associated with RT3,13,14 for the majority of patients with a negative PET scan after 3 cycles of R-CHOP and to improve the outcome in the minority of patients with a positive interim PET scan.
METHODS
Patients
S1001 was a phase II NCTN study for previously untreated patients with nonbulky (< 10 cm) stage I/II CD20-positive DLBCL. Since the WHO classification underwent a change over the study period, new categories of high-grade B-cell lymphoma (HGBL) with or without MYC and BCL2 or/and BCL6 rearrangements were also eligible. Staging was based on both CT and PET/CT scans. Patients with primary mediastinal, HIV-associated, post-transplantation, testicular, CNS, primary cutaneous, and indolent lymphoma were excluded. Patients, including those whose disease was grossly resected at diagnosis, could have either measurable or evaluable disease. Patients had to have a WHO performance status of 0-2, adequate organ function, a left ventricular ejection fraction of at least the lower level of normal, and a negative bone marrow biopsy within 6 weeks of registration. Patients had to be at least 18 years old, with no upper age limit. The protocol had to be approved by institutional review boards. All patients provided written informed consent in accordance with their institutional policies and according to the Declaration of Helsinki. The trial was registered at ClinicalTrials.gov (ClinicalTrials.gov identifier: NCT01359592).
All patients received 3 cycles of standard R-CHOP treatment given every 3 weeks, with rituximab 375 mg/m2 IV, cyclophosphamide 750 mg/m2 IV, doxorubicin 50 mg/m2 IV, vincristine 1.4 mg/m2 (capped at 2 mg) IV, and prednisone 100 mg a day by mouth for 5 days. Patients had an iPET between day 15 and 18 of cycle 3, which was centrally reviewed in real time at Imaging and Radiation Oncology Core Rhode Island. Those with a negative iPET, defined as Deauville 1-3, proceeded with 1 additional cycle of R-CHOP. Patients with a positive iPET (Deauville 4-5) initiated 36 Gy of involved field radiation therapy (IFRT), plus an additional boost to fluorodeoxyglucose (FDG)-avid areas of up to 9 Gy, within 5 weeks of cycle 3 of R-CHOP. Three to 6 weeks after completing IFRT, patients received ibritumomab tiuxetan administered per standard protocol, with rituximab 250 mg/m2 on day 1 and day 7, 8, or 9, and ibritumomab tiuxetan 0.4 mCi/kg on day 7, 8, or 9, after rituximab. A final PET scan was performed 12 weeks after treatment completion. Patients were observed with examination and testing, including CT scans every 6 months for the first 2 years and then annually for up to 7 years or death, whichever came first.
Pathology Procedures
Pathology was centrally reviewed by 2 expert hematopathologists after the study completed enrollment. The cell of origin (COO) was first assessed on immunohistochemistry by the Hans algorithm15 and subsequently by Nanostring using Lymph2Cx.16 If not performed locally, additional immunohistochemistry slides were stained to establish the double protein expressor (DPE) status, defined as having both positive MYC staining of at least 40% and BCL2 staining of at least 50% of malignant cells.17 Similarly, additional fluorescence in situ hybridization (FISH) studies for MYC, BCL2, and BCL6 using the LSI dual color break-apart probes (Abbott Molecular, Des Plaines, IL) were performed centrally as needed to establish the diagnosis of HGBL with or without MYC and BCL2 or/and BCL6 rearrangements. In 9 cases, outside FISH results were confirmed with a central FISH study.
Statistical Plan
The primary end point was the 5-year PFS rate, whereas secondary end points included OS rate, PFS and OS rates for iPET-positive and iPET-negative subgroups, toxicity, and response rates. Assuming a 10% ineligibility rate, 155 enrolled patients were needed to obtain 140 eligible patients. This would be sufficient to estimate the 5-year PFS rate to within 6% (95% CI), to test the historical 5-year PFS estimate of 85% against an alternative hypothesis of 93%. Using an exact binomial test, this design had a type I error of .057 and 93% power.
Analysis was performed on all eligible patients. PFS and OS were estimated using the Kaplan-Meier method.18 Adjustment for prognostic factors for PFS and OS was performed using Cox regression analysis.19 The cumulative incidence of progression was estimated accounting for the competing risk of nonlymphoma death.20 Toxicity was assessed according to National Cancer Institute Common Toxicity Criteria, version 4. Eligible patients receiving at least 1 dose of drug were included in the assessment of adverse events. Response was assessed using revised Cheson criteria.21
RESULTS
Patient Characteristics
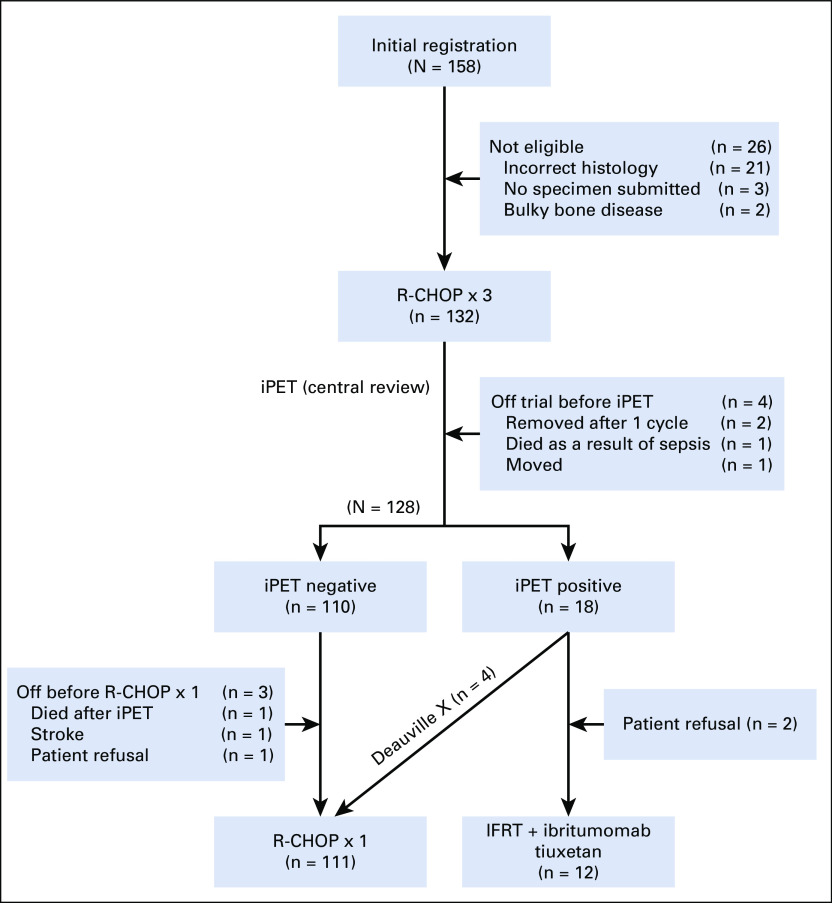
S1001 was activated on July 15, 2011, and accrual was completed on June 1, 2016. Of 158 patients enrolled, 26 were ineligible because of incorrect histology (the majority [21] had concurrent indolent or follicular lymphoma grade 3B), lack of diagnostic tissue submission for central pathology review (3), or bulky evaluable bone disease (2; Fig 1).
FIG 1.
CONSORT diagram. IFRT, involved field radiation therapy; iPET, interim positron emission tomography/computed tomography scan; R-CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone.
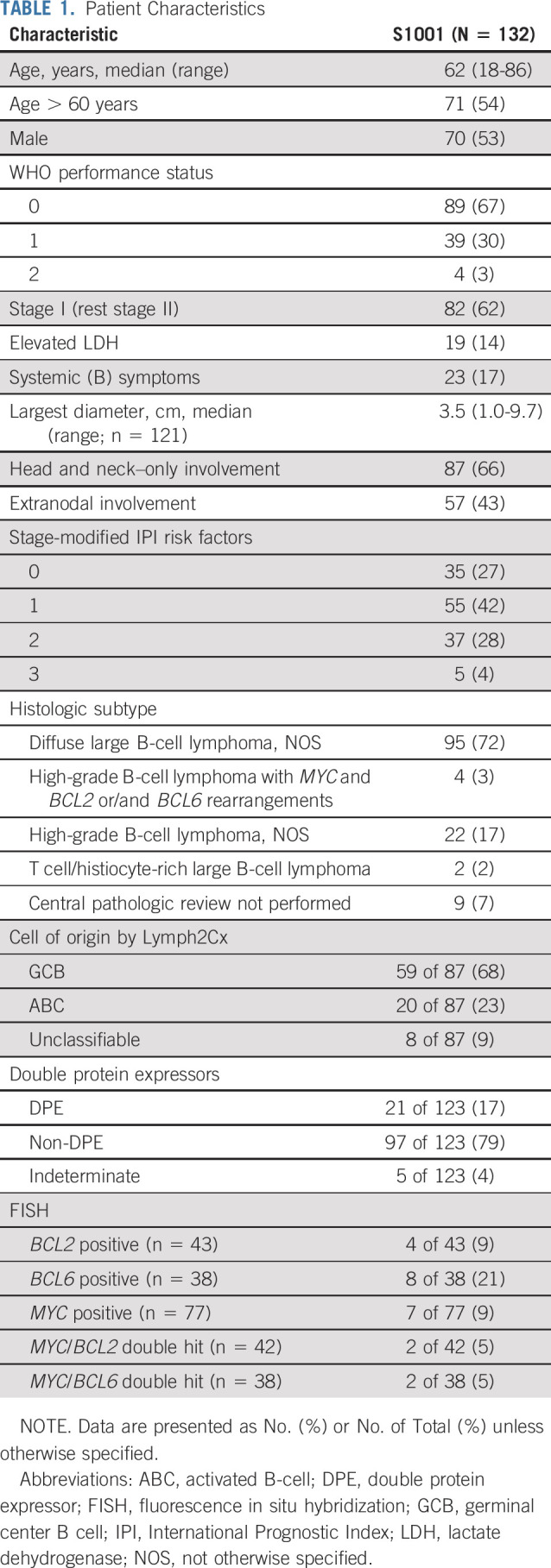
The clinical and disease characteristics of the 132 eligible patients are summarized in Table 1. The median age was 62 years, 62% had stage I disease, 17% had B symptoms, 14% had elevated lactate dehydrogenase (LDH), 43% had extranodal involvement, 66% had exclusive involvement of the head and neck region, and 10% had their disease fully resected at baseline. smIPI was 0 in 27%, 1 in 42%, 2 in 28%, and 3 in 4% of the patients. Overall, 72% of the patients had DLBCL, NOS not otherwise specified (NOS); 17% had HGBL, NOS; and 3% had HGBL with MYC and BCL2 or/and BCL6 rearrangements (“double-hit” lymphoma [DHL] or “triple-hit” lymphoma). COO by Lymph2Cx was assessable in 87 patients; 68% had germinal cen-ter B-cell (GCB), 23% had activated B-cell (ABC), and 9% had unclassifiable. DPE was present in 16%. None of the patients with DHL (2 with MYC/BCL2 and 2 with MYC/BCL6 rearrangements) had dual protein overexpression.
TABLE 1.
Patient Characteristics
Treatment
Of the 132 eligible patients, 128 had central review of their interim PET scan, of which 110 were iPET negative (Fig 1). Only 18 were iPET positive, 4 because of infection (Deauville X), which were treated as iPET negative with 1 additional cycle of R-CHOP. Of 14 patients (11%) with truly positive iPET, 2 refused radiation and 12 received IFRT followed by ibritumomab tiuxetan. Eight of the 12 patients (67%) converted from partial response (PR) to complete response (CR) after IFRT plus ibritumomab tiuxetan, and 4 (33%) had PR. Overall, CR was 92%, PR was 4%, and stable disease (SD) was 1% (with 4 [3%] unevaluable, 3 because the patient was coming off treatment before assessment and 1 because the patient had inadequate measurements on follow-up).
Median time from the date of diagnosis to the date of treatment initiation (the diagnosis-to-treatment interval)22 was 31 days, similar to the 32 days reported in the FLYER trial in limited-stage DLBCL.23 Ninety-eight percent of patients completed R-CHOP as planned. Two iPET-negative patients did not receive a subsequent cycle of R-CHOP (1 patient refused and 1 patient had a stroke). Radiation was initiated at a median of 30 days after R-CHOP (range, 23-44 days). There were no major radiation administration deviations or known ibritumomab tiuxetan deviations.
Safety
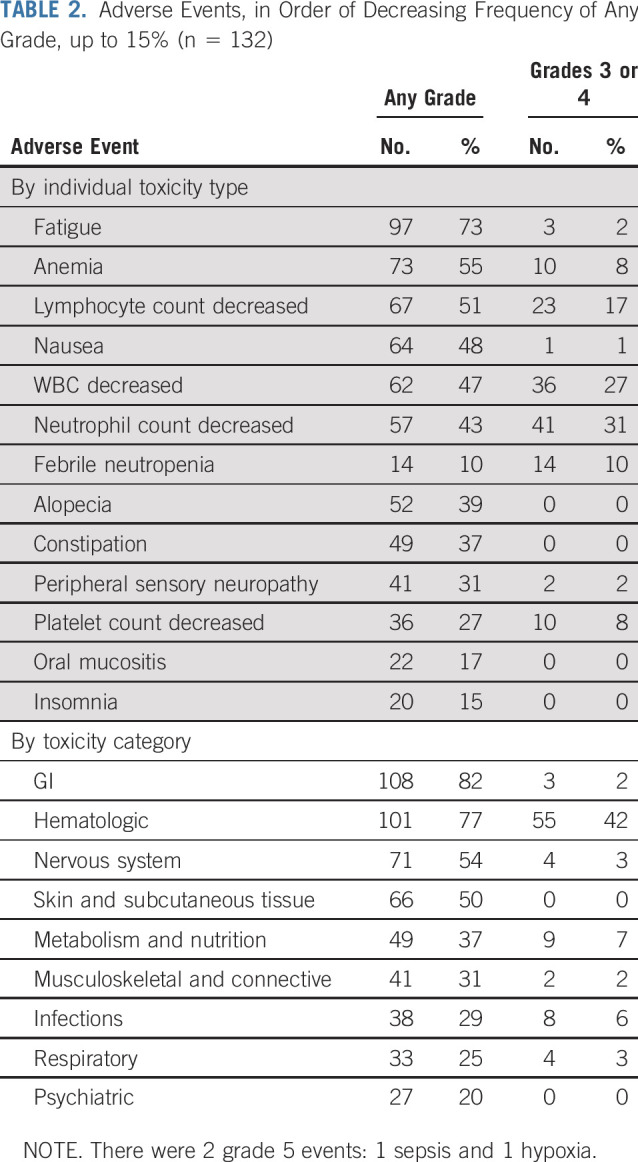
Adverse events are summarized in Table 2. Among the 132 patients, 1 patient died as a result of sepsis and 1 as a result of hypoxia. Thirteen patients (10%) had febrile neutropenia, 57 (31%) had grade 3-4 neutropenia, 10 (8%) had grade 3 anemia, 10 (8%) had grade 3-4 thrombocytopenia, 2 (2%) had grade 3 lung infection, 3 (2%) had grade 3 urinary tract infection, and 2 (2%) had grade 3 peripheral neuropathy. Of the 12 patients who received IFRT followed by ibritumomab tiuxetan, 2 had grade 3-4 neutropenia, 3 had grade 3-4 thrombocytopenia, and 2 experienced radiation dermatitis.24
TABLE 2.
Adverse Events, in Order of Decreasing Frequency of Any Grade, up to 15% (n = 132)
Outcomes
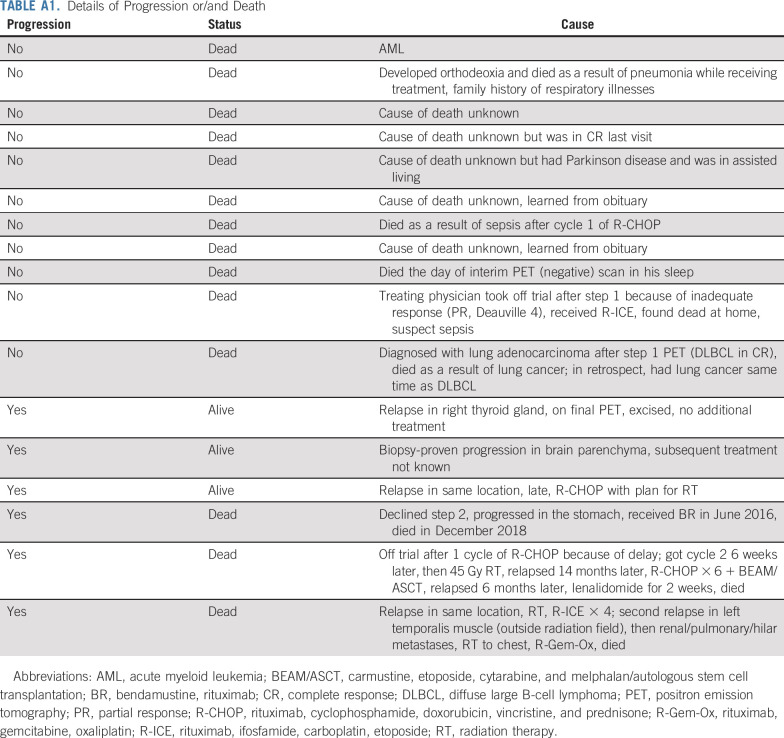
With a median follow-up of 4.92 years (range, 1.1 to 7.7 years), only 6 patients have progressed and 3 have died as a result of lymphoma. Of the 6 patients who progressed, 4 were iPET negative and received R-CHOP × 4, 1 was iPET positive but declined radiation, and 1 went off treatment after 1 cycle of R-CHOP because of treatment delay. One of the 6 patients progressed in CNS. There was no primary refractory disease. The median time to progression was 1.1 years (range, 0.2-6.2 years), with a continuous pattern of relapse. Of the 3 patients who had progression but remained alive, 1 had excision, 1 was retreated with R-CHOP, and in 1, the details of additional treatment were unknown. Eleven patients have died as a result of nonlymphoma causes, including 1 patient as a result of acute myeloid leukemia (AML) (in the iPET-negative arm) and 1 as a result of lung adenocarcinoma diagnosed on iPET (all deaths are listed in Appendix Table A1, online only). The median age of patients who died as a result of nonlymphoma causes was 80 years (range, 56-86 years). All 4 patients with DHL had a negative iPET and maintain remission.
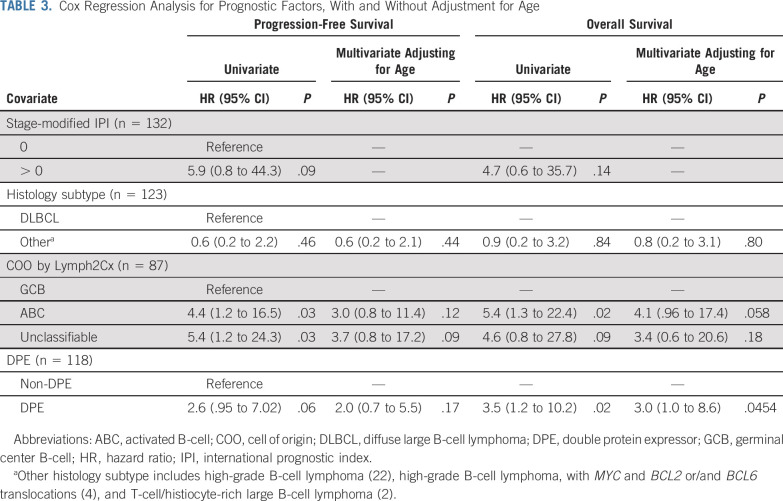
The 5-year PFS estimate is 87% (95% CI, 79% to 92%), and the OS estimate is 89% (95% CI, 82% to 94%; Figs 2A and 2B). iPET-positive and iPET-negative patients had similar outcomes, with PFS of 86% versus 89% and OS of 85% versus 91%, respectively, as shown by the landmark analysis from the time of iPET (Fig 3). Three of 30 patients with Deauville 3 iPET relapsed, compared with 1 in 80 with Deauville 1-2, but the difference was not statistically significant. Although histology (DLBCL v HGBL, NOS) did not predict outcomes, COO, DPE, and smIPI were prognostic of PFS and OS. Five-year PFS by smIPI was 97% for smIPI of 0, 86% for smIPI of 1-2, and 30% for smIPI of 3. GCB had a 5-year PFS of 95% versus 72% for ABC and 49% for unclassifiable. DPE patients had a 5-year PFS of 70% versus 89% for non-DPE patients. However, COO, DPE, and smIPI are also known to correlate with more advanced age.17,25 Using a Cox regression model adjusting for age, DPE retained statistical significance for OS (P = .045) but not for PFS (Table 3).
FIG 2.
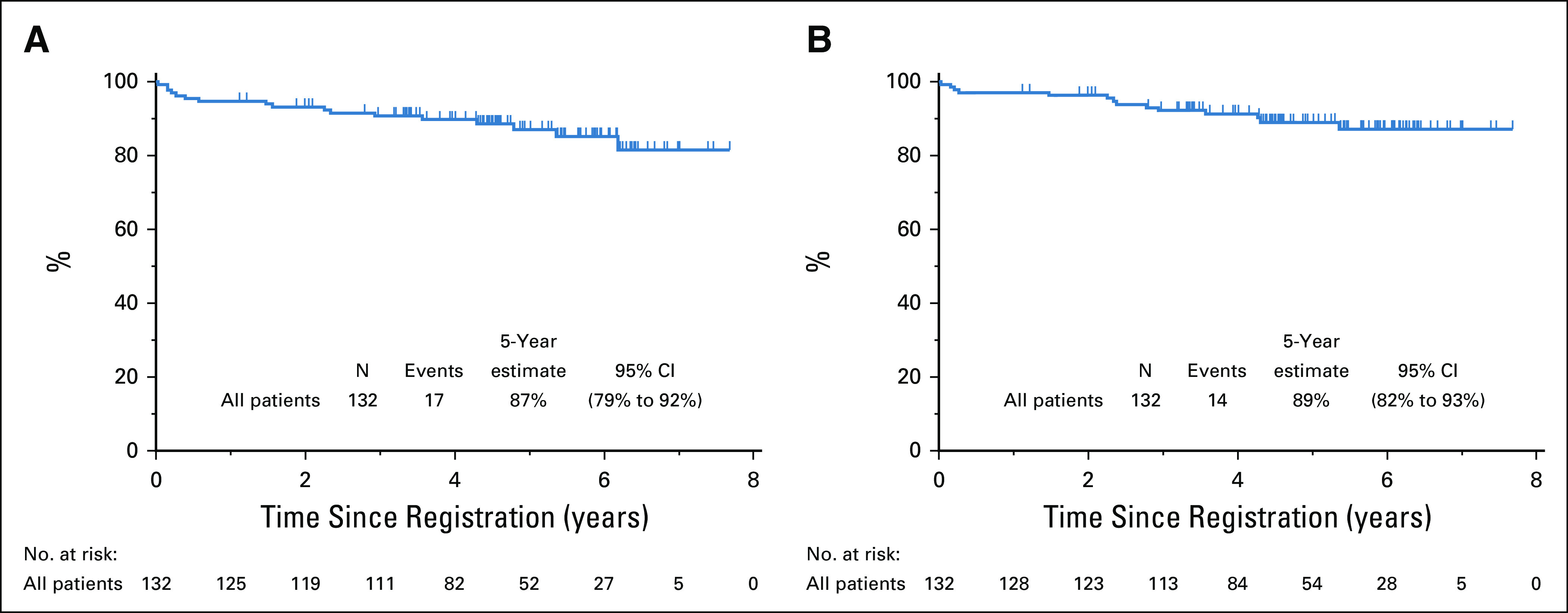
(A) Progression-free survival and (B) overall survival estimates.
FIG 3.
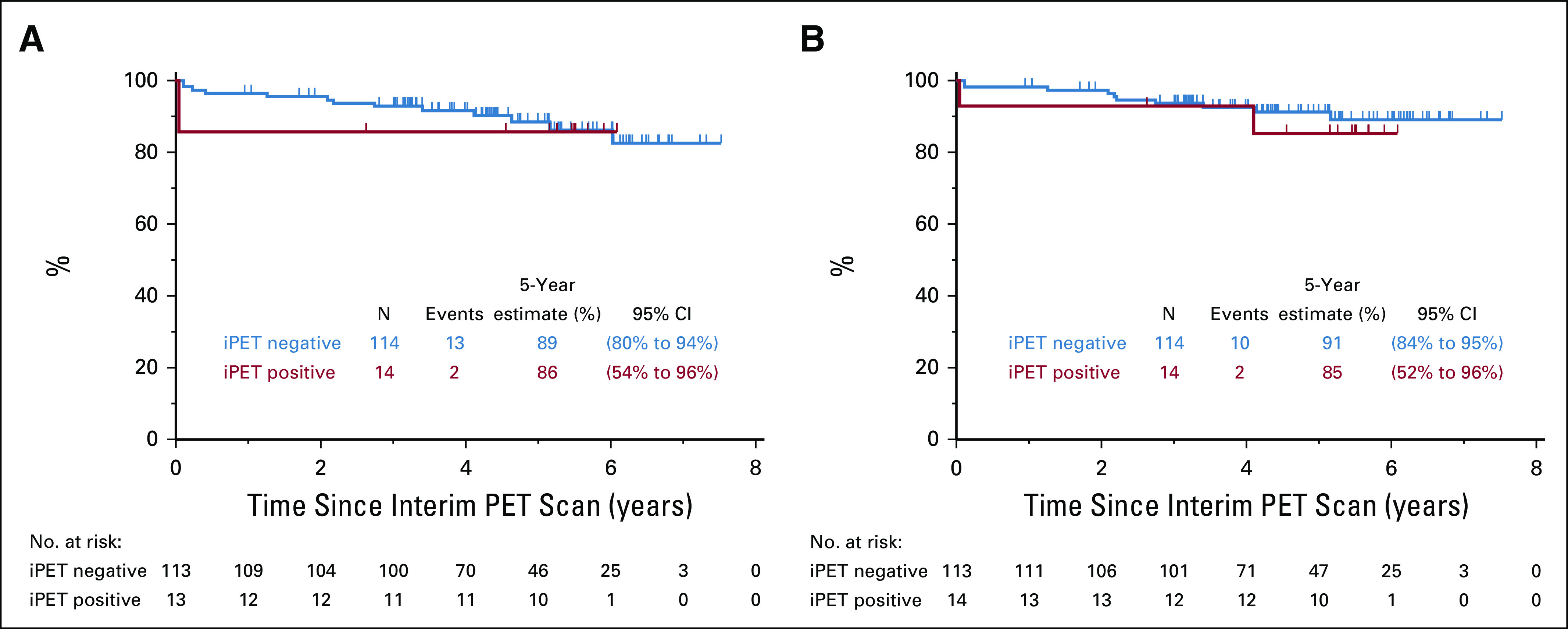
Landmark analysis at interim positron emission tomography (iPET)/computed tomography scan. (A) Progression-free survival. (B) Overall survival.
TABLE 3.
Cox Regression Analysis for Prognostic Factors, With and Without Adjustment for Age
Considering that the majority of events in the study were driven by nonlymphoma deaths, competing risk modeling was performed in an exploratory analysis. The model showed that the 5-year cumulative incidence of lymphoma progression, with death as a result of other causes as a competing risk, was 4.5% (95% CI, 1.6% to 9.9%), whereas the 5-year cumulative incidence of progression or death as a result of other causes was 12.8% (95% CI, 7.4% to 19.8%).
Because of a high proportion of exclusive head and neck involvement, the sites of presentation from the prior SWOG study S0313 were reviewed and showed that 29 of 43 patients with DLBCL (67%) had head and neck–only involvement, confirming this finding. Patients with head and neck–only involvement had a lower probability of elevated LDH (9% v 24%, P = .034) and of B symptoms (10% v 31%, P = .007) than did other patients. In addition, the median diameter of the largest lymph node was smaller in such patients: 3 cm versus 5.2 cm in others (P = .0002). Neither head and neck–only presentation nor extranodal involvement predicted outcome.
DISCUSSION
To our knowledge, NCTN S1001 is the largest prospective study in the United States of limited-stage DLBCL in the rituximab era, with the best NCTN results reported in this disease subset. Patients who were iPET negative after 3 cycles of R-CHOP (89%) received 1 additional cycle of R-CHOP, whereas patients who were iPET positive (11%) received IFRT followed by ibritumomab tiuxetan, with similarly excellent long-term outcomes. With a median follow-up of almost 5 years, only 6 patients in total have experienced disease progression, and only 3 have died as a result of lymphoma.
Our results are similar to those reported by a BC Cancer retrospective experience of PET-adapted therapy.12 S1001 had a lower rate of iPET positivity (11% v 18%) than that reported by BC Cancer, likely because of Deauville 3 being classified as iPET negative rather than positive in S1001, without showing any higher risk of relapse in patients with Deauville 3 iPET (3 out of 30). There are other trials prospectively investigating PET-directed therapy in DLBCL, including OPTIMAL > 60 (ClinicalTrials.gov identifier: NCT01478542) and LNH 2009-1B (ClinicalTrials.gov identifier: NCT01285765). Our data demonstrate the fea-sibility of real-time PET analysis and support the timing of scans at between days 15 and 18 of the third cycle of R-CHOP.
Several large European rituximab-based studies in limited-stage DLBCL have focused on patients with more favorable risk than those treated in S1001. The MabThera International Trial defined a cohort of 101 patients with favorable outcome to 6 cycles of R-CHOP or R-CHOP with etoposide (R-CHOEP) × 6 with radiation to bulky disease; with a median age of 47 years (all < 60 years), IPI of 0, and disease bulk < 7.5 cm, this group had a 6-year PFS rate of 90% and OS of 95%.26 The FLYER trial randomly assigned patients with a median age 48 years, age-adjusted IPI (aaIPI) of 0, and bulk < 7.5 cm to 6 cycles of R-CHOP or 4 cycles of R-CHOP plus 2 additional rituximab doses, and showed similar PFS (3-year, 94% v 96%) and OS (98% v 99%),23 establishing the role of R-CHOP × 4 without radiation for these younger, favorable-risk patients. LYSA/GOELAMS 02-03 randomly assigned patients who achieved CR after 4 cycles of R-CHOP administered every 14 days, with smIPI of 0 and bulk < 7 cm, to observation (n = 76) or to 40 Gy of IFRT (n = 82).27 Including those patients with an smIPI of 1 who received an additional 2 cycles of R-CHOP before RT resulted in a 5-year event-free survival (EFS) of 89% versus 92% and a 5-year OS of 92% versus 96%.
In comparison with the patients in these trials, patients in S1001 were considerably older, with a median age of 62 years; 73% had an elevated smIPI, with 14% having elevated LDH and 17% having systemic symptoms, making our results applicable to all patients with limited-stage DLBCL, as opposed to the younger and more favorable–risk patients in the European studies. Given our relatively long follow-up, we observed several nonlymphoma deaths, particularly in older patients. Indeed, our observed 5-year PFS rate of 87% did not meet our original goal of 93%, primarily because of the competing risk of death as a result of nonlymphoma causes (12.8% at 5 years) occurring in patients at a median age of 80 years. Considering these competing risks of death in an older population over a prolonged time period, we feel that the traditional end point of PFS does not adequately reflect the observed treatment effects, which was supported by our competing risk modeling.
Remarkably, no patient in S1001 who received RT has relapsed to date, despite having positive iPET. We used a relatively high radiation dose (36 Gy plus a 9 Gy boost v 30-35 Gy in other studies) and a larger radiation field (IFRT v involved-site RT) for these patients, which is no longer standard. In addition, ibritumomab tiuxetan is not approved in DLBCL. However, ibritumomab tiuxetan could have eliminated residual disease through the crossfire of radioisotope.28 Given the small number of patients who required radiation in our study, additional studies are needed to confirm this favorable outcome in patients who had a positive iPET and to better understand the degree to which radiation and radioimmunotherapy contributed to favorable outcomes.
As part of S1001, we demonstrated a distinct biology of limited-stage DLBCL, such as a predominance of GCB origin (68%) as defined rigorously by Lymph2Cx, confirming retrospective evidence using immunohistochemistry surrogates for GCB.29,30 Similarly, DHL, which carries an unfavorable prognosis in advanced-stage DLBCL, did not portend a worse outcome in S1001, similar to the findings of previously published retrospective studies.29,31,32 Although double-protein expression predicted worse OS in Cox regression analysis, the importance of this finding with so few lymphoma-related events must also be interpreted with caution. HGBL, NOS, was present in 17% of S1001 patients and was not prognostic. Only 1 of 6 patients progressed in CNS, despite prohibition of CNS prophylaxis, demonstrating that limited-stage DLBCL does not routinely require CNS prophylaxis, with the exception of testicular DLBCL, which was excluded from the study.
Head and neck involvement at such a high proportion (66%) has not been reported previously in studies of patients with limited-stage DLBCL, although this definition includes both nodal and extranodal disease. This finding was confirmed on review of S0313 (67%; unpublished data). Head and neck–only presentation had more favorable features, such as a lower rate of elevated LDH and B symptoms, and having a smaller median diameter of the largest lymph node could suggest that its presentation at a palpable location resulted in earlier detection.
In summary, S1001 showed excellent outcomes in patients with limited-stage DLBCL, including older patients and those with moderate disease bulk. Together with the FLYER results in younger, more favorable risk–patients, the findings of our study have established that R-CHOP × 4 is a new standard, less morbid approach in limited-stage DLBCL for the absolute majority of patients, reserving radiation for the small subset of patients with interim PET-positive disease. The results were also favorable in subgroups defined by age, smIPI, cell of origin, and histology and were marginally unfavorable for DPE.
ACKNOWLEDGMENT
We thank the patients; the clinicians enrolling and taking care of patients, including our colleagues at ECOG-ACRIN Cancer Research Group and Alliance for Clinical Trials in Oncology; SWOG Operations and Statistical Center, and the multidisciplinary team: hematopathologists, radiation oncologists, and nuclear medicine physicians.
Appendix
TABLE A1.
Details of Progression or/and Death
SUPPORT
Supported by National Institutes of Health/National Cancer Institute Grant Nos. CA180803, CA180819, CA180820, CA180821, and CA180888, and by Spectrum Pharmaceuticals, Inc.
AUTHOR CONTRIBUTIONS
Conception and design: Daniel O. Persky, Louis S. Constine, Michael L. LeBlanc, Richard I. Fisher, Thomas P. Miller
Provision of study material or patients: Daniel O. Persky, Deborah M. Stephens, Steven I. Park, Nancy L. Bartlett, Lode J. Swinnen, Paul M. Barr, Jerome D. Winegarden III, Louis S. Constine, John P. Leonard, Brad S. Kahl, Richard I. Fisher, Sonali M. Smith, Thomas P. Miller, Jonathan W. Friedberg
Collection and assembly of data: Daniel O. Persky, Hongli Li, Deborah M. Stephens, Thomas J. Fitzgerald, Joo Y. Song, Michael L. LeBlanc, Lisa M. Rimsza, Jonathan W. Friedberg
Data analysis and interpretation: Daniel O. Persky, Hongli Li, Deborah M. Stephens, Steven I. Park, Nancy L. Bartlett, Lode J. Swinnen, Paul M. Barr, Louis S. Constine, John P. Leonard, Brad S. Kahl, Joo Y. Song, Richard I. Fisher, Lisa M. Rimsza, Sonali M. Smith, Thomas P. Miller, Jonathan W. Friedberg
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Positron Emission Tomography–Directed Therapy for Patients With Limited-Stage Diffuse Large B-Cell Lymphoma: Results of Intergroup National Clinical Trials Network Study S1001
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Daniel O. Persky
Consulting or Advisory Role: MorphoSys, Debiopharm Group, Bayer, Cellectar, Kymera, Celgene
Research Funding: Merck (Inst)
Deborah M. Stephens
Honoraria: Genentech
Consulting or Advisory Role: Pharmacyclics/Janssen, Karyopharm Therapeutics
Research Funding: Acerta Pharma, Gilead Sciences, Karyopharm Therapeutics, Verastem, Juno Therapeutics
Steven I. Park
Consulting or Advisory Role: Bristol Myers Squibb, Rafael Pharmaceuticals, G1 Therapeutics, Teva
Speakers' Bureau: Gilead Sciences, Seattle Genetics
Research Funding: Seattle Genetics, Bristol Myers Squibb
Nancy L. Bartlett
Consulting or Advisory Role: Seattle Genetics
Research Funding: Seattle Genetics (Inst), Genentech (Inst), Kite Pharma (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), Immune Design (Inst), Forty Seven (Inst), Janssen (Inst), Pharmacyclics (Inst), Millennium (Inst), ADC Therapeutics (Inst), Autolus (Inst)
Lode J. Swinnen
Consulting or Advisory Role: Pharmacyclics, Celgene
Research Funding: AbbVie/Pharmacyclics (Inst)
Paul M. Barr
Consulting or Advisory Role: Pharmacyclics, AbbVie, Seattle Genetics, Genentech, Celgene, Novartis, Infinity Pharmaceuticals, Janssen, Merck, TG Therapeutics, MorphoSys, AstraZeneca
Research Funding: Pharmacyclics (Inst), AstraZeneca (Inst)
Louis S. Constine
Honoraria: UpToDate, Springer, Lippincott
Travel, Accommodations, Expenses: Particle Therapy Cooperative Group of North America
John P. Leonard
Consulting or Advisory Role: Celgene, Bristol Myers Squibb, Gilead Sciences, Epizyme, Bayer, Genentech/Roche, ADC Therapeutics, MEI Pharma, AstraZeneca, Merck, Morphosys, Nordic Nanovector, Karyopharm Therapeutics, Sutro, Miltenyi, Regeneron, Bayer, Akcea, Sandoz, Nordic Nanovector, Genmab
Research Funding: Celgene (Inst), Alliance for Clinical Trials in Oncology (Inst), Takeda (Inst), Pfizer (Inst), National Cancer Institute (Inst), Janssen Oncology (Inst)
Travel, Accommodations, Expenses: BeiGene
Brad S. Kahl
Consulting or Advisory Role: Celgene, Juno Therapeutics, AbbVie, Pharmacyclics, Acerta Pharma, ADC Therapeutics, Pharmacyclics, Genentech, Roche, AstraZeneca, BeiGene, Bayer, MEI Pharma, TG Therapeutics, Kite/Gilead, MorphoSys, Janssen
Research Funding: Genentech (Inst), Acerta Pharma (Inst), ADC Therapeutics (Inst), Celgene (Inst)
Travel, Accommodations, Expenses: Celgene, Juno Therapeutics, Genentech/Roche, AbbVie, Millennium, Seattle Genetics
Richard I. Fisher
Honoraria: Prime Oncology, Imedex
Consulting or Advisory Role: Celgene, AstraZeneca, Portola Pharmaceuticals, BeiGene
Expert Testimony: Hoffmann-LaRoche
Lisa M. Rimsza
Consulting or Advisory Role: Celgene
Patents, Royalties, Other Intellectual Property: Co-inventor on patent undergoing commercialization by Nanostring
Sonali M. Smith
Consulting or Advisory Role: Kite Pharma, AbbVie/Genentech, Celgene, TG Therapeutics, Bayer, Seattle Genetics, Janssen Oncology
Research Funding: Celgene, Pharmacyclics/Janssen, Acerta Pharma/AstraZeneca, Forty Seven, Portola Pharmaceuticals, TG Therapeutics, Novartis
Other Relationship: Karyopharm Therapeutics
Jonathan W. Friedberg
Consulting or Advisory Role: Bayer, Astellas Pharma, Portola
Research Funding: Seattle Genetics (Inst), Kite Pharma (Inst)
Patents, Royalties, Other Intellectual Property: Patent on bone marrow microenvironment signals (I)
Travel, Accommodations, Expenses: Roche
No other potential conflicts of interest were reported.
REFERENCES
- 1.Morton LM, Wang SS, Devesa SS, et al. Lymphoma incidence patterns by WHO subtype in the United States, 1992-2001. Blood. 2006;107:265–276. doi: 10.1182/blood-2005-06-2508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller TP, Dahlberg S, Cassady JR, et al. Chemotherapy alone compared with chemotherapy plus radiotherapy for localized intermediate- and high-grade non-Hodgkin’s lymphoma. N Engl J Med. 1998;339:21–26. doi: 10.1056/NEJM199807023390104. [DOI] [PubMed] [Google Scholar]
- 3.Stephens DM, Li H, LeBlanc ML, et al. Continued risk of relapse independent of treatment modality in limited-stage diffuse large B-cell lymphoma: Final and long-term analysis of Southwest Oncology Group Study S8736. J Clin Oncol. 2016;34:2997–3004. doi: 10.1200/JCO.2015.65.4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller TP. The limits of limited stage lymphoma. J Clin Oncol. 2004;22:2982–2984. doi: 10.1200/JCO.2004.05.926. [DOI] [PubMed] [Google Scholar]
- 5.Shenkier TN, Voss N, Fairey R, et al. Brief chemotherapy and involved-region irradiation for limited-stage diffuse large-cell lymphoma: An 18-year experience from the British Columbia Cancer Agency. J Clin Oncol. 2002;20:197–204. doi: 10.1200/JCO.2002.20.1.197. [DOI] [PubMed] [Google Scholar]
- 6.Persky DO, Unger JM, Spier CM, et al. Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–2263. doi: 10.1200/JCO.2007.13.6929. [DOI] [PubMed] [Google Scholar]
- 7.Persky DO, Miller TP, Unger JM, et al. Ibritumomab consolidation after 3 cycles of CHOP plus radiotherapy in high-risk limited-stage aggressive B-cell lymphoma: SWOG S0313. Blood. 2015;125:236–241. doi: 10.1182/blood-2014-06-584623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pfreundschuh M, Trümper L, Osterborg A, et al. CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: A randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 9.Haioun C, Itti E, Rahmouni A, et al. [18F]fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) in aggressive lymphoma: An early prognostic tool for predicting patient outcome. Blood. 2005;106:1376–1381. doi: 10.1182/blood-2005-01-0272. [DOI] [PubMed] [Google Scholar]
- 10.Spaepen K, Stroobants S, Dupont P, et al. Early restaging positron emission tomography with ( 18)F-fluorodeoxyglucose predicts outcome in patients with aggressive non-Hodgkin’s lymphoma. Ann Oncol. 2002;13:1356–1363. doi: 10.1093/annonc/mdf256. [DOI] [PubMed] [Google Scholar]
- 11.Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J Clin Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sehn LH, Scott DW, Villa D, et al: Long-term follow-up of a PET-guided approach to treatment of limited-stage diffuse large B-cell lymphoma (DLBCL) in British Columbia (BC). Blood 134:401, 2019.
- 13.Hodgson DC. Long-term toxicity of chemotherapy and radiotherapy in lymphoma survivors: Optimizing treatment for individual patients. Clin Adv Hematol Oncol. 2015;13:103–112. [PubMed] [Google Scholar]
- 14.Mudie NY, Swerdlow AJ, Higgins CD, et al. Risk of second malignancy after non-Hodgkin’s lymphoma: A British Cohort Study. J Clin Oncol. 2006;24:1568–1574. doi: 10.1200/JCO.2005.04.2200. [DOI] [PubMed] [Google Scholar]
- 15.Hans CP, Weisenburger DD, Greiner TC, et al. Confirmation of the molecular classification of diffuse large B-cell lymphoma by immunohistochemistry using a tissue microarray. Blood. 2004;103:275–282. doi: 10.1182/blood-2003-05-1545. [DOI] [PubMed] [Google Scholar]
- 16.Scott DW, Wright GW, Williams PM, et al. Determining cell-of-origin subtypes of diffuse large B-cell lymphoma using gene expression in formalin-fixed paraffin-embedded tissue. Blood. 2014;123:1214–1217. doi: 10.1182/blood-2013-11-536433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson NA, Slack GW, Savage KJ, et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30:3452–3459. doi: 10.1200/JCO.2011.41.0985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 19.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 20.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 21.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25:579–586. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 22.Maurer MJ, Ghesquières H, Link BK, et al. Diagnosis-to-treatment interval is an important clinical factor in newly diagnosed diffuse large B-cell lymphoma and has implication for bias in clinical trials. J Clin Oncol. 2018;36:1603–1610. doi: 10.1200/JCO.2017.76.5198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Poeschel V, Held G, Ziepert M, et al. Four versus six cycles of CHOP chemotherapy in combination with six applications of rituximab in patients with aggressive B-cell lymphoma with favourable prognosis (FLYER): A randomised, phase 3, non-inferiority trial. Lancet. 2020;394:2271–2281. doi: 10.1016/S0140-6736(19)33008-9. [DOI] [PubMed] [Google Scholar]
- 24.Persky D, Li H, Stephens DM, et al. A phase II intergroup trial of PET-directed therapy for limited stage diffuse large B-cell lymphoma (DLBCL): SWOG study S1001 (NCT01359592) Blood 1301553.2017. 28778864 [Google Scholar]
- 25.Mareschal S, Lanic H, Ruminy P, et al. The proportion of activated B-cell like subtype among de novo diffuse large B-cell lymphoma increases with age. Haematologica. 2011;96:1888–1890. doi: 10.3324/haematol.2011.050617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pfreundschuh M, Kuhnt E, Trümper L, et al. CHOP-like chemotherapy with or without rituximab in young patients with good-prognosis diffuse large-B-cell lymphoma: 6-year results of an open-label randomised study of the MabThera International Trial (MInT) Group. Lancet Oncol. 2011;12:1013–1022. doi: 10.1016/S1470-2045(11)70235-2. [DOI] [PubMed] [Google Scholar]
- 27.Lamy T, Damaj G, Soubeyran P, et al. R-CHOP 14 with or without radiotherapy in nonbulky limited-stage diffuse large B-cell lymphoma. Blood. 2018;131:174–181. doi: 10.1182/blood-2017-07-793984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morschhauser F, Illidge T, Huglo D, et al. Efficacy and safety of yttrium-90 ibritumomab tiuxetan in patients with relapsed or refractory diffuse large B-cell lymphoma not appropriate for autologous stem-cell transplantation. Blood. 2007;110:54–58. doi: 10.1182/blood-2007-01-068056. [DOI] [PubMed] [Google Scholar]
- 29.Barraclough A, Alzahrani M, Ettrup MS, et al. COO and MYC/BCL2 status do not predict outcome among patients with stage I/II DLBCL: A retrospective multicenter study. Blood Adv. 2019;3:2013–2021. doi: 10.1182/bloodadvances.2019000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar A, Lunning MA, Zhang Z, et al. Excellent outcomes and lack of prognostic impact of cell of origin for localized diffuse large B-cell lymphoma in the rituximab era. Br J Haematol. 2015;171:776–783. doi: 10.1111/bjh.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grass GD, Mills MN, Ahmed KA, et al. Radiotherapy for early stage diffuse large B-cell lymphoma with or without double or triple hit genetic alterations. Leuk Lymphoma. 2019;60:886–893. doi: 10.1080/10428194.2018.1506586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Torka P, Kothari SK, Sundaram S, et al. Outcomes of patients with limited-stage aggressive large B-cell lymphoma with high-risk cytogenetics. Blood Adv. 2020;4:253–262. doi: 10.1182/bloodadvances.2019000875. [DOI] [PMC free article] [PubMed] [Google Scholar]