Abstract
Due to their unpredictability and variable effects, injectable anesthetic regimens in laboratory rodent species warrant refinement. In our study we sought to evaluate alfaxalone, which has gained recent popularity in veterinary medicine, alone and in combination with dexmedetomidine to evaluate their anesthetic ability in Sprague–Dawley rats when administered intraperitoneally. Three doses of alfaxalone only and 4 dose combinations of alfaxalone-dexmedetomidine were tested in males and female rats. The time to induction, anesthetic duration, pulse rate, respiratory rate, temperature, and time to recovery were recorded by a blind observer. The level of anesthesia induced by the various anesthetic protocols was assessed by using pedal withdrawal reflex to a noxious stimulus and scored according to the response. Dependent on the treatment group, atipamezole or saline was administered intraperitoneally once animals reached 60 min of anesthesia. Regardless of the dose, alfaxalone alone achieved only a sedative level of anesthesia, whereas all alfaxalone-dexmedetomidine combinations led to a surgical level of anesthesia in all animals. Anesthesia regimens using alfaxalone alone and in combination with dexmedetomidine demonstrated sex-associated differences, with female rats maintaining longer durations of sedation or anesthesia than their male counterparts. Both male and female rats displayed decreases in physiologic parameters consistent with the effects of dexmedetomidine. Given the results described herein, we recommend 20 mg/kg alfaxalone for sedation and 30 mg/kg alfaxalone combined with 0.05 mg/kg dexmedetomidine for surgical anesthesia in female rats. Appropriate doses of alfaxalone only and alfaxalone-dexmedetomidine for male rats were not determined in this study and need further evaluation.
Abbreviation: LORR, loss of righting reflex
The suitability of anesthetic regimen for use in laboratory rodents depends on many factors, including the type of procedure to be conducted, desired anesthetic duration and recovery time, animal species, health status of the animal, and research objectives. The most common practices for delivering anesthetics to laboratory rodents is via the injectable and inhalant routes. Although inhalant agents are used most commonly, they pose safety risks to users through gas exposure due to ill-fitting masks or system leaks, operator error, or operator neglect.30 In view of documented contraindicated scientific outcomes to the use of inhalant agents, injectable anesthetics are used as an alternative.33,39,42,49,57 Injectable anesthetics have led to successful induction and maintenance in laboratory rodents and continue to be used by investigators for a multitude of reasons. Preferences for the use of injectable anesthetics include the lack of ancillary vaporizer equipment, lower initial cost, and the ability to provide multimodal anesthesia. In addition, injectable anesthetics offer advantages when multiple animals are needed to be anesthetized at one time or when there is procedural incompatibility (e.g., oral procedures, nasal instillation) with the use of inhalant delivery systems.46
Currently, the most widely used injectable anesthetic protocol in laboratory rodent species involves the use of ketamine with xylazine.16,18,22 However, this combination has been noted to provide an inconsistent anesthetic depth, variability in induction and recovery times, prolonged sedation, corneal opacities, muscle necrosis, and unwanted secondary effects sometimes leading to death.2,5,21,22,29,32,48,50,54 Drug combinations such as propofol–medetomidine–fentanyl, dexmedetomidine–tiletamine–zolazepam–butorphanol, and others have been explored as alternatives to ketamine–xylazine anesthesia, with variable outcomes.1,4-6,54 In an effort to refine laboratory rodent anesthesia and improve animal welfare, we investigated the use of 2 injectable anesthetics, alfaxalone and dexmedetomidine.
Alfaxalone (3-α-hydroxy-5-α-pregnane-11, 20-dione) is a neuroactive steroid anesthetic that functions as a GABAA agonist.51 It is a Schedule IV controlled substance, and a DEA license is needed for its procurement. In addition to a single-use preparation (Alfaxan, Jurox, Kansas City, MO), a newer formulation (Alfaxan Multidose, Jurox), which has a shelf life of 28 d after opening, is commercially available. Both formulations are reported to have the same anesthetic profile as the original off-market preparation41 and are approved for the induction and maintenance of general anesthesia in cats, dogs, and rabbits by intravenous administration. The use of alfaxalone has many advantages, including a wide margin of safety, smooth induction and recovery, painless injection, and absence of tissue reaction when administered extravascularly. In addition, alfaxalone is rapidly eliminated from the body, thus allowing for repeated dose titration without accumulation, causes minimal cardiovascular and pulmonary effects, and is compatible with other anesthetic agents.14,25,34-36,40 Reported adverse effects include hypothermia, apnea, hypotension, hypoxemia, variable anesthesia quality, vocalization, muscle tremors, and jerking movements.8,14,31,35,36
Dexmedetomidine is a specific and selective α2-adrenoceptor agonist that provides both analgesia and sedation.19,24 It is a widely used anesthetic in veterinary medicine, and its anesthetic effects have been well characterized.37,38 Although dexmedetomidine has been noted to cause cardiac and respiratory depression, these effects are quickly reversible by using atipamezole, an α2-adrenergic receptor antagonist.38 The selectivity of dexmedetomidine allows for better control and predictability and aids in minimizing unwanted side effects.19,24 When used in low concentrations, it is anesthetic-sparing, allowing dose reduction of other coadministered anesthetic agents.43,45,52 This reduction contributes to balanced anesthesia and allows for a safer anesthetic event.
The goal of the current study was to evaluate the anesthetic effects of intraperitoneally administered alfaxalone alone and in combination with dexmedetomidine. Although alfaxalone administered intravenously—but not intraperitoneally—was shown to provide a surgical anesthetic plane in rats, the use of higher doses of alfaxalone had not yet been published when we designed our study.31 We did not consider alternative routes of administration in light of the commonality of intraperitoneal dosing in rats and the risk of exceeding recommended dose volumes established by many institutions. We focused on determining whether rats reached a sedative or a surgical anesthetic plane after intraperitoneal administration of alfaxalone or alfaxalone–dexmedetomidine and sought to establish doses suitable for use in male and female rats. We hypothesized that 1) the intraperitoneal administration of alfaxalone alone would result in sedation characterized by the loss of righting reflex (LORR) for 60 min and 2) a combination of alfaxalone and dexmedetomidine given intraperitoneally would result in a surgical plane of anesthesia, characterized by the loss of all monitored reflexes, for at least 60 min, with physiologic parameters returning to normal after the administration of atipamezole.
Materials and Methods
Animals and housing.
All procedures were approved by the IACUC of the University of Florida, an AAALAC-accredited facility. Animals were maintained according to the Guide for the Care and Use of Laboratory Animals.27 A total of 44 Sprague–Dawley rats (Hsd:Sprague–Dawley SD, Envigo, Indianapolis, IN; weight, 200 to 300 g) were used for this study. Rats were housed in same-sex pairs, were allowed a 1-wk acclimation period after arrival at the facility, and were housed on autoclaved corncob bedding (1/8-in., Teklad, Envigo, Indianapolis, IN) under ventilated cage conditions. Rats were fed a standard commercial rodent diet (2918, Envigo, Indianapolis, IN), and reverse-osmosis–purified water was provided automatically ad libitum. The rats were maintained in a temperature-controlled room (70 to 77 °F [21.1 to 25.0 °C]), with a 12:12-h light:dark cycle, 10 to 15 air changes hourly, and 30% to 70% relative humidity. Rats were sentinel-tested to be SPF for coronavirus, Hantaan virus, Kilham rat virus, lymphocytic choriomeningitis virus, mouse adenovirus, pneumonia virus of mice, rat minute virus, rat parvovirus, reovirus type 3, Sendai virus, Theiler murine encephalomyelitis virus, Toolan H1 virus, CAR bacillus, Citrobacter rodentium, Clostridium piliforme, Corynebacterium kutscheri, Mycoplasma pulmonis, Pasteurella pneumotropica, Salmonella spp., Streptobacillus moniliformis, Encephalitozoon cuniculi, fur mites, and pinworms (Syphacia spp. and Aspicularis spp.)
Pilot study.
A pilot study was performed to determine clinically relevant dose ranges of alfaxalone (10 mg/mL, Alfaxan Multidose, Jurox) alone and in combination with dexmedetomidine (0.5 mg/mL; Dexdomitor, Zoetis, Parsippany, NJ). Doses of alfaxalone (20, 25, 30, 35, 40, and 50 mg/kg) were administered intraperitoneally to animals in dose groups (n = 2 [1 female and 1 male per dose group; A20, A25, A30, A35, A40, and A50, respectively), which were monitored for the induction and depth of anesthesia (sedation or surgical plane of anesthesia. Based on the doses that had the ability to induce an acceptable level of anesthesia, alfaxalone was then combined with dexmedetomidine (0.05, 0.1, or 0.5 mg/kg IP; male dose groups: A20D0.1, A25D0.5, A25D0.1, A30D0.05, A30D0.1, and A40D0.1; female dose groups: A20D0.05, A20D0.1, A25D0.1, A25D0.5, A30D0.05) for evaluation. The doses of dexmedetomidine were chosen in light of previously published ranges for rats.7,18,54 For safety reasons, doses for both protocols were evaluated in a stepwise fashion from the lowest to highest dose. Between doses, animals underwent a 1-wk (or longer) washout period.
Study design.
Given the results of the pilot study, we investigated 3 dose levels of alfaxalone as a sole agent (A20, A30, and A40) and 4 alfaxalone–dexmedetomidine combinations (A20D0.1, A25D0.05, A25D0.1, and A30D0.05). For the current study, animals were allocated to groups blocked by sex (12 rats per same-sex dose group) and randomized by treatment by using a random number generator. Each rat was dosed 4 or 5 times and never received the same drug or drug combination more than once. The same observer was used throughout the study and was blind to anesthetic treatment. A 1-wk (or longer) washout period was used between dose administrations.
Drug administration and health assessment.
Prior to drug administration, each rat received a physical examination, consisting of evaluation of hydration status, mentation, and mucous membrane color. The drugs and reversal agents were prepared and the filled syringes given to the blinded observer to administer to each animal. For dexmedetomidine reversal, atipamezole (Antisedan 5.0 mg/mL; Zoetis, Parsippany, NJ) was administered intraperitoneally at the same volume as the calculated dose of dexmedetomidine. To keep the observer blinded to the treatment, animals that received alfaxalone only were ‘reversed’ with intraperitoneal saline (0.9% NaCl, Hospira, Lake Forest, IL) in the same fashion as atipamezole.
Each alfaxalone and alfaxalone–dexmedetomidine dose was injected intraperitoneally into the lower right quadrant by using a 25-gauge needle. After drug administration, rats were placed into a separate cage to monitor induction quality, and the time to LORR was recorded. Induction quality was assessed to determine the presence of spontaneous movement (i.e., facial twitching, generalized twitching, chewing, head shaking, jerking, muscle fasciculations) and was evaluated from the time of drug administration until the LORR.
Upon LORR, rats were transferred to a recirculating warm-water blanket that was set to 37 °C to prevent hypothermia. Prior to the placement of monitoring equipment, both eyes were lubricated with ophthalmic ointment (Puralube Vet Ointment, Dechra, Overland Park, KS) to prevent corneal desiccation. The return of righting reflex or spontaneous movement were assessed every 5 min until the rat could maintain itself in ventral recumbency.
Physiologic monitoring.
Once unconscious, physiologic parameters were recorded every 5 min for a maximum of 60 min. Mucous membrane color was recorded based on direct observation of the oral mucosa. A pulse oximeter probe (Physiosuite, Kent Scientific, Torrington, CT) was placed on the interdigital space of a hindlimb to monitor SpO2 and pulse rate. A rectal probe was used to measure core body temperature. Respiratory rate was monitored visually. Rats were allowed to breathe room air.
Anesthetic quality, depth, and duration.
Induction time was measured as the time from drug administration until LORR; the first physiologic recording was obtained at 5 min after LORR. The duration of anesthesia was measured as the time of LORR until all reflexes were regained or the rat was able to right itself. Recovery was measured as the time from atipamezole administration until the animal was able to maintain ventral recumbency. The time to recovery was not defined for alfaxalone-only dose groups, given that alfaxalone is a nonreversible agent; therefore, only the duration of anesthesia was considered. Duration and recovery were defined among groups after the observer was unblinded to the drug administered, and recovery was determined only for alfaxalone–dexmedetomidine dose groups.
Anesthetic depth parameters were scored every 5 min up to 60 min concurrently with physiologic parameters. Anesthetic depth was characterized as either sedation or surgical anesthesia. Sedation was defined as loss of consciousness with retained responsiveness to noxious stimuli. A surgical anesthetic plane was defined as loss of consciousness with continuous nonresponsiveness to noxious stimuli. Anesthetic depth was assessed using the pedal withdrawal response which was evaluated by pinching the toe webbing of either hind foot in an alternating fashion using a pair of hemostats. A response scoring system that was developed inhouse was used. Responses were scored on a 3-point system (0 to 2) to correlate presence and speed of movement with positive or negative response to noxious stimuli. A positive response correlated with pedal withdrawal in the event of noxious stimuli, whereas a lack of response meant there was no pedal withdrawal to noxious stimuli. Descriptions of the scoring system used to assess anesthetic score and depth are provided in Figure 1.
Figure 1.
Anesthetic depth was scored (scale, 0 to 2) every 5 min. All animals were scored by the same observer, who was blinded to the assigned treatment. Pedal withdrawal reflex responses correlated with anesthetic depth: 0 (awake) or 2 (surgical plane).
Statistical analysis.
All statistical calculations were performed by using the statistical toolbox in MatLab (MathWorks, Natick, MA). Data were evaluated for normal distribution prior to running any other analyses. The effects of alfaxalone or alfaxalone–dexmedetomidine dose, animal sex, and their interaction were evaluated by using ANOVA to test the effects of these factors on the duration of chemical restraint and recovery time. Significance was identified at a P value less than 0.05, and additional posthoc testing was not performed.
Results
Animal health.
The general appearance of all rats throughout the study remained unremarkable, with animals sustaining normal physical examinations during assessments. All rats maintained normal body weights, which matched the vendor's normal standard growth curve.
Pilot study.
In all of the alfaxalone and alfaxalone–dexmedetomidine dose groups, all animals became sedated or anesthetized, except for one male rat in the A25 group. Induction quality remained consistently smooth among all dose groups, with no evidence of excessive jerking, shaking, or muscle fasciculations that interfered with induction quality. Both anesthetic protocols resulted in rapid induction times, with LORR occurring within minutes (0 to 9 min) after intraperitoneal injection. The duration of anesthesia (sedation) in the alfaxalone-only dose groups (i.e., A20, A25, A30, A35, A40, A50) ranged from 84 to 244 min in female rats and 0 min to 159 min in males (Table 1). For alfaxalone–dexmedetomidine, the duration of anesthesia (sedation or surgical) ranged from 75 to 123 min in female rats (i.e., A20D0.05, A20D0.1, A25D0.1, A25D0.5, A30D0.05) compared with 59 to 198 min in male rats (Table 2). Physiologic parameters remained stable, with animals maintaining normal pulse rates (250 to 480 bpm) and normal temperature (35 to 38 °C). Bradypnea (fewer than 75 breaths per min; range: 66 to 115 bpm) was noted, with no overt clinical consequences seen.17,23,26 Hypoxia (SpO2 less than 90%; approximately 88 mm Hg) was seen in the A15 female dose group, but all other dose groups were normoxic (SpO2 greater than 90%). All animals recovered normally. Anesthetic depth (sedation or surgical plane of anesthesia) was assessed by using the pedal withdrawal response but the duration of either plane of anesthesia was not quantified for alfaxalone–dexmedetomidine combinations.
Table 1.
Pilot study: results for alfaxalone as a sole agent
| Dose | Sex | Body temperature (°C) | SpO2 (mm Hg) | Pulse rate (bpm) | Respiratory rate (breaths per min) | Induction time (min) | Duration (min) |
| A15 | Female | 37 | 88 | 342 | 85 | 3 | 36 |
| Male | 37 | 93 | 449 | 103 | 3 | 47 | |
| A20 | Female | 36 | 90 | 403 | 80 | 5 | 101 |
| Male | 36 | 93 | 389 | 114 | 3 | 14 | |
| A25 | Female | 36 | 96 | 384 | 76 | 3 | 84 |
| Male | 0 | 0 | 0 | 0 | 0 | 0 | |
| A30 | Female | 36 | 95 | 355 | 79 | 2 | 90 |
| Male | 37 | 97 | 382 | 115 | 3 | 47 | |
| A35 | Female | 35 | 96 | 356 | 75 | 2 | 112 |
| Male | 36 | 93 | 415 | 108 | 2 | 44 | |
| A40 | Female | 37 | 92 | 382 | 77 | 1 | 99 |
| Male | 37 | 96 | 400 | 106 | 1 | 59 | |
| A50 | Female | 32 | 91 | 272 | 55 | 2 | 244 |
| Male | 36 | 97 | 327 | 77 | 1 | 159 |
Subscripted numeral indicates dose of alfaxalone (A) in mg/kg IP. Each dose was tested on 1 male rat and 1 female rat. Time to induction was defined as the time from injection to LORR; duration was defined as the time from LORR until the animal regained consciousness and was able to maintain sternal recumbency.
Table 2.
Pilot study: alfaxalone–dexmedetomidine combination
| Dose | Sex | Body temperature (°C) | SpO2 (mm Hg) | Pulse rate (bpm) | Respiratory rate (breaths per min) | Induction time (min) | Duration (min) |
| A20D0.05 | Female | 37 | 97 | 264 | 71 | 8 | 75 |
| A20D0.1 | Female | 37 | 94 | 263 | 77 | 2 | 123 |
| A25D0.1 | Female | 36 | 96 | 271 | 56 | 1 | 77 |
| A25D0.5 | Female | 37 | 98 | 250 | 76 | 1 | 92 |
| A30D0.05 | Female | 36 | 89 | 268 | 68 | 3 | 98 |
| A20D0.1 | Male | 37 | 99 | 399 | 102 | 9 | 78 |
| A25D0.1 | Male | 37 | 95 | 310 | 72 | 1 | 59 |
| A25D0.5 | Male | 36 | 93 | 229 | 64 | 1 | 82 |
| A30D0.05 | Male | 33 | 92 | 220 | 86 | 7 | 73 |
| A30D0.1 | Male | 37 | 81 | 301 | 71 | 2 | 159 |
| A40D0.1 | Male | 36 | 87 | 475 | 78 | 4 | 198 |
Subscripted numerals indicate doses of alfaxalone (A) and dexmedetomidine (D) in mg/kg IP. Each dose combination was tested on 1 rat. Time to induction was defined as the time from injection to LORR; duration was defined as the time from LORR until the animal regained consciousness and was able to maintain sternal recumbency.
We used these results to choose doses for further investigation. The doses of alfaxalone included the lowest doses that produced sedation for approximately 60 min. Although doses below 40 mg/kg IP did not meet that criterion for male rats, we opted to use the same doses in the experimental study as for females, to compare sex associated differences. Consequently, we tested one lower dose combination (A20D0.05) in female rats and 2 higher dose combinations (A30D0.1, A40D0.1) in males; the remainder of the doses evaluated were the same for both sexes. Doses for the drug combinations were selected in a similar fashion to alfaxalone doses, but the ability of a dose to produce surgical anesthesia was considered. The dose groups that met that criterion were A20, A30, A40, A20D0.1, A25D0.05, A25D0.1, and A30D0.05.
Physiologic parameters.
In the alfaxalone-only dose groups (A20, A30, and A40), membrane color remained pink at all time points. The induction time for alfaxalone-only dose groups ranged from 1 to 2 min. The recovery time for alfaxalone was not determined in the pilot study. Pulse rates (309 to 499 bpm) remained within normal physiologic ranges. Animals did experience a degree of bradypnea (< 75 bpm; range: 61 to 140 bpm) and hypothermia (< 35.9 °C; range: 33 °C- 37 °C) at selected time points for both males and females across all 3 dose groups (Tables 3 and 4). A decline in temperature during the anesthetic event did not affect recovery. All animals remained normoxic (SpO2 greater than 90%) across dose groups.
Table 3.
Experimental study: alfaxalone as a sole agent
| Dose | Sex | Body temperature (°C) | SpO2 (mm Hg) | Pulse rate (bpm) | Respiratory rate (breaths per min) | Induction time (min) | Duration of sedation (min) |
| A20 | Female | 37 ± 0.7 | 97 ± 3 | 410 ± 38 | 88 ± 18 | 2 ± 0.5 | 44 ± 10 |
| Male | 36 ± 0.5 | 98 ± 1 | 429 ± 31 | 91 ± 13 | 2 ± 1 | 13 ± 8 | |
| A30 | Female | 36 ± 0.5 | 98 ± 1 | 367 ± 15 | 73 ± 6 | 2 ± 0.5 | 59 ± 2 |
| Male | 36 ± 0.9 | 97 ± 0.9 | 427 ± 18 | 90 ± 19 | 2 ± 0.7 | 38 ± 16 | |
| A40 | Female | 36 ± 0.5 | 98 ± 1 | 357 ± 20 | 68 ± 5 | 1 ± 0.4 | 60 ± 0 |
| Male | 36 ± 0.3 | 99 ± 0.4 | 410 ± 45 | 96 ± 11 | 1 ± 0.4 | 33 ± 9 |
Subscripted numeral indicates dose of alfaxalone (A) in mg/kg IP. Time to induction was defined as the time from injection to LORR; duration of sedation was defined as the time from LORR with a positive pedal withdrawal reflex to a noxious stimulus. Data are reported as mean ± 1 SD (maximum, 60 min; n = 8 per group).
Table 4.
Experimental study: alfaxalone–dexmedetomidine combination
| Dose | Sex | Body temperature (°C) | SpO2 (mm Hg) | Pulse rate (bpm) | Respiratory rate (breaths per min) | Induction time (min) | Duration of surgical anesthesia (min) | Recovery time (min) |
| A20D0.1 | Female | 36 ± 0.5 | 97 ± 2 | 271 ± 12 | 64 ± 3 | 1 ± 0.5 | 51 ± 21 | 12 ± 13 |
| Male | 36 ± 0.6 | 94 ± 5 | 270 ± 12 | 82 ± 4 | 2 ± 0.6 | 23 ± 27 | 3 ± 2 | |
| A25D0.1 | Female | 36 ± 0.3 | 93 ± 4 | 275 ± 12 | 58 ± 5 | 2 ± 0.7 | 59 ± 2 | 8 ± 6 |
| Male | 36 ± 0.5 | 96 ± 2 | 255 ± 13 | 69 ± 4 | 2 ± 1 | 51 ± 9 | 8 ± 11 | |
| A25D0.05 | Female | 37 ± 0.5 | 95 ± 3 | 296 ± 12 | 79 ± 3 | 2 ± 0.7 | 53 ± 12 | 8 ± 4 |
| Male | 36 ± 0.3 | 98 ± 1 | 270 ± 11 | 68 ± 4 | 2 ± 0.7 | 31 ± 13 | 8 ± 15 | |
| A30D0.05 | Female | 36 ± 0.6 | 87 ± 6 | 288 ± 12 | 56 ± 4 | 1 ± 0.8 | 53 ± 21 | 17 ± 10 |
| Male | 36 ± 0.5 | 98 ± 1 | 270 ± 12 | 72 ± 4 | 1 ± 0.8 | 44 ± 21 | 3 ± 4 |
Subscripted numerals indicate doses of alfaxalone (A) and dexmedetomidine (D) in mg/kg IP. Time to induction was defined as the time from injection to LORR; duration of surgical anesthesia was defined as the time from LORR with absence of pedal withdrawal response to a noxious stimulus. Recovery time was defined as the time from the administration of atipamezole until return of the righting reflex. Data are reported as mean ± 1 SD (maximum, 60 min; n = 8 per group (except n = 7 for female A30D0.05 group).
Similar to the animals in the alfaxalone-only dose groups, all rats in the alfaxalone–dexmedetomidine dose groups (A20D0.1, A25D0.05, A25D0.1, A30D0.05) had pink mucous membranes at all time points. The induction time in the alfaxalone–dexmedetomidine dose groups ranged from 1 to 2 min; recovery time ranged from 3 to 17 min. Pulse rates (245 to 315 bpm) and respiratory rates (49 to 85 breaths per minute) varied, with lower ranges below published normal physiologic ranges; however, no overt clinical signs were associated with this finding. Failure of the pulse oximeter to accurately obtain a peripheral pulse and SpO2 reading occurred when the pulse rate dropped below approximately 240 bpm for individual time points. This problem was addressed by adjusting the sensor to a different limb until a stable reading was obtained. The parameter was not recorded when no reading was obtained by the next 5-min time point. No animals became hypothermic (temperature less than 35 °C), despite rectal body temperature decreasing by 1 to 2 °C over the 60-min duration. Hypoxia (SpO2 less than 90%) was noted from pulse oximeter readings at variable time points in all 4 dose groups, with an average of approximately 87% SpO2 in the A30D0.05 female dose group. This finding was consistent with the expected physiologic effects due to administration of dexmedetomidine. No overt clinical signs were associated with these findings. Hypoxia resolved without medical intervention.
Anesthetic depth and duration.
In the alfaxalone-only dose groups (A20, A30, and A40), a majority (47 of 48) of the rats had a positive pedal withdrawal response to the noxious stimulus, indicating that they were only sedated. One female in the A40 group lacked a pedal withdrawal response at the 10-, 15-, and 35-min time points, but had a positive response for the remainder of the event (total, 60 min).
A majority (59 of 64) of the animals in the alfaxalone-dexmedetomidine dose groups (A20D0.1, A25D0.05, A25D0.1, A30D0.05) reached a surgical plane of anesthesia, defined as the lack of a pedal withdrawal in response to noxious stimulus and a reflex score of 2. Three male rats in the A20D0.1 dose group and one in the A30D0.05 dose group reached sedation only. One female in the A30D0.05 dose group did not become anesthetized after administration and remained fully awake.
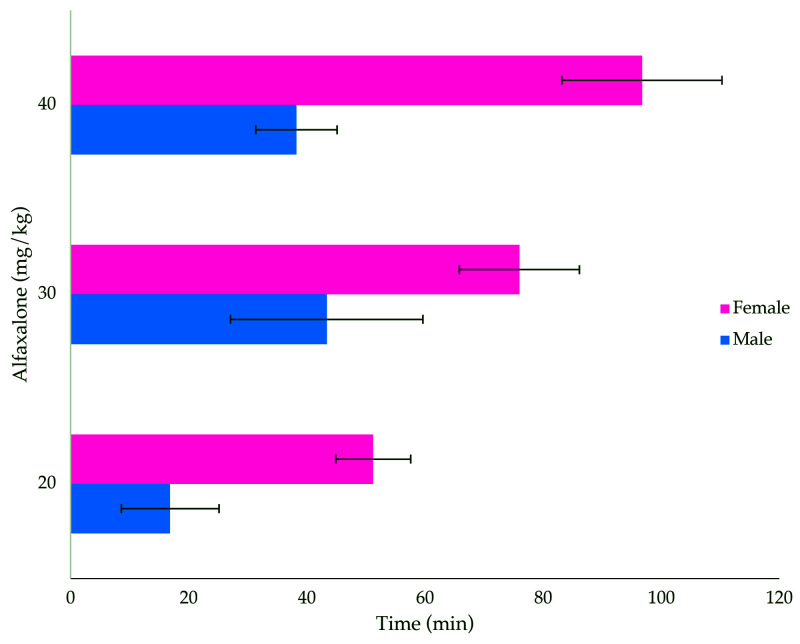
The duration of sedation due to alfaxalone alone was significantly affected by dose (P < 10−10) and animal sex (P < 10−16), with a dose×sex interaction effect (P = 0.0022; Figure 2). All animals became sedated, characterized as an anesthetic score of 1. The duration of sedation corresponded with the dose administered, with higher doses resulting in longer sedation times. Overall, female rats in all 3 dose groups remained sedated longer than did their male counterparts.
Figure 2.
Duration of sedation (min; mean ± 1 SD; n = 8 per group) in male and female rats treated with 3 doses of alfaxalone (20, 30, and 40 mg/kg) administered intraperitoneally. Significant differences were found between sexes (ANOVA, P < 10−16) and doses (P < 10−10), and there was a dose×sex interaction (P = 0.0022).
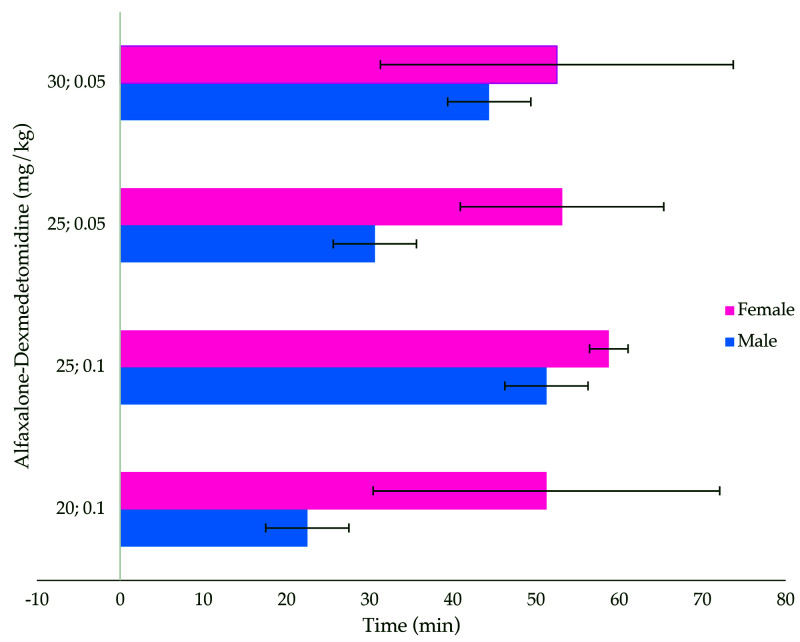
The duration of surgical anesthesia produced by using alfaxalone–dexmedetomidine was significantly affected by dose (P = 0.0045) and animal sex (P < 10−6), but there was no dose-sex interaction effect (P = 0.15; Figure 3). A majority (58 of 64 [91%]) of the animals across all 4 dose combinations reached a surgical plane of anesthesia deemed by reaching an anesthetic score of 2. Females across these dose groups predominantly reached this anesthetic score for 60 min. While a surgical plane of anesthesia was achieved by animals in the A20D0.1 dose group, some males (2 of 8) only reached a level of sedation (score of 1).
Figure 3.
Duration of surgical anesthesia (min; mean ± 1 SD; n = 8 per group) in male and female rats treated with alfaxalone (20, 25, and 30 mg/kg) and dexmedetomidine (0.05 and 0.1 mg/kg) intraperitoneally. Surgical anesthesia was defined as a lack of response to noxious stimuli and the inability to return to sternal position. The duration of anesthesia was significantly affected by dose (P = 0.0045) and sex (P < 10−6).
Neither alfaxalone–dexmedetomidine dose, animal sex, or their interaction significantly affected recovery time (P = 0.97, P = 0.03, and P = 0.22, respectively).
Discussion
The primary aim of this study was to determine whether alfaxalone alone or in combination with dexmedetomidine resulted in sedation or a surgical plane of anesthesia for at least 60 min in male and female rats. Previous studies using alfaxalone at various doses in rodents have documented variability in anesthetic depth, duration of anesthesia, and adverse events; we considered all of these reports when evaluating the results from our study.3,8,12,31 Although similar studies have been conducted in rats previously, we found that repeating a dose-finding study using alternative doses of a new formulation (Alfaxan Multidose) alfaxalone and as alfaxalone–dexmedetomidine combinations was warranted to find a dose that would be suitable for use in both male and female rats. Our study revealed that the level of anesthesia that animals achieved varied in both protocols. Our results also indicated relevant sex-associated and dose-related differences with regard to the plane and duration of anesthesia achieved.
Overall, alfaxalone alone provided a level of sedation (score 1) over the dosage range of 20 to 40 mg/kg IP. The exception was one female rat in the A40 group that had a negative pedal withdrawal response at 3 different time points, deeming her to have reached a surgical anesthetic plane. This outcome was not a consistent finding in the present study but has been seen in others that explored the effects of alfaxalone.20,28,56 Variation arose from the use of different species, routes of administration, doses administered, and definitions of anesthetic depth. Due to the lack of this outcome in other animals dosed with alfaxalone in our study, we conclude that, when used as a sole agent, intraperitoneal alfaxalone provides only sedation in rats.
We also assessed the duration of sedation: the higher the dose of alfaxalone administered, the longer the duration of anesthesia. Sex-associated differences in sedation duration were apparent within dose groups. Specifically, female rats experienced a significantly longer duration of sedation when given the same dose of alfaxalone as their male counterparts. Sex-associated differences have also been seen in other studies that explored the use of alfaxalone in a variety of species.3,11,13,15,31,44,55 Potential causes of this phenomenon, such as pharmacokinetics and formulation-dependent factors, have been analyzed. Sex-associated variations in drug metabolism, bioavailability, distribution, and excretion have been investigated as well.15,31,47,53 A study of the older formulation of alfaxalone, alphadalone, suggested that male rodents need markedly (i.e., more than 4fold) higher doses to produce similar effects to those females when the drug was administered intraperitoneally.15 Another study suggested that 3 times the alfaxalone dose was required to attain a similar duration of sedation in males as in females.3 Because the previously cited study3 was not yet published during the implementation of our study design, we explored only a 2fold increased, maximal dose (i.e., A40) for the male rats in our study. The A40 dose failed to produce similar effects in both sexes, thus demonstrating that higher doses should be used in male rats to produce comparable effects to those in females.
Dose groups receiving alfaxalone combined with dexmedetomidine, delivered intraperitoneally, allowed animals to reach a surgical level of anesthesia (score 2); however, anesthetic duration varied across groups. This outcome is in contrast to a study that explored alfaxalone only; alfaxalone with medetomidine and butorphanol; and medetomidine, midazolam, and butorphanol in combination and administered subcutaneously or intraperitoneally (control) in mice.25 Those authors found that alfaxalone (40, 60, and 80 mg/kg), medetomidine, and butorphanol administered subcutaneously resulted in surgical anesthesia whereas comparable intraperitoneal doses did not.25 We found intraperitoneal dosing to be suitable for producing a surgical plane of anesthesia in rats. Using alternative dosing routes was beyond the scope of our current study but should be considered in the future.
Sex-associated differences in the duration of surgical anesthesia also emerged in the alfaxalone–dexmedetomidine dose groups. Overall, female rats had longer durations of surgical anesthesia than males. This result was similar to another study in rats that that compared alfaxalone (25 mg/kg in females and 75 mg/kg in males) and dexmedetomidine (0.05 mg/kg) combinations in rats.3 Contrary to the previous study, we saw comparable surgical anesthetic durations between the males and females in the A25D0.1 dose group. This dose combination used a low dose of alfaxalone with an increased dose of dexmedetomidine. In addition to the sedative, hypnotic, analgesic properties of dexmedetomidine, it is known to be anesthetic-sparing.9,19,37,38 This property allows the doses of other coadministered anesthetics, like alfaxalone, to be reduced. We found that the combination of alfaxalone and dexmedetomidine enabled our rats to experience a balanced anesthetic event,10 where not only surgical anesthesia was achieved and maintained for a considerable amount of time but also both sexes of animals remained physiologically stable and could undergo reversal of anesthetic effects. Our results show that equivalent doses of alfaxalone–dexmedetomidine combinations can be used in both sexes to produce similar effects.
During evaluation of the alfaxalone–dexmedetomidine doses, some of our animals experienced peripheral vasoconstriction and bradycardia. In addition, some hypoxia was appreciated at various time points across all dose groups and overall in the A30D0.05 female rats. Although these are well-characterized adverse effects of dexmedetomidine, this phenomenon likely triggered failure of the pulse rate and SpO2 probe to gauge a signal once an animal approached or fell below a pulse rate of less than 240 bpm.19,37,38 Once rats reached our desired 60-min duration, these parameters were quickly reversed with the use of intraperitoneal atipamezole. Despite the reduction in physiologic parameters, none of our animals displayed any undesirable clinical consequences that might have warranted medical intervention; therefore, we did not see the value of using 100% oxygen in these animals. Its use would take away the desire and practicality of using an injectable anesthetic agent dependent on its intended use. Nonetheless, a full evaluation and risk assessment should be conducted prior to administering this drug combination.
Surgical tolerance was not considered in the present study, which only assessed surgical anesthetic depth by using the pedal withdrawal reflex. Although this reflex is the optimal one for assessing anesthetic depth in rodents,4,5 sole use of this method leaves room for misinterpretation of results. Therefore, alfaxalone and alfaxalone–dexmedetomidine regimens for rodents should be analyzed in a surgical setting. Another limitation of the current study was the lack of assessing higher doses of alfaxalone in male rats. Including higher doses would have enabled us to determine alfaxalone and dexmedetomidine doses for male rats that produced effects similar to those in females.
In conclusion, we suggest that alfaxalone is suitable for short-term (i.e., 60 min or less) or noninvasive (i.e., imaging, sedated assessments, nonpainful procedures) procedures that require only mild sedation. The sex of the animal should be considered prior to choosing the appropriate dose to ensure that the desired duration is achieved. Our data showed that alfaxalone at 20 mg/kg IP is ideal for the use in female rats, whereas higher doses (50 to 80 mg/kg IP) should be explored in male rats to produce sedation longer than 40 min. Alfaxalone–dexmedetomidine provided a more predictable anesthetic outcome, with rats reaching a surgical anesthetic depth. We recommend the A25D0.1, dose in light of its ability to provide consistent and comparable anesthesia in both sexes of rats.
Acknowledgments
We thank Dr Kevin Otto (University of Florida) for the use of his laboratory space and equipment, Elliot Dirr (University of Florida) for assistance in this project, Dr Irene Cooke (University of Florida) for financial support of this project, and Lesa Howell and Alex Macleod for ensuring that all agents used were procured and available when needed.
References
- 1.Alves HC, Valentim AM, Olsson IAS, Antunes LM. 2009. Intraperitoneal anaesthesia with propofol, medetomidine and fentanyl in mice. Lab Anim 43:27–33. 10.1258/la.2008.007036. [DOI] [PubMed] [Google Scholar]
- 2.Amouzadeh HR, Sangiah S, Qualls CW, Cowell RL, Mauromoustakos A. 1991. Xylazine-induced pulmonary edema in rats. Toxicol Appl Pharmacol 108:417–427. 10.1016/0041-008X(91)90088-V. [DOI] [PubMed] [Google Scholar]
- 3.Arenillas M, Gomez de Segura IA. 2018. Anaesthetic effects of alfaxalone administered intraperitoneally alone or combined with dexmedetomidine and fentanyl in the rat. Lab Anim 52:588–598. 10.1177/0023677218764214. [DOI] [PubMed] [Google Scholar]
- 4.Arras M, Autenried P, Rettich A, Spaeni D, Rülicke T. 2001. Optimization of intraperitoneal injection anesthesia in mice: drugs, dosages, adverse effects, and anesthesia depth. Comp Med 51:443–456. [PubMed] [Google Scholar]
- 5.Buitrago S, Martin TE, Tetens-Woodring J, Belicha-Villanueva A, Wilding GE. 2008. Safety and efficacy of various combinations of injectable anesthetics in BALB/c mice. J Am Assoc Lab Anim Sci 47:11–17. [PMC free article] [PubMed] [Google Scholar]
- 6.Cagle LA, Franzi LM, Epstein SE, Kass PH, Last JA, Kenyon NJ. 2017. Injectable anesthesia for mice: combined effects of dexmedetomidine, tiletamine-zolazepam, and butorphanol. Anesthesiol Res Pract 2017:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carpenter J, Marion C. 2017. Exotic animal formulary, 5th ed. St Louis (MO): Elsevier. [Google Scholar]
- 8.Doerning CM, Bradley MP, Lester PA, Nowland MH. 2018. Effects of subcutaneous alfaxalone alone and in combination with dexmedetomidine and buprenorphine in guinea pigs (Cavia porcellus). Vet Anaesth Analg 45:658–666. 10.1016/j.vaa.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Doze VA, Chen BX, Maze M. 1989. Dexmedetomidine produces a hypnotic-anesthetic action in rats via activation of central α2 adrenoceptors. Anesthesiology 71:75–79. 10.1097/00000542-198907000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Dugdale A. 2010. Veterinary anaesthesia: Principles to practice. 1st ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 11.Erickson RL, Blevins CE, De Souza Dyer C, Marx JO. 2019. Alfaxalone–xylazine anesthesia in laboratory mice (Mus musculus). J Am Assoc Lab Anim Sci 58:30–39. 10.30802/AALAS-JAALAS-18-000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eshar D, Huckins GL, Shrader TC, Beaufrère H. 2019. Comparison of intramuscular administration of alfaxalone-ketamine-dexmedetomidine and alfaxalone-butorphanol-midazolam in naked mole-rats (Heterocephalus glaber). Am J Vet Res 80:1089–1098. 10.2460/ajvr.80.12.1089. [DOI] [PubMed] [Google Scholar]
- 13.Estes KS, Brewster ME, Webb AI, Bodor N. 1990. A non- surfactant formulation for alfaxalone based on an amorphous cyclodextrin: Activity studies in rats and dogs. Int J Pharm 65:101–107. 10.1016/0378-5173(90)90014-U. [DOI] [Google Scholar]
- 14.Ferré PJ, Pasloske K, Whittem T, Ranasinghe MG, Li Q, Lefebvre HP. 2006. Plasma pharmacokinetics of alfaxalone in dogs after an intravenous bolus of Alfaxan-CD RTU. Vet Anaesth Analg 33:229–236. 10.1111/j.1467-2995.2005.00264.x. [DOI] [PubMed] [Google Scholar]
- 15.Fink G, Sarkar DK, Dow RC, Dick H, Borthwick N, Malnick S, Twine M. 1982. Sex difference in response to alphaxalone anaesthesia may be oestrogen dependent. Nature 298:270–272. 10.1038/298270a0. [DOI] [PubMed] [Google Scholar]
- 16.Flecknell P. 2009. Laboratory animal anaesthesia, 3rd ed. Burlington (MA): Elsevier. [Google Scholar]
- 17.Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT. editors. 2015. Laboratory animal medicine. 3rd ed San Diego (CA): Academic Press; 10.1016/B978-0-12-409527-4.00001-8 [DOI] [Google Scholar]
- 18.Gaertner DJ, Hallman TM, Hankenson FC, Batchelder MA. 2008. Anesthesia and analgesia for laboratory rodents, p 249–250. Chapter 10. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed. Burlington (MA): Elsevier. [Google Scholar]
- 19.Gertler R, Brown HC, Mitchell DH, Silvius EN. 2001. Dexmedetomidine: a novel sedative-analgesic agent. Proc (Bayl Univ Med Cent) 14:13–21. 10.1080/08998280.2001.11927725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giral M, García-Olmo DC, Gómez-Juárez M, Gómez de Segura IA. 2014. Anaesthetic effects in the ferret of alfaxalone alone and in combination with medetomidine or tramadol: A pilot study. Lab Anim 48:313–320. 10.1177/0023677214539150. [DOI] [PubMed] [Google Scholar]
- 21.Giroux MC, Hélie P, Burns P, Vachon P. 2015. Anesthetic and pathological changes following high doses of ketamine and xylazine in Sprague Dawley rats. Exp Anim 64:253–260. 10.1538/expanim.14-0088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CJ, Knight J, Precious S, Simpkin S. 1981. Ketamine alone and combined with diazepam or xylazine in laboratory animals: a 10 year experience. Lab Anim 15:163–170. 10.1258/002367781780959107. [DOI] [PubMed] [Google Scholar]
- 23.Grimm KA, Lamont LA, Tanquilli WJ, Greene SA, Robertson SA, editors. 2015. Veterinary anesthesia and analgesia: The 5th ed of Lumb and Jones. Ames (IA): Wiley Publishing. [Google Scholar]
- 24.Grosu I, Lavand'homme P. 2010. Use of dexmedetomidine for pain control. F1000 Med Rep 2:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higuchi S, Yamada R, Hashimoto A, Miyoshi K, Yamashita K, Ohsugi T. 2016. Evaluation of a combination of alfaxalone with medetomidine and butorphanol for inducing surgical anesthesia in laboratory mice. Jpn J Vet Res 64:131–139. [PubMed] [Google Scholar]
- 26.Hrapkiewicz K, Colby LA, Denison P. 2013. Clinical laboratory animal medicine: an introduction, 4th ed. Ames (IA): Wiley–Blackwell. [Google Scholar]
- 27.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 28.Knotek Z, Hrdá A, Knotková Z, Trnková Š, Babák V. 2013. Alfaxalone anaesthesia in the green iguana (Iguana iguana). Acta Vet Brno 82:109–114. 10.2754/avb201382010109. [DOI] [Google Scholar]
- 29.Koehn D, Meyer KJ, Syed NA, Anderson MG. 2015. Ketamine/xylazine-induced corneal damage in mice. PLoS One 10:1–12. 10.1371/journal.pone.0132804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lahvic N, Liu M. 2019. Waste gas scavenging system. StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. [Google Scholar]
- 31.Lau C, Ranasinghe MG, Shiels I, Keates H, Pasloske K, Bellingham MC. 2013. Plasma pharmacokinetics of alfaxalone after a single intraperitoneal or intravenous injection of Alfaxan® in rats. J Vet Pharmacol Ther 36:516–520. 10.1111/jvp.12055. [DOI] [PubMed] [Google Scholar]
- 32.Levin-Arama M, Abraham L, Waner T, Harmelin A, Steinberg DM, Lahav T, Harlev M. 2016. Subcutaneous compared with intraperitoneal KetamineXylazine for anesthesia of mice. J Am Assoc Lab Anim Sci 55:794–800. [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J, Wang P, Zhang X, Zhang W, Gu G. 2014. Effects of different concentration and duration time of isoflurane on acute and long-term neurocognitive function of young adult C57BL/6 mouse. Int J Clin Exp Pathol 7:5828–5836. [PMC free article] [PubMed] [Google Scholar]
- 34.Michou JN, Leece EA, Brearley JC. 2012. Comparison of pain on injection during induction of anaesthesia with alfaxalone and two formulations of propofol in dogs. Vet Anaesth Analg 39:275–281. 10.1111/j.1467-2995.2012.00709.x. [DOI] [PubMed] [Google Scholar]
- 35.Muir W, Lerche P, Wiese A, Nelson L, Pasloske K, Whittem T. 2008. Cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in dogs. Vet Anaesth Analg 35:451–462. 10.1111/j.1467-2995.2008.00406.x. [DOI] [PubMed] [Google Scholar]
- 36.Muir W, Lerche P, Wiese A, Nelson L, Pasloske K, Whittem T. 2009. The cardiorespiratory and anesthetic effects of clinical and supraclinical doses of alfaxalone in cats. Vet Anaesth Analg 36:42–54. 10.1111/j.1467-2995.2008.00428.x. [DOI] [PubMed] [Google Scholar]
- 37.Murrell JC, Hellebrekers LJ. 2005. Medetomidine and dexmedetomidine: A review of cardiovascular effects and antinociceptive properties in the dog. Vet Anaesth Analg 32:117–127. 10.1111/j.1467-2995.2005.00233.x. [DOI] [PubMed] [Google Scholar]
- 38.Naaz S, Ozair E. 2014. Dexmedetomidine in current anaesthesia practice—a review. J Clin Diagn Res 8:GE01–GE04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Farrell RA, Foley AG, Buggy DJ, Gallagher HC. 2018. Neurotoxicity of inhalation anesthetics in the neonatal rat brain: effects on behavior and neurodegeneration in the piriform cortex. Anesthesiol Res Pract 2018:1–9. 10.1155/2018/6376090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parkinson L, Mans C. 2017. Anesthetic and postanesthetic effects of alfaxalone-butorphanol compared with dexmedetomidine-ketamine in Chinchillas (Chinchilla lanigera). J Am Assoc Lab Anim Sci 56:290–295. [DOI] [PubMed] [Google Scholar]
- 41.Pasloske K, Ranasinghe MG, Sauer S, Hare J. 2018. The bioequivalence of a single intravenous administration of the anesthetic alfaxalone in cyclodextrin versus alfaxalone in cyclodextrin plus preservatives in cats. J Vet Pharmacol Ther 41:437–446. 10.1111/jvp.12485. [DOI] [PubMed] [Google Scholar]
- 42.Rosenholm M, Paro E, Antila H, Vöikar V, Rantamäki T. 2017. Repeated brief isoflurane anesthesia during early postnatal development produces negligible changes on adult behavior in male mice. PLoS One 12:1–16. 10.1371/journal.pone.0175258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sharma P, Gombar S, Ahuja V, Jain A, Dalal U. 2017. Sevoflurane sparing effect of dexmedetomidine in patients undergoing laparoscopic cholecystectomy: A randomized controlled trial. J Anaesthesiol Clin Pharmacol 33:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siriarchavatana P, Ayers JD, Kendall LV. 2016. Anesthetic activity of alfaxalone compared with ketamine in mice. J Am Assoc Lab Anim Sci 55:426–430. [PMC free article] [PubMed] [Google Scholar]
- 45.Song J, Ji Q, Sun Q, Gao T, Liu K, Li L. 2016. The opioid-sparing effect of intraoperative dexmedetomidine infusion after craniotomy. J Neurosurg Anesthesiol 28:14–20. 10.1097/ANA.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 46.Southam DS, Dolovich M, O'Byrne PM, Inman MD. 2002. Distribution of intranasal instillations in mice: effects of volume, time, body position, and anesthesia. Am J Physiol Lung Cell Mol Physiol 282:L833–L839. 10.1152/ajplung.00173.2001. [DOI] [PubMed] [Google Scholar]
- 47.Tanaka E. 1999. Gender-related differences in pharmacokinetics and their clinical significance. J Clin Pharm Ther 24:339–346. 10.1046/j.1365-2710.1999.00246.x. [DOI] [PubMed] [Google Scholar]
- 48.Turner PV, Albassam MA. 2005. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp Med 55:175–182. [PubMed] [Google Scholar]
- 49.Valentim AM, Alves HC, Olsson IAS, Antunes LM. 2008. The effects of depth of isoflurane anesthesia on the performance of mice in a simple spatial learning task. J Am Assoc Lab Anim Sci 47:16–19. [PMC free article] [PubMed] [Google Scholar]
- 50.Veilleux-Lemieux D, Castel A, Carrier D, Beaudry F, Vachon P. 2013. Pharmacokinetics of ketamine and xylazine in young and old Sprague–Dawley rats. J Am Assoc Lab Anim Sci 52:567–570. [PMC free article] [PubMed] [Google Scholar]
- 51.Visser SAG, Smulders CJGM, Gladdines WWFT, Irth H, Van Der Graaf PH, Danhof M. 2000. High-performance liquid chromatography of the neuroactive steroids alphaxalone and pregnanolone in plasma using dansyl hydrazine as fluorescent label: Application to a pharmacokinetic-pharmacodynamic study in rats. J Chromatogr B Biomed Sci Appl 745:357–363. 10.1016/S0378-4347(00)00296-6. [DOI] [PubMed] [Google Scholar]
- 52.Walia C, Gupta R, Kaur M, Mahajan L, Kaur G, Kaur B. 2018. Propofol sparing effect of dexmedetomidine and magnesium sulfate during BIS targeted anesthesia: A prospective, randomized, placebo controlled trial. J Anaesthesiol Clin Pharmacol 34:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Warne LN, Beths T, Whittem T, Carter JE, Bauquier SH. 2015. A review of the pharmacology and clinical application of alfaxalone in cats. Vet J 203:141–148. 10.1016/j.tvjl.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Wellington D, Mikaelian I, Singer L. 2013. Comparison of ketamine-xylazine and ketamine-dexmedetomidine anesthesia and intraperitoneal tolerance in rats. J Am Assoc Lab Anim Sci 52:481–487. [PMC free article] [PubMed] [Google Scholar]
- 55.White KL, Paine S, Harris J. 2017. A clinical evaluation of the pharmacokinetics and pharmacodynamics of intravenous alfaxalone in cyclodextrin in male and female rats following a loading dose and constant rate infusion. Vet Anaesth Analg 44:865–875. 10.1016/j.vaa.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Whittem T, Pasloske KS, Heit MC, Ranasinghe MG. 2008. The pharmacokinetics and pharmacodynamics of alfaxalone in cats after single and multiple intravenous administration of Alfaxan® at clinical and supraclinical doses. J Vet Pharmacol Ther 31:571–579. 10.1111/j.1365-2885.2008.00998.x. [DOI] [PubMed] [Google Scholar]
- 57.Zhu C, Gao J, Karlsson N, Li Q, Zhang Y, Huang Z, Li H, Kuhn HG, Blomgren K. 2010. Isoflurane anesthesia induced persistent, progressive memory impairment, caused a loss of neural stem cells, and reduced neurogenesis in young, but not adult, rodents. J Cereb Blood Flow Metab 30:1017–1030. 10.1038/jcbfm.2009.274. [DOI] [PMC free article] [PubMed] [Google Scholar]