Abstract
In this study, adult intact male and female (n = 10) naked mole rats (Heterocephalus glaber) were anesthetized by using a combination of ketamine (20 mg/kg IM), and alfaxalone (4.0 mg/kg IM). Induction and recovery times were recorded. Vital parameters, including heart rate, respiratory rate, and reflexes, were monitored every 5 min during the anesthetic period. Anesthetic induction was smooth and rapid. Induction time was significantly longer in male rats (median, 325 s; range, 180 to 385 s) than in females (median, 145 s; range, 118 to 180 s). In addition, overall duration of loss of righting reflex was shorter in male mole rats (median, 50 min; range, 36 to 65 min) than females (median, 70 min; range, 60 to 85 min). Males largely had intact withdrawal reflexes, whereas females showed variable loss of both forelimb and hindlimb withdrawal reflexes. Neither recovery time (mean ± 1 SD, 16 ± 13 min) nor vital parameters differed between sexes. None of animals showed any anesthesia-related adverse responses. According to these findings, intramuscular AK is a safe and effective protocol that provides brief, light anesthesia in male naked mole rats and deeper anesthesia in females. We recommend adding analgesics when this AK protocol is used for pain-inducing or invasive procedures, and further studies evaluating higher doses and different combinations are indicated.
Abbreviations: K, ketamine; LPR, loss of palpebral reflex; LRR, loss of righting reflex; M, midazolam
Naked mole rats (Heterocephalus glaber) are members of the family Bathyergidae and are subterranean rodents native to subSaharan Africa. They live in colonies comprising as many as 300 animals and are eusocial, an unusual trait among mammals. In addition, naked mole rats possess several unique physiologic adaptations that have made them models in biomedical and behavioral research.2,15 Mole rats are used in aging research due to their remarkable longevity compared with other rodent species; in cancer resistance studies given their low prevalence of cancers; to study hypoxia resistance because of their physiologic adaptations (including a low basal metabolic rate, high hematocrit, and hemoglobin with high oxygen affinity) that enable them to survive in hypoxic and hypercapnic burrow environments; and in somatosensory research due to unique characteristics of pain perception.7,15,61 Naked mole rats are also used in behavioral studies due to their unusual social structure as well as to study endocrine and neuronal regulation of reproduction, given that mole rat colonies have a single breeding female and only a few breeding males.41,52
Chemical immobilization or general anesthesia is often necessary for examination or diagnostic procedures in mole rats.23 Inhalant anesthetic agents are commonly used in anesthetic protocols due to their fast induction and recovery properties.10,21 However, inhalant anesthesia administered by facemask or induction chamber can be an occupational health concern for staff due to increased exposure to waste gases not to mention stressful for patients.14,50,54,55,62 In addition, inhalant agents have been reported to cause respiratory irritation, increased salivation, lacrimation, and bronchosecretion as well as hypotension and hypothermia in rodent species.14 Furthermore, inhalant anesthesia may limit the ability to perform certain procedures or when necessary equipment is unavailable (for example, field settings). In such cases, injectable anesthetic protocols may be preferable or required.9,20,21
Given the unique physiologic adaptations of naked mole rats, especially their low basal metabolic rate, extrapolation of anesthetic doses reported for other rodent species of similar size may be unsafe and is not recommended.22 Previously reported injectable anesthesia protocols in mole rat species commonly include combinations of ketamine (15 to 100 mg/kg) and xylazine (0.6 to 8 mg/kg), with little information regarding anesthetic variables.2,13,23,29,30,44-46 Due to the small body size of naked mole rats, regimens that involve subcutaneous, intramuscular, or intraperitoneal administration might be practical.58 Appropriate combinations of injectable agents ideally involve short-acting anesthetics, with wide safety margins, and preferably are reversible.2,21,26,40 Therefore, additional studies regarding the safety and efficacy of injectable anesthetic regimens in naked mole rats are required.
Alfaxalone (3α-hydroxy-5α-pregnane-11, 20-dione) is used as an induction anesthesia agent that, although intended primarily for intravenous administration, can also be administered via the intramuscular and subcutaneous routes.3,12,14,47,57 Alfaxalone is a neuroactive steroid molecule that acts on GABA receptors, resulting in centrally mediated muscle relaxation and anesthesia.59 The anesthetic profile of alfaxalone resembles that of propofol, and the effects of alfaxalone may be potentiated when the drug is combined with other sedatives and analgesics.1,31,34,47 Addition of ketamine, a centrally acting N-methyl-D-aspartate receptor antagonist, may provide a deeper level of anesthesia and some analgesia and may permit more invasive procedures or result in a longer duration or better quality of immobilization, compared with alfaxalone only.8,42,53
Injectable combinations of alfaxalone (2 mg/kg), ketamine (20 mg/kg), and dexmedetomidine (0.02 mg/kg; AKD) and of alfaxalone (2 mg/kg), butorphanol (2 mg/kg), and midazolam (1 mg/kg; ABM) were reported to provide safe and effective anesthesia in naked mole rats.18 The objective of this study was to determine the physiologic effects, including anesthetic parameters and vital signs, of AK in naked mole rats. We hypothesized that AK would achieve safe and effective anesthesia in naked mole rats, regardless of their sex or body weight.
Materials and Methods
Animals and facility.
This study was reviewed and approved by the IACUC at Kansas State University (IACUC no. 4128.1) and Lincoln Children's Zoo Ethics Committee. Intact male and female zoo-born naked mole rats (n = 10; age, 1.5 to 2.5 y; body weight [mean ± 1 SD], 43.9 ± 6.7 g [range: females, 37 to 46 g; males, 46 to 58 g]) were included in the study. The mole rats were group-housed in a climate-controlled room (temperature, approximately 28 °C; relative humidity, 50% to 60%) with a 14.5:9.5-h light:dark cycle. The enclosure consisted of a series of 23 clear acrylic chambers and tubes with cottonwood shavings. The diet comprised rice cereal reconstituted with water, romaine lettuce, apple, carrot, yam, and corn; food was not withheld prior to anesthesia. The mole rats included in the study were evaluated as part of their annual health examination.
Experimental procedure.
The doses used in the present study were determined based on the lowest effective and practical drug volume used in preliminary experiments. An adequate plane of anesthesia was defined as a loss of righting, palpebral, and withdrawal reflexes with a stable heart rate.
In the current study, 10 naked mole rats (5 male and 5 female) were placed in individual clear plastic animal containers and moved from the group housing location to a designated procedure room (temperature, approximately 25 °C). The anesthetic protocol consisted of alfaxalone (4 mg/kg IM; Jurox, Kansas City, MO), ketamine hydrochloride (20 mg/kg IM; Ketaset, Hospira, Lake Forest, IL) into the thigh musculature in a single injection, by using 0.3-mL insulin syringes (U-100 BD Ultra-Fine Short Insulin Syringe; Becton Dickinson, Franklin Lakes, NJ) with 8-mm, 31-gauge needles. After each mole rat was injected, it was placed in a clear plastic container and closely monitored during anesthetic induction. The animals were returned to individual clear plastic containers during recovery in a heated incubator for active heat support. All animals were anesthetized within 8 h of each other and monitored by the same primary clinicians.
Anesthetic induction time was defined as the time from drug injection to the loss of righting reflex (LRR). Immediately after LRR was observed the mole rats were removed from the plastic container and positioned in sternal recumbency, eye lubricant was topically applied bilaterally. The naked mole rats were allowed to spontaneously breathe room air. Additional reflexes, including palpebral, forelimb withdrawal, and hindlimb withdrawal, were monitored every 5 min during the anesthetic period. Reflexes were scored on a 0 to 2 scale, with 0 indicating an intact reflex, 1 a reduced reflex, and 2 an absent reflex. Both withdrawal reflexes were assessed by pinching the metacarpal or metatarsal region with plastic forceps and observing the response. Pinching was performed by the same investigator for each naked mole rat and applied twice to confirm observed response. LRR was assessed by gently rolling each naked mole rat into lateral recumbency and evaluating attempts to right itself into stern recumbency. Loss of the palpebral reflex (LPR) was evaluated by gently touching the rostral canthus of the eye twice with a cotton-tipped applicator. A deep (or surgical) plane of anesthesia was defined as the loss of at least 3 monitored reflexes.19 Vital signs, including heart rate and respiratory rate, were measured every 5 min. Initial recordings of heart rate and respiratory rate were considered baseline measurements to assess physiologic changes under anesthesia. Heart rate was determined via an Doppler probe over the thorax (model 811B Doppler Flow Detector, Parks Medical Electronics, Las Vegas, NV). Respiratory rate was measured by direct visualization of chest movements. All animals were placed on intermittent ECG for evaluation of cardiac rhythm. SpO2 was measured by handheld pulse oximeters placed over the distal limbs. Anesthesia was determined to be complete when animals started moving or attempted to right themselves. Full recovery was determined as return of all reflexes with purposeful movement as compared with random or uncoordinated movement, which suggests a reduced level of sedation. The quality of anesthetic induction and recovery was scored for each anesthetic trial by using the same scoring system (1 to 4) previously described for rabbits: 1 indicated a smooth induction, and 4 indicated a poor induction.3
Statistical analysis.
Heart rate and respiratory rate were assessed over time by using linear mixed models, with time, sex, and weight as fixed effects and individual naked mole rats as the random effect. Time was treated as a factor to compare with baseline. Residual plots were used to assess linearity, homogeneity of variances, normality, and outliers. In addition, quantile plots were performed on the residuals for normality assessment. Posthoc analysis was performed by using the Tukey adjustment. Differences in induction, anesthesia, and recovery times were assessed by using Wilcoxon tests. R (http://www.R-project.org/) was used for statistical analysis, with an α value of 0.05 for statistical significance.
Results
Induction and recovery.
Median induction time was 180 s (range, 118 to 385 s), median anesthesia duration was 60 min (36 to 85 min), and median time to full recovery was 18 min (4 to 42 min). The induction time was significantly (P = 0.016) longer in males (median, 325 s; range, 180 to 385 s) than in females (145 min; 118 to 180 s). Anesthetic duration was significantly (P = 0.036) shorter in males (median, 50 min; range, 36 to 65 min) than females (70 min; 60 to 85 min). The time to full recovery time did not differ between sexes (P = 1). Induction and recovery quality scores ranged from 1 to 2 (smooth to adequate). No anesthesia-related adverse events were observed in any of the treated animals.
Physiologic data.
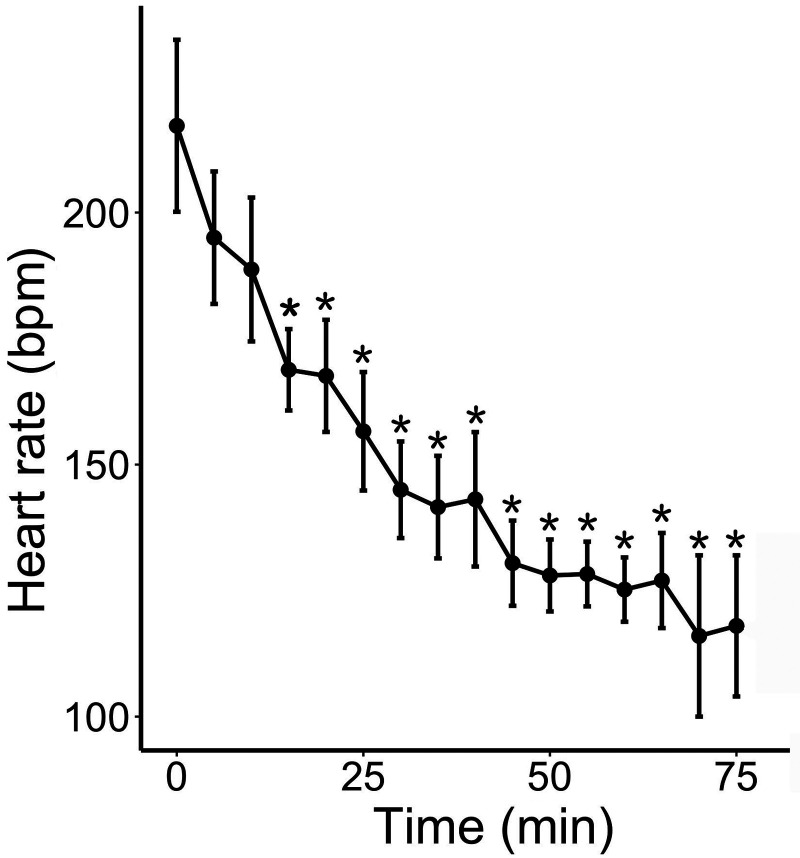
Heart rate decreased significantly (P < 0.001) over time after anesthesia (Figure 1) and was lower than baseline starting at 15 min (P = 0.0026) and throughout the rest of the anesthesia. There was no effect of sex (P = 0.12) or weight (P = 0.10) on measured heart rates.
Figure 1.
Heart rate (bpm; mean ± SEM) over time in 10 naked mole rats anesthetized with intramuscular AK. Time 0 represents anesthetic induction (loss of the righting reflex); *, P < 0.05 relative to baseline (time 0).
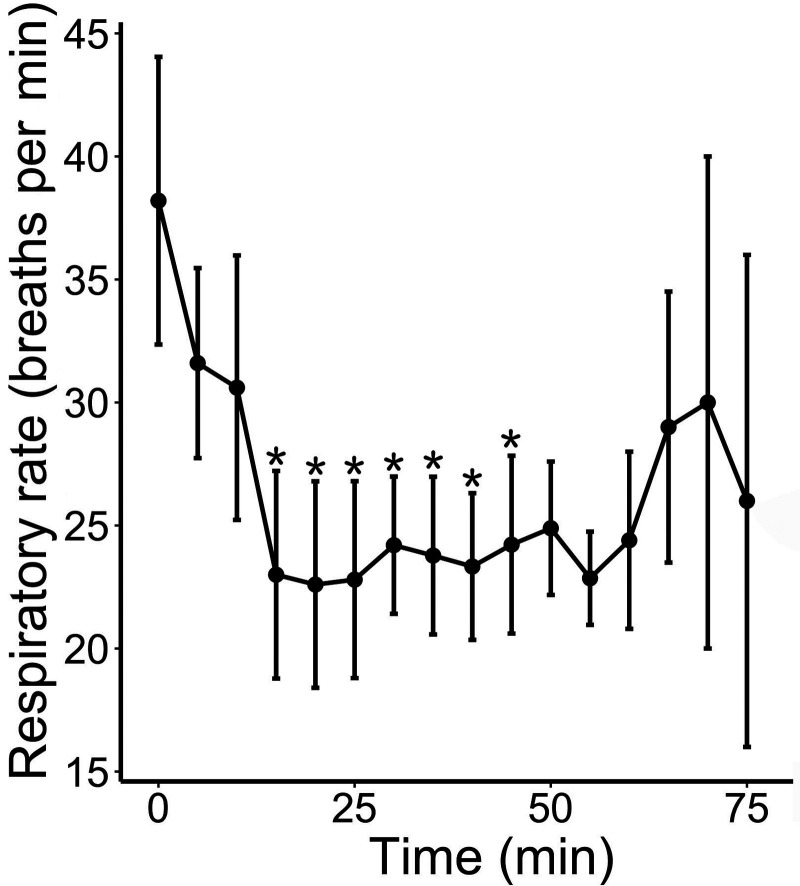
Respiratory rate significantly decreased over time after anesthesia (P < 0.009; Figure 2) and was lower than baseline respiratory rate starting at 15 min (P = 0.015) and until 45 min included (all P < 0.045). There was no effect of sex (P = 0.51) or weight (P = 0.66) on measured respiratory rates. We attempted to measure SpO2 but were largely unsuccessful despite the use of 2 different handheld pulse oximeters placed over various parts of all 4 limbs. No significant abnormalities were noted on ECG.
Figure 2.
Respiratory rate (breaths per min; mean ± SEM) over time in 10 naked mole rats anesthetized with intramuscular AK. Time 0 represents anesthetic induction (loss of the righting reflex). *, P < 0.05 relative to baseline (time 0).
Reflexes.
All animals exhibited LRR and LPR, which lasted throughout the duration of anesthesia (Table 1). Under anesthesia, male mole rats largely showed intact withdrawal reflexes, whereas females showed variable loss of both forelimb and hindlimb withdrawal reflexes.
Table 1.
Numbers of male (total n= 5) and female (total n= 5) naked mole rats anesthetized with intramuscular AK for which various reflexes were absent at various time points after anesthetic induction
| Males |
Females |
|||||||
| Time (min) | Righting | Palpebral | Forelimb withdrawal | Hindlimb withdrawal | Righting | Palpebral | Forelimb withdrawal | Hindlimb withdrawal |
| 0 | 5 | 5 | 0 | 0 | 5 | 5 | 5 | 5 |
| 5 | 5 | 5 | 1 | 0 | 5 | 5 | 4 | 5 |
| 10 | 5 | 5 | 0 | 0 | 5 | 5 | 4 | 5 |
| 15 | 5 | 5 | 0 | 0 | 5 | 5 | 4 | 5 |
| 20 | 5 | 5 | 1 | 0 | 5 | 5 | 2 | 3 |
| 25 | 5 | 5 | 0 | 0 | 5 | 5 | 2 | 1 |
| 30 | 5 | 5 | 0 | 0 | 5 | 5 | 1 | 1 |
| 35 | 4 | 4 | 0 | 0 | 5 | 5 | 1 | 0 |
| 40 | 4 | 4 | 0 | 0 | 5 | 5 | 1 | 0 |
| 45 | 4 | 4 | 0 | 0 | 5 | 5 | 1 | 0 |
| 50 | 1 | 4 | 0 | 0 | 4 | 4 | 0 | 0 |
| 55 | 1 | 2 | 0 | 0 | 4 | 4 | 0 | 0 |
| 60 | 1 | 1 | 0 | 0 | 4 | 4 | 0 | 0 |
| 65 | 1 | 3 | 3 | 0 | 0 | |||
| 70 | 2 | 3 | 0 | 0 | ||||
| 75 | 2 | 2 | 0 | 0 | ||||
| 80 | 1 | 1 | 0 | 0 | ||||
| 85 | 0 | 1 | 0 | 0 | ||||
Discussion
In the study reported here, naked mole rats were anesthetized with an AK combination that appeared to provide safe and effective immobilization but with some differences in the anesthetic effects attributable to the sex of the tested naked mole rats. These differences should be taken into consideration when using this AK anesthesia protocol in this species.
Injectable anesthesia protocols in mole rats are rarely described as part of literature publications; thus, these protocols provide limited information regarding the anesthetic parameters, including induction time, length of recovery, and vital parameters.2,13,23,29,30,34,35,39,46,56 In one study, naked mole rats were anesthetized with tribromoethanol (300 mg), with buprenorphine (0.05 mg/kg) administered as postoperative analgesia.46 In other studies, naked mole rats anesthetized by using intraperitoneal ketamine– xylazine (80 mg/kg and 16 mg/kg, respectively) or ketamine–xylazine (100 mg/kg and 2 mg/kg, respectively) recovered without complications.13,30 Although these protocols were effective, many studies did not assess physiologic or anesthetic parameters.29,35,46,56,63
In addition, not all anesthetic agents are readily available in all countries or states. Finally, some injectable anesthetic agents are contraindicated in various disease conditions and when evaluating specific physiologic parameters. For example, due to their profound cardiovascular effects and vasoconstrictive properties, α2-agonists may be contraindicated when evaluating cardiovascular function or obtaining blood. For all species, having several options for safe and effective anesthesia is important for tailoring the anesthesia to the experimental needs. Treating naked mole rats with ketamine (15 to 20 mg/kg) and xylazine (0.6 to 1.0 mg/kg), alfaxalone–ketamine–dexmeditomidine, or alfaxalone–butorphanol–midazolam achieved adequate anesthesia with good recovery.19,29 We used a similar dose of ketamine (20 mg/kg IP) but a higher dose of alfaxalone (4 mg/kg compared with 2 mg/kg IP) in the present study.
The induction of anesthesia in the current protocol was smooth and rapid (median, 180 s) in all animals. This duration is slightly longer than the induction time (median, 150 s) observed previously with the alfaxalone–ketamine–dexmeditomidine protocol.19 In addition, induction time was significantly longer and anesthetic duration significantly shorter in male mole rats. We also noted significant differences between sexes in regard to loss of reflexes. Lateral recumbency (or LRR) after drug administration in rodents is considered a marker of anesthesia onset.16 In the present study, all treated animals showed LRR, with females exhibiting longer duration of LRR than males, which was not observed in similar previous studies.19 These data suggest that this regimen can provide at least brief immobilization in this species, depending on the animal's sex.
The absence or marked suppression of withdrawal reflexes has been suggested to indicate a deep level or onset of deep (surgical) anesthesia.21 Administration of AK did not provide a consistent surgical plane of anesthesia, as indicated by the variable responses to toe pinch. In rodents, the hindlimb withdrawal reflex may be lost at a lighter plane of anesthesia than the forelimb withdrawal reflex.21 In the current study, these reflexes showed sex-associated differences: males exhibited no loss of the hindlimb withdrawal reflex and minimal loss of forelimb withdrawal reflex, and females exhibited loss of both hindlimb and forelimb withdrawal reflexes, with forelimb reflexes being suppressed slightly longer, with variable response, and showing return of withdrawal reflex in all females by 45 min. In addition to intragroup variability, there were differences in the observed responses between forelimb and hindlimb withdrawal reflexes within individual animals, suggesting that both reflexes should be tested to determine the level of anesthesia before invasive or painful procedures are initiated. Regardless, response to a toe pinch might be an indicator of the depth of anesthesia alone, and the potential antinociceptive effect of AK in naked mole rats should be further evaluated. In a previous study using AK supplemented with dexmedetomidine, all tested animals exhibited loss of withdrawal reflexes.18 Supplemental drugs (anesthetics, analgesics, or both) or increases in the doses of alfaxalone and ketamine might be required for invasive and painful procedures.
The sex-associated differences in the current study may be due to use of alfaxalone. Sex-associated differences in the pharmacokinetics and pharmacodynamics of alfaxalone have been demonstrated in rats.2,19,60 One study found that males required a 3-fold higher dose than females when administered intraperitoneally.2 The higher concentration of the endogenous GABA modulator (3α-hydroxydihydroprogesterone) in females has been proposed as a possible mechanism for sex-associated differences.2 In another study, analysis of time–concentration profiles indicated that alfaxalone was more eliminated readily from males than females, suggesting that the difference in activity between sexes was related to differences in clearance.4 The current study's findings are consistent with those previously reported in other rodent species and suggest that naked mole rats also exhibit sex-associated differences. However, note that the female naked mole rats in our study had lower body weights, and it is possible that the small volumes of the administered injectable drugs could have led to subtle differences in accuracy of dosing and explain the greater anesthetic effects in the female rats. However, we noted no differences between sexes in regard to heart rate, respiratory rate, recovery time, and recovery scores. In a study that evaluated 2 alfaxalone-containing anesthetic protocols (alfaxalone–ketamine–dexmeditomidine and alfaxalone–butorphanol–midazolam),19 no sex-associated differences were observed, perhaps due to the lower alfaxalone dose (2 mg/kg) or supplemental agents used. Regardless, the sex-related differences in anesthesia responses in the current study should be considered when using this AK immobilization protocol in naked mole rats.
LPR usually indicates a deep plane of anesthesia but can be difficult to assess or may appear variably in small rodents.20,28 In our current study, unanesthetized mole rats had reliable and consistent palpebral reflex responses on stimulation with a cotton-tipped applicator. All mole rats showed LPR and LRR concurrently, and a few individual animals also showed LPR while recovering their righting reflex. Although not specifically discussed, a previous study evaluating anesthetic protocols in naked mole rats found LPR in more animals than LRR, possibly suggesting similar effects to those we observed.19 Palpebral reflex is elicited by touching the periocular skin and is important for protection of the eye; generally palpebral reflex is thought be absent in unconscious animals; however, there is variability between species.28 In most species, palpebral reflex is often present at a light plane of anesthesia, is expected to return prior to righting reflex, and is used to assess anesthetic depth.28 Naked mole rats are burrowing rodents with reduced visual capacity and an expanded somatosensory system.7,29,45 The physiologic adaptions in subterranean naked mole rats may affect their palpebral reflex, which therefore may not provide a reliable indicator for anesthetic depth in this species.
In this study, both heart rate and respiratory rate decreased with time under anesthesia; this generally occurs with deep anesthesia and has been reported previously in naked mole rats and other species.2,19,33,43,47,48,49,51 Pulse oximetry largely failed in the current study. Pulse oximetry can result in inaccurate measurements of SpO2 as a result of decreased tissue perfusion and low oxygen saturation levels, especially in cases of vasoconstriction and hypothermia, which are expected responses in anesthetized animals.9 Pulse oximeters measure arterial hemoglobin oxygen saturation at 2 wavelengths of light: a red wavelength and an infrared wavelength. Oxygen-saturated hemoglobin absorbs more infrared and less red light than deoxygenated hemoglobin.9 To achieve a percentage saturation, the red:infrared wavelength ratio is compared with calibration curves programmed into the pulse oximeter. These curves reference the human oxygenation curve; naked mole rats might have a different oxygenation pattern. As shown in other species, pulse oximeters can be inaccurate when used beyond their original calibration reference.9,32,38 The hemoglobin in naked mole rats has higher affinity for oxygen than does human hemoglobin;15 consequently, mole rats have a leftward shift in their oxygenation curve, which could mean that pulse oximetry readings in naked mole rats overestimate blood oxygenation.9 Further studies involving arterial pO2, blood gas measurements, and other pulse oximetry technologies are required to determine whether the respiratory rate we measured is associated with hypoxemia and other blood gas abnormalities in mole rats under AK anesthesia. Although the animals in this study made a full recovery after the procedure, supplemental oxygen should be considered when using the described AK protocol in naked mole rats.
Mole rats in the current study had no observable adverse effects associated with the AK protocol, and all animals made a full recovery and were returned to their main holding area within 2 to 3 h from the end of the procedure. Alfaxalone in other species has been associated with various perianesthetic adverse effects. Mice anesthetized with alfaxalone alone or a combination of alfaxalone and xylazine displayed popcorn-like jumping movements after injection and intense scratching of the face, hyperresponsiveness to noise or touch, and marked limb jerking during recovery.18,53 Rats anesthetized with alfaxalone (intraperitoneally or intravenously) experienced short transitory dose-dependent apnea, and several of these rats also displayed facial twitching during induction and recovery.36 Guinea pigs anesthetized with alfaxalone alone or in combination with dexmedetomidine or buprenorphine had dose-dependent respiratory depression and twitching or bruxism at higher doses.14 Tremors, twitching, and rolling were observed in most of the chinchillas anesthetized with alfaxalone–butorphanol but in none of those anesthetized with dexmedetomidine–ketamine.47 Dogs anesthetized with alfaxalone alone showed adverse responses including apnea, tachypnea, hypotension, hypoxia, and excitement.12,43,51,57 In another study, dogs anesthetized with alfaxalone combined with butorphanol or medetomidine displayed excitement, paddling, twitching, apnea, and cyanosis.37 Coadministration of several drugs in an anesthetic ‘cocktail’ can be used to decrease the dose of the main agent (which was alfaxalone in the present study), thus minimizing its potential adverse effects.10,21 Naked mole rats anesthetized with either alfaxalone–ketamine–dexmeditomidine or alfaxalone–butorphanol–midazolam did not show these adverse behavioral effects.19 The AK doses in the current protocol were on the lower end of the range compared with those reported in other rodents and may account for the lack of adverse effects seen in other species. Future studies using higher AK doses are needed to assess the potential associated adverse effects.
This study has several limitations. Dosing of the anesthetic drugs was a challenge, given the small size of the subjects. The volume administered was less than 0.04 mL per site. This volume exceeds the 0.05 mL/kg recommended according to substance administration guidelines for intramuscular injections.57 However, this dose limitation is often impractical in small rodent species such as naked mole rats. Recommendations for other small rodents often exceed this dose, and the volume used in the current study is below those recommended for other rodent species. For example, the recommended volume for intramuscular injection into an adult degu (average weight, 260 g) is 0.5 mL, which is approximately 2.5 mL/kg.11 Another limitation of our current study was the small sample size, which could have led to type II error for some parameters. However, previous studies evaluating anesthesia in other species have used similar sample sizes.22,23 Future studies might include a larger sample size, to reduce the likelihood of type II error. Hematologic parameters, including venous blood gases, were not measured in the current study and may be worthwhile in future studies, to evaluate for acid–base disorders and hypoxemia with this protocol. In addition, systemic arterial blood pressure would be beneficial, to better evaluate cardiovascular function. Additional studies comparing the anesthetic parameters of isoflurane and our AK protocol would be useful for assessing the safety and efficacy of the respective protocols.
In conclusion, the AK regimen evaluated in this study appeared to be a safe and effective method for brief immobilization of mole rats. This regimen provided rapid and smooth induction and recovery with no apparent adverse effects. The data in this study suggest that the AK protocol provides a short light anesthesia in males, and a longer and deeper anesthesia in females. However, anesthetic depth of individual animals must still be determined before painful, invasive, or surgical procedures are performed in naked mole rats anesthetized with this AK combination. Further studies evaluating higher doses or additional combinations would be beneficial in the future.
Acknowledgments
Support for this project was provided by the KSU-CVM MCAT grant. We also thank the keeper staff at the Lincoln Children's Zoo and John Doyal, Emma Bishop-Young, Justin Yuen, Carolyn Mark, and Tori Matta for their assistance with this study.
References
- 1.Arenillas M, Gomez de Segura IA. 2018. Anaesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab Anim 52:588–598. 10.1177/0023677218764214. [DOI] [PubMed] [Google Scholar]
- 2.Artwohl J, Hill T, Corner C, Park T. 2002. Naked mole rats: unique opportunities and husbandry challenges. Lab Anim (NY) 31:32–36. [DOI] [PubMed] [Google Scholar]
- 3.Bradley MP, Doerning CM, Nowland MH, Lester PA. 2019. Intramuscular administration of alfaxalone alone and in combination for sedation and anesthesia of rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 58:216–222. 10.30802/AALAS-JAALAS-18-000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brewster ME, Anderson WR, Webb A, Bodor N, Pop E. 1996. Anesthetic activity and pharmacokinetics of the neurosteroid alfaxalone formulated in 2-hydroxypropyl-β-cyclodextrin in the rat. In: Szejtli J, Szente L. editors. Proceedings of the Eighth International Symposium on Cyclodextrins. p 499–502. Springer, Dordrecht: 10.1007/978-94-011-5448-2_110 [DOI] [Google Scholar]
- 5.Bencze M, Behuliak M, Zicha J. 2013. The impact of four different classes of anesthetics on the mechanisms of blood pressure regulation in normotensive and spontaneously hypertensive rats. Physiol Res 62:471–478. [DOI] [PubMed] [Google Scholar]
- 6.Browning GR, Eshar D, Beaufrère H. 2019. Comparison of dexmedetomidine–ketamine–midazolam and isoflurane for anesthesia of black-tailed prairie dogs (Cynomys ludovicianus). J Am Assoc Lab Anim Sci 58:50–57. 10.30802/AALAS-JAALAS-18-000001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buffenstein R, Park T, Hanes M, Artwohl JE. 2012. Naked mole rat, p 1055–1074. In: Suckow MA, Stevens KA, Wilson RP, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. Waltham (MA): Elsevier. [Google Scholar]
- 8.Buisman M, Wagner MC, Hasiuk MMM, Prebble M, Law L, Pang DSJ. 2015. Effects of ketamine and alfaxalone on application of a feline pain assessment scale. J Feline Med Surg 18:643–651. 10.1177/1098612X15591590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan ED, Chan MM, Chan MM. 2013. Pulse oximetry: understanding its basic principles facilitates appreciation of its limitations. Respir Med 107:789–799. 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Chinnadurai SK, Strahl-Heldreth D, Fiorello CV, Harms CA. 2016. Best-practice guidelines for field-based surgery and anesthesia of free-ranging wildlife. I. Anesthesia and analgesia. J Wildl Dis 52 2 Suppl:S14–S27. 10.7589/52.2S.S14. [DOI] [PubMed] [Google Scholar]
- 11.Colby LA, Rush HG, Mahoney MM, Lee TM. 2012. Degu, p 1031–1053. Chapter 44. In: Suckow MA, Stevens KA, Wilson RP, editors. The laboratory rabbit, guinea pig, hamster, and other rodents. Waltham (MA): Elsevier; 10.1016/B978-0-12-380920-9.00044-4 [DOI] [Google Scholar]
- 12.Costa D, Leiva M, Moll X, Aguilar A, Peña T, Andaluz A. 2015. Alfaxalone versus propofol in dogs: a randomised trial to assess effects on peri-induction tear production, intraocular pressure and globe position. Vet Rec 176:73–73. 10.1136/vr.102621. [DOI] [PubMed] [Google Scholar]
- 13.Crish SD, Dengler-Crish CM, Catania KC. 2006. Central visual system of the naked mole-rat (Heterocephalus glaber). Anat Rec A Discov Mol Cell Evol Biol 288:205–212. 10.1002/ar.a.20288. [DOI] [PubMed] [Google Scholar]
- 14.Doerning CM, Bradley MP, Lester PA, Nowland MH. 2018. Effects of subcutaneous alfaxalone alone and in combination with dexmedetomidine and buprenorphine in guinea pigs (Cavia porcellus). Vet Anaesth Analg 45:658–666. 10.1016/j.vaa.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 15.Donnelly TM, Bergin I, Ihrig M. 2015. Biology and diseases of other rodents, p 328–330. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning K, Whary M, editors. Laboratory animal medicine, 3rd ed Amsterdam: Elsevier. [Google Scholar]
- 16.d'Ovidio D, Marino F, Noviello E, Lanaro E, Monticelli P, Adami C. 2018. Sedative effects of intramuscular alfaxalone in pet guinea pigs (Cavia porcellus). Vet Anaesth Analg 45:183–189. 10.1016/j.vaa.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Erhardt W, Lendl C, Hipp R, Schindele M, Blümel G. 1989. Pulse oximetry—a non-invasive method for direct and continuous monitoring of oxygen saturation and pulse rate—comparative studies with blood gas analysis and hemoreflectometry in the dog, swine and sheep. Berl Munch Tierarztl Wochenschr 102:289–292. [PubMed] [Google Scholar]
- 18.Erickson RL, Blevins CE, Souza Dyer CD, Marx JO. 2019. Alfaxalone–xylazine anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 58:30–39. 10.30802/AALAS-JAALAS-18-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eshar D, Huckins GL, Shrader TC, Beaufrère H. 2019. Comparison of intramuscular administration of alfaxalone-ketamine-dexmedetomidine and alfaxalone-butorphanol-midazolam in naked mole rats (Heterocephalus glaber). Am J Vet Res 80:1089–1098. 10.2460/ajvr.80.12.1089. [DOI] [PubMed] [Google Scholar]
- 20.Fink G, Sarkar DK, Dow RC, Dick H, Borthwick N, Malnick S, Twine M. 1982. Sex difference in response to alphaxalone anaesthesia may be oestrogen dependent. Nature 298:270–272. 10.1038/298270a0. [DOI] [PubMed] [Google Scholar]
- 21.Flecknell PA, Thomas AA. 2015. Comparative anesthesia and analgesia of laboratory animals, p 754–763. In: Grimm KA, Lamont LA, Tranquilli WJ, Greene SA, editors. Veterinary anesthesia and analgesia. Oxford, England: Wiley Blackwell. [Google Scholar]
- 22.Fox L, Snyder LB, Mans C. 2016. Comparison of dexmedetomidine-ketamine with isoflurane for anesthesia of chinchillas (Chinchilla lanigera). J Am Assoc Lab Anim Sci 55:312–316. [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia Montero A, Burda H, Begall S. 2015. Chemical restraint of African mole-rats (Fukomys sp.) with a combination of ketamine and xylazine. Vet Anaesth Analg 42:187–191. 10.1111/vaa.12180. [DOI] [PubMed] [Google Scholar]
- 24.Gil AG, Silvan G, Villa A, Illera JC. 2012. Heart and respiratory rates and adrenal response to propofol or alfaxalone in rabbits. Vet Rec 170:444. [DOI] [PubMed] [Google Scholar]
- 25.Giral M, García-Olmo DC, Gómez-Juárez M, Gómez de Segura IA. 2014. Anaesthetic effects in the ferret of alfaxalone alone and in combination with medetomidine or tramadol: a pilot study. Lab Anim 48:313–320. 10.1177/0023677214539150. [DOI] [PubMed] [Google Scholar]
- 26.Hahn N, Eisen RJ, Eisen L, Lane RS. 2005. Ketamine-medetomidine anesthesia with atipamezole reversal: practical anesthesia for rodents under field conditions. Lab Anim (NY) 34:48–51. 10.1038/laban0205-48. [DOI] [PubMed] [Google Scholar]
- 27.Haupt M, Bennett NC, Oosthuizen MK. 2017. Locomotor activity and body temperature patterns over a temperature gradient in the highveld mole-rat (Cryptomys hottentotus pretoriae). plos one 12:1–17. 10.1371/journal.pone.0169644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heard DJ. 2007. Monitoring. p 83–91. Chapter 6. In: West G, Heard D, Caulkett N, editors. Zoo animal and wildlife immobilization and anesthesia. Oxford (United Kingdom): Blackwell. [Google Scholar]
- 29.Henry EC, Sarko DK, Catania KC. 2008. Central projections of trigeminal afferents innervating the face in naked mole-rats (Heterocephalus glaber). Anat Rec (Hoboken) 291:988–998. 10.1002/ar.20714. [DOI] [PubMed] [Google Scholar]
- 30.Hetling JR, Baig-Silva MS, Comer CM, Pardue MT, Samaan DY, Qtaishat NM, Pepperberg DR, Park TJ. 2005. Features of visual function in the naked mole rat Heterocephalus glaber. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 191:317–330. 10.1007/s00359-004-0584-6. [DOI] [PubMed] [Google Scholar]
- 31.Higuchi S, Yamada R, Hashimoto A, Miyoshi K, Yamashita K, Ohsugi T. 2016. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn J Vet Res 64:131–139. [PubMed] [Google Scholar]
- 32.Jacobson JD, Miller MW, Matthews NS, Hartsfield SM, Knauer KW. 1992. Evaluation of accuracy of pulse oximetry in dogs. Am J Vet Res 53:537–540. [PubMed] [Google Scholar]
- 33.Jaspar N, Mazzarelli M, Tessier C, Milic-Emili J. 1983. Effect of ketamine on control of breathing in cats. J Appl Physiol Respir Environ Exerc Physiol 55:851–859. 10.1152/jappl.1983.55.3.851. [DOI] [PubMed] [Google Scholar]
- 34.Khenissi L, Nikolayenkova-Topie O, Broussaud S, Touzot-Jourde G. 2017. Comparison of intramuscular alfaxalone and ketamine combined with dexmedetomidine and butorphanol for castration in cats. J Feline Med Surg 19:791–797. 10.1177/1098612X16657951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer B, Buffenstein R. 2004. The pancreas of the naked mole rat (Heterocephalus glaber): an ultrastructural and immunocytochemical study of the endocrine component of thermoneutral and cold acclimated animals. Gen Comp Endocrinol 139:206–214. 10.1016/j.ygcen.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Lau C, Ranasinghe MG, Shiels I, Keates H, Pasloske K, Bellingham MC. 2013. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan in rats. J Vet Pharmacol Ther 36:516–520. 10.1111/jvp.12055. [DOI] [PubMed] [Google Scholar]
- 37.Maddern K, Adems VJ, Hill NAT, Leece EA. 2010. Alfaxalone induction dose following administration of medetomidine and butorphanol in the dog. Vet Anaesth Analg 37:7–13. 10.1111/j.1467-2995.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- 38.Matthews NS, Hartke S, Allen JC., Jr 2003. An evaluation of pulse oximeters in dogs, cats and horses. Vet Anaesth Analg 30:3–14. 10.1046/j.1467-2995.2003.00121.x. [DOI] [PubMed] [Google Scholar]
- 39.McMullen CA, Andrade FH, Crish SD. 2010. Underdeveloped extraocular muscles in the naked mole-rat (Heterocephalus glaber). Anat Rec (Hoboken) 293:918–923. 10.1002/ar.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Molina AM, Moyano MR, Serrano-Rodriguez JM, Lora AJ, Serrano-Caballero JM. 2015. Analyses of anaesthesia with ketamine combined with different sedatives in rats. Vet Med (Praha) 60:368–375. 10.17221/8384-VETMED. [DOI] [Google Scholar]
- 41.Molteno AJ, Kalló I, Bennett NC, King JA, Coen CW. 2004. A neuroanatomical and neuroendocrinological study into the relationship between social status and the GnRH system in cooperatively breeding female Damaraland mole rats, Reproduction. 127:13-21. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz KA, Robertson SA, Wilson DV. 2017. Alfaxalone alone or combined with midazolam or ketamine in dogs: intubation dose and select physiologic effects. Vet Anaesth Analg 44:766–774. 10.1016/j.vaa.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Muir W, Lerche P, Wiese A, Nelson L, Pasloske K, Whittem T. 2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet Anaesth Analg 35:451–462. 10.1111/j.1467-2995.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 44.Negroni J, Nevo E, Cooper HM. 1997. Neuropeptidergic organization of the suprachiasmatic nucleus in the blind mole rat (Spalax ehrenbergi). Brain Res Bull 44:633–639. 10.1016/S0361-9230(97)00306-7. [DOI] [PubMed] [Google Scholar]
- 45.Němec P, Burda H, Peichl L. 2004. Subcortical visual system of the African mole-rat Cryptomys anselli: to see or not to see? Eur J Neurosci 20:757–768. 10.1111/j.1460-9568.2004.03510.x. [DOI] [PubMed] [Google Scholar]
- 46.Park KK, Luo X, Mooney SJ, Yungher BJ, Belin S, Wang C, Holmes MM, He Z. 2017. Retinal ganglion cell survival and axon regeneration after optic nerve injury in naked mole-rats. J Comp Neurol 525:380–388. 10.1002/cne.24070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parkinson L, Mans C. 2017. Anesthetic and postanesthetic effects of alfaxalone–butorphanol compared with dexmedetomidine–ketamine in chinchillas (Chinchilla lanigera). J Am Assoc Lab Anim Sci 56:290–295. [DOI] [PubMed] [Google Scholar]
- 48.Ribas T, Bublot I, Junot S, Beaufrère H, Rannou B, Gagnière P, Cadoré JL, Pariaut R. 2015. Effects of intramuscular sedation with alfaxalone and butorphanol on echocardiographic measurements in healthy cats. J Feline Med Surg 17:530–536. 10.1177/1098612X14551187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rodrigo-Mocholí D, Belda E, Bosmans T, Laredo FG. 2016. Clinical efficacy and cardiorespiratory effects of intramuscular administration of alfaxalone alone or in combination with dexmedetomidine in cats. Vet Anaesth Analg 43:291–300. 10.1111/vaa.12304. [DOI] [PubMed] [Google Scholar]
- 50.Säre H, Ambrisko TD, Moens Y. 2011. Occupational exposure to isoflurane during anaesthesia induction with standard and scavenging double masks in dogs, pigs and ponies. Lab Anim 45:191–195. 10.1258/la.2011.010128. [DOI] [PubMed] [Google Scholar]
- 51.Seo JI, Han SH, Choi R, Han J, Lee L, Hyun C. 2015. Cardiopulmonary and anesthetic effects of the combination of butorphanol, midazolam and alfaxalone in Beagle dogs. Vet Anaesth Analg 42:304–308. 10.1111/vaa.12223. [DOI] [PubMed] [Google Scholar]
- 52.Sherman PW, Jarvis JUM, Braude SH. 1992. Naked mole rats. Sci Am 267:72–78. 10.1038/scientificamerican0892-72. [DOI] [Google Scholar]
- 53.Siriarchavatana P, Ayers JD, Kendall LV. 2016. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 55:426–430. [PMC free article] [PubMed] [Google Scholar]
- 54.Smith JC, Bolon B. 2002. Atmospheric waste isoflurane concentrations using conventional equipment and rat anesthesia protocols. Contemp Top Lab Anim Sci 41:10–17. [PubMed] [Google Scholar]
- 55.Smith JC, Bolon B. 2006. Isoflurane leakage from non-rebreathing rodent anaesthesia circuits: comparison of emissions from conventional and modified ports. Lab Anim 40:200–209. 10.1258/002367706776318999. [DOI] [PubMed] [Google Scholar]
- 56.Streicher S, Boyles JG, Oosthuizen MK, Bennett NC. 2011. Body temperature patterns and rhythmicity in free-ranging subterranean Damaraland mole rats, Fukomys damarensis. PLoS One 6:e26346 10.1371/journal.pone.0026346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tamura J, Ishizuka T, Fukui S, Oyama N, Kawase K, Miyoshi K, Sano T, Pasloske K, Yamashita K. 2015. The paramacological effects of the anesthetic alfaxalone after intramuscular administration to dogs. J Vet Med Sci 77:289–296. 10.1292/jvms.14-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Turner PV, Brabb T, Pekow C, Vasbinder MA. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J Am Assoc Lab Anim Sci 50:600–613. [PMC free article] [PubMed] [Google Scholar]
- 59.Visser SA, Smulders CJ, Gladdines WW, Irth H, van der Graaf PH, Danhof M. 2000. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnenolone in plasma using dansyl hydrazine as fluorescent label: application to a pharmacokinetic–pharmacodynamic study in rats. J Chromatogr B Biomed Sci Appl 745:357–363. 10.1016/S0378-4347(00)00296-6. [DOI] [PubMed] [Google Scholar]
- 60.White KL, Paine S, Harris J. 2017. A clinical evaluation of the pharmacokinetics and pharmacodynamics of intravenous alfaxalone in cyclodextrin in male and female rats following a loading dose and constant rate infusion. Vet Anaesth Analg 44:865–875. 10.1016/j.vaa.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 61.Widmer HR, Hoppeler H, Nevo E, Taylor CR, Weibel ER. 1996. Working underground: respiratory adaptations in the blind mole rat. Proc Natl Acad Sci USA 94:2062–2067. 10.1073/pnas.94.5.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wolforth J, Dyson MC. 2011. Flushing induction chambers used for rodent anesthesia to reduce waste anesthetic gas. Lab Anim (NY) 40:76–83. 10.1038/laban0311-76. [DOI] [PubMed] [Google Scholar]
- 63.Xiao J. 2007. A new coordinate system for rodent brain and variability in the brain weights and dimensions of different ages in the naked mole rat. J Neurosci Methods 162:162–170. 10.1016/j.jneumeth.2007.01.007. [DOI] [PubMed] [Google Scholar]