Abstract
PURPOSE
The Children’s Oncology Group (COG) protocol AALL0434 evaluated the safety and efficacy of multi-agent chemotherapy with Capizzi-based methotrexate/pegaspargase (C-MTX) in patients with newly diagnosed pediatric T-cell lymphoblastic lymphoma (T-LL) and gained preliminary data using nelarabine in high-risk patients.
PATIENTS AND METHODS
The trial enrolled 299 patients, age 1-31 years. High-risk (HR) patients had ≥ 1% minimal detectable disease (MDD) in the bone marrow at diagnosis or received prior steroid treatment. Induction failure was defined as failure to achieve a partial response (PR) by the end of the 4-week induction. All patients received the augmented Berlin-Frankfurt-Muenster (ABFM) C-MTX regimen. HR patients were randomly assigned to receive or not receive 6 5-day courses of nelarabine incorporated into ABFM. Patients with induction failure were nonrandomly assigned to ABFM C-MTX plus nelarabine. No patients received prophylactic cranial radiation; however, patients with CNS3 disease (CSF WBC ≥ 5/μL with blasts or cranial nerve palsies, brain/eye involvement, or hypothalamic syndrome) were ineligible.
RESULTS
At end-induction, 98.8% of evaluable participants had at least a PR. The 4-year event-free survival (EFS) and overall survival (OS) were 84.7% ± 2.3% and 89.0% ± 2.0%. The 4-year disease-free survival (DFS) from end-induction was 85.9% ± 2.6%. There was no difference in DFS observed between the HR and standard-risk groups (P = .29) or by treatment regimen (P = .55). Disease stage, tumor response, and MDD at diagnosis did not demonstrate thresholds that resulted in differences in EFS. Nelarabine did not show an advantage for HR patients. CNS relapse occurred in only 4 patients.
CONCLUSION
COG AALL0434 produced excellent outcomes in one of the largest trials ever conducted for patients with newly diagnosed T-LL. The COG ABFM regimen with C-MTX provided excellent EFS and OS without cranial radiation.
INTRODUCTION
Lymphoblastic lymphoma makes up approximately 20% of childhood non-Hodgkin lymphoma. More than 80% of patients have a precursor T-cell immunophenotype (T-cell lymphoblastic lymphoma, T-LL).1 Identifying prognostic factors has been challenging, because age, sex, race, or cytogenetic abnormalities have not been found to be prognostically significant.2-7 However, modern treatments have resulted in event-free survival (EFS) spanning 80%-90% for high-stage patients (III-IV).8-12 Salvage rates are dismal for relapsed patients.13
CONTEXT
Key Objectives
Children, adolescents, and young adults with newly diagnosed T-cell lymphoblastic lymphoma (T-LL) were enrolled in AALL0434 to evaluate the Children’s Oncology Group augmented Berlin-Frankfurt-Muenster regimen using Capizzi-style methotrexate (MTX) plus pegaspargase rather than high-dose MTX. A high-risk T-LL subpopulation was also randomly assigned to receive the regimen with or without nelarabine.
Knowledge Generated
Using this regimen, which did not require cranial radiation, the overall survival and disease-free survival rates were comparable or superior to expected. Furthermore, the treatment-related mortality rate was low, and no patient developed a secondary malignancy. High-risk patients had comparable outcomes to standard-risk patients regardless of the use of nelarabine, suggesting that pegaspargase contained within Capizzi-style MTX was an important component of the therapy.
Relevance
This regimen can therefore provide the backbone to build future therapies for T-LL to further improve outcomes while minimizing treatment-related sequelae.
Prior studies have reported that the amount of minimally detectable disease (MDD) in the bone marrow measured by flow cytometry at diagnosis is prognostically important.7,14 Our previous trial (A5971; ClinicalTrials.gov identifier: NCT00004228) found that patients with ≥ 1% MDD had an inferior outcome compared with patients with < 1% MDD.
Nelarabine is a water-soluble prodrug of ara-G, a synthetic deoxyguanosine derivative that is resistant to cleavage by endogenous purine nucleoside phosphorylase and is cytotoxic to T-lymphoblasts.15 Initial studies have demonstrated that it is highly active in T-cell leukemia/lymphoma and can be combined safely with chemotherapy in newly diagnosed pediatric T-cell acute lymphoblastic leukemia (T-ALL).16-18 We therefore sought to explore whether the addition of nelarabine could improve outcomes in a high-risk (HR) T-LL group.
Recent studies have demonstrated that leukemia-based therapy is an effective strategy in the treatment of T-LL.8-10,12 Two different methotrexate (MTX) intensification strategies are used commonly: high-dose MTX with leucovorin rescue (HD-MTX), and Capizzi-style MTX, escalating intravenous MTX without leucovorin rescue plus pegaspargase (C-MTX).15,19 Recent T-LL trials have used modifications of ALL-BFM-90 with HD-MTX instead of C-MTX, which typically results in 2 fewer doses of pegaspargase.
Children’s Oncology Group (COG) AALL0434 was a phase III trial developed for children, adolescents, and young adults 1-30.99 years old with T-ALL. The trial featured a 2 × 2 pseudo-factorial randomization for patients with T-ALL using the COG augmented Berlin-Frankfurt-Muenster (ABFM) regimen comparing C-MTX versus HD-MTX in patients with intermediate and high-risk T-ALL also randomly assigned to receive or not receive nelarabine.15,19,20 Results of the T-ALL population showed that C-MTX had a superior disease-free survival (DFS) and overall survival (OS) to HD-MTX.20,21 The addition of nelarabine further improved the DFS.21 The previous COG T-LL study (A5971) failed to demonstrate that HD-MTX improved the outcome for these patients. Furthermore, the Pediatric Oncology Group demonstrated that l-asparaginase was important in T-cell malignancies,22 Thus, we wished to examine whether the C-MTX ABFM regimen, which includes a total of 7 doses of pegaspargase, would be efficacious for pediatric T-LL, compared with 5 doses of pegaspargase for HD-MTX.
PATIENTS AND METHODS
Patient Population
Enrollment of patients with T-ALL began in January 2007, with the addition of T-LL enrollment in September 2010. Study accrual was completed in July 2014. All patients fulfilled the diagnosis for T-LL using institutional standards on the basis of WHO criteria. The diagnosis was confirmed by central review (S.L.P., R.R.M.). Patients with Murphy stage II-IV disease were eligible. Subjects with Down syndrome were ineligible; patients found to have the Philadelphia chromosome were not eligible for postinduction therapy in this study. Risk assignment was not based on cytogenetics,2-4 genomic alterations,5,6 or the early T-precursor phenotype.7
MDD status was achieved using flow cytometry of bone marrow specimens obtained at diagnosis, analyzed at the University of Washington (B.L.W.) using established methodologies.23 Before receiving systemic therapy, CSF examination established the CNS disease status; CNS1 (no blasts in the CSF), CNS2 (CSF WBC < 5/μL with blasts), and CNS3 (CSF WBC ≥ 5/μL with blasts or cranial nerve palsies, brain/eye involvement, or hypothalamic syndrome).19,24 Patients with T-LL with CNS3 disease or gross involvement of the testes were ineligible to participate. Our previous study, A5971, only had 12 patients of 266 with CNS disease at presentation and no patients with testicular disease. Given the low number of expected patients, the patients were not eligible for enrollment.
AALL0434 was approved by the National Cancer Institute, Cancer Therapy Evaluation Program, US Food and Drug Administration, and Pediatric Central Institutional Review Board (IRB), and by IRBs at each participating center. In accordance with the Declaration of Helsinki, informed consent/assent was obtained before study entry.
Study Design
The treatment assignments for participants with T-LL in AALL0434 were based on their risk status established at diagnosis (Data Supplement). High-risk (HR) patients were defined as patients with ≥ 1% MDD in the bone marrow or who had received steroid pretreatment for > 48 hours before diagnosis, potentially masking the extent of bone marrow disease. All others were designated standard risk (SR).
All SR patients with T-LL received the COG ABFM regimen, arm A. Patients assigned to the HR category were randomly assigned to receive arm A or the same regimen with 6 5-day courses of nelarabine (arm B).20 The study used 2 consents, one for induction and a second for the postinduction therapy, when the HR patients were randomly assigned their treatment (Appendix Table A1, online only).15
Arm A began with a 28-day, prednisone-based, 4-drug induction, followed by an ABFM consolidation phase (Table A1). This was followed by an 8-week interim maintenance (IM) phase, where patients received C-MTX with escalating doses of intravenous MTX without leucovorin plus 2 doses of pegaspargase, vincristine (5 doses), and intrathecal MTX (2 doses). After completion of IM, patients received a single delayed intensification (DI) phase. Details of pegaspargase dosing were not captured for these patients. All patients then received maintenance therapy for 2 years after the start of IM, (Table A1). No patient received cranial radiation therapy (CRT).
Patients assigned to arm B received therapy identical to arm A, with the addition of nelarabine. Nelarabine was administered as 5-day courses (consolidation, days 1-5 and 29-33, in DI, days 29-33, and 3 courses in maintenance on days 29-33 of the first 3 cycles; Table A1).
Treatment-related adverse events were graded using Common Terminology Criteria for Adverse Events version 4. Toxicities associated with nelarabine, including CNS toxicity, peripheral neuropathy, and rhabdomyolysis, were monitored with immediate notification of the study chair.
Disease Evaluation
Disease evaluations were performed at the end of induction, consolidation, and at the end of therapy, using the radiologic imaging modalities to stage the disease at diagnosis. Additional radiologic monitoring was not anticipated to affect the ultimate number of patients with progressive disease and was not required. Responses were determined by the treating institution, and nuclear imaging was not required for evaluation. The following were used to classify responses at the end of the 4-week induction:
Complete response (CR): disappearance of all evidence of disease.
Complete response unconfirmed (CRu): a lymph node mass > 1.5 cm that regressed by > 75% in sum of the products of the greatest perpendicular diameters (SPD), or any lesions that had decreased by > 75%.
Partial response (PR): a > 50% decrease in the SPD of disease and no new lesions.
No response (NR): failure to qualify for a PR and no new lesions.
Progressive disease (PrD): > 25% increase in the SPD or appearance of new lesion(s) with the first measurement at the end of induction.
Induction failure (IF) was defined as NR or PrD at the day-29 evaluation.
Patients deemed to have NR were nonrandomly assigned to arm B (C-MTX plus nelarabine) to begin consolidation therapy as soon as possible without waiting until day 36 or count recovery. Patients with PrD after induction were removed from protocol therapy. Evaluations of persistent masses did not require additional imaging. Relapse was defined as any recurrence of disease.
Statistical Analysis
EFS was defined as time from study enrollment (first consent) to first event (IF, induction death, relapse, second malignant neoplasm, remission death) or date of last contact for those who were event free. DFS was defined as time from postinduction random assignment (second consent) to first event or date of last contact for those who were disease-free. OS was defined as time from study enrollment to death or date of last contact for those who were alive. OS for the randomly assigned cohorts was defined as time from postinduction random assignment to death or date of last of contact for those who were alive.
The patients with T-LL were stratified and analyzed separately from analyses for the patients with ALL. As expected when the study was amended to include the patients with T-LL, there was insufficient power for any formal comparison of outcomes between randomized regimens (± nelarabine). Outcomes of the randomly assigned cohort are descriptive in nature only.
Data current as of June 30th, 2018 are included in this report. Survival rates were estimated by using the method of Kaplan-Meier with standard errors of Peto et al.25,26 Survival rates and hazard ratios are presented as number (95% CI). Two-sided log-rank tests were used for comparison of survival curves. Proportions were compared between groups using a χ2 test or Fisher’s exact test. A P < .05 was considered significant for all comparisons. All analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC). Graphics were generated with R Version 2.13.1.27
RESULTS
Participants
AALL0434 enrolled 299 patients with T-LL (2010-2014). Seventeen were subsequently deemed ineligible/unevaluable, leaving 282 patients who were evaluable for induction (Appendix Table A2, online only), including 95 SR and 180 HR patients, 3 patients with IF, and 4 unknowns (Fig 1). Of these, 82 SR patients, 121 HR patients and 2 patients defined as IF continued on postinduction therapy. Reasons for the 77 patients coming off protocol therapy at the end of induction are summarized in Table A3 (online only). The design of the study required a second consent at the end of induction, which included the random assignment of the HR patients to nelarabine. Sixty-one percent of these 77 patients refused additional protocol therapy.
FIG 1.
CONSORT diagram. Arm A, Capizzi-based methotrexate/pegaspargase, Arm B: Capizzi-based methotrexate/pegaspargase plus nelarabine; T-LL, T-cell non-Hodgkin lymphoma.
Table 1 summarizes characteristics of the patients who continued postinduction therapy on protocol by risk-group assignment. Staging information was not submitted for 77 patients, and stage was given as indeterminate for 11 patients. Fifty-seven (47%) of the HR patients were classified as HR solely because of prior steroid exposure, despite having an MDD at diagnosis of < 1% in the bone marrow. Twenty-one (17%) of the HR patients who received steroids before enrollment still had an MDD ≥ 1%, and 38 (31%) who did not receive steroid pretreatment were HR because they had MDD ≥ 1%.
TABLE 1.
Patient Characteristics Receiving Postinduction Therapy
Outcomes Defined by Risk Groups and Early Response
At the end of induction (EOI), 98.8% of all eligible, evaluable patients achieved at least a partial response (30.8% CR, 34.0% CRu, 34.0% PR). Median follow-up was 4.9 years. The 4-year EFS and OS for the whole cohort (including those who did not continue protocol therapy postinduction) was 84.7% ± 2.3% and 89.0% ± 2.0%, respectively (Fig 2A). Four-year DFS from the end of induction for all patients who continued with postinduction therapy in the study was 85.9 ± 2.6% (Fig 2B). The 2 patients with T-LL IF assigned to C-MTX plus nelarabine completed therapy and were event free.
FIG 2.

(A) Event-free survival (EFS) and overall survival (OS) curves. Four-year EFS and OS for all patients with T-cell non-Hodgkin lymphoma were 84.7% ± 2.3% and 89.0% ± 2.0% (n = 282), respectively. (B) Overall disease-free survival (DFS) from the end of induction; 4-year DFS was 85.9% ± 2.6% (n = 203).
There were 10 events within the SR group and 23 events in the HR group (11 in arm A and 12 in arm B; Table A4, online only). There were 3 remission deaths in the SR group and 2 in the HR group (1 in each arm). Progressive disease after induction occurred in 5 patients; 3 of these patients had a PR and 2 had CRu at the EOI. Relapse occurred in 5 SR and 17 HR patients (7 in arm A and 10 in arm B). Four patients had CNS relapses. Four-year DFS was 85.0% ± 3.4% for SR patients compared with 87.4% ± 4.0% for HR patients (P = .2866; Fig 3A). There was no significant difference in DFS when comparing SR versus HR arm A vs HR arm B cohorts (Appendix Fig A1, online only). Furthermore, patients with MDD levels in the bone marrow at diagnosis of < 1% had an EFS 82.4% ± 3.1% compared with 89.5% ± 3.3% for those with an MDD ≥ 1% (P = .3084; Fig 3B).
FIG 3.

(A) Disease-free survival (DFS) for high-risk versus standard-risk groups; 4-year DFS was 85.0% ± 3.4% (n = 121) versus 87.4% ± 4.0% (n = 82; P = .2866). (B) Event-free survival (EFS) for minimal detectable disease (MDD) < 1% versus MDD ≥ 1% detected in the bone marrow at diagnosis: 4-year EFS, 82.4% ± 3.1% (n = 176) versus 89.5% ± 3.3% (n = 97; P = .3084). T-LL, T-cell non-Hodgkin lymphoma.
EFS for different ages (patients < 10 years, 82.6% ± 3.6%; 10-16 years, 86.1% ± 3.5%, v > 16 years old, 86.4% ± 5.2%; P = .4360), stages (stage II, 88.9% ± 7.7%; III, 87.6% ± 2.9%; IV, 95.4% ± 3.3%; P = .197), or responses (CR, 92.2% ± 3.3%; CRu, 84.4% ± 4.1%; PR, 81.0% ± 4.5%; P = .217) were not significantly different (Appendix Fig A2, online only).
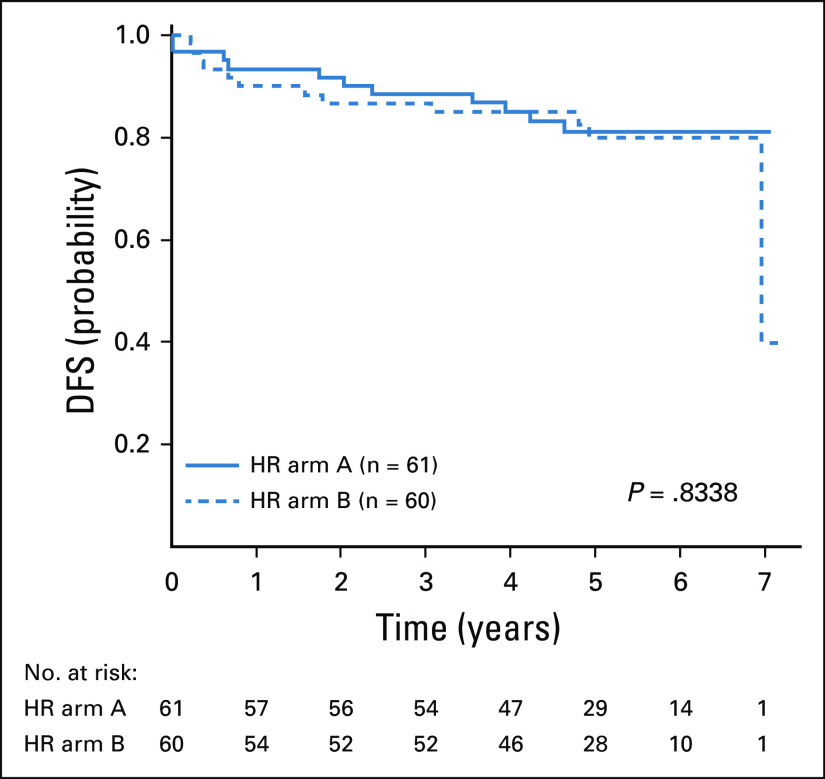
Comparison of the 2 HR treatment arms did not demonstrate a significant difference in DFS (ie, C-MTX, 85.1% ± 4.8%; and C-MTX plus nelarabine, 85.0% ± 4.9%; P = .834; Fig 4). There was also no difference in DFS (82.7% ± 6.3%, 83.3% ± 6.0%) or OS (87.8% ± 5.3%, 85.7% ± 5.6%) when comparing the C-MTX and C-MTX plus nelarabine arms for those patients with prior steroid exposure (P = .954 and P = .761), respectively. Finally, DFS comparison of MDD < 1% versus ≥ 1% for the subset of HR patients also was not significantly different (Appendix Fig A3, online only). Thus, HR assignment because of steroid exposure or MDD ≥ 1 had comparable DFS and OS outcomes.
FIG 4.
Disease-free survival (DFS) for high-risk (HR) patients by randomly assigned arm: no nelarabine (arm A) versus nelarabine (arm B); 4-year DFS was 85.1% ± 4.8% (n = 61) versus 85.0% ± 4.9% (n = 60; P = .8338), respectively.
Toxicities
There were only 5 nonrelapse deaths. In the SR group there were 3 deaths (fungal infection during consolidation [n = 1]; unknown causes during DI [n = 1]; hemophagocytic lymphohistiocytosis [n = 1]). In the HR group there were 2 deaths (arm-A patient from pancreatitis during maintenance therapy [n = 1]; arm-B patient from cerebral edema attributed to pegaspargase during DI, 34 days from the last dose of nelarabine [n = 1]). One benign tumor was observed with no other secondary malignancies.
Targeted neurotoxicity reporting was performed because of prior experience with nelarabine.17,28,29 There was no significant difference in grade 1-4 CNS toxicity in HR patients randomly assigned to either C-MTX or CMTX plus nelarabine (P = .06; Table 2). However, for peripheral motor and sensory neuropathy, patients receiving nelarabine had a significantly higher rate of grade 1-3 toxicity, (P = .03 and .005). Neither group experienced grade 4 sensory toxicity, but 3 patients in arm A and 5 patients in arm B experienced grade 3 toxicity. There were no differences in either peripheral motor or sensory neuropathy within age groups. (Appendix Tables A5 and A6).
TABLE 2.
Summary of Toxicities

No other nelarabine-associated toxicities were observed in patients with T-LL, including rhabdomyolysis. There were no significant differences in infection risk between the patients who received nelarabine versus those who did not (P = .857).
DISCUSSION
AALL0434 assembled one of the largest prospective studies for pediatric patients with T-LL and provided excellent DFS and OS outcomes. The use of C-MTX is in contrast to recent studies that have used HD-MTX and CRT, which are both associated with significant toxicity.30-35
Nelarabine failed to demonstrate a difference in outcome despite the reported success in T-ALL but was not powered to detect a benefit. However, given previous reports of activity in T-LL with nelarabine, it is likely that this agent is active. The addition of nelarabine into this leukemia protocol backbone for T-LL was safe, with only a modest increase in peripheral sensory neuropathy. Given the acceptable toxicity profile, and the compelling evidence that nelarabine is active in T-ALL, using nelarabine for future trials should be considered, particularly if larger studies could clarify its role.
Risk categories were assigned based on the MDD at diagnosis, which was shown to be associated with a worse outcome in COG A5971.7 Despite these previous findings, there was no difference in outcome when comparing the HR to SR subjects who were assigned the same C-MTX therapy. This finding is also in contrast to other published reports that MDD is of prognostic importance.14 Hence, it appears that the C-MTX ABFM therapy may have negated the prognostic impact of MDD. In A5971, MDD was assessed using a BFM backbone containing HD-MTX. Although 2 different preparations were used for each trial (l-asparaginase for A5971 and pegaspargase for ALL0434), the asparaginase exposure of C-MTX in AALL0434 was approximately 30% greater than the HD-MTX in A5971.7,36 Given the superior outcome of patients with T-ALL receiving C-MTX compared with HD-MTX on AALL0434,20 C-MTX may have improved the outcome of the HR T-LL population.
Risk factors correlating with recurrence in T-LL have been difficult to identify.14,37-39 This problem is especially important, given the dismal outcome of relapsed patients.13,40 We were unable to identify any significant differences in outcome when examining variables previously associated with inferior prognosis. Thus, continued efforts are needed to identify new prognostic factors in T-LL that can be used in future trials evaluating novel agents.
Overall, this study represents one of the largest prospective trials for the treatment of newly diagnosed pediatric T-LL with outcomes that are either comparable or superior to other trials for this disease.9,12,36-38,41-43 Previous trials with higher EFS have included cranial radiation for high-stage patients irrespective of their CNS status.8 Cranial radiation is associated with a panoply of long-term toxicities in childhood cancer survivors.44-46 This trial demonstrated that the COG ABFM C-MTX regimen can achieve a low CNS recurrence rate (1.97%) without prophylactic CRT. However, because patients with CNS3 with T-LL were excluded from this trial, we cannot comment on its role for these patients. Despite this limitation, the observed toxicities of this treatment regimen were relatively low, with 1 benign tumor to date and a nonrelapse mortality rate of only 1.8%. The outstanding outcomes achieved from this trial provide the basis on which future therapies can be built.
ACKNOWLEDGMENT
We thank the Cancer Therapeutic Evaluation Program for its support of AALL0434, the study’s Data and Safety Monitoring Committee, and the Pediatric Central Institutional Review Board. We also thank all participating Children’s Oncology Group investigators and the subjects and their families for participating in the study; this report would not have been possible without them.
Appendix
FIG A1.
Disease-free survival (DFS) comparison of standard-risk (SR) versus high-risk (HR) arm A versus HR arm B.
FIG A2.
Event-free survival (EFS) comparing age groups, < 10 years (n = 123), 10-16 years (n = 104), ≥ 16 years (n = 55; P = .4360).
FIG A3.
Disease-free survival (DFS) comparison of minimal detectable disease (MDD) < 1% versus ≥ 1% for the subset of high-risk (HR) patients is given below.
TABLE A1.
Two-Stage Consenting Process and Details of Therapies
TABLE A2.
Patients Ineligible or Inevaluable for Induction Therapy
TABLE A3.
Reasons for Removal From Protocol at the End of Induction
TABLE A4.
T-LL Disease-Free Survival Events for Standard and High Risk

TABLE A5.
Peripheral Motor Neuropathy

TABLE A6.
Peripheral Sensory Neuropathy

PRIOR PRESENTATION
Presented in part at the 61st American Society of Hematology Annual Meeting, San Diego, CA, December 7-10, 2018.
SUPPORT
Supported by National Institutes of Health Grants No. U10 CA98543, U10 CA98413, U10 CA180886, 1U24-CA196173, and U10 CA180899; the St. Baldrick’s Foundation; M.L.L. is the Benioff Chair of Children’s Health and the Deborah and Arthur Ablin Endowed Chair for Pediatric Molecular Oncology at Benioff Children’s Hospital. E.A.R. is a KiDS of NYU Foundation Professor at NYU Langone Health. S.P.H. is the Jeffrey E. Perelman Distinguished Chair in Pediatrics at The Children’s Hospital of Philadelphia.
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Robert J. Hayashi, Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Sherrie L. Perkins, Elizabeth A. Raetz, Naomi J. Winick, William L. Carroll, Stephen P. Hunger, Catherine M. Bollard
Administrative support: Megan S. Lim
Provision of study material or patients: Stuart S. Winter, William L. Carroll
Collection and assembly of data: Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Zhiguo Chen, Brent L. Wood, David T. Teachey, Sherrie L. Perkins, Rodney R. Miles
Data analysis and interpretation: Robert J. Hayashi, Stuart S. Winter, Kimberly P. Dunsmore, Meenakshi Devidas, Zhiguo Chen, Brent L. Wood, Michelle L. Hermiston, David T. Teachey, Sherrie L. Perkins, Mignon L. Loh, Naomi J. Winick, William L. Carroll, Stephen P. Hunger, Megan S. Lim, Thomas G. Gross, Catherine M. Bollard
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Successful Outcomes of Newly Diagnosed T Lymphoblastic Lymphoma: Results From Children’s Oncology Group AALL0434
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert J. Hayashi
Consulting or Advisory Role: Magenta Therapeutics
Stuart D. Winter
Honoraria: Jazz Pharmaceuticals
Consulting or Advisory Role: Jazz Pharmaceuticals
Travel, Accommodations, Expenses: Jazz Pharmaceuticals
Kimberly P. Dunsmore
Employment: Dexcom (I)
Stock and Other Ownership Interests: Dexcom (I)
Travel, Accommodations, Expenses: Dexcom (I)
Meenakshi Devidas
Honoraria: PSI, Novartis
Brent L. Wood
Honoraria: Amgen, Seattle Genetics, AbbVie, Janssen, Amgen, Astellas Pharma
Consulting or Advisory Role: Sysmex
Research Funding: Amgen (Inst), Seattle Genetics (Inst), Pfizer (Inst), Juno Therapeutics (Inst), BiolineRx (Inst), Biosight (Inst), Stemline Therapeutics (Inst), Janssen Oncology (Inst), Novartis
Travel, Accommodations, Expenses: Amgen
Michelle L. Hermiston
Consulting or Advisory Role: Novartis, Sobi
Patents, Royalties, Other Intellectual Property: Spouse has patents pending for platform technology with application to oncology, diagnostics, and anti-infections, and for anti-bleeding technology (I)
David T. Teachey
Consulting or Advisory Role: Janssen
Research Funding: Novartis (Inst)
Elizabeth A. Raetz
Research Funding: Pfizer (Inst)
Other Relationship: Celgene
Mignon L. Loh
Consulting or Advisory Role: MediSix Therapeutics
William L. Carroll
Other Relationship: Amgen
Stephen Hunger
Stock and Other Ownership Interests: Amgen, Merck (I), Amgen (I), Pfizer (I)
Honoraria: Amgen
Consulting or Advisory Role: Novartis
Megan S. Lim
Consulting or Advisory Role: Seattle Genetics, EUSA Pharma
Travel, Accommodations, Expenses: Seattle Genetics, EUSA Pharma
Catherine M. Bollard
Leadership: Mana Therapeutics, Cabaletta Bio, Catamaran Bio
Stock and Other Ownership Interests: Mana Therapeutics, Neximmune, Torque, Caballeta Bio
Consulting or Advisory Role: Torque, NexImmune, Cellectis, Cabaletta Bio
Patents, Royalties, Other Intellectual Property: TAA-specific T cells and HIV specific T cells
Open Payments Link: https://openpaymentsdata.cms.gov/physician/381202
No other potential conflicts of interest were reported.
REFERENCES
- 1.Minard-Colin V, Brugières L, Reiter A, et al. Non-Hodgkin lymphoma in children and adolescents: Progress through effective collaboration, current knowledge, and challenges ahead. J Clin Oncol. 2015;33:2963–2974. doi: 10.1200/JCO.2014.59.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heerema NA, Sather HN, Sensel MG, et al. Frequency and clinical significance of cytogenetic abnormalities in pediatric T-lineage acute lymphoblastic leukemia: A report from the Children’s Cancer Group. J Clin Oncol. 1998;16:1270–1278. doi: 10.1200/JCO.1998.16.4.1270. [DOI] [PubMed] [Google Scholar]
- 3.Lones MA, Heerema NA, Le Beau MM, et al. Chromosome abnormalities in advanced stage lymphoblastic lymphoma of children and adolescents: A report from CCG-E08. Cancer Genet Cytogenet. 2007;172:1–11. doi: 10.1016/j.cancergencyto.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 4.Balbach ST, Makarova O, Bonn BR, et al. Proposal of a genetic classifier for risk group stratification in pediatric T-cell lymphoblastic lymphoma reveals differences from adult T-cell lymphoblastic leukemia. Leukemia. 2016;30:970–973. doi: 10.1038/leu.2015.203. [DOI] [PubMed] [Google Scholar]
- 5.Callens C, Baleydier F, Lengline E, et al. Clinical impact of NOTCH1 and/or FBXW7 mutations, FLASH deletion, and TCR status in pediatric T-cell lymphoblastic lymphoma. J Clin Oncol. 2012;30:1966–1973. doi: 10.1200/JCO.2011.39.7661. [DOI] [PubMed] [Google Scholar]
- 6.Bonn BR, Rohde M, Zimmermann M, et al. Incidence and prognostic relevance of genetic variations in T-cell lymphoblastic lymphoma in childhood and adolescence. Blood. 2013;121:3153–3160. doi: 10.1182/blood-2012-12-474148. [DOI] [PubMed] [Google Scholar]
- 7.Coustan-Smith E, Sandlund JT, Perkins SL, et al. Minimal disseminated disease in childhood T-cell lymphoblastic lymphoma: A report from the children’s oncology group. J Clin Oncol. 2009;27:3533–3539. doi: 10.1200/JCO.2008.21.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reiter A, Schrappe M, Ludwig WD, et al. Intensive ALL-type therapy without local radiotherapy provides a 90% event-free survival for children with T-cell lymphoblastic lymphoma: A BFM group report. Blood. 2000;95:416–421. [PubMed] [Google Scholar]
- 9.Uyttebroeck A, Suciu S, Laureys G, et al. Treatment of childhood T-cell lymphoblastic lymphoma according to the strategy for acute lymphoblastic leukaemia, without radiotherapy: Long term results of the EORTC CLG 58881 trial. Eur J Cancer. 2008;44:840–846. doi: 10.1016/j.ejca.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Sun XF, Xia ZJ, Zhen ZJ, et al. Intensive chemotherapy improved treatment outcome for Chinese children and adolescents with lymphoblastic lymphoma. Int J Clin Oncol. 2008;13:436–441. doi: 10.1007/s10147-008-0771-5. [DOI] [PubMed] [Google Scholar]
- 11.Burkhardt B, Woessmann W, Zimmermann M, et al. Impact of cranial radiotherapy on central nervous system prophylaxis in children and adolescents with central nervous system-negative stage III or IV lymphoblastic lymphoma. J Clin Oncol. 2006;24:491–499. doi: 10.1200/JCO.2005.02.2707. [DOI] [PubMed] [Google Scholar]
- 12.Sandlund JT, Pui CH, Zhou Y, et al. Effective treatment of advanced-stage childhood lymphoblastic lymphoma without prophylactic cranial irradiation: Results of St Jude NHL13 study. Leukemia. 2009;23:1127–1130. doi: 10.1038/leu.2008.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burkhardt B, Reiter A, Landmann E, et al. Poor outcome for children and adolescents with progressive disease or relapse of lymphoblastic lymphoma: A report from the Berlin-Frankfurt-Muenster group. J Clin Oncol. 2009;27:3363–3369. doi: 10.1200/JCO.2008.19.3367. [DOI] [PubMed] [Google Scholar]
- 14.Mussolin L, Buldini B, Lovisa F, et al. Detection and role of minimal disseminated disease in children with lymphoblastic lymphoma: The AIEOP experience. Pediatr Blood Cancer. 2015;62:1906–1913. doi: 10.1002/pbc.25607. [DOI] [PubMed] [Google Scholar]
- 15.Winter SS, Dunsmore KP, Devidas M, et al. Safe integration of nelarabine into intensive chemotherapy in newly diagnosed T-cell acute lymphoblastic leukemia: Children’s Oncology Group Study AALL0434. Pediatr Blood Cancer. 2015;62:1176–1183. doi: 10.1002/pbc.25470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunsmore KP, Devidas M, Linda SB, et al. Pilot study of nelarabine in combination with intensive chemotherapy in high-risk T-cell acute lymphoblastic leukemia: A report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2753–2759. doi: 10.1200/JCO.2011.40.8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zwaan CM, Kowalczyk J, Schmitt C, et al. Safety and efficacy of nelarabine in children and young adults with relapsed or refractory T-lineage acute lymphoblastic leukaemia or T-lineage lymphoblastic lymphoma: Results of a phase 4 study. Br J Haematol. 2017;179:284–293. doi: 10.1111/bjh.14874. [DOI] [PubMed] [Google Scholar]
- 18.Hefazi M, Litzow MR. Recent advances in the biology and treatment of T cell acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13:265–274. doi: 10.1007/s11899-018-0455-9. [DOI] [PubMed] [Google Scholar]
- 19.Larsen EC, Devidas M, Chen S, et al. Dexamethasone and high-dose methotrexate improve outcome for children and young adults with high-risk B-acute lymphoblastic leukemia: A report from Children’s Oncology Group study AALL0232. J Clin Oncol. 2016;34:2380–2388. doi: 10.1200/JCO.2015.62.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winter SS, Dunsmore KP, Devidas M, et al. Improved survival for children and young adults with T-lineage acute lymphoblastic leukemia: Results from the Children’s Oncology Group AALL0434 methotrexate randomization. J Clin Oncol. 2018;36:2926–2934. doi: 10.1200/JCO.2018.77.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunsmore KP, Winter S, Devidas M, et al. COG AALL0434: A randomized trial testing nelarabine in newly diagnosed t-cell malignancy. J Clin Oncol. 2018;36(15_suppl; abstr 10500) doi: 10.1200/JCO.20.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amylon MD, Shuster J, Pullen J, et al. Intensive high-dose asparaginase consolidation improves survival for pediatric patients with T cell acute lymphoblastic leukemia and advanced stage lymphoblastic lymphoma: A Pediatric Oncology Group study. Leukemia. 1999;13:335–342. doi: 10.1038/sj.leu.2401310. [DOI] [PubMed] [Google Scholar]
- 23.Wood BL. Flow cytometric monitoring of residual disease in acute leukemia. Methods Mol Biol. 2013;999:123–136. doi: 10.1007/978-1-62703-357-2_8. [DOI] [PubMed] [Google Scholar]
- 24.Steinherz PG. CNS leukemia: Problem of diagnosis, treatment, and outcome. J Clin Oncol. 1995;13:310–313. doi: 10.1200/JCO.1995.13.2.310. [DOI] [PubMed] [Google Scholar]
- 25.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 26.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35:1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. R Foundation: The R project for statistical computing. http://www.r-project.org.
- 28.Kuhlen M, Bleckmann K, Möricke A, et al. Neurotoxic side effects in children with refractory or relapsed T-cell malignancies treated with nelarabine based therapy. Br J Haematol. 2017;179:272–283. doi: 10.1111/bjh.14877. [DOI] [PubMed] [Google Scholar]
- 29.Kawakami M, Taniguchi K, Yoshihara S, et al. Irreversible neurological defects in the lower extremities after haploidentical stem cell transplantation: Possible association with nelarabine. Am J Hematol. 2013;88:853–857. doi: 10.1002/ajh.23502. [DOI] [PubMed] [Google Scholar]
- 30.Chow EJ, Liu W, Srivastava K, et al. Differential effects of radiotherapy on growth and endocrine function among acute leukemia survivors: A childhood cancer survivor study report. Pediatr Blood Cancer. 2013;60:110–115. doi: 10.1002/pbc.24198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schuitema I, de Sonneville L, Kaspers G, et al. Executive dysfunction 25 years after treatment with cranial radiotherapy for pediatric lymphoid malignancies. J Int Neuropsychol Soc. 2015;21:657–669. doi: 10.1017/S1355617715000788. [DOI] [PubMed] [Google Scholar]
- 32.Green DM, Zhu L, Wang M, et al. Effect of cranial irradiation on sperm concentration of adult survivors of childhood acute lymphoblastic leukemia: A report from the St. Jude Lifetime Cohort Study. Hum Reprod. 2017;32:1192–1201. doi: 10.1093/humrep/dex082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schmiegelow K. Advances in individual prediction of methotrexate toxicity: A review. Br J Haematol. 2009;146:489–503. doi: 10.1111/j.1365-2141.2009.07765.x. [DOI] [PubMed] [Google Scholar]
- 34.Bhojwani D, Sabin ND, Pei D, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014;32:949–959. doi: 10.1200/JCO.2013.53.0808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard SC, McCormick J, Pui CH, et al. Preventing and managing toxicities of high-dose methotrexate. Oncologist. 2016;21:1471–1482. doi: 10.1634/theoncologist.2015-0164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abromowitch M, Sposto R, Perkins S, et al. Shortened intensified multi-agent chemotherapy and non-cross resistant maintenance therapy for advanced lymphoblastic lymphoma in children and adolescents: Report from the Children’s Oncology Group. Br J Haematol. 2008;143:261–267. doi: 10.1111/j.1365-2141.2008.07320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Landmann E, Burkhardt B, Zimmermann M, et al. Results and conclusions of the European Intergroup EURO-LB02 trial in children and adolescents with lymphoblastic lymphoma. Haematologica. 2017;102:2086–2096. doi: 10.3324/haematol.2015.139162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillon M, Aricò M, Mussolin L, et al. Long-term results of the AIEOP LNH-97 protocol for childhood lymphoblastic lymphoma. Pediatr Blood Cancer. 2015;62:1388–1394. doi: 10.1002/pbc.25469. [DOI] [PubMed] [Google Scholar]
- 39.Sunami S, Sekimizu M, Takimoto T, et al. Prognostic impact of intensified maintenance therapy on children with advanced lymphoblastic lymphoma: A report from the Japanese Pediatric Leukemia/Lymphoma Study Group ALB-NHL03 study. Pediatr Blood Cancer. 2016;63:451–457. doi: 10.1002/pbc.25824. [DOI] [PubMed] [Google Scholar]
- 40.Mitsui T, Mori T, Fujita N, et al. Retrospective analysis of relapsed or primary refractory childhood lymphoblastic lymphoma in Japan. Pediatr Blood Cancer. 2009;52:591–595. doi: 10.1002/pbc.21941. [DOI] [PubMed] [Google Scholar]
- 41.Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: A randomized study by the Children’s Oncology Group (POG 9404) Blood. 2011;118:874–883. doi: 10.1182/blood-2010-06-292615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gao Y-J, Pan C, Tang J-Y, et al. Clinical outcome of childhood lymphoblastic lymphoma in Shanghai China 2001-2010. Pediatr Blood Cancer. 2014;61:659–663. doi: 10.1002/pbc.24848. [DOI] [PubMed] [Google Scholar]
- 43.Bergeron C, Coze C, Segura C, et al. Treatment of childhood T-cell lymphoblastic lymphoma-long-term results of the SFOP LMT96 trial. Pediatr Blood Cancer. 2015;62:2150–2156. doi: 10.1002/pbc.25699. [DOI] [PubMed] [Google Scholar]
- 44.Mostoufi-Moab S, Seidel K, Leisenring WM, et al. Endocrine abnormalities in aging survivors of childhood cancer: A report from the Childhood Cancer Survivor Study. J Clin Oncol. 2016;34:3240–3247. doi: 10.1200/JCO.2016.66.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mueller S, Sear K, Hills NK, et al. Risk of first and recurrent stroke in childhood cancer survivors treated with cranial and cervical radiation therapy. Int J Radiat Oncol Biol Phys. 2013;86:643–648. doi: 10.1016/j.ijrobp.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krull KR, Zhang N, Santucci A, et al. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood. 2013;122:550–553. doi: 10.1182/blood-2013-03-487744. [DOI] [PMC free article] [PubMed] [Google Scholar]