Abstract
Intraperitoneal (IP) injection is a common route of anesthetic administration in mice. Ketamine-xylazine (KX) anesthesia is one of the most widely used IP protocols, but has limitations. Etomidate is an alternative to ketamine that has been used in both human and veterinary medicine yet has not been widely studied in mice. The purpose of this study was to evaluate etomidate-xylazine (EX) anesthesia as an alternative to KX. We hypothesized that EX would be as safe and effective as KX, with both sex- and strain-dependent differences. Male and female Crl:CD1(ICR), C57BL/6NCrl, BALB/cJ and NU/J mice were given a single IP dose of ketamine 100 mg/kg and xylazine 10 mg/kg or etomidate 20 mg/kg and xylazine 10 mg/kg. Sedation times were similar between KX and EX, with CD1 mice exhibiting shorter sedation times. Surgical anesthesia was achieved in 44% of EX mice, compared with 4% of KX mice. C57BL/6NCrl mice were significantly more likely to achieve surgical anesthesia when given EX (94%) or KX (18%) than were other strains. In all strains except C57BL/6NCrl mice, females were more likely to reach surgical anesthesia than males. Several mice experienced an adverse hyperexcitement response during induction, with BALB/cJ (79%) and NU/J (87%) mice given EX significantly more likely than other strains to experience hyperexcitement. EX and KX protocols had no overall differences in lowest respiration rate, lowest systolic blood pressure, lowest rectal temperature, or levels of acidosis, although the lowest heart rates were significantly higher with EX, indicating that EX and KX have similar safety profiles. Thus, EX and KX administration were associated with several significant physiologic differences when comparing sexes or individual strains. Our results indicate that EX is an equally effective sedative and a more effective surgical anesthetic than KX; however, EX is only recommended for invasive procedures in C57BL/6 mice due to the high rate of hyperexcitement and inconsistent surgical depth seen in other strains. Further study is needed to optimize EX for use in multiple mouse strains.
Abbreviations: EX, etomidate/xylazine; KX, ketamine/xylazine; HR, heart rate; IP, intraperitoneal; PWR, pedal withdrawal reflex; RT, rectal temperature; RR, respiration rate; SBP, systolic blood pressure
Intraperitoneal (IP) injection is a frequent route of administration for anesthesia to mice, although relatively few anesthetic protocols are in use or widely studied. Although inhalants provide a more easily adjustable anesthetic plane than injectables, inhalants also require expensive equipment and can impede access to anatomic sites needed for some procedures. Barbiturates and nonpharmaceutical grade anesthetics were the injectable standards for many years in laboratory animal medicine, but they have become less common because safer options are now available. Barbiturates are known to have a narrow safety margin and are associated with dose-related respiratory and cardiovascular depression which worsens with time.3,17 OLAW and the Guide recommend that nonpharmaceutical grade drugs should be avoided to prevent potential toxicities that could threaten animal health.24,35 Ketamine-based combinations, in particular ketamine/xylazine, are frequently used to provide injectable anesthetia.40
Ketamine-based protocols have limitations in anesthetic efficacy and study applications. Reported dose ranges for ketamine can vary widely, depending on desired anesthetic depth. Doses of ketamine/xylazine recommended for inducing surgical anesthesia in mice have produced variable results, with some studies showing a continued response to noxious stimuli after standard dosing.3,4,8,9 Higher doses have been associated with higher mortality rates.3,30 Even when ketamine is effective, some researchers may consider its use to be a confounding variable due to its effects on the immune system. Ketamine is reported to have several anti-inflammatory effects, including reduced function of cytokines, lymphocytes, neutrophils, and NK cells.31 Ketamine has also been associated with increased tumor metastasis.33 Anesthetic protocol selection is further complicated by the issue that anesthetic outcomes can vary considerably based on experimental use, sex, or strain of the animals used in experiments.7,15,19,27,32,39 For these reasons, continued investigation of alternative IP anesthetic protocols would be beneficial.
Etomidate is a potential alternative to ketamine, yet limited studies have been published on its use in rodents. Etomidate is a γ-aminobutryic acid (GABA) receptor agonist that is primarily used as an anesthetic in human and small animal veterinary medicine. This hypnotic agent has been preferred for its wide therapeutic index and minimal effects on heart rate, respiration, and blood pressure.17,20,21,37 Etomidate would be easier for researchers to use than ketamine because it is not classified as a DEA controlled substance, thus removing the complexity of obtaining a DEA license and reducing the effort associated with complying to increasingly strict federal regulations.2 Its availability as a pharmaceutical grade drug also provides reliable manufacturing to high standards. Possible drawbacks to using etomidate as an IP anesthetic include reports of pain or myoclonus during induction after intravenous administration.11,28 Currently, in a research setting, etomidate is most commonly used as an intravenous bolus or continuous rate infusion. However, these methods of anesthetic delivery require training, skill, and specialized equipment.1,5,37 IP injection is a simpler technique that requires less training or equipment.
Studies on appropriate dose, safety, and efficacy of etomidate as an IP anesthetic in mice have been limited. Previous studies have shown that etomidate, when used as a sole agent in an IP anesthetic protocol, does not produce adequate anesthesia for surgical procedures.21,29 One group that deemed IP etomidate as adequate for surgical anesthesia did not assess reflex responses to determine if appropriate anesthetic depth had been achieved.20 Adding potent opioids to an IP etomidate protocol has been shown to be sufficient for surgical anesthesia; however this combination did not eliminate the myoclonus seen at induction.21 Combining etomidate with xylazine, which is known to provide muscle relaxation and analgesia, could increase the anesthetic efficacy of etomidate and potentially reduce the level of myoclonus seen in previous protocols. However, this combination has not yet been tested.
The purpose of this study was to compare the anesthetic, respiratory, and cardiovascular effects of 2 IP anesthetic combinations, etomidate/xylazine (EX) and ketamine/xylazine (KX), in both males and females of 4 commonly used mouse strains. We hypothesized that EX would be equivalent to or better than KX in anesthetic efficacy and safety. We also expected that efficacy and safety would vary by strain and sex for both of these anesthetic protocols.
Materials and Methods
All animals were housed in an AAALAC International accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals.24 All animal procedures were reviewed and approved by Emory University's Institutional Animal Care and Use Committee.
Animals and Housing.
Male and female Crl:CD1(ICR) and C57BL/6NCrl mice were purchased from Charles River Laboratories, Wilmington, MA. Male and female BALB/cJ and NU/J mice were purchased from The Jackson Laboratory, Bar Harbor, ME (JAX stock no. 000651 and no. 002019 respectively). All animals were purchased at 8 wk of age and were used for the experiment by 10 wk of age. After their arrival to the animal facility, mice were divided by sex and strain, housed 4 to 5 mice per cage, and allowed to acclimate for at least 72 h before experimental manipulation. Animals were maintained with their original cage mates until experiments had been completed for all animals in the cage. Mice were housed on 1/8 in. corncob bedding (Bed-o'Cobs, The Andersons, Maumee, OH) in polycarbonate caging with Lifespans (cage no. 10027, Lab Products, Seaford, DE) and 2 × 2 cotton squares (Ancare Corp., Bellmore, NY) for enrichment. Mice were fed LabDiet 5053, Irradiated Laboratory Rodent Diet (LabDiet, St Louis, MO) and provided autoclaved reverse osmosis-filtered water in bottles ad libitum. All cage components, including bedding, cage furnishings, and enrichment were autoclaved before use. Room environment was maintained at 68 to 72 °F (20 to 22 °C), 30% to 70% humidity, on a 12:12 light:dark cycle. All animals were included in a colony health surveillance program to exclude: Sendai Virus; Mouse Hepatitis Virus; Mouse Minute Virus; Mouse Parvovirus 1; Mouse Parvovirus 2; NS-1; Theiler Virus (GDVII or TMEV); Epizootic Diarrhea of Infant Mice; Lymphocytic Choriomeningitis Virus; Polyoma Virus; Mouse Cytomegalovirus; Ectromelia Virus; K Virus; Mycoplasma Pulmonis; Mouse Adenovirus 1 and 2; Pneumonia Virus of Mice; Reovirus; Fur mites; Pinworms.
Experimental Design.
A 3-factor prospective experiment was conducted to compare KX and EX protocols. Male and female mice of each strain (Crl:CD1 n = 19 male, 23 female; C57BL/6NCrl n = 16 male, 18 female; BALB/cJ n = 19 male, 18 female and NU/J n = 17 male, 17 female), aged 8 to 10 wk were used. Power analysis determined that 16 mice per cohort of strain and sex would provide 80% or more power to detect statistically significant differences in outcomes; however additional mice were added to some cohorts due to difficulty in data collection. Difficulties primarily occurred during blood collection (See “Blood Collection” for details).
Three to 4 animals were sedated concurrently for monitoring. Animals selected for testing at a given time were randomized by strain, sex, time of day, date, cage number and anesthetic protocol. Testing was performed between 0800 and 1500 over the course of one month. Anesthetic protocols in this experiment were KX (ketamine 100 mg/kg and xylazine 10 mg/kg) or EX (etomidate 20 mg/kg and xylazine 10 mg/kg. The individual performing IP injection and subjective measurements was blind to the anesthetic protocol administered to each mouse (detailed description in Monitoring of Anesthesia). After the experiment, mice were maintained for a minimum of 2 wk to assess for delayed morbidity or mortality. Animals were transferred to an institutional teaching protocol or euthanized via CO2 asphyxiation at the end of study, with the entire cage of animals removed from the study at one time.
Anesthetic Drugs.
Anesthetic drugs used in this study include ketamine (KetaVed 100 mg/mL, Vedco St. Josephs, MO), xylazine (AnaSed 20 mg/mL, Akorn), and etomidate (Amidate 2 mg/mL, Hopsira, Lake Forest, IL). Dosage for KX anesthesia was based on common published usage4,17,26 and the current facility recommended dose to induce surgical anesthesia. Dosage for EX was determined by a small pilot study (data not shown), in which female C57BL/6NCrl mice were found to lose righting reflex and recover within an hour. Drugs were diluted using bacteriostatic water (Hospira, Lakeforest, IL) to a concentration that resulted in doses of 10 mL/kg KX, and 10.5 mL/kg EX; the similar injection volumes rendered the observer blind to the drug combination that was injected. All mice were weighed within 24 h before the anesthetic procedure and dosed according to body weight.
Monitoring of Anesthesia.
Definitions of time periods used for analysis are listed in Figure 1. The time of IP injection was recorded as time 0. After the injection, mice were observed continuously for loss of righting reflex. Loss of righting reflex was determined by an inability to return to sternal recumbency after being placed in dorsal recumbency. When the reflex was lost, mice were placed in dorsal recumbency on a surgical drape that covered a water-circulating heating pad. The heating pad was prewarmed and set to 107 °F (41.7 °C) for thermoregulatory support. Sterile eye lubricant was applied.
Figure 1.
Anesthetic time period definitions.
Multiple physiologic measurements and pedal withdrawal reflex (PWR) were recorded every 5 min until return of righting reflex. Measurements included systolic blood pressure (SBP), rectal temperature (RT), heart rate (HR), and respiration rate (RR). For blood pressure measurements, a noninvasive blood pressure monitoring cuff was positioned at the base of the tail (CODA High Throughput System Noninvasive Blood Pressure System, Kent Scientific, Torrington, CT). Tail cuff size was chosen based on weight according to manufacturer's recommendations in which mice weighing less than 25g were given extra small tail cuffs and mice weighing greater than or equal to 25g were given small tail cuffs. A lubricated mouse rectal probe was inserted to monitor core body temperature (Microtherma 2 thermometer, Thermoworks, Lindon, UT). A piece of tape was applied to each probe to indicate a standardized probe insertion depth. A Soft Touch Paw Sensor and MouseSTAT System (Kent Scientific, Torrington, CT) were used to monitor HR of all mice. Respiration rate was visually assessed and recorded.
PWR was assessed on the digits of the hind limbs using 2 methods. Two methods were used to determine depth of anesthesia and to reduce variability between subjects, ensuring a consistent and accurate assessment of PWR. The first method, a traditional manual finger pinch, was performed by the same blinded observer throughout the study. The second method was performed by a second observer via mechanical pinch applied by a modified set of 5” curved Semken-Taylor tissue forceps. A wound clip was used as a spacer to prevent the forceps from closing completely, leaving a 1 mm gap to minimize tissue trauma and to standardize the amount of pressure administered. This gap was measured daily at the start of procedures. Each method (forceps compared with finger) was performed by separate observers that were blinded to the PWR of the opposing method. Both methods were performed at each 5 min time point. A surgical depth of anesthesia was defined as a negative response to both PWR assessment methods. A negative response to one method, but not both, was considered inadequate anesthetic depth for surgical procedures. Return of PWR by either method was also recorded.
Physiologic and reflex monitoring continued until return of the righting reflex, as determined by spontaneous righting from the dorsal to supine position. Mice that regained righting reflex were then placed and centered on a 2 × 2 cotton square in a clean cage. Mice were monitored every 3 min for return of purposeful movement, defined as the ability to move the entire body (excluding the tail) off the cotton square. Time of return to purposeful movement was noted and the mouse was considered fully recovered. Throughout the recovery process half of the cage was positioned over an electric heating pad for thermoregulatory support. A single observer who was blind to the anesthetic combination performed the following for all animals: IP injection, determination of loss of righting reflex, manual toe pinch, RR, and determination of return of purposeful movement. Any measurement or task completed mechanically was recorded by a second observer, who varied and was not always blind to treatment. These included HR readings, RT, and mechanical forceps pinch. SBP was automatically documented by software provided with the CODA High Throughput System Noninvasive Blood Pressure System.
Blood Collection.
Forty minutes after the IP injection, mice were restrained for submandibular blood collection. Two hundred microliters of venous blood were collected for blood gas analysis. If an adequate volume of blood could not be collected after 2 submandibular punctures (one per side), no further collection attempts were made. Instead a naïve animal underwent the entire experiment to make up for the lack of data and further strengthen statistical analysis in other parameters. pH and pCO2 were measured using the Stat Profile Prime Plus Critical Care Analyzer, Nova Biomedical. After return of purposeful movement, all mice were administered 1 mL of warmed subcutaneous 0.9% saline for blood volume support.
Statistical Analysis.
Descriptive statistics are reported for duration of loss and recovery of PWR, lowest SBP and time to lowest SBP. SBP was based on mice with at least 2 measurements of BP.
All statistical analysis were 2-sided, with an α of 0.05. When correction for multiple comparisons was required, an α of 0.01 was used.
Hyperexcitement and loss of PWR were categorized as 2 levels, “yes” or “no”. Levels of acidosis were classified into 2 categories: normal-mild acidosis (7.45 ≥ pH greater than or equal to 7.25) or moderate-severe acidosis (pH less than or equal to 7.24). To examine the association between anesthetic protocol, strain, sex with hyperexcitement, loss of PWR and acidosis, overall (pooled) and stratified χ2 or Fisher exact tests were conducted.
To examine the association between pCO2 and 3 factors of interest (treatment (2 levels, KX or EX), strain (4 levels) and sex (2 levels)), generalized linear regression models were fit starting with the fully saturated model containing treatment, strain, sex, all 2-way interactions combinations, and the 3-way interaction of treatment by strain by sex. Backward selection procedure was used to determine which main effects and interaction terms remained in a final model at α level equal to 0.05. A similar modeling strategy was used for sedation.
The uncontrolled association between pCO2 and pH was assessed via computing Pearson correlation coefficient. Next a generalized linear model is fit with pCO2 as the dependent variable, and pH, protocol, strain, sex, protocol along with the 2-way and 3-way interactions. Backward selection procedure was used to determine which main effects and interaction terms remained in a final model at α level equal to 0.05.
The analysis of lowest HR, RR and RT and time to lowest HR, RR and RT included mice that had at least 2 measurements taken. The lowest value was considered as dependent variable in multiple regression models as described for pCO2 and sedation.
All statistical analysis was conducted in the statistical software package SAS, version 9.4. Tests and models had 2-sided alternative hypothesis and α level equal to 0.05. If interaction terms were significant in the generalized linear models, pairwise combinations were examined. To control for multiple comparisons, α level was considered as 0.01 for these post hoc comparisons.
Results
Induction.
Summary statistics, by strain, sex, and treatment, are shown in Table 1. All animals achieved loss of righting reflex after IP injection. Five mice were excluded from analysis due to lack of recorded time for loss of righting reflex. Induction times were similar for both EX and KX (1.9 ± 0.5 min for EX and 1.8 ± 0.6 min KX, inclusive of all 4 strains and both sexes).
Table 1.
Effect of protocol on venous pH and pCO2 in mice
| Sex | Protocol | N obs | Mean pH ±SD | Mean pCO2 ±SD mm Hg | Normal 7.35 < pH ≤7.45 | Mild Acidosis 7.25 <pH ≤ 7.35 | Moderate Acidosis 7.15 <pH ≤ 7.25 | Severe Acidosis pH ≤ 7.15 | |
| Balb/c | |||||||||
| EX | 16 | 7.351 ± 0.029 | 32.619 ± 4.872 | 7(43.8%) | 9(56.3%) | . | . | ||
| KX | 17 | 7.350 ± 0.036 | 35.835 ± 7.528 | 9(52.9%) | 8(47.1%) | . | . | ||
| F | EX | 8 | 7.336 ± 0.026 | 32.450 ± 5.600 | 1(12.5%) | 7(87.5%) | . | . | |
| KX | 8 | 7.380 ± 0.022 | 30.088 ± 3.835 | 7(87.5%) | 1(12.5%) | . | . | ||
| M | EX | 8 | 7.366 ± 0.024 | 32.788 ± 4.409 | 6(75.0%) | 2(25.0%) | . | . | |
| KX | 9 | 7.324 ± 0.023 | 40.944 ± 6.170 | 2(22.2%) | 7(77.8%) | . | . | ||
| C57BL/6 | |||||||||
| EX | 17 | 7.320 ± 0.087 | 37.300 ± 6.709 | 8(47.1%) | 7(41.2%) | 1(5.9%) | 1(5.9%) | ||
| KX | 17 | 7.338 ± 0.043 | 42.900 ± 8.068 | 7(41.2%) | 10(58.8%) | . | . | ||
| F | EX | 9 | 7.287 ± 0.110 | 36.544 ± 7.685 | 2(22.2%) | 5(55.6%) | 1(11.1%) | 1(11.1%) | |
| KX | 9 | 7.365 ± 0.023 | 38.656 ± 5.435 | 6(66.7%) | 3(33.3%) | . | . | ||
| M | EX | 8 | 7.357 ± 0.027 | 38.150 ± 5.816 | 6(75.0%) | 2(25.0%) | . | . | |
| KX | 8 | 7.308 ± 0.041 | 47.675 ± 8.111 | 1(12.5%) | 7(87.5%) | . | . | ||
| CD1 | |||||||||
| EX | 16 | 7.323 ± 0.051 | 39.406 ± 5.188 | 3(18.8%) | 11(68.8%) | 2(12.5%) | . | ||
| KX | 17 | 7.362 ± 0.053 | 39.091 ± 6.252 | 12(70.6%) | 4(23.5%) | 1(5.9%) | . | ||
| F | EX | 8 | 7.320 ± 0.056 | 38.525 ± 5.538 | 1(12.5%) | 6(75.0%) | 1(12.5%) | . | |
| KX | 9 | 7.346 ± 0.060 | 40.716 ± 6.110 | 6(66.7%) | 2(22.2%) | 1(11.1%) | . | ||
| M | EX | 8 | 7.326 ± 0.050 | 40.288 ± 5.023 | 2(25.0%) | 5(62.5%) | 1(12.5%) | . | |
| KX | 8 | 7.382 ± 0.039 | 37.263 ± 6.281 | 6(75.0%) | 2(25.0%) | . | . | ||
| NU | |||||||||
| EX | 16 | 7.294 ± 0.054 | 37.769 ± 6.380 | 2(12.5%) | 11(68.8%) | 3(18.8%) | . | ||
| KX | 17 | 7.334 ± 0.056 | 42.624 ± 7.644 | 7(41.2%) | 8(47.1%) | 2(11.8%) | . | ||
| F | EX | 8 | 7.299 ± 0.054 | 39.000 ± 5.739 | 1(12.5%) | 6(75.0%) | 1(12.5%) | . | |
| KX | 9 | 7.373 ± 0.031 | 39.767 ± 6.896 | 7(77.8%) | 2(22.2%) | . | . | ||
| M | EX | 8 | 7.289 ± 0.058 | 36.538 ± 7.130 | 1(12.5%) | 5(62.5%) | 2(25.0%) | . | |
| KX | 8 | 7.291 ± 0.045 | 45.838 ± 7.544 | . | 6(75.0%) | 2(25.0%) | . | ||
| Total | |||||||||
| F | EX | 33 | 7.310 ± 0.069 | 36.627 ± 6.497 | 5(15.2%) | 24(72.7%) | 3(9.1%) | 1(3.0%) | |
| KX | 35 | 7.365 ± 0.038 | 37.513 ± 6.880 | 26(74.3%) | 8(22.9%) | 1(2.9%) | . | ||
| M | EX | 32 | 7.334 ± 0.051 | 36.941 ± 6.080 | 15(46.9%) | 14(43.8%) | 3(9.4%) | . | |
| KX | 33 | 7.326 ± 0.049 | 42.870 ± 7.866 | 9(27.3%) | 22(66.7%) | 2(6.1%) | . | ||
| All | |||||||||
| EX | 65 | 7.322 ± 0.062 | 36.782 ± 6.248 | 20(30.8%) | 38(58.5%) | 6(9.2%) | 1(1.5%) | ||
| KX | 68 | 7.346 ± 0.048 | 40.112 ± 7.800 | 35(51.5%) | 30(44.1%) | 3(4.4%) | . |
N obs: number of mice captured in measuring pH.
EX: Etomidate/Xylazine; F: female; KX:Ketamine/Xylazine; M: Male
Hyperexcitement.
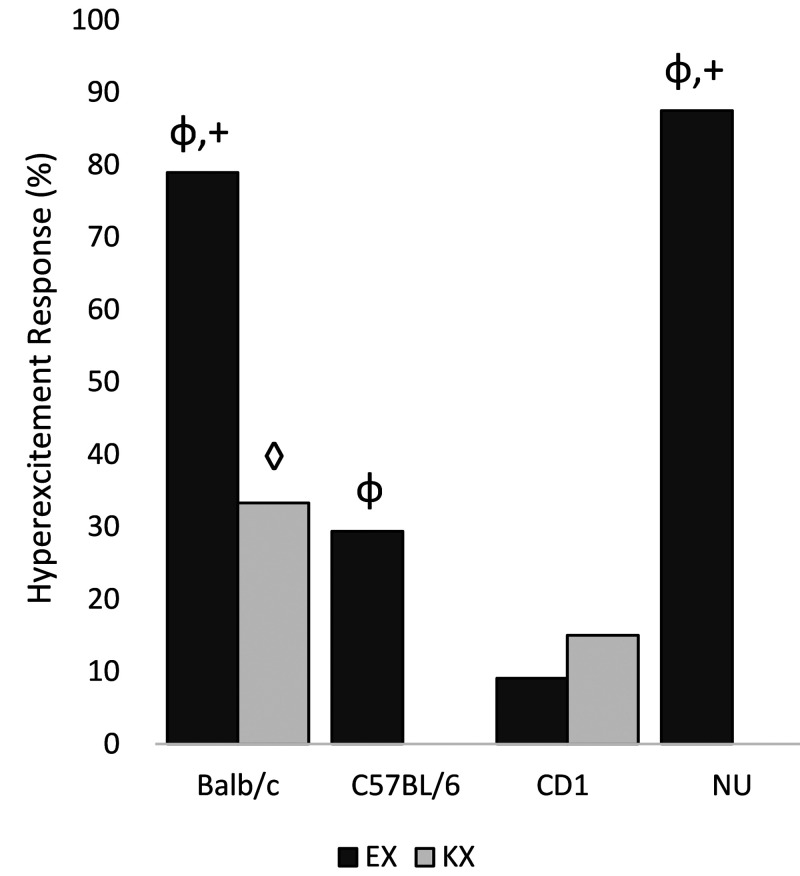
Data are shown in Figure 2. Several mice (49% EX, 12% KX) experienced an adverse reaction in the form of hyperexcitement during induction of anesthesia. Hyperexcitement was characterized by 4-legged vertical hopping, muscle shivering, and/or myoclonic jerking of one or more legs. This behavior typically occurred within 1 min of injection and ceased by 30 s before loss of righting reflex. All animals that experienced this behavior recovered normally, with no lingering gait abnormalities or neurologic deficits on visual observation.
Figure 2.
Percentage of mice that exhibited hyperexcitement on induction. φ: EX is more likely to cause hyperexcitement than KX in Balb/c, C57BL/6, and NU mice (P < 0.05). +: Balb/c and NU mice administered EX are more likely to experience hyperexcitement than C57Bl/6 and CD1 mice administered EX (P < 0.0001). ◊: Balb/c mice administered KX had higher rates of hyperexcitement than other strains administered KX (P = 0.0039).
When stratified by strain, the proportion of mice showing hyperexcitement was significantly higher with EX as compared with KX for strain BALB/c (EX: 79%, KX 33%, P = 0.0051), C57BL/6 (EX: 29%, KX: 0%, P = 0.0445) and NU (EX: 87%, KX: 0%, P < 0.0001), but not for strain CD1 (EX: 9%, KX:15%, P = 0.6560). When stratified by sex, hyperexcitement is significantly more likely to occur during with EX rather than KX in both females (EX: 46%, KX: 14%, P = 0.0020) and males (EX: 51%, KX: 11%, P = 0.0002). When comparing the 4 strains, stratified by treatment, mice that received EX showed significantly different proportions of hyperexcitement (BALB/c, 79%; C57BL/6, 21%; CD1, 9%; NU, 87%, P < 0.0001), with BALB/c and NU mice having higher rates than C57BL/6 and CD1. Mice that received KX also showed significant strain effects on proportions of hyperexcitement (BALB/c, 33%; C57BL/6, 0%; CD1, 15%; NU, 0%, P = 0.0039), with BALB/c having significantly higher rates than the other strains. When stratifying by sex, the proportions of hyperexcitement for females are significantly different among the 4 strains (P < 0.0001), yet for males the strain effect is not significant (P = 0.0861). When comparing the 2 sexes, no significant difference is found overall or stratified by strain.
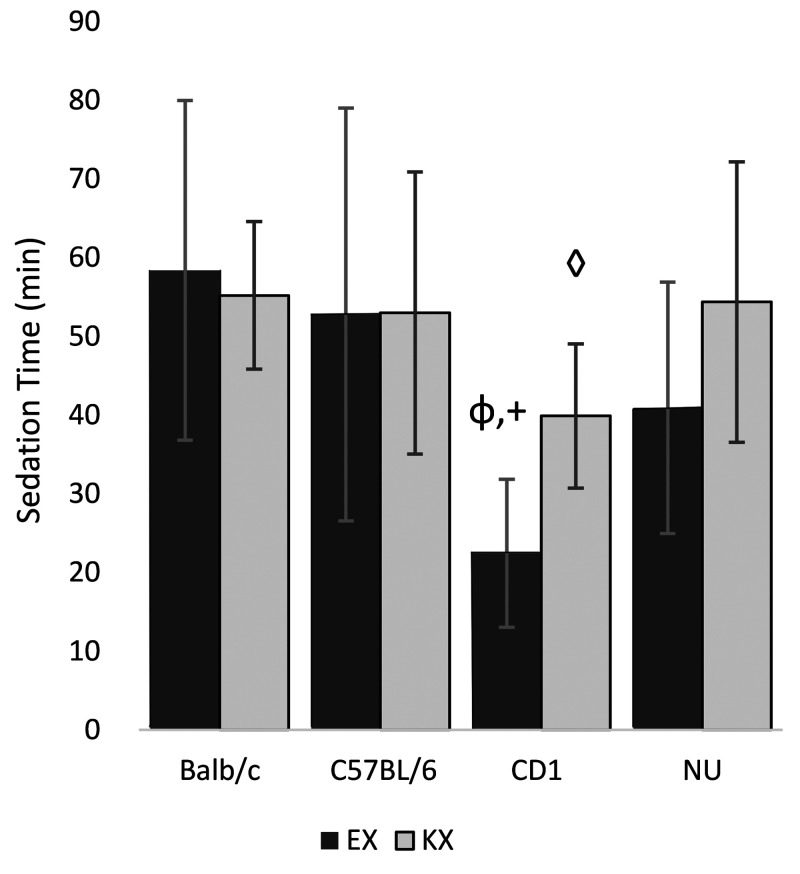
Sedation.
Data are shown in Figure 3. Six mice were excluded from analysis due to mortality (n = 1) or lack of recorded time for loss of righting reflex (n = 5). Overall, mice given EX had shorter sedation times (43 ± 24 min) than did mice given KX (52 ± 15 min). The statistically significant terms in the final generalized linear model are treatment, strain, sex, treatment by strain interaction and strain by sex interaction. Because the primary focus of this study is to compare the 2 drug treatments, the estimated mean from the term “treatment*strain” are presented. α = 0.01 is used for correction of multiple comparison.
Figure 3.
Mean sedation time ± SD φ: CD1 mice administered EX had shorter sedation times compared with other strains administered EX. (P < 0.01). +: CD1 mice administered EX had shorter sedation times than CD1 mice administered KX (P = 0.003). ◊: CD1 mice administered KX had shorter sedation times when compared with other strains administered KX. (P < 0.01).
Combining data from both sexes, with data controlled for sex, CD1 mice given EX had significantly shorter estimated mean sedation times than did those given KX (EX: 22 min, KX: 40 min, estimated difference = -17, SE = 5, P = 0.0003). Comparing the 4 strains given EX, including controlled data from both sexes, CD1 mice (22 min) had significantly shorter mean sedation times than did other strains (BALB/c = 59 min, estimated difference = -36 min, SE = 5, P < 0.0001; C57BL/6 = 53 min., estimated difference= -30 min., SE = 5, P < 0.0001; NU= 41 min, estimated difference= -19 min., SE = 5, P = 0.0002). Comparing the 4 strains when given KX, controlling for sex, CD1 mice (40 min) have statistically shorter mean sedation times than did the other strains (BALB/c = 54 min, estimated difference = -15 min., SE = 5, P = 0.0030; C57BL/6 = 54 min., estimated difference = -16, SE = 5, P = 0.0023; NU = 41 min, estimated difference = -14 min, SE = 5, P = 0.0063). In summary, the estimated mean sedation time for CD1 mice given EX is significantly shorter than for KX, and for both treatments, CD1 mice have significantly shorter estimated mean sedation times than other strains.
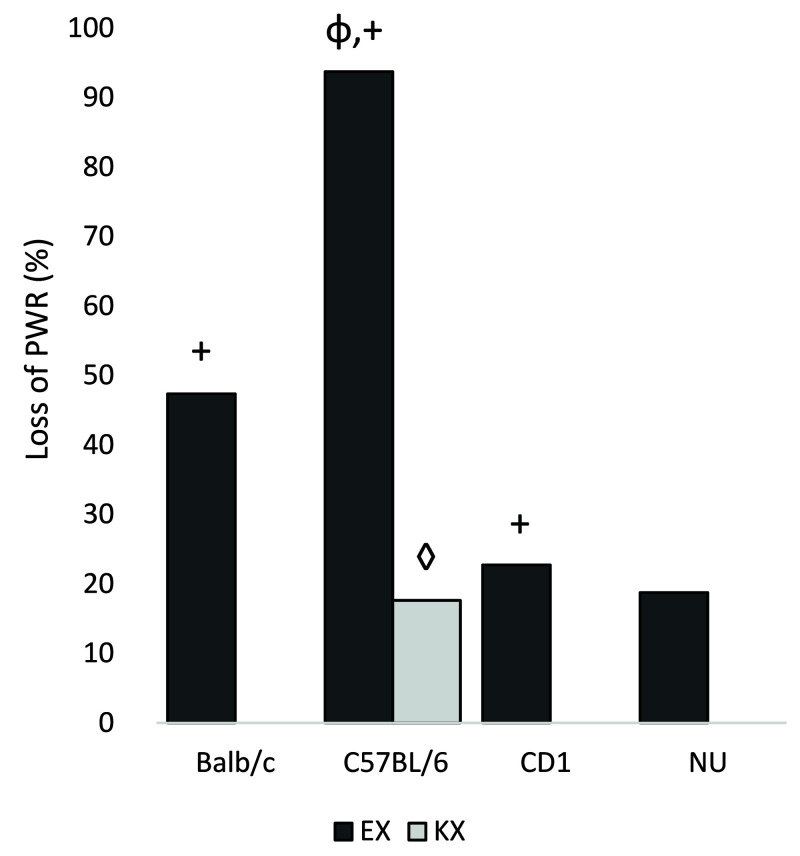
Loss of Pedal Withdraw Reflex.
Findings are shown in Figure 4. Two mice were excluded from analysis due to loss of recorded data for results of forceps or finger pinch. When comparing the 2 treatments, mice given EX (44%) were significantly more likely to lose PWR than were mice given KX (44% and 4%, respectively; P < 0.0001). When stratified by strain, mice given EX are significantly more likely to lose PWR than are mice given KX, as follows: BALB/c, EX:47%, KX:0%, P = 0.0012; CD1, EX: 23%, KX:0%, P = 0.049, and C57BL/6, EX: 94%, KX; 18% P < 0.0001. A similar trend was also seen in NU mice (EX: 19%, KX: 0%) (P = 0.0936).
Figure 4.
Percentage of mice with PWR loss. Φ: C57BL/6 mice administered EX were more likely to lose PWR than other strains administered EX (P < 0.001). +: Balb/c, C57BL/6, and CD1 mice administered EX were more likely to lose PWR than mice administered KX. (P < 0.05) ◊: C57BL/6 mice administered KX were more likely to lose PWR than other strains administered KX (P = 0.0228).
Stratifying by sex, both males and females given EX were significantly more likely to lose PWR than were mice given KX. When comparing strains, stratified by treatment, mice given EX had statistically different proportions of PWR loss (BALB/c:47%, C57BL/6:94%, CD1:23%, NU: 19%, P < 0.0001), with C57BL/6 and BALB/c mice achieving higher proportions. When given KX, the proportions of PWR loss are also significantly different among the 4 strains (BALB/c:0%, C57BL/6:18%, CD1:0%, NU:0%, P = 0.0228), with C57BL/6 achieving the highest proportion. Stratifying by sex and strain, males of the 4 strains had significantly different proportions (P < 0.0001) with C57BL/6 males achieving the highest proportion in both EX and KX treatments, while females did not show significant strain differences. When comparing loss of PWR between males and females, no statistically significant differences were found overall, or when the data were stratified by strain or treatment.
Duration of loss PWR.
PWR was assessed at 5-min intervals; therefore, an exact time of loss or return of PWR was not accurately determined. We assumed that a mouse that lost PWR at one time point was at a surgical anesthetic depth for > 0 min and less than or equal to 5 min; a mouse that lost PWR at 2 time points was at surgical anesthetic depth for greater than 5 min and less than or equal to 10 min; and so on. C57BL/6 males and females given EX had the longest median loss of PWR, with 4 time points and 2.5 time points respectively (approximately 15 to 20 min and 10 to 12.5 min). BALB/c mice given EX had a median loss of PWR for 2 time points (approx. 5 to 10 min). Both CD1 and NU mice given EX had a median loss of PWR for 1 time point (approx. 5 min or less). C57BL/6 males given KX had a median loss of PWR for 1 time point (approx. 5 min or less).
Recovery from anesthesia.
One mouse was excluded from analysis due to death. Times from recovery of the righting reflex to return of purposeful movement had large variations. The majority of mice became ambulatory and regained purposeful movement at the same time as return of righting reflex (recovery time of 0 min). The longest recovery time was more than 30 min. Overall, mice given EX had longer recovery times (6.5 ± 10.9 min) compared with mice given KX (2.6 ± 4.8 min). Male NU mice given EX had the longest average recovery time (16.4 ± 16.3 min) and male BALB/c mice given KX had the shortest average recovery time (0.4 ± 0.7 min).
Mortality.
Mortality for both protocols was zero (KX = 0%, n = 0) or very low (EX = 1.35%, n = 1). The single female C57BL/6 mouse that died under EX anesthesia had an abnormality noted on induction. As the observer retracted the plunger to ensure proper needle placement prior to injecting the anesthetic, 0.2 mL of clear fluid was aspirated. No exclusion criteria were set regarding IP injection, so the needle was redirected, and EX anesthesia administered. This mouse died 59 min after EX administration. Gross necropsy revealed an additional 1.4 mL of free fluid in the abdominal cavity. The volume of fluid administered during IP injection was 0.2 mL of anesthetic in addition to the 0.2 mL of aspirated fluid. Bladder integrity was tested by inflating the bladder with a dilute mixture of Hemacolor dye and saline. No rupture or leaks were noted. Cytology of the abdominal fluid revealed rare intact lymphocytes and macrophages. Histopathology revealed mild, focal adrenal cortical necrosis and hemorrhage. All other organs examined were unremarkable. All mice were observed daily for a minimum of 2 wk after anesthetic administration. No additional adverse effects were noted.
Acidosis/Venous pH.
Data are shown in Table 1. Only one mouse, the female C57BL/6 that died, showed severe acidosis (pH = 7.023). Fourteen mice were excluded from analysis because inadequate blood volumes were collected for blood gas measurement. Overall, no significant difference was found between likelihood of moderate-severe acidosis when comparing KX and EX protocols. Comparing the strains, when stratified by sex, male NU mice (25%) were significantly more likely to experience moderate-severe acidosis than males of other strains. (BALB/c: 0%, C57BL/6: 0%, CD1: 6%, P = 0.0235).
pCO2.
Data are shown in Table 1. Fourteen mice were excluded from analysis because inadequate blood volumes were collected for blood gas measurement. The statistically significant terms retained in the generalized linear model are treatment, strain, sex, their 2-way interactions, and their 3-way interaction. The estimated mean from the term “protocol*strain*sex” is presented in the following paragraph. α = 0.01 is used for correction of multiple comparisons.
Analysis in the complete model (treatment, sex, and strain) revealed that male mice given EX had significantly lower pCO2 averages than male mice given KX: BALB/c (EX:33 mm Hg, KX: 41 mm Hg, estimated difference -8, SE = 3, P = 0.0078), NU (EX: 37 mm Hg, KX: 46 mm Hg, estimated difference = -9, SE = 3, P = 0.0033), and C57BL/6 (EX: 38 mm Hg, KX: 48 mm Hg, estimated difference = -10, SE = 3, P = 0.0026), Among the females, BALB/c mice given KX (30 mm Hg) had significantly lower average pCO2 than females of other strains, as follows: CD1 (41 mm Hg, estimated difference=-11,SE = 3, P = 0.0006), NU (40 mm Hg, estimated difference = -10, SE = 3, P = 0.0017), and C57BL/6 (39 mm Hg, estimated difference = -9, SE = 3, P = 0.0053). Male CD1 mice given KX (37 mm Hg) had significantly lower average pCO2 than did NU (46 mm Hg, estimated difference = -9, SE = 3, P = 0.0066) and C57BL/6 (48 mm Hg, estimated difference = -10, SE = 3, P = 0.0011). When comparing males to females within strain, the mean pCO2 was significantly lower for the females than for the males among BALB/c mice given KX (F: 30 mm Hg, M: 41 mm Hg, estimated difference = -11, SE = 3, P = 0.0005) and C57BL/6 mice given KX (F:39 mm Hg, M: 48 mm Hg, estimated difference = -9, SE = 3, P = 0.0034).
Lowest Heart Rate (HR).
Data are shown in Table 2. Thirteen mice were excluded for having fewer than 2 time points, and an additional 3 mice were excluded for being outliers in the dataset. Their values were 446/min, 567/min and 596/min, resulting in a total of 16 mice excluded from analysis. The significant terms in the final model are treatment, strain and sex;.α = 0.01 is used to indicate statistical significance when comparing strains. When data were controlled for strain and sex, the estimated mean lowest HR for EX is 271 bpm and for KX is 255 bpm (estimated difference = 164, SE = 4, P < 0.0001) This indicates that the average lowest HR after EX administration is significantly higher than after KX, although both are lower than published normal ranges (low normal = 310 bpm).23,42 When data were controlled for treatment and sex, BALB/c mice have the lowest estimated mean lowest HR when compared with other strains. The estimated mean lowest HR for BALB/c mice (250 bpm) is significantly lower than CD1 (270 bpm, estimated difference = -19, SE = 6, P = 0.0008) and NU mice (265 bpm, estimated difference = -15, SE = 6, P = 0.0096). A statistical trend for this difference is also seen when BALB/c mice are compared with C57BL/6 (264 bpm, estimated difference = -14, SE = 6, P = 0.0150).
Table 2.
Summary statistics for lowest value and time to lowest value for HR and RR.
| Sex | Protocol | N | Lowest HR (beats/min) | Time to Lowest HR (min.) | Lowest RR (breaths/min) | Time to Lowest RR (min) | |
| BALB/c | |||||||
| EX | 19 | 254.06 ± 19.03(16) | 37.31 ± 21.47(16) | 115.79 ± 13.05(19) | 21.84 ± 18.29(19) | ||
| KX | 18 | 248.91 ± 3.56(11) | 11.36 ± 10.52(11) | 111.67 ± 8.57(18) | 27.11 ± 14.92(18) | ||
| F | EX | 10 | 257.63 ± 20.50(8) | 27.88 ± 19.92(8) | 114.00 ± 13.50(10) | 26.80 ± 18.63(10) | |
| KX | 8 | 248.17 ± 4.79(6) | 11.83 ± 14.43(6) | 110.00 ± 7.56(8) | 24.38 ± 14.86(8) | ||
| M | EX | 9 | 250.50 ± 18.08(8) | 46.75 ± 19.67(8) | 117.78 ± 13.02(9) | 16.33 ± 17.23(9) | |
| KX | 10 | 249.80 ± 1.10(5) | 10.80 ± 3.96(5) | 113.00 ± 9.49(10) | 29.30 ± 15.38(10) | ||
| C57BL/6 | |||||||
| EX | 17 | 278.00 ± 31.02(17) | 35.35 ± 22.09(17) | 102.35 ± 13.93(17) | 21.24 ± 18.85(17) | ||
| KX | 17 | 251.00 ± 10.81(16) | 24.13 ± 14.65(16) | 114.12 ± 12.28(17) | 34.82 ± 22.86(17) | ||
| F | EX | 9 | 292.11 ± 35.64(9) | 25.56 ± 19.76(9) | 101.11 ± 18.33(9) | 18.78 ± 23.34(9) | |
| KX | 9 | 257.13 ± 12.10(8) | 14.88 ± 6.24(8) | 115.56 ± 15.09(9) | 29.11 ± 12.89(9) | ||
| M | EX | 8 | 262.13 ± 14.29(8) | 46.38 ± 20.16(8) | 103.75 ± 7.44(8) | 24.00 ± 13.16(8) | |
| KX | 8 | 244.88 ± 4.29(8) | 33.38 ± 15.00(8) | 112.50 ± 8.86(8) | 41.25 ± 30.26(8) | ||
| CD1 | |||||||
| EX | 22 | 274.18 ± 28.78(17) | 13.35 ± 7.57(17) | 93.33 ± 13.90(21) | 11.33 ± 6.35(21) | ||
| KX | 20 | 265.75 ± 26.74(20) | 19.65 ± 11.52(20) | 103.00 ± 13.02(20) | 17.40 ± 8.25(20) | ||
| F | EX | 12 | 269.10 ± 23.63(10) | 14.20 ± 8.85(10) | 90.00 ± 14.14(12) | 12.08 ± 7.15(12) | |
| KX | 11 | 269.18 ± 33.86(11) | 16.55 ± 3.75(11) | 100.00 ± 10.95(11) | 17.64 ± 6.86(11) | ||
| M | EX | 10 | 281.43 ± 35.59(7) | 12.14 ± 5.67(7) | 97.78 ± 13.02(9) | 10.33 ± 5.34(9) | |
| KX | 9 | 261.56 ± 15.13(9) | 23.44 ± 16.37(9) | 106.67 ± 15.00(9) | 17.11 ± 10.13(9) | ||
| NU | |||||||
| EX | 16 | 277.00 ± 26.65(16) | 31.88 ± 13.75(16) | 95.00 ± 7.30(16) | 8.88 ± 5.80(16) | ||
| KX | 18 | 254.22 ± 9.83(18) | 22.56 ± 14.89(18) | 113.33 ± 10.85(18) | 21.50 ± 11.35(18) | ||
| F | EX | 8 | 288.00 ± 28.40(8) | 34.50 ± 16.41(8) | 97.50 ± 8.86(8) | 9.38 ± 4.21(8) | |
| KX | 9 | 257.22 ± 12.79(9) | 20.78 ± 9.95(9) | 114.44 ± 14.24(9) | 17.89 ± 7.75(9) | ||
| M | EX | 8 | 266.00 ± 20.96(8) | 29.25 ± 10.95(8) | 92.50 ± 4.63(8) | 8.38 ± 7.33(8) | |
| KX | 9 | 251.22 ± 4.60(9) | 24.33 ± 19.10(9) | 112.22 ± 6.67(9) | 25.11 ± 13.58(9) | ||
| Total | |||||||
| F | EX | 39 | 276.71 ± 29.91(35) | 24.89 ± 17.53(35) | 100.26 ± 16.46(39) | 16.85 ± 16.15(39) | |
| KX | 37 | 259.47 ± 21.88(34) | 16.44 ± 8.81(34) | 109.46 ± 13.53(37) | 21.95 ± 11.41(37) | ||
| M | EX | 35 | 264.48 ± 24.52(31) | 34.32 ± 20.52(31) | 103.24 ± 13.87(34) | 14.68 ± 12.79(34) | |
| KX | 36 | 252.35 ± 10.63(31) | 24.23 ± 16.61(31) | 111.11 ± 10.36(36) | 27.86 ± 19.56(36) | ||
| All | |||||||
| EX | 74 | 270.97 ± 27.99(66) | 29.32 ± 19.43(66) | 101.64 ± 15.28(73) | 15.84 ± 14.62(73) | ||
| KX | 73 | 256.08 ± 17.68(65) | 20.15 ± 13.59(65) | 110.27 ± 12.02(73) | 24.86 ± 16.13(73) |
Mean ± SD(n) for each outcome is presented, n represents captured mice.
EX: Etomidate/Xylazine; F: female; KX:Ketamine/Xylazine; M: Male
Time to lowest HR.
Data are shown in Table 2. The selected model for time to lowest HR retains the predictors of treatment, strain, sex, treatment*strain, strain*sex. The estimated mean for term “treatment*strain” is presented, and α = 0.01 is applied for correction of multiple comparisons. When data were controlled for sex, BALB/c mice given EX (37 min) had significantly longer average times to lowest HR than did BALB/c mice given KX (12 min, estimated difference = 25, SE = 6, P < 0.0001). When comparing strains, again using data controlled for sex, CD1 mice given EX (13.603 min) had significantly shorter mean time to lowest HR when compared with other strains (BALB/c = 37 min, estimated difference = -24, SE = 5, P < 0.0001; NU = 32 min., estimated difference = -18, SE = 5, P = 0.0005; C57BL/6 = 36 min., estimated difference = -22, SE =5, P < 0.0001).
To summarize, the HR-related data show that BALB/c mice had lower heart rates than other strains, yet BALB/c mice given EX took significantly longer to reach their lowest heart rate than did those given KX.
Lowest Respiration Rate (RR).
Data are shown in Table 2. One mouse was excluded for having less than 2 recorded time points due to an extremely short sedation time. The statistical model for lowest RR uses the terms of treatment, strain, and treatment*strain. The estimated mean from term “treatment*strain” are presented. α = 0.01 is used for correction of multiple comparisons.
When comparing treatments, the estimated mean lowest RR for NU mice given EX (95 breaths per min) is significantly lower than NU mice given KX (113 breaths per min, estimated difference = -18, SE = 4, P < 0.0001); the estimated mean lowest RR for C57BL/6 mice given EX (102 breaths per min) is significantly lower than C57BL/6 mice given KX (114 breaths per min, estimated difference = -12, SE = 4, P = 0.0048).
When comparing strains, BALB/c mice given EX (116 breaths per min) had a mean lowest RR that was significantly higher than in the other strains given EX (CD1 = 93 breaths per min, estimated difference = 22, SE = 4, P < 0.0001; NU = 95 breaths per min, estimated difference = 21, SE = 4, P < 0.0001; C57BL/6 = 102 breaths per min, estimated difference = 13, SE = 4, P = 0.0010); CD1 mice given KX (103 breaths per min) had an estimated mean lowest RR that was significantly lower than NU mice (113 breaths per min, estimated difference=-10, SE = 4, P = 0.0087) and C57BL/6 mice (114 breaths per min, estimated difference = -11, SE = 4, P = 0.0055) given KX. A similar trend was detected when comparing CD1 mice given KX to BALB/c mice given KX (112 per min, estimated difference = -9, P = 0.0237), however this does not pass the test of significance. All estimated mean lowest RR, regardless of strain, sex, or treatment, were below published normal ranges (normal: 163 breaths per min).18,42
Time to lowest RR.
Data are shown in Table 2. The selected model for time to lowest RR includes the variables of treatment and strain. The estimated mean from terms “treatment” and “strain” are presented. Correction for multiple comparison (α = 0.01) is applied when comparing strains.
When data were controlled for strain, the estimated mean time to lowest RR was significantly shorter in mice given EX (16 min) than in mice given KX (25 min, estimated difference = -9, SE = 2, P = 0.0002). When comparing among strains, regardless of sex or treatment, BALB/c mice (25 min) had a significantly longer mean time to lowest RR than CD1 (14 min, estimated difference = 10, SE = 3, P = 0.0023) and NU mice (15 min, estimated difference = 9, SE = 3, P = 0.0077). C57BL/6 mice (28 min) had a significantly longer estimated mean time to lowest RR than did CD1 (14 min, estimated difference = 14, SE = 3, P < 0.0001) or NU mice (15 min, estimated difference = 13, SE = 3.488, P = 0.0004). NU mice had the shortest times to mean lowest RR.
Lowest Rectal Temperature.
Data are shown in Table 3. One mouse was excluded for having less than 2 recorded time points due to an extremely short sedation time. The model for lowest RT uses the terms treatment, strain, sex and treatment*strain. The estimated mean from term “treatment*strain” are presented.
Table 3.
Summary statistics for lowest value and time to lowest value for RT and SBP.
| Sex | Protocol | N | Lowest RT (°F) | Time to Lowest RT (min.) | Lowest SBP (mm Hg) | Time to Lowest SBP (min) | |
| BALB/c | |||||||
| EX | 19 | 93.75 ± 1.40(19) | 46.32 ± 15.05(19) | 69.37 ± 20.98(19) | 33.79 ± 23.65(19) | ||
| KX | 18 | 93.11 ± 1.11(18) | 47.56 ± 10.07(18) | 70.22 ± 19.60(18) | 28.44 ±17.76(18) | ||
| F | EX | 10 | 93.85 ± 1.36(10) | 38.60 ± 6.98(10) | 78.90 ± 20.95(10) | 24.80 ± 11.44(10) | |
| KX | 8 | 93.29 ± 1.44(8) | 42.13 ± 7.30(8) | 78.25 ± 22.17(8) | 17.00 ± 14.40(8) | ||
| M | EX | 9 | 93.64 ± 1.53(9) | 54.89 ± 17.25(9) | 58.78 ± 16.02(9) | 43.78 ± 29.97(9) | |
| KX | 10 | 92.97 ± 0.82(10) | 51.90 ± 10.14(10) | 63.80 ± 15.49(10) | 37.60 ± 15.01(10) | ||
| C57BL/6 | |||||||
| EX | 17 | 92.90 ± 1.93(17) | 40.06 ± 16.74(17) | 81.71 ± 21.20(17) | 17.65 ± 10.52(17) | ||
| KX | 17 | 92.05 ± 1.62(17) | 51.24 ± 17.02(17) | 90.06 ± 16.83(17) | 16.41 ± 14.18(17) | ||
| F | EX | 9 | 93.46 ± 2.19(9) | 35.561 ± 5.22(9) | 84.22 ± 20.60(9) | 18.11 ± 7.87(9) | |
| KX | 9 | 93.22 ± 1.30(9) | 38.44 ± 5.41(9) | 97.67 ± 10.06(9) | 11.33 ± 3.71(9) | ||
| M | EX | 8 | 92.28 ± 1.49(8) | 45.13 ± 17.90(8) | 78.88 ± 22.91(8) | 17.13 ± 13.48(8) | |
| KX | 8 | 90.74 ± 0.57(8) | 65.63 ± 13.48(8) | 81.50 ± 19.33(8) | 22.13 ± 19.32(8) | ||
| CD-1 | |||||||
| EX | 22 | 96.30 ± 2.14(21) | 22.05 ± 9.46(21) | 62.67 ± 10.94(18) | 17.22 ± 8.04(18) | ||
| KX | 20 | 93.51 ± 2.56(20) | 35.00 ± 8.42(20) | 64.95 ± 9.41(20) | 25.85 ± 11.79(20) | ||
| F | EX | 12 | 95.85 ± 2.17(12) | 25.25 ± 9.52(12) | 61.91 ± 7.63(11) | 18.91 ± 7.94(11) | |
| KX | 11 | 93.90 ± 1.46(11) | 34.82 ± 8.36(11) | 65.36 ± 9.07(11) | 23.64 ± 11.97(11) | ||
| M | EX | 10 | 96.90 ± 2.06(9) | 17.78 ± 7.95(9) | 63.86 ± 15.48(7) | 14.57 ± 8.04(7) | |
| KX | 9 | 93.02 ± 3.52(9) | 35.22 ± 9.00(9) | 64.44 ± 10.35(9) | 28.56 ± 11.66(9) | ||
| NU | |||||||
| EX | 16 | 91.17 ± 1.73(16) | 36.88 ± 17.26(16) | 78.69 ± 21.95(16) | 17.00 ± 13.64(16) | ||
| KX | 18 | 90.34 ± 2.10(18) | 49.78 ± 15.24(18) | 70.83 ± 19.99(18) | 28.28 ± 17.91(18) | ||
| F | EX | 8 | 91.65 ± 2.05(8) | 38.38 ± 22.42(8) | 90.88 ± 19.13(8) | 17.38 ± 16.48(8) | |
| KX | 9 | 91.17 ± 1.63(9) | 40.11 ± 15.06(9) | 83.11 ± 19.48(9) | 17.56 ± 12.12(9) | ||
| M | EX | 8 | 90.69 ± 1.32(8) | 35.38 ± 11.43(8) | 66.50 ± 18.09(8) | 16.63 ± 11.26(8) | |
| KX | 9 | 89.51 ± 2.28(9) | 59.44 ± 7.52(9) | 58.56 ± 11.44(9) | 39.00 ± 16.63(9) | ||
| Total | |||||||
| F | EX | 39 | 93.92 ± 2.43(39) | 33.74 ± 14.61(39) | 77.76 ± 20.15(38) | 19.95 ± 11.07(38) | |
| KX | 37 | 92.94 ± 1.76(37) | 38.57 ± 9.72(37) | 80.32 ± 19.28(37) | 17.73 ± 11.70(37) | ||
| M | EX | 35 | 93.49 ± 2.81(34) | 38.18 ± 19.66(34) | 66.84 ± 19.06(32) | 23.94 ± 21.77(32) | |
| KX | 36 | 91.62 ± 2.57(36) | 52.67 ± 14.93(36) | 66.58 ± 16.17(36) | 32.25 ± 16.54(36) | ||
| All | |||||||
| EX | 74 | 93.72 ± 2.60(73) | 35.81 ± 17.17(73) | 72.77 ± 20.27(70) | 21.77 ± 16.81(70) | ||
| KX | 73 | 92.29 ± 2.28(73) | 45.52 ± 14.35(73) | 73.55 ± 19.00(73) | 24.89 ± 15.96(73) |
Mean ±SD(n) for each outcome is presented, n represents captured mice.
For SBP, cuff size is not grouped.
EX: Etomidate/Xylazine; F: female; KX:Ketamine/Xylazine; M: Male.
When comparing treatments within strains, independent of sex, CD1 mice given EX had an estimated mean lowest RT of 96.2 °F (35.7 °C), 2.8 °F (1.5 °C) higher than the mean lowest RT of CD1 mice given KX (93.5 °F/34.1 °C), SE = 0.6, P < 0.0001. When comparing strains, CD1 mice given EX also had a significantly higher lowest mean RT than did other strains given EX (BALB/c= 93.7 °F/34.3 °C, estimated difference = 2.5 °F/1.4 °C, SE = 0.6, P < 0.0001; NU = 91.2 °F/32.9 °C, estimated difference = 5.1 °F/2.8 °C, SE = 0.6, P < 0.0001; C57BL/6 = 92.9 °F/33.8 °C, estimated difference = 3.4 °F/1.9 °C, SE = 0.6, P < 0.0001). Conversely, the estimated mean lowest temperature for NU mice was significantly lower than in other strains, regardless of treatment. The estimated mean lowest temperature for NU mice given EX was 91.2 °F/32.9 °C, which is significantly lower than other strains given EX: BALB/c (93.7 °F/34.3 °C, estimated difference= -2.6 °F/-1.4 °C, SE = 0.6, P < 0.0001), CD1 (96.2 °F/35.7 °C, estimated difference= -5.1 °F/-2.8 °C, SE = 0.6, P < 0.0001) and C57BL/6 (92.9 °F/33.8 °C), estimated difference= -1.7 °F/-0.9 °C, SE = 0.6, P = 0.0093). The estimated mean lowest temperature for NU mice given KX was 90.3 °F/32.4 °C, which is significantly lower than in other strains given KX: BALB/c (93.2 °F/34.0 °C, estimated difference = -2.8 °F/-1.6 °C, SE = 0.6, P < 0.0001), CD1 (93.5 °F/34.1 °C, estimated difference = -3.1 °F/-1.7 °C, SE = 0.6, P < 0.0001) and C57BL/6 (92.0 °F/ 33.355 °C, estimated difference = -1.7 °F/-0.9 °C, SE = 0.6, P = 0.0080). All estimated mean lowest RT are below published normal ranges (normal: 98.8 to 99.3 °F/37.1 to 37.4 °C).18,42
Time to lowest RT.
Data are shown in Table 2. The selected model for time to lowest RT contains terms of treatment, strain, sex, treatment*sex and strain*sex. As the primary intent was to compare the 2 anesthetic treatments, the estimated mean from term “treatment*sex” is presented. Male mice given EX have significantly shorter mean time (39 min) to lowest RT than do male mice given KX (53 min, estimated difference = 14, SE = 3, P < 0.0001).
Lowest SBP.
Data are shown in Table 3. Four mice were excluded because they did not have at least 2 recorded time points for analysis. The mean lowest SBP for EX was 72.77 mm Hg and for KX was 73.55 mm Hg. Cuff size affected blood pressure recordings, despite our following manufacturing instructions. Mice assigned extra small cuffs had higher SBP than mice assigned small cuffs (S: 61.400 mm Hg, XS: 86.147 mm Hg).
Time to lowest SBP.
Data are shown in Table 3. Four mice were excluded because they did not have at least 2 recorded time points for analysis. Time to lowest SBP had huge variation within treatment (22 ± 17 min and 25 ± 16 min for EX and KX, respectively, with all strains and both genders included in the analysis.
Association between pCO2 and pH.
When including all treatments, strains and sexes in the analysis, the Pearson correlation coefficient between pCO2 and pH is -0.4071, P < 0.0001. Based on the multivariable regression model, with every 0.01 unit increase in pH, pCO2 significantly falls by 0.6122 mm Hg [SE = 0.1120, 95% CI (-0.8338, -0.3906); P < 0.0001].
Discussion
KX and EX protocols were both effective for sedation of BALB/c, C57BL/6 mice and NU mice, with a range of approximately 40 to 60 min of sedation time. CD1 mice had significantly shorter sedation times, although this was most pronounced for EX, producing an average sedation time of only 22 min. CD1 mice given KX also had significantly shorter sedation times than other strains. These times are consistent with other studies of BALB/c and CD1 mice given KX anesthesia.4,9,30 Overall, females had slightly lower sedation times for a given anesthetic treatment than did males. This was consistent among all strains and both treatments, with the exception of EX administered to CD1 and NU mice. However, males and females of CD1 and NU mice were not statistically different. Recovery was smooth, uneventful and occurred within approximately 10 min of resuming the righting reflex for all treatment groups.
The only protocol that produced reliable surgical anesthesia was EX in C57BL/6 mice, in which 93% of C57BL/6 (100% in males, 87% in females) lost pedal withdraw reflex for 12 to 20 min. EX was also more likely to produce surgical anesthesia than KX in all other strains, but only in 20% to 50% of mice depending on strain. With the exception of C57BL/6 mice, females were more likely to reach surgical anesthesia than males. This contradicts previous findings.30 This finding seems contradictory to the lower sedation times exhibited by females. The combination of etomidate and xylazine had not been evaluated prior to the current study, but when used as sole agent, etomidate did not produce surgical anesthesia in previous studies.21,29 Our results suggest that the addition of xylazine increased the likelihood of achieving surgical anesthesia, but was not consistent enough for invasive procedures in any strain except C57BL/6 at the current dose.
Despite multiple recommendations for the current dose of KX anesthesia as a surgical anesthetic, few mice (3 of 72) given KX lost pedal withdraw reflex in our study.16,17,23,26 Our results with KX are not unique. In one study, BALB/c mice that received the same dose of KX did not reach adequate surgical anesthesia.4 Several other studies used similar KX protocols with the similar results (100 mg/kg K, 20 mg/kg X in male HanIbm:NMRI and 95 mg/kg K, 7 mg/kg X in CD1 mice).3,9 Several reasons could account for our study's lack of surgical anesthesia under KX, contradicting anecdotal information of efficacy and common practice of use. As our study confirms, surgical efficacy can vary depending on strain. Also, dose recommendations and usage can vary widely, with the use of higher doses resulting in more consistent surgical anesthesia.3 Finally, significant variability is present in the methods used to determine surgical anesthesia. Anesthesia in laboratory animals is defined as a “state of unconsciousness, analgesia, muscle relaxation, and a-reflexia.”27 A state of unconsciousness is difficult to assess in animals, with EEG being the most accurate yet generally impractical method.22 Therefore, operators must analyze other parameters that, when combined, suggest a state of unconsciousness. Loss of spinal reflexes and analgesia can be assessed based on reaction to a noxious stimulus, as is done with pedal withdraw reflex. Previous studies have suggested that level of consciousness cannot be directly correlated with loss of reflexes;22 however, pedal withdraw reflex still remains the most noninvasive and accurate way to determine surgical anesthetic depth for most researchers.3 If reflexes remain, the animal cannot be said to have reached a surgical plane of anesthesia. The current study minimized the risk of erroneously determining that mice had reached surgical anesthesia by using 2 PWR methods. However, the use of 2 methods resulted in several instances in which mice were negative for one method but not for the other. If only one method had been used in this study, the frequency and duration of surgical anesthesia would have been higher, possibly explaining the variance from previous literature concerning KX efficacy.
Several mice experienced an adverse reaction during anesthetic induction in the form of hyperexcitement before loss of righting reflex. This reaction was significantly higher in mice that received EX (49%) as compared with KX (12%), with the most occurrences seen in BALB/c (79%) and NU (87%) mice given EX. Fewer of these reactions occurred in C57BL/6 mice given EX, with approximately one third of subjects experiencing hyperexcitement. Myoclonic spasms and hyperexcitement have been previously reported with the use of etomidate in both mice and humans.13,14,20,21 The addition of xylazine to our anesthetic protocol did not eliminate this effect. Pain and thrombophlebitis have also been associated with intravenous administration of etomidate and may be a contributing factor to the hyperexcitement reaction noted after IP injection.11,28 The pain of intravenous etomidate administration is attributed to the use of propylene glycol as a solvent, which is the formulation used in the current study.10 While irritation after IP injection cannot be completely ruled out, the authors believe that pain is unlikely to be associated with the hyperexcitement seen during induction. If pain were a contributing factor for hyperexcitement, all or most of the animals that received etomidate would have exhibited hyperexcitement, which was not the case for C57BL/6 and CD1 mice in this study. In addition, other clinical symptoms of pain in mice, such as blephorospasm, hunched posture, piloerection, and a reluctance to move, were not observed during the induction period and have not been reported in previous IP etomidate literature. Although no mice exhibited lingering adverse effects from the hyperexcitement reaction, the potential for secondary injury must be considered when using EX anesthesia.
Respiratory status was evaluated by measuring respiratory rate, PvCO2 and venous pH. Although measurement of arterial blood gases would have been preferable to venous samples to determine respiratory status, the amount of blood required for blood gas analysis would have been difficult to obtain from an artery without invasive methods. Instead, venous blood was chosen to avoid the confounding variable of invasive arterial blood collection. pH values between venous and arterial samples have previously shown to be comparable, with arterial pH typically 0.03 higher than venous pH.6 PvO2 was not evaluated because of the larger expected difference between venous and arterial oxygen. To evaluate clinically relevant differences in blood gas values, we divided venous pH values into 2 categories: normal-mild acidosis (7.45 ≥ pH greater than or equal to 7.25) or moderate-severe acidosis (pH less than or equal to 7.24). Acidosis commonly occurs during anesthesia as a result of decreased alveolar ventilation or due to reduced tissue perfusion.12 Injectable anesthetics, including the ones used in this study, are known to induce respiratory depression and peripheral vasoconstriction.17,25,38 Acidosis can affect several organ systems, but has its most pronounced impact on the cardiovascular system. At mild levels of acidosis, cardiac output is reduced and arterial vasodilation develops, resulting in mild hypotension. During moderate acidosis, cardiac output can increase due to catecholamine release. In severe acidosis (< 7.1), a resistance to catecholamines develops, resulting in severe hypotension, cardiovascular dysfunction, decreased cardiac output, and arrhythmias that can lead to death.34,36,41 The majority of mice in both treatment groups were in the normal-mild acidosis ranges (89% EX, 96% KX), and only one mouse had severe acidosis. No significant difference was found between likelihood of developing moderate-severe acidosis when comparing KX and EX. In one significant strain-associated difference in acidosis, male NU mice were more likely to exhibit moderate-severe acidosis than were males of other strains, regardless of treatment (EX pH = 7.29 ± 0.06; KX pH = 7.29 ± 0.04).
Lowest average respiratory rates were below normal for all treated mice in this study. Overall, EX mice exhibited lower RR than KX mice (with the exception of BALB/c mice). However, this did not result in a significant difference between the treatments. NU mice given EX were the quickest to reach their lowest RR (9 ± 6 min.) Because of this, the higher rate of moderate acidosis noted in male NU mice could be attributed to experiencing low RR for a potentially longer period of time. The low respiratory rates and the negative correlation between pCO2 and pH suggest that respiratory acidosis (as opposed to metabolic acidosis) is occurring in these mice. If arterial blood gas sampling had been performed, pH would likely be higher and pCO2 likely lower for these animals, given the normal relationship between arterial and venous blood gas sampling.6 Future studies evaluating arterial blood gases could be useful to better characterize the respiratory effects of EX compared with KX.
Heart rate (HR) analysis revealed that mice given EX had significantly higher HR at their nadir when compared with mice given KX; however, all lowest mean HR values (245 to 295 bpm) were below normal range (310 to 840 bpm).18,42 Time to lowest mean HR was significantly shorter in CD1 mice as compared with other strains, as would be expected considering the significantly shorter sedation times of CD1 mice. Etomidate and ketamine are both reported to have minimal cardiovascular effects.17,37 The bradycardia recorded in this study is most likely a result of administering equal doses of an α2-agonist, xylazine, in both protocols, which is known to decrease HR.17,25 This effect is also the most likely reason that no significant differences were noted between EX and KX in average lowest systolic blood pressure (SBP), although the significantly higher HR of mice given EX may indicate that etomidate has less cardiovascular impact than ketamine. More precise analysis of etomidate effects on the cardiovascular system could be performed with the use of indwelling arterial catheters or telemetry systems. However, such methodology was beyond the scope of this study.43
All mice exhibited marked hypothermia under sedation despite being placed on a heating pad. Hypothermia is a known effect of xylazine and tends to persist despite the provision of thermoregulatory support.3,17 Hypothermia was especially significant in NU mice, regardless of treatment (mean lowest RT: EX, 91.2 °F/32.9 °C; KX, 90.3 °F/32.4 °C). Sedation times of NU mice did not vary significantly from other strains, so the lower temperatures are most likely attributed to the lack of insulating fur inherent in the NU phenotype. CD1 mice maintained significantly higher rectal temperature (RT) at their lowest average when compared with other strains, which may be attributed to their short sedation time. Low sedation times could have attenuated the effect of anesthetically suppressed thermoregulation that often occurs during anesthesia.
Mortality was low for both KX and EX protocols. No deaths occurred in mice given KX, which is consistent with mortality reported in previous studies.3,4,8,9 One death occurred in a C57BL/6 female given EX, resulting in an EX mortality rate of 1.35%. Although the definitive cause of death in this mouse could not be determined, it was probably not due to the EX itself. More likely the animal had a preexisting condition, given the large amount of ascites found during necropsy, in conjunction with the fluid aspirated during induction. Although the bladder could have been penetrated during anesthetic injection, bladder rupture seems unlikely as the source of ascites because of the normal bladder integrity on necropsy and the large volume of fluid recovered. The only lesion found, mild adrenocortical necrosis and hemorrhage, can be caused by multiple conditions such as stress, ischemia, inflammation, systemic neoplasia, and exogenous chemicals. Etomidate has been known to cause an acute adrenocortical insufficiency in humans. However, we are not aware of reports that associate etomidate with adrenal necrosis and hemorrhage.13 The low mortality in this study provides further support for etomidate's wide therapeutic index.20,21
In summary, our hypothesis that etomidate/xylazine will be equal to or greater than ketamine/xylazine in anesthetic efficacy and safety is supported by our data. EX is as effective as KX at producing sedation in all 4 strains tested, although CD1 mice exhibited significantly shorter sedation times with both treatments. At doses tested, EX was found to be a more effective surgical anesthetic than KX; however, the durations of surgical anesthesia were short and not consistent for any strain except C57BL/6 mice. Safety profiles were similar between the 2 protocols, with low mortality and no significant differences between SBP, RR, RT, or the likelihood of developing moderate-severe acidosis. As expected, sex and strain related differences were identified, including: decreased sedation times in CD1 mice, more pronounced hypothermia in NU mice, and higher likelihood of achieving surgical anesthesia in females as compared with males.
We conclude that EX could be used in all tested strains for short, nonpainful procedures that require immobility. However, EX should be used with caution in some strains, such as BALB/c and NU mice, due to their higher likelihood of hyperexcitement on induction. EX would be particularly useful in C57BL/6 mice, which achieve consistent levels of surgical anesthesia for 15 to 20 min and show a low frequency of hyperexcitement on induction. Finally, researchers should be aware of previously reported physiologic effects after etomidate administration, such as acute adrenal insufficiency, which could potentially confound some experiments. In the future, testing a wider range of etomidate-xylazine doses could provide useful information on the frequency and duration of surgical anesthesia. Adding an opioid or benzodiazepine to the current etomidate protocol could further reduce the hyperexcitement seen on induction and increase the duration of surgical anesthesia. Further study is needed to refine and optimize the use of etomidate as an injectable anesthetic.
Acknowledgments
The authors would like to thank Leela Greeter RLAT, Andrey Krasnopeyev ALAT, Jhanay Jones and Alicia Szewczyk BS for their contribution and participation in this project. We would also like to thank Emory's Division of Animal Resources veterinary and husbandry staff for their care of our mice. Finally, we would like to acknowledge our mice and their contribution to biomedical research. This work was supported by Emory University departmental funds.
References
- 1.Amlong CA, Perkins MG, Houle TT, Miller KW, Pearce RA. 2016. Contrasting effects of the γ-aminobutyric acid type a receptor β3 subunit N265M mutation on loss of righting reflexes induced by etomidate and the novel anesthetic barbiturate R-mTFD–MPAB. Anesth Analg 123:1241–1246. 10.1213/ANE.0000000000001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andreae MH, Rhodes E, Bourgoise T, Carter GM, White RS, Indyk D, Sacks H, Rhodes R. 2016. An ethical exploration of barriers to research on controlled drugs. Am J Bioeth 16:36–47. 10.1080/15265161.2016.1145282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 4.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 5.Butovas S, Rudolph U, Jurd R, Schwarz C, Antkowiak B. 2010. Activity patterns in the prefrontal cortex and hippocampus during and after awakening from etomidate anesthesia. Anesthesiology 113:48–57. 10.1097/ALN.0b013e3181dc1db7. [DOI] [PubMed] [Google Scholar]
- 6.Byrne AL, Bennett M, Chatterji R, Symons R, Pace NL, Thomas PS. 2014. Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis. Respirology 19:168–175. 10.1111/resp.12225. [DOI] [PubMed] [Google Scholar]
- 7.Cagle LA, Franzi LM, Epstein SE, Kass PH, Last JA, Kenyon NJ. 2017. Injectable anesthesia for mice: combined effects of dexmedetomidine, tiletamine-zolazepam, and butorphanol. Anesthesiol Res Pract 2017:9161040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chaves AA, Weinstein DM, Bauer JA. 2001. Non-invasive echocardiographic studies in mice: influence of anesthetic regimen. Life Sci 69:213–222. 10.1016/S0024-3205(01)01123-7. [DOI] [PubMed] [Google Scholar]
- 9.Dholakia U, Clark-Price SC, Keating SCJ, Stern AW. 2017. Anesthetic effects and body weight changes associated with ketamine-xylazine-lidocaine administered to CD-1 mice. PLoS One 12:1–11. 10.1371/journal.pone.0184911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Doenicke A, Kugler A, Vollmann N. 1990. Venous tolerance to etomidate in lipid emulsion or propylene glycol (hypnomidate). Can J Anaesth 37:823–824. 10.1007/BF03006547. [DOI] [PubMed] [Google Scholar]
- 11.Doenicke AW, Roizen MF, Kugler J, Kroll H, Foss J, Ostwald P. 1999. Reducing myoclonus after etomidate. Anesthesiology 90:113–119. 10.1097/00000542-199901000-00017. [DOI] [PubMed] [Google Scholar]
- 12.Ellison RG, Ellison LT, Hamilton WF. 1955. Analysis of respiratory acidosis during anesthesia. Ann Surg 141:375–382. 10.1097/00000658-195503000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erdoes G, Basciani RM, Eberle B. 2014. Etomidate—a review of robust evidence for its use in various clinical scenarios. Acta Anaesthesiol Scand 58:380–389. 10.1111/aas.12289. [DOI] [PubMed] [Google Scholar]
- 14.Erhardt W, Hebestedt A, Aschenbrenner G, Pichotka B, Blümel G. 1984. A comparative study with various anesthetics in mice (pentobarbitone, ketamine-xylazine, carfentanyl-etomidate). Res Exp Med (Berl) 184:159–169. 10.1007/BF01852390. [DOI] [PubMed] [Google Scholar]
- 15.Filibeck U, Castellano C. 1980. Strain dependent effects of ketamine on locomotor activity and antinociception in mice. Pharmacol Biochem Behav 13:443–447. 10.1016/0091-3057(80)90252-X. [DOI] [PubMed] [Google Scholar]
- 16.Flecknell P. 2016. Anaesthesia of common laboratory species: special considerations, p 193–256. Chapter 5. In: Flecknell P, editor. Laboratory animal anaesthesia, 4th ed Boston: Academic Press. [Google Scholar]
- 17.Flecknell P, Lofgren JLS, Dyson MC, Marini RR, Michael Swindle M, Wilson RP. 2015. Preanesthesia, anesthesia, analgesia, and euthanasia, p 1135–1200. Chapter 24. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT. editors. Laboratory animal medicine, 3rd ed Boston (MA): Academic Press. [Google Scholar]
- 18.Foster HL, Small JD, Fox JG, Hutton JJ. 1985. The mouse in biomedical research. Volume 3. Normative biology, immunology, and husbandry. Q Rev Biol 60:251–251. 10.1086/414412. [DOI] [Google Scholar]
- 19.Gargiulo S, Greco A, Gramanzini M, Esposito S, Affuso A, Brunetti A, Vesce G. 2012. Mice anesthesia, analgesia, and care, Part I: anesthetic considerations in preclinical research. ILAR J 53:E55–E69. 10.1093/ilar.53.1.55. [DOI] [PubMed] [Google Scholar]
- 20.Gomwalk NE, Healing TD. 1981. Etomidate: a valuable anaesthetic for mice. Lab Anim 15:151–152. 10.1258/002367781780959099. [DOI] [PubMed] [Google Scholar]
- 21.Green CJ, Knight J, Precious S, Simpkin S. 1981. Metomidate, etomidate and fentanyl as injectable anaesthetic agents in mice. Lab Anim 15:171–175. 10.1258/002367781780958919. [DOI] [PubMed] [Google Scholar]
- 22.Haberham ZL, van den Brom WE, Venker-van Haagen AJ, Baumans V, de Groot HN, Hellebrekers LJ. 1999. EEG evaluation of reflex testing as assessment of depth of pentobarbital anaesthesia in the rat. Lab Anim 33:47–57. 10.1258/002367799780578570. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrandt IJ, Su H, Weber WA. 2008. Anesthesia and other considerations for in vivo imaging of small animals. ILAR J 49:17–26. 10.1093/ilar.49.1.17. [DOI] [PubMed] [Google Scholar]
- 24.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): The National Academies Press. [Google Scholar]
- 25.Janssen CF, Maiello P, Wright MJ, Jr, Kracinovsky KB, Newsome JT. 2017. Comparison of atipamezole with yohimbine for antagonism of xylazine in mice anesthetized with ketamine and xylazine. J Am Assoc Lab Anim Sci 56:142–147. [PMC free article] [PubMed] [Google Scholar]
- 26.Koehn D, Meyer KJ, Syed NA, Anderson MG. 2015. Ketamine/Xylazine-induced corneal damage in mice. PLoS One 10:1–12. 10.1371/journal.pone.0132804. Published correction. PloS One 2015. doi:10.1371/journal.pone.0132804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kohn DF, Wixson SK, White WJ, Benson GJ, editors. 1997. Anesthesia and analgesia in laboratory animals. New York (NY): Academic Press. [Google Scholar]
- 28.Kosarek L, Hart SR, Schultz L, Digiovanni N. 2011. Increase in venous complications associated with etomidate use during a propofol shortage: an example of clinically important adverse effects related to drug substitution. Ochsner J 11:143–146. [PMC free article] [PubMed] [Google Scholar]
- 29.Lairez O, Lonjaret L, Ruiz S, Marchal P, Franchitto N, Calise D, Fourcade O, Mialet-Perez J, Parini A, Minville V. 2013. Anesthetic regimen for cardiac function evaluation by echocardiography in mice: comparison between ketamine, etomidate and isoflurane versus conscious state. Lab Anim 47:284–290. 10.1177/0023677213496236. [DOI] [PubMed] [Google Scholar]
- 30.Levin-Arama M, Abraham L, Waner T, Harmelin A, Steinberg DM, Lahav T, Harlev M. 2016. Subcutaneous compared with intraperitoneal ketaminexylazine for anesthesia of mice. J Am Assoc Lab Anim Sci 55:794–800. [PMC free article] [PubMed] [Google Scholar]
- 31.Liu FL, Chen TL, Chen RM. 2012. Mechanisms of ketamine-induced immunosuppression. Acta Anaesthesiol Taiwan 50:172–177. 10.1016/j.aat.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 32.Meier A, Gross ETE, Schilling JM, Seelige R, Jung Y, Santosa E, Searles S, Lin T, Tu XM, Patel HH, Bui JD. 2018. Isoflurane impacts murine melanoma growth in a sex-specific, immune-dependent manner. Anesth Analg 126:1910–1913. 10.1213/ANE.0000000000002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melamed R, Bar-Yosef S, Shakhar G, Shakhar K, Ben-Eliyahu S. 2003. Suppression of natural killer cell activity and promotion of tumor metastasis by ketamine, thiopental, and halothane, but not by propofol: mediating mechanisms and prophylactic measures. Anesth Analg 97:1331–1339. 10.1213/01.ANE.0000082995.44040.07. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell JH, Wildenthal K, Johnson RL., Jr 1972. The effects of acid-base disturbances on cardiovascular and pulmonary function. Kidney Int 1:375–389. 10.1038/ki.1972.48. [DOI] [PubMed] [Google Scholar]
- 35.National Institutes of Health. [Internet]. 2017. PHS policy on humane care and use of laboratory animals. Frequently asked questions. [Cited 23 July 2019]. Available at: https://olaw.nih.gov/guidance/faqs#.
- 36.Orchard CH, Cingolani HE. 1994. Acidosis and arrhythmias in cardiac muscle. Cardiovasc Res 28:1312–1319. 10.1093/cvr/28.9.1312. [DOI] [PubMed] [Google Scholar]
- 37.Petrinovic MM, Hankov G, Schroeter A, Bruns A, Rudin M, von Kienlin M, Kunnecke B, Mueggler T. 2016. A novel anesthesia regime enables neurofunctional studies and imaging genetics across mouse strains. Sci Rep 6:1–12. 10.1038/srep24523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwarzkopf TM, Horn T, Lang D, Klein J. 2013. Blood gases and energy metabolites in mouse blood before and after cerebral ischemia: the effects of anesthetics. Exp Biol Med (Maywood) 238:84–89. 10.1258/ebm.2012.012261. [DOI] [PubMed] [Google Scholar]
- 39.Siriarchavatana P, Ayers JD, Kendall LV. 2016. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 55:426–430. [PMC free article] [PubMed] [Google Scholar]
- 40.Stokes EL, Flecknell PA, Richardson CA. 2009. Reported analgesic and anaesthetic administration to rodents undergoing experimental surgical procedures. Lab Anim 43:149–154. 10.1258/la.2008.008020. [DOI] [PubMed] [Google Scholar]
- 41.Teplinsky K, O'Toole M, Olman M, Walley KR, Wood LD. 1990. Effect of lactic acidosis on canine hemodynamics and left ventricular function. Am J Physiol 258:H1193–H1199. [DOI] [PubMed] [Google Scholar]
- 42.Whary MT, Baumgarth N, Fox JG, Barthold SW. 2015. Biology and diseases of mice, p 43–149. Chapter 3. In: Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. Laboratory animal medicine 3rd ed Boston (MA): Academic Press. [Google Scholar]
- 43.Zhao X, Ho D, Gao S, Hong C, Vatner DE, Vatner SF. 2011. Arterial pressure monitoring in mice. Curr Protoc Mouse Biol 1:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]