Abstract
PURPOSE
Patients with advanced endometrial carcinoma have limited treatment options. We report final primary efficacy analysis results for a patient cohort with advanced endometrial carcinoma receiving lenvatinib plus pembrolizumab in an ongoing phase Ib/II study of selected solid tumors.
METHODS
Patients took lenvatinib 20 mg once daily orally plus pembrolizumab 200 mg intravenously once every 3 weeks, in 3-week cycles. The primary end point was objective response rate (ORR) at 24 weeks (ORRWk24); secondary efficacy end points included duration of response (DOR), progression-free survival (PFS), and overall survival (OS). Tumor assessments were evaluated by investigators per immune-related RECIST.
RESULTS
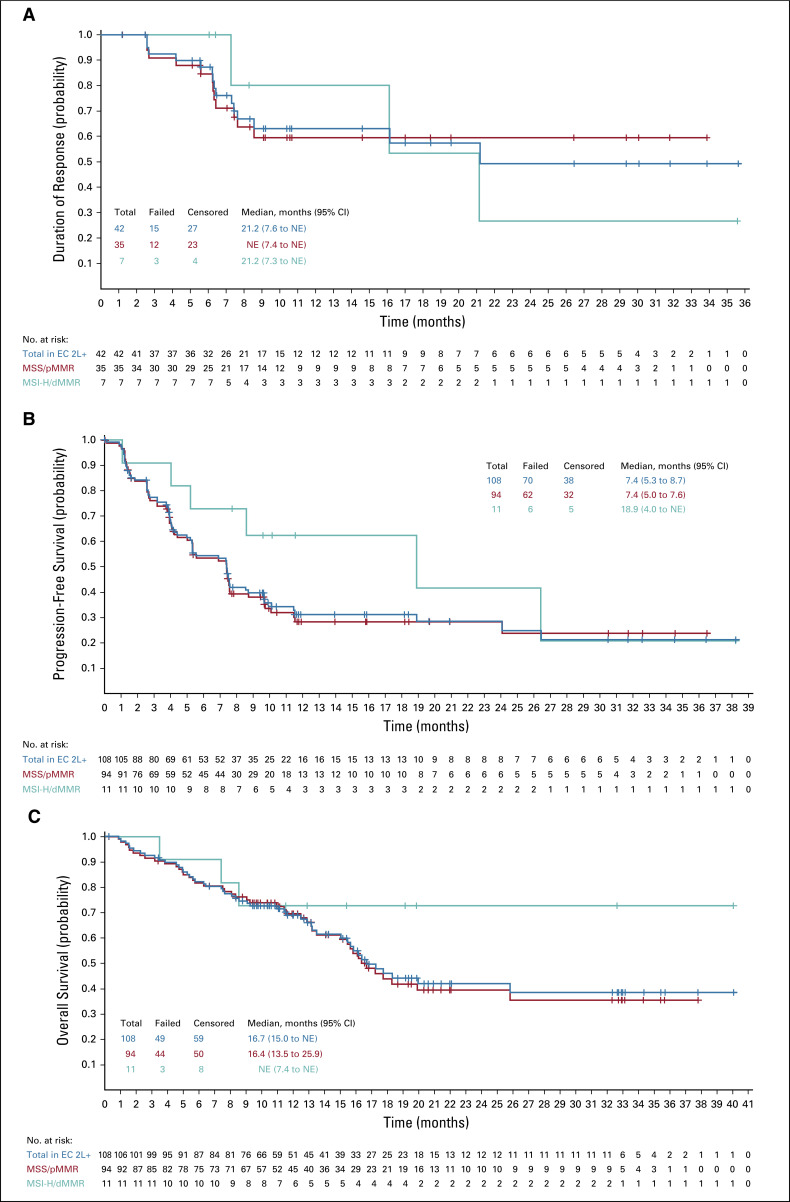
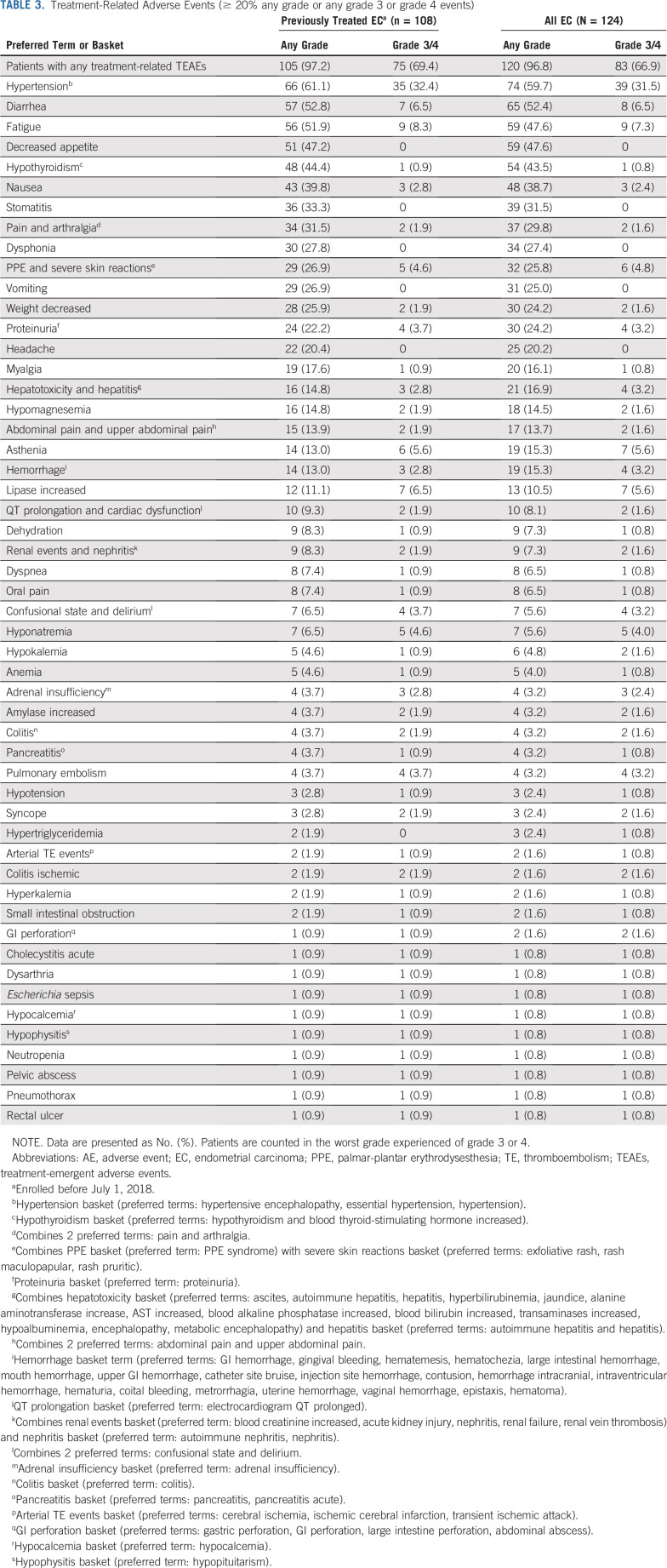
At data cutoff, 108 patients with previously treated endometrial carcinoma were enrolled, with a median follow-up of 18.7 months. The ORRWk24 was 38.0% (95% CI, 28.8% to 47.8%). Among subgroups, the ORRWk24 (95% CI) was 63.6% (30.8% to 89.1%) in patients with microsatellite instability (MSI)–high tumors (n = 11) and 36.2% (26.5% to 46.7%) in patients with microsatellite-stable tumors (n = 94). For previously treated patients, regardless of tumor MSI status, the median DOR was 21.2 months (95% CI, 7.6 months to not estimable), median PFS was 7.4 months (95% CI, 5.3 to 8.7 months), and median OS was 16.7 months (15.0 months to not estimable). Grade 3 or 4 treatment-related adverse events occurred in 83/124 (66.9%) patients.
CONCLUSION
Lenvatinib plus pembrolizumab showed promising antitumor activity in patients with advanced endometrial carcinoma who have experienced disease progression after prior systemic therapy, regardless of tumor MSI status. The combination therapy had a manageable toxicity profile.
INTRODUCTION
The incidence and disease-related mortality of endometrial cancer, the most common gynecologic cancer in the United States, continues to increase.1-4 Although early-stage endometrial carcinoma is associated with a favorable 5-year relative survival rate (96%),5 the rate is 18% in patients with distant metastases.5
Paclitaxel plus carboplatin is standard first-line treatment of advanced, recurrent, and metastatic endometrial carcinoma.6,7 Until recently, only 2 other therapies were specifically approved in the metastatic setting.8 Megestrol acetate is approved for the palliative treatment of advanced endometrial carcinoma, regardless of platinum use.9 Pembrolizumab, a monoclonal antibody targeting programmed death receptor-1 (PD-1), is broadly (ie, tissue agnostic) approved for microsatellite instability–high (MSI-H)/mismatch-repair–deficient (dMMR) solid tumors that have progressed after prior therapy and have no satisfactory alternative treatment options.10 Accordingly, pembrolizumab is used for metastatic MSI-H endometrial carcinoma after front-line chemotherapy failure.
MSI-H tumors with high mutational burdens are more susceptible to checkpoint inhibitors,11 and the mutational burden in MSI-H endometrial cancers is particularly high.12 Pembrolizumab has demonstrated efficacy in patients with MSI-H endometrial cancer. In a phase II study of pembrolizumab monotherapy in patients with previously treated advanced MSI-H/dMMR noncolorectal cancer, results from the endometrial cohort (n = 49) demonstrated an objective response rate (ORR) of 57.1% (95% CI, 42.2% to 71.2%), with a median progression-free survival (PFS) of 25.7 months (95% CI, 4.9 months to not reached).13,14 However, MSI-H endometrial cancers comprise only 16% of recurrent disease cases.15 In a phase Ib study of pembrolizumab for advanced programmed death ligand-1 (PD-L1)–positive endometrial cancer (in patients whose disease progressed after standard therapy or for whom standard therapy was not appropriate), 18 of 19 patients with evaluable tumor samples had microsatellite-stable (MSS) cancer.16 For all patients in the efficacy analysis (n = 23), the ORR was 13% (95% CI, 2.8% to 33.6%), with median PFS of 1.8 months (95% CI, 1.6 to 2.7 months), suggesting that pembrolizumab monotherapy may be less effective in patients with MSS tumors.
Lenvatinib is an oral multikinase inhibitor that targets vascular endothelial growth factor receptors 1-3, fibroblast growth factor receptors 1-4, platelet-derived growth factor receptor-α, RET, and KIT.17-19 In a phase II study of lenvatinib monotherapy for advanced, previously treated endometrial cancer, the ORR was 14.3% (as assessed by independent imaging review [IIR] per RECIST version 1.120), and the median PFS was 5.4 months.21
The combination of immune checkpoint inhibitors with lenvatinib has been evaluated in preclinical mouse xenograft studies.22-24 In these studies, lenvatinib plus PD-1/PD-L1 signal inhibitors had more potent antitumor activity than either agent alone.22-24 KEYNOTE-146/Study 111 (a phase Ib/II study) evaluated this combination in patients with advanced tumors.25 In an interim cohort analysis (activity data cutoff: December 15, 2017; median study follow-up: 13 months) of patients with advanced endometrial cancer who were selected regardless of PD-L1 status, histology, or tumor MSI status, lenvatinib plus pembrolizumab demonstrated promising efficacy: objective response at week 24, assessed using immune-related RECIST (irRECIST), was achieved by 39.6% of the 53 patients (investigator assessment; 45.3%, IIR).26 Here we present the final primary efficacy analysis results of the cohort of patients from KEYNOTE-146/Study 111 with advanced endometrial cancer.
METHODS
Study Design and Patients
KEYNOTE-146/Study 111 is an ongoing multinational, open-label, single-arm study (ClinicalTrials.gov identifier: NCT02501096) of lenvatinib plus pembrolizumab in patients with selected solid tumors (ie, non–small-cell lung cancer, renal cell carcinoma, endometrial carcinoma, urothelial carcinoma, squamous cell carcinoma of the head and neck, or melanoma). Eligibility criteria for the endometrial cohort have been published,26 and a brief overview is provided in the Data Supplement.
This study was conducted in accordance with the guidelines of Good Clinical Practice (defined by the International Council on Harmonization) and the principles of the Declaration of Helsinki; the protocol was approved by the institutional review board or ethics committee at each participating center, and all patients provided written informed consent.
Procedures and Study End Points
On the basis of the phase Ib dose-finding results,25 patients were administered lenvatinib 20 mg once daily orally and pembrolizumab 200 mg intravenously once every 3 weeks in 3-week cycles (maximum of 35 pembrolizumab treatments).
The primary end point in this study was ORR at week 24 (ORRWK24). Responses were confirmed by a second assessment ≥ 4 weeks after initial response. Secondary end points included ORR, duration of response (DOR), PFS, overall survival (OS), disease control rate (DCR; defined as the proportion of patients with a best overall response of complete response [CR], partial response [PR], or stable disease), and clinical benefit rate (CBR; defined as the proportion of patients with CR, PR, or durable stable disease [defined as stable disease ≥ 23 weeks]). Tumor responses for primary and secondary end points were assessed by the investigator per irRECIST.27
Prespecified exploratory end points included tumor responses per irRECIST27 and RECIST version 1.120 by IIR (assessed by BioTel Research, BioTelemetry), and antitumor activity according to PD-L1 status. Activity by tumor histology and MSI status were post hoc exploratory analyses. Adverse events (AEs) were assessed according to Common Terminology Criteria for Adverse Events version 4.03.
For all patients, tumor assessments (investigator assessment and IIR) were completed at baseline, every 6 weeks for the first 24 weeks, and every 9 weeks thereafter. PD-L1 status was determined using an investigational version of the PD-L1 immunochemistry 22C3pharmDx28 and a provisional combined positive score (defined as the number of staining tumor and immune cells relative to total tumor cells) cutoff of 1. Central testing to determine MSI was conducted using the MSI Analysis System, and central testing for mismatch repair (MMR) status was conducted using the Ventana MMR Immunohistochemical Assay. Available data regarding known MSI/MMR status based on local testing per institutional guidelines was also collected.
Toxicity was managed by supportive medications, treatment interruption, dose reduction (lenvatinib only; re-escalation was not allowed), and/or treatment discontinuation in accordance with predefined dose-modification guidelines. Information regarding treatment discontinuation of either lenvatinib or pembrolizumab and patient assessments during the follow-up period of this study can be found in the Data Supplement.
Statistical Methods
The phase II portion was designed to expand to 20 patients with endometrial cancer on the basis of efficacy and safety results. A protocol amendment allowed further expansion to a total sample size of ∼120 patients after predetermined criteria of 2 interim analyses were met. Details regarding sample size determination can be found in the Data Supplement.
Efficacy analyses focused on patients who previously received systemic treatment; the primary analysis was planned for patients from the full analysis set who completed 8 cycles of treatment and had week 24 tumor assessments or who had discontinued early because of progressive disease, unacceptable toxicity, withdrawn consent, or study termination by the sponsor at the time of data cutoff. According to an addendum to the statistical analyses (made before database lock), data cutoff was when ≥ 100 patients with histologically confirmed, previously treated endometrial carcinoma had sufficient follow-up to provide a median follow-up of ≥ 12 months, and ≥ 6 months of follow-up after initial objective response for all responders.
Additional efficacy analyses are reported for previously treated and treatment-naïve patients (Data Supplement), regardless of their follow-up time at data cutoff. These efficacy analyses were based on the intention-to-treat population (ie, all patients with endometrial cancer who entered the study treatment period). Safety analyses were based on the safety set, defined as all patients (previously treated and treatment naïve) who received any amount of study drug, irrespective of their follow-up time at data cutoff. The most common treatment-related AEs were reported by preferred terms or “baskets” (ie, groups of related preferred terms). Treatment-emergent prespecified AEs, serious treatment-related AEs, and deaths were reported by preferred terms.
Statistical analyses were performed using SAS (SAS Institute) version 9.4. ORRWK24 and the exact 95% CIs were calculated with the Clopper-Pearson method, as were 95% CIs for ORR, DCR, and CBR. Patients who did not have an evaluable tumor assessment were included in the denominator for the calculation of ORR. Medians of PFS and OS (and their median follow-up times) and DOR were estimated with the Kaplan-Meier method, and 95% CIs were calculated with a generalized Brookmeyer and Crowley method. Probabilities of patients achieving a DOR ≥ 6 months or ≥ 12 months were calculated using the Kaplan-Meier product-limit method and the Greenwood formula.
RESULTS
Patients
When enrollment (starting September 10, 2015) reached 118 patients (July 1, 2018), end-of-enrollment notifications were sent to study sites. At the time of notification, 7 additional patients who had completed screening were allowed to enroll in the endometrial carcinoma cohort (Data Supplement). Of the 125 enrolled patients, 1 had a major protocol violation—her primary tumor was determined to be a uterine leiomyosarcoma.
Analysis of the primary end point focused on the 108 patients with endometrial carcinoma who enrolled before July 1, 2018 and had previously received systemic therapy; these patients met all previously described conditions for the primary analysis at time of data cutoff (January 10, 2019; Data Supplement). The median follow-up for these patients was 18.7 months (95% CI, 13.1 to 20.3 months), and 29 (26.9%) patients were receiving ongoing study treatment with at least 1 study drug at data cutoff. The most common histologic subtypes of disease were endometrioid adenocarcinoma (50.9%; International Federation of Gynecology and Obstetrics [FIGO] grade 1 or 2, 28.7%; FIGO grade 3, 22.2%) and serous carcinoma (32.4%; Table 1). Nearly half (49.1%) of the patients were PD-L1 positive. Ninety-four (87.0%) and 11 (10.2%) patients were MSS or MMR proficient (pMMR) and MSI-H or dMMR, respectively (Table 1).
TABLE 1.
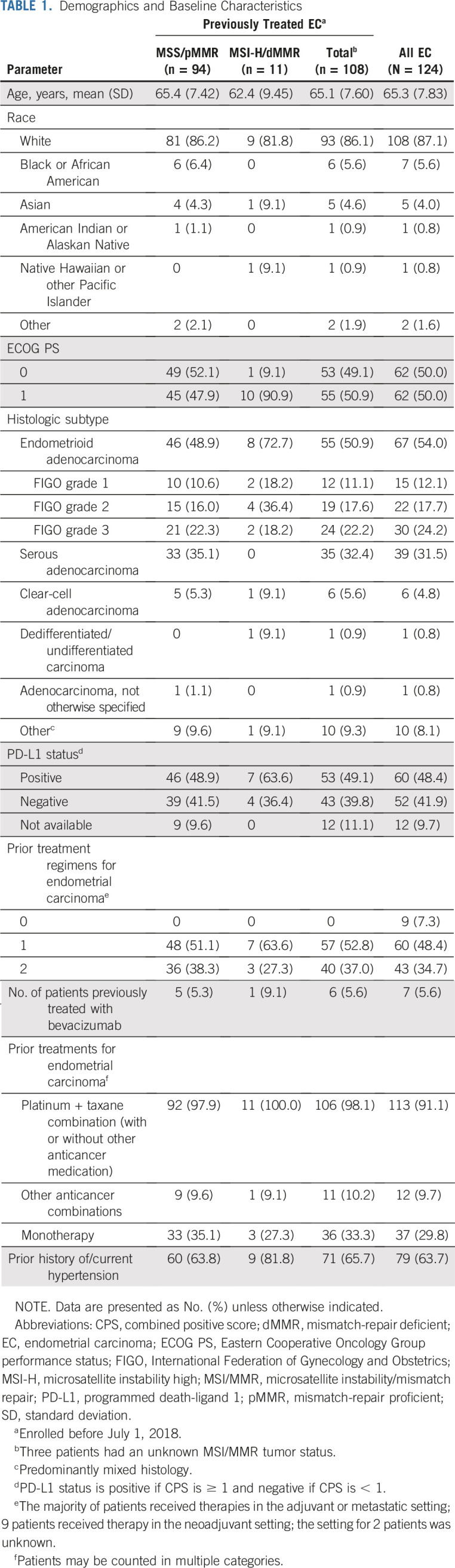
Demographics and Baseline Characteristics
Efficacy
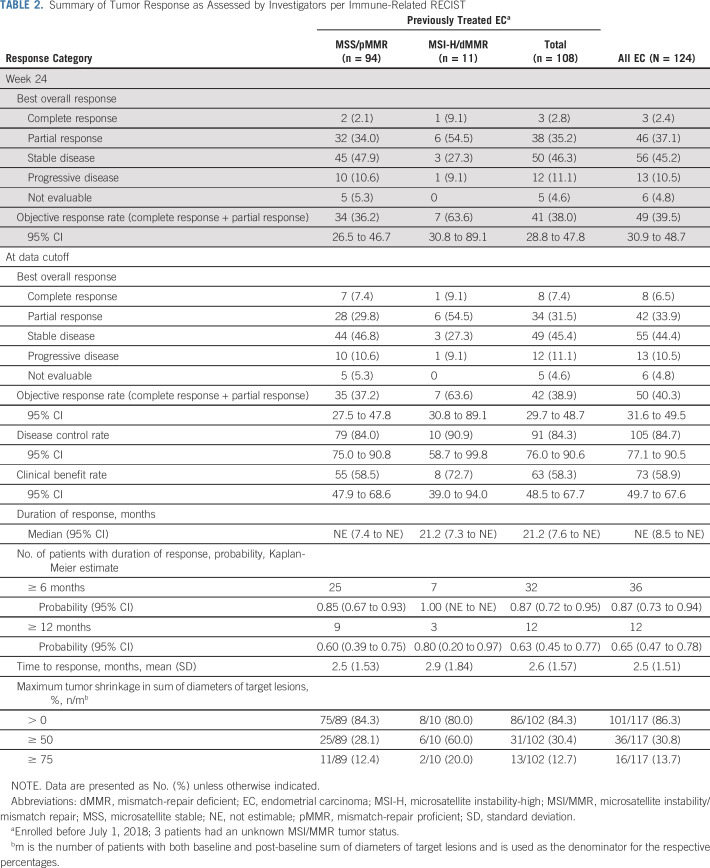
The primary end point, ORRWK24, was 38.0% (41/108 patients; 95% CI, 28.8% to 47.8%; Table 2). In the 102 patients with evaluable tumor assessments by investigators per irRECIST, the sum of diameters of target lesions decreased (any size) from baseline in 86 (84.3%) patients (Fig 1; Table 2); 31 (30.4%) had a maximum decrease of ≥ 50% and 13 (12.7%) had a maximum decrease of ≥ 75% (Table 2).
TABLE 2.
Summary of Tumor Response as Assessed by Investigators per Immune-Related RECIST
FIG 1.
Percentage change in sum of diameters of target lesions from baseline to post-baseline nadir by microsatellite instability/mismatch-repair (MSI/MMR) status (by investigator assessment; using immune-related RECIST). dMMR, MMR deficient; m, the number of previously treated patients with both baseline and at least 1 postbaseline target lesion assessment; MSI-H, MSI-high; pMMR, MMR proficient; PD-1, programmed death receptor-1; PD-L1, programmed death-ligand 1.
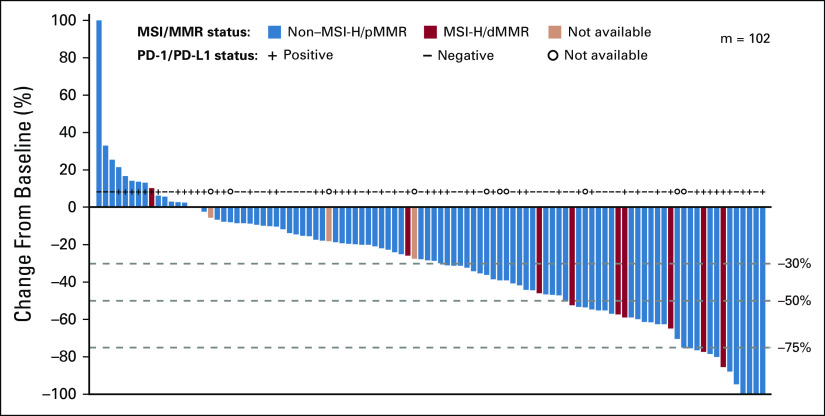
Regarding secondary end points, the ORR of patients previously treated for endometrial carcinoma was 38.9% (42/108, 95% CI, 29.7% to 48.7%; CR, 8/108, 7.4%, 95% CI, 3.3% to 14.1%; PR, 34/108, 31.5%, 95% CI, 22.9% to 41.1%) and the median DOR was 21.2 months (95% CI, 7.6 months to not estimable [NE]; Table 2; Fig 2A). Among responders, the Kaplan-Meier estimate of the probability of a DOR ≥ 6 months was 87% (95% CI, 72% to 95%), and the Kaplan-Meier estimate of the probability of a DOR ≥ 12 months was 63% (95% CI, 45% to 77%; Table 2). Median PFS was 7.4 months (95% CI, 5.3 to 8.7 months; median follow-up: 11.9 months; 95% CI, 9.9 to 18.4 months; Fig 2B) and median OS was 16.7 months (95% CI, 15.0 months to NE; Fig 2C).
FIG 2.
Kaplan-Meier plot of (A) duration of response, (B) progression-free survival, and (C) overall survival assessed by the investigator per immune-related RECIST (for A and B groups only; not appropriate for C) in patients with endometrial cancer previously treated with systemic therapies. dMMR, mismatch-repair deficient; EC 2L+, endometrial cancer second-line (or greater) treatment MSI-H, microsatellite instability high; MSS, microsatellite stable; NE, not estimable; pMMR, mismatch-repair proficient.
Tumor responses were similar irrespective of investigator or IIR and tumor response criteria (Data Supplement). Of note, 35.8% (19/53; 95% CI, 23.1% to 50.2%) of patients with PD-L1–positive tumors and 39.5% (17/43; 95% CI, 25.0% to 55.6%) of patients with PD-L1–negative tumors had objective responses by investigator assessment per irRECIST (Data Supplement). For patients with MSS/pMMR tumors, ORR—as assessed by investigators per irRECIST—was 37.2% (35/94; 95% CI, 27.5% to 47.8%). For patients with MSI-H/dMMR tumors, ORR was 63.6% (7/11; 95% CI, 30.8% to 89.1%; Table 2). Median DOR was NE (95% CI, 7.4 months to NE) for patients with MSS/pMMR tumors and 21.2 months (95% CI, 7.3 months to NE) for patients with MSI-H/dMMR tumors (Table 2; Fig 2A). Details regarding median PFS and OS by tumor MSI status are shown in Figures 2B and 2C, respectively. Additional details of responses by MSI and PD-L1 status are shown in the Data Supplement; the percentage changes in the sums of diameters of target lesions at post-baseline nadir by histologic subtype are shown in Figure 3 (by investigator assessment; using irRECIST) and the Data Supplement (by IIR; using RECIST version 1.1). Tumor responses by histologic subtypes are shown in the Data Supplement.
FIG 3.
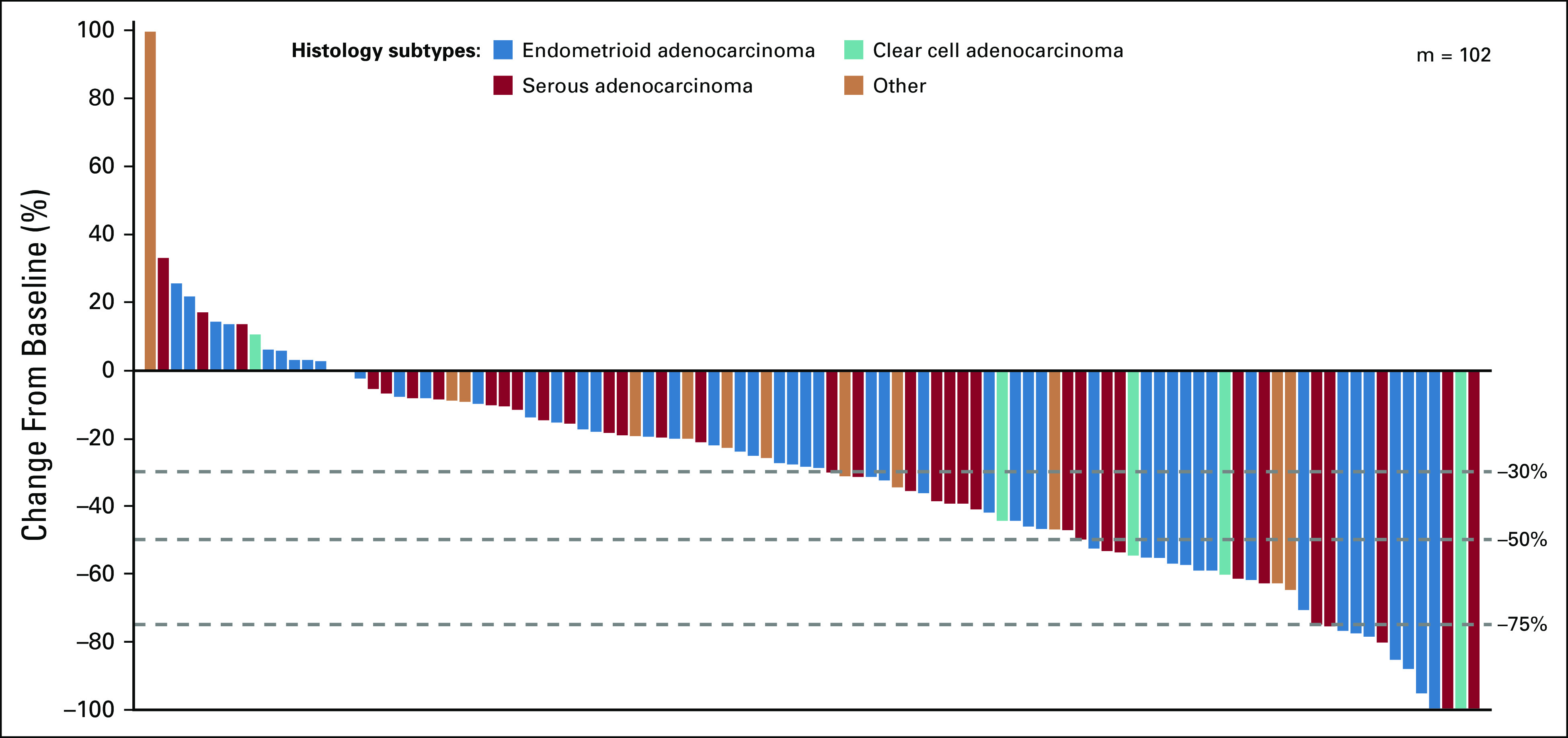
Percentage change in sum of diameters of target lesions from baseline to post-baseline nadir by histologic subtype (by investigator assessment; using immune-related RECIST). m, the number of previously treated patients with both baseline and at least 1 postbaseline target lesion assessment.
Safety
Mean duration of treatment with lenvatinib plus pembrolizumab was 8.5 months (8.2 months, lenvatinib and 7.3 months, pembrolizumab). Any-grade treatment-related AEs occurred in 120 (96.8%) patients. Grade 3 or 4 treatment-related AEs occurred in 83 (66.9%) patients (Table 3). Overall, 22 (17.7%) patients discontinued 1 or both study drugs because of treatment-related AEs (19 [15.3%] discontinued lenvatinib, 15 [12.1%] discontinued pembrolizumab, and 11 [8.9%] discontinued both study drugs). Treatment-related AEs led to dose interruptions of lenvatinib and/or pembrolizumab in 87 (70.2%) patients and dose reductions of lenvatinib in 78 (62.9%) patients. The mean dose intensity of lenvatinib was 14.4 mg/day (standard deviation [SD], 4.3), and the mean dose of lenvatinib received as a percentage of the planned dose was 71.9% (SD, 21.4). The mean duration of treatment with lenvatinib at each dose level is reported in the Data Supplement; 11 (8.9%) patients remained on lenvatinib for ≥ 6 months after cycle 1 day 1 with no dose reductions.
TABLE 3.
Treatment-Related Adverse Events (≥ 20% any grade or any grade 3 or grade 4 events)
The most common any-grade treatment-related AEs and the most common grade 3 or 4 treatment-related AEs are reported in Table 3 and the Data Supplement, respectively. Seventy-one patients (57.3%) experienced treatment-emergent, prespecified AEs associated with pembrolizumab. Hypothyroidism was the most frequent of these AEs, occurring in 59 (47.6%) patients.
Serious treatment-related AEs are reported in the Data Supplement. Fifty-one deaths occurred during this study: 16 while receiving study drug and 35 during follow-up—with a median time from last dose to death of 171 days (29/35 patients discontinued because of radiologic progression; cause of death during follow-up was not recorded). Of note, 4 deaths were considered caused by treatment-emergent AEs (1 each due to GI perforation, intestinal obstruction, general physical health deterioration, and metabolic encephalopathy) not deemed treatment related, and 2 deaths were judged to be treatment related (1 due to sepsis caused by an Escherichia coli infection and 1 due to intracranial hemorrhage).
DISCUSSION
Advanced endometrial carcinomas have a poor prognosis; the continued annual increase in incidence and disease-related mortality of endometrial cancer1,3,29 underscores a dire need to improve therapeutics for this malignancy.
Nearly 84% of patients with recurrent endometrial carcinoma have MSS or microsatellite-indeterminate tumors.15 Although pembrolizumab is effective for MSI-H disease (objective response, 28/49 [57.1%] patients),13,14 it appears less effective for MSS disease (best response was PR, 2/18 patients).16 Similarly, in advanced/recurrent previously treated endometrial carcinoma, investigational PD-1 monoclonal antibody dostarlimab (formerly TSR-042) had greater efficacy in patients with MSI-H tumors compared with patients with MSS tumors (ORR, [confirmed and unconfirmed responses] 50.0% and 19.1%, respectively).30 ORRs observed with other investigational PD-L1 monoclonal antibodies have had similar trends in previously treated endometrial cancer (avelumab: 27%, patients with dMMR tumors; 6%, patients with pMMR tumors; durvalumab: 43%, patients with dMMR tumors; 3%, patients with pMMR tumors).31,32 Moreover, antiangiogenic and antimicrotubule medications have limited efficacy (ORR, 14%-16%; median PFS, 3.4-4.2 months) in recurrent/advanced endometrial carcinoma.33,34
Our study had comparable results (ORRWK24, 38.0%; ORR, 38.9%; median PFS, 7.4 months in patients with previously treated endometrial cancer) to the recently published interim analysis of lenvatinib plus pembrolizumab in endometrial cancer (ORRWK24, 39.6%; ORR, 39.6%; median PFS, 7.4 months)26 but with a longer follow-up time; as such, this suggests that the combination of lenvatinib plus pembrolizumab has favorable efficacy compared with that of previously reported therapies in similar populations.30,33,34 Importantly, tumor responses in our study were similar regardless of the tumor response criteria used and whether evaluated by investigator or IIR. Lenvatinib plus pembrolizumab also demonstrated a robust depth of response; 84% of patients with evaluable tumor assessments had decreased tumor lesions (any size) from baseline, and 30% had a maximum decrease of ≥ 50%. Even more compelling, ORR in patients with difficult-to-treat MSS disease was 37.2%. Although the mechanistic basis for the encouraging efficacy of lenvatinib plus pembrolizumab in advanced endometrial carcinoma requires additional study, experimental models indicate lenvatinib modulates cancer immunity by decreasing the suppressive tumor-associated macrophage population,22,35 which may allow T cells reinvigorated by pembrolizumab to have enhanced antitumor activity.
The safety profile of lenvatinib plus pembrolizumab was generally similar to previously reported profiles of each monotherapy (lenvatinib, 24 mg/d; pembrolizumab, 10 mg/kg every 2 or 3 weeks).13,14,16,21,36-38 Although hypothyroidism occurred in a greater proportion of patients in our study than in previous reports of either monotherapy (48% [treatment-related, 43%] v ≤ 37%, respectively),36,37 there was only 1 occurrence of grade 3 hypothyroidism. Overall, the rate of grade 3/4 treatment-related AEs was similar in our study to the interim analysis of lenvatinib plus pembrolizumab in endometrial cancer (67% v 68%, respectively).26 Timely identification of treatment-related AEs and their management throughout this study with dose interruptions and reductions may have facilitated treatment continuation, as only 17.7% of patients discontinued study treatment(s) because of treatment-related AEs. On average, patients received 11 pembrolizumab treatments, with only 4 patients reaching the maximum number of allowable doses (n = 3, 35 treatments; and n = 1, 36 treatments [allowable before protocol amendment 4]).
This study is limited by its nonrandomized nature. Moreover, exploratory analyses regarding PD-L1 status should be viewed with caution, as the cutoff for PD-L1 positivity is not definitive, and it is not known if PD-L1 status is a positive prognostic indicator in endometrial cancer. Additional limitations include the absence of a quality-of-life analysis as well as a lack of biomarker assessments to characterize patients susceptible to checkpoint inhibitors (ie, tumor mutational burden and mutation-associated neoantigens); however, biomarker analyses are planned. It should be noted that although this trial may be considered small, its size (key efficacy analysis, n = 108; overall population, n = 124) is comparable to the collective size of trials that led to the approval of pembrolizumab in patients with MSI-H cancers (n = 149).39
Overall, the results of this study are encouraging and showed compelling efficacy and an acceptable safety profile in patients with advanced endometrial carcinoma. As a result of this study, lenvatinib plus pembrolizumab was granted accelerated approval for the treatment of patients with advanced endometrial carcinoma that is not MSI-H or dMMR, who have disease progression after prior systemic therapy, and who are not candidates for curative surgery or radiation.40 In addition, two phase III trials of lenvatinib plus pembrolizumab in advanced endometrial carcinoma are currently underway (ClinicalTrials.gov identifier: NCT03884101 [v carboplatin plus paclitaxel in the first-line setting] and ClinicalTrials.gov identifier: NCT03517449 [v doxorubicin or paclitaxel in previously treated patients]).
ACKNOWLEDGMENT
Jeffrey K. Bratz, PhD, of Oxford PharmaGenesis, Newtown, PA, provided editing services on the text and assisted with the collation and input of author changes during revision drafts. He also assisted with the preparation for submission to Journal of Clinical Oncology.
PRIOR PRESENTATION
Presented at the ESMO Congress 2019, Barcelona, Spain, September 27-October 1, 2019.
SUPPORT
Supported by Eisai Inc., Woodcliff Lake, NJ, USA, and Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Vicky Makker, Matthew H. Taylor, Carol Aghajanian, Daniel E. Stepan, Corina E. Dutcus, Emmett V. Schmidt, Pallavi Sachdev, Robert Shumaker
Administrative support: Antonio Casado Herraez
Provision of study material or patients: Carol Aghajanian, Ana Oaknin, James Mier, Allen L. Cohn, Raquel Bratos, Christopher DiSimone, Mark Messing, Antonio Casado Herraez
Collection and assembly of data: Vicky Makker, Matthew H. Taylor, Ana Oaknin, James Mier, Allen L. Cohn, Margarita Romeo, Raquel Bratos, Marcia S. Brose, Christopher DiSimone, Mark Messing, Daniel E. Stepan, Corina E. Dutcus, Pallavi Sachdev, Antonio Casado Herraez
Data analysis and interpretation: Vicky Makker, Matthew H. Taylor, Ana Oaknin, Allen L. Cohn, Margarita Romeo, Raquel Bratos, Marcia S. Brose, Mark Messing, Daniel E. Stepan, Corina E. Dutcus, Jane Wu, Emmett V. Schmidt, Robert Orlowski, Pallavi Sachdev, Antonio Casado Herraez
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Lenvatinib Plus Pembrolizumab in Patients With Advanced Endometrial Cancer
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Vicky Makker
Honoraria: Eisai, Merck
Consulting or Advisory Role: Eisai, Merck, Karyopharm Therapeutics, Takeda, ArQule
Research Funding: Lilly, AstraZeneca, Eisai, Merck, Takeda, Bristol-Myers Squibb
Travel, Accommodations, Expenses: Eisai, Merck, Karyopharm Therapeutics
Other Relationship: IBM
Matthew H. Taylor
Consulting or Advisory Role: Bristol-Myers Squibb, Eisai, Array BioPharma, Loxo, Bayer, ArQule, Blueprint Medicines, Novartis, Sanofi/Genzyme
Speakers' Bureau: Bristol-Myers Squibb, Eisai
Carol Aghajanian
Consulting or Advisory Role: Tesaro, Clovis Oncology, Immunogen, Roche, Mersana Therapeutics, Eisai
Research Funding: Genentech/Roche (Inst), AbbVie (Inst), Clovis Oncology (Inst), AstraZeneca (Inst)
Ana Oaknin
Consulting or Advisory Role: Roche, AstraZeneca, PharmaMar, Clovis Oncology, Tesaro, Immunogen, Genmab
Research Funding: AbbVie Deutschland (Inst), Ability Pharmaceuticals (Inst), Advaxis (Inst), Aeterna Zentaris (Inst), Amgen (Inst), Aprea Therapeutics (Inst), Clovis Oncology (Inst), Eisai (Inst), F. Hoffmann–La Roche (Inst), Regeneron Pharmaceuticals (Inst)
Travel, Accommodations, Expenses: AstraZeneca, Clovis Oncology, PharmaMar, Roche
Allen L. Cohn
Honoraria: Bristol-Myers Squibb, Ipsen
Speakers' Bureau: Bristol-Myers Squibb, Ipsen
Margarita Romeo
Consulting or Advisory Role: Tesaro/Glaxo, AstraZeneca, Roche
Research Funding: Eisai (Inst), Aprea Therapeutics (Inst), AstraZeneca (Inst)
Travel, Accommodations, Expenses: Pfizer, MSD
Marcia S. Brose
Honoraria: Bayer
Consulting or Advisory Role: Bayer, AstraZeneca, Eisai, Blueprint Medicines, Loxo, Novartis, Genzyme, Bristol-Myers Squibb
Research Funding: Bayer (Inst), Eisai (Inst), Novartis (Inst), Roche/Genentech (Inst), Exelixis (Inst), Blueprint Medicines (Inst), Loxo (Inst)
Mark Messing
Leadership: US Oncology
Daniel E. Stepan
Employment: Eisai
Corina E. Dutcus
Employment: Eisai
Jane Wu
Employment: Eisai
Emmett V. Schmidt
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme
Travel, Accommodations, Expenses: Merck Sharp & Dohme
Robert Orlowski
Employment: Merck Sharp & Dohme
Stock and Other Ownership Interests: Merck Sharp & Dohme, Celgene, OncoSec, Nektar, Bluebird Bio
Research Funding: Merck Sharp & Dohme
Pallavi Sachdev
Employment: Eisai
Employment: Eisai
Travel, Accommodations, Expenses: Eisai, Eisai
Robert Shumaker
Employment: Eisai
Employment: RRD International (I)
Antonio Casado Herraez
Consulting or Advisory Role: Roche Spain, PharmaMar, EISAI, Merck Sharp & Dohme
Research Funding: PharmaMar (Inst)
Travel, Accommodations, Expenses: PharmaMar, Roche, Lilly Spain
Other Relationship: Lilly (Inst)
No other potential conflicts of interest were reported.
REFERENCES
- 1.Cote ML, Ruterbusch JJ, Olson SH, et al. The growing burden of endometrial cancer: A major racial disparity affecting black women. Cancer Epidemiol Biomarkers Prev. 2015;24:1407–1415. doi: 10.1158/1055-9965.EPI-15-0316. [DOI] [PubMed] [Google Scholar]
- 2.Constantine GD, Kessler G, Graham S, et al. Increased incidence of endometrial cancer following the Women’s Health Initiative: An assessment of risk factors. J Womens Health (Larchmt) 2019;28:237–243. doi: 10.1089/jwh.2018.6956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society Key statistics for endometrial cancer. https://www.cancer.org/cancer/endometrial-cancer/about/key-statistics.html
- 5.American Cancer Society Endometrial cancer survival rates, by stage. https://www.cancer.org/cancer/endometrial-cancer/detection-diagnosis-staging/survival-rates.html
- 6. National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology: Uterine Neoplasms. Version 3.2019. https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
- 7. Miller D, Filiaci V, Fleming G, et al: Late-breaking abstract 1: Randomized phase III noninferiority trial of first line chemotherapy for metastatic or recurrent endometrial carcinoma: A Gynecologic Oncology Group study. Gynecol Oncol 125:771, 2012 (abstr) [Google Scholar]
- 8.Makker V, Green AK, Wenham RM, et al. New therapies for advanced, recurrent, and metastatic endometrial cancers. Gynecol Oncol Res Pract. 2017;4:19. doi: 10.1186/s40661-017-0056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 1996;14:357–361. doi: 10.1200/JCO.1996.14.2.357. [DOI] [PubMed] [Google Scholar]
- 10. Keytruda (pembrolizumab) [package insert]. Whitehouse Station, NJ, Merck Sharp & Dohme, 2018.
- 11.Arora E, Masab M, Mittar P, et al. Role of immune checkpoint inhibitors in advanced or recurrent endometrial cancer. Cureus. 2018;10:e2521. doi: 10.7759/cureus.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. doi: 10.1038/nature12113. Cancer Genome Atlas Research Network, Kandoth C, Schultz N, et al: Integrated genomic characterization of endometrial carcinoma. Nature 497:67-73, 2013 [Erratum: Nature 500:242, 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Malley D, Marabelle A, De Jesus-Acosta A, et al. Pembrolizumab in patients with MSI-H advanced endometrial cancer from the KEYNOTE-158 study. Ann Oncol. 2019;30(suppl 5; abstr 1044P):V425–V426. doi: 10.1200/JCO.21.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: Results from the Phase II KEYNOTE-158 study. J Clin Oncol. 2020;38:1–10. doi: 10.1200/JCO.19.02105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soumerai TE, Donoghue MTA, Bandlamudi C, et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin Cancer Res. 2018;24:5939–5947. doi: 10.1158/1078-0432.CCR-18-0412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ott PA, Bang YJ, Berton-Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1-positive endometrial cancer: Results from the KEYNOTE-028 study. J Clin Oncol. 2017;35:2535–2541. doi: 10.1200/JCO.2017.72.5952. [DOI] [PubMed] [Google Scholar]
- 17.Matsui J, Yamamoto Y, Funahashi Y, et al. E7080, a novel inhibitor that targets multiple kinases, has potent antitumor activities against stem cell factor producing human small cell lung cancer H146, based on angiogenesis inhibition. Int J Cancer. 2008;122:664–671. doi: 10.1002/ijc.23131. [DOI] [PubMed] [Google Scholar]
- 18.Okamoto K, Kodama K, Takase K, et al. Antitumor activities of the targeted multi-tyrosine kinase inhibitor lenvatinib (E7080) against RET gene fusion-driven tumor models. Cancer Lett. 2013;340:97–103. doi: 10.1016/j.canlet.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Tohyama O, Matsui J, Kodama K, et al. Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor that targets multiple receptor tyrosine kinases in preclinical human thyroid cancer models. J Thyroid Res. 2014;2014:638747. doi: 10.1155/2014/638747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 21. Vergote I, Teneriello M, Powell MA, et al: A phase II trial of lenvatinib in patients with advanced or recurrent endometrial cancer: Angiopoietin-2 as a predictive marker for clinical outcomes. J Clin Oncol 31, 2013 (15 suppl; abstr 5520)
- 22. Kato Y, Tabata K, Hori Y, et al: Effects of lenvatinib on tumor-associated macrophages enhance antitumor activity of PD-1 signal inhibitors. Mol Cancer Ther 14, 2015 (12 suppl 2; abstr A92)
- 23. Kato Y, Bao X, Macgrath S, et al: Lenvatinib mesilate (LEN) enhanced antitumor activity of a PD-1 blockade agent by potentiating Th1 immune response. Ann Oncol 27, 2016 (suppl 6; abstr 2PD)
- 24.Kimura T, Kato Y, Ozawa Y, et al. Immunomodulatory activity of lenvatinib contributes to antitumor activity in the Hepa1-6 hepatocellular carcinoma model. Cancer Sci. 2018;109:3993–4002. doi: 10.1111/cas.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Taylor M, Dutcus CE, Schmidt E, et al: A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients with selected solid tumors [abstract]. Ann Oncol 27, 2016 (suppl 6; abstr 776PD)
- 26.Makker V, Rasco D, Vogelzang NJ, et al. Lenvatinib plus pembrolizumab in patients with advanced endometrial cancer: An interim analysis of a multicentre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2019;20:711–718. doi: 10.1016/S1470-2045(19)30020-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seymour L, Bogaerts J, Perrone A, et al. iRECIST: Guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18:e143–e152. doi: 10.1016/S1470-2045(17)30074-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. doi: 10.1097/PAI.0000000000000408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eheman C, Henley SJ, Ballard-Barbash R, et al. Annual Report to the Nation on the status of cancer, 1975-2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer. 2012;118:2338–2366. doi: 10.1002/cncr.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Oaknin A, Duska LR, Sullivan RJ, et al: Preliminary safety, efficacy, and pharmacokinetic/pharmacodynamic characterization from GARNET, a phase I/II clinical trial of the anti–PD-1 monoclonal antibody, TSR-042, in patients with recurrent or advanced MSI-h and MSS endometrial cancer. Gynecol Oncol 154:17, 2019 (suppl 1, abstr 33) [Google Scholar]
- 31.Konstantinopoulos PA, Luo W, Liu JF, et al. Phase II study of avelumab in patients with mismatch repair deficient and mismatch repair proficient recurrent/persistent endometrial cancer. J Clin Oncol. 2019;37:2786–2794. doi: 10.1200/JCO.19.01021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Antill YC, Kok PS, Robledo K, et al: Activity of durvalumab in advanced endometrial cancer (AEC) according to mismatch repair (MMR) status: The phase II PHAEDRA trial (ANZGOG1601). J Clin Oncol 37, 2019 (15 suppl; abstr 5501)
- 33.Aghajanian C, Sill MW, Darcy KM, et al. Phase II trial of bevacizumab in recurrent or persistent endometrial cancer: A Gynecologic Oncology Group study. J Clin Oncol. 2011;29:2259–2265. doi: 10.1200/JCO.2010.32.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMeekin S, Dizon D, Barter J, et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with advanced endometrial cancer. Gynecol Oncol. 2015;138:18–23. doi: 10.1016/j.ygyno.2015.04.026. [DOI] [PubMed] [Google Scholar]
- 35.Kato Y, Tabata K, Kimura T, et al. Lenvatinib plus anti-PD-1 antibody combination treatment activates CD8+ T cells through reduction of tumor-associated macrophage and activation of the interferon pathway. PLoS One. 2019;14:e0212513. doi: 10.1371/journal.pone.0212513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motzer RJ, Hutson TE, Glen H, et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open-label, multicentre trial. Lancet Oncol. 2015;16:1473–1482. doi: 10.1016/S1470-2045(15)00290-9. [DOI] [PubMed] [Google Scholar]
- 37.Robert C, Schachter J, Long GV, et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–2532. doi: 10.1056/NEJMoa1503093. [DOI] [PubMed] [Google Scholar]
- 38.Schlumberger M, Tahara M, Wirth LJ, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 39.Marcus L, Lemery SJ, Keegan P, et al. FDA approval summary: Pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res. 2019;25:3753–3758. doi: 10.1158/1078-0432.CCR-18-4070. [DOI] [PubMed] [Google Scholar]
- 40. Lenvima (lenvatinib) [package insert]. Woodcliff Lake, NJ, Eisai, 2019.