Abstract
Recently, the zebrafish has gained in popularity as a vertebrate animal model for biomedical research. Commercial zebrafish housing systems are available and are designed to maximize stocking density of fish for a given space, but these systems are expensive and purchasing them may not be feasible for emerging laboratories with limited funding. In this article, we describe the construction of a simple and affordable recirculating zebrafish housing system. This system can be constructed in 3 working days, with materials readily available in hardware stores. The cost for construction of the system was only 3,000 MYR (750 USD). The system consists of a water reservoir, a supply line that delivers water to the shelves holding the zebrafish tanks, and a drainage line that receives water from both the supply line and the shelves containing the fish tanks and returns this water to the reservoir. This system also has a 3-stage filtration process to ensure that clean water is delivered to the zebrafish tank. The system can house up to 360 zebrafish. This low-cost housing system may make research using zebrafish feasible some laboratories.
The zebrafish (Danio rerio) is rapidly becoming a popular vertebrate model for research into pharmacogenetics, toxicology, obesity, cardiovascular disease, hematology, neuroscience, cancer, and developmental biology.5,8,9 The advantages of the zebrafish over traditional rodent species include ease of maintenance, optical transparency during development, rapid ex utero development, a fully sequenced genome, and a high degree of genetic homology with mammals.6,9,16 Due to these advantages, the use of zebrafish as an experimental animal model has gained tremendous popularity among researchers. Our review of the number of publications on zebrafish in PubMed revealed 249 publications in 2000 which increase to 4,049 publications in 2010 and rose to 8,220 in 2018.
Zebrafish are tropical freshwater fish of the Cyprinidae family and are naturally found in the rivers and ponds of Pakistan, India, Bangladesh, Nepal, and Myanmar.11,20 The adult fish measure approximately 4 to 5 cm long, have a cylindrical body, and a distinct color pattern alternating light and dark horizontal stripes.17 Adult females may spawn every 2 to 3 d, with each spawning producing hundreds of eggs.17 The zebrafish embryo develops rapidly, with primary organs forming within the first day postfertilization and will reach sexual maturity within 2 to 3 mo.7,17
Maintaining an appropriate environment for the zebrafish becomes progressively more challenging as the scale of the operation increases.13 Commercial companies offer compact zebrafish housing systems with UV disinfection and filtration systems to reduce maintenance and “self-cleaning” tanks. The purchase of a commercial stand-alone rack that can accommodate 15 × 10 L tanks (equivalent to housing 750 to 1,800 adult fish) costs between 40,000 to 100,000 MYR (10,000 to 20,000 USD). This cost is a barrier to the use of zebrafish models, particularly for a new researcher who wishes to venture into this area.
The most common zebrafish housing arrangements are recirculating systems. These systems minimize water usage by recycling water and maintain water quality through a series of filtration systems.13 In a recirculating system, water is kept in a reservoir before it is supplied to the individual tanks through the supply line. Excess water flows into the drainage line and to the water reservoir. The water goes through a filtration system where wastes are removed before the water is returned to the tanks.3 We designed a recirculating zebrafish housing system using readily available components and accessories. The construction of an affordable zebrafish housing system as described in this study is ideal for a laboratory that may lack the ability to perform zebrafish studies without a low-cost housing option.
Materials and Methods
Materials.
All materials used in the construction of this zebrafish housing system are listed in Table 1. We purchased materials from a hardware and an aquarium store. The arrangement of various functional units, such as the water reservoir and filtration unit, water supply line, and water drainage line, are shown in Figures 1 and 2.
Table 1.
List of important components required to build zebrafish housing system.
| Components | Remarks | Quantity required |
| Rack | To hold all components of the zebrafish housing system | 1 |
| PVC pipe 1/2” | Use for shelf feeding line | User define |
| 1/2” PVC valve socket end | 3 | |
| 1/2” PVC fitting end cap | 3 | |
| 1/2” PVC fitting equal tee | 12 | |
| 1/2” water tap | 12 | |
| PVC pipe 3/4” | Use for water supply line | User define |
| 3/4” PVC valve socket end | 1 | |
| 3/4” PVC fitting equal elbow | 3 | |
| 3/4” PVC fitting equal tee | 1 | |
| PVC fitting reducing tee 3/4” x 1/2” | To connect water supply line and shelf feeding line | 3 |
| PVC pipe 1 1/2” | Use for water drainage line | User define |
| 1 1/2” PVC elbow fitting 45° | 1 | |
| 1 1/2” PVC fitting end cap | 3 | |
| 1 1/2” PVC fitting equal tee | 15 | |
| PVC fitting reducing socket 1 1/2” x 3/4” | To connect water supply line and drainage line | 1 |
| Pipeline filter equipped with: | 2 | |
| - fiber cartridge | Use for particulate filtration | |
| - activated carbon cartridge | To absorb volatile organic contaminants | |
| 100 L polyethylene tank | Use as water reservoir tank | 1 |
| Submergible water pump | 1 | |
| Aquarium air pump | To supply aeration to the water reservoir tank | 1 |
| 11W UV sterilizer for aquarium | Use for disinfection | 1 |
| Bio balls and ceramic rings | Required for nitrification and denitrification process | 1 |
| Reusable cotton sponge | Use for particulate filtration | 1 |
| Nylon net bag | To hold bio balls in the water reservoir tank | 1 |
| 5 mm acrylic sheet | ||
| PVC fitting V-tank connector 1/2” | Draining outlet of zebrafish tank | 12 |
| 1/2” PVC fitting equal elbow | 12 |
Figure 1.
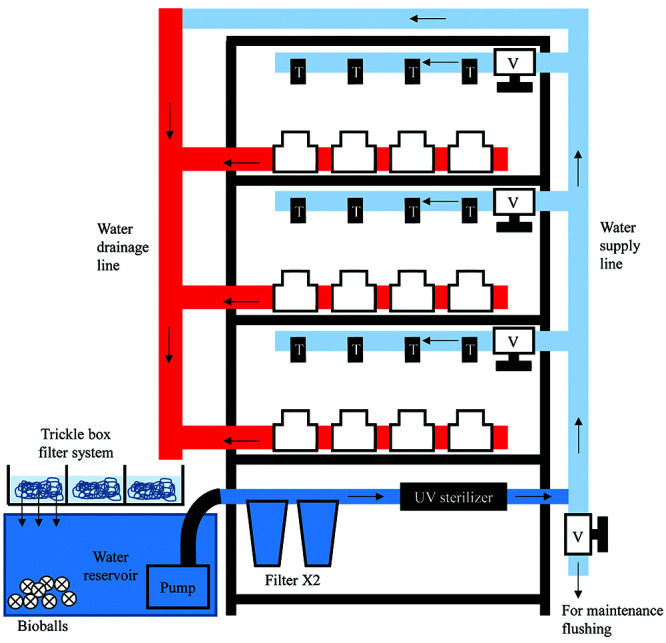
Front view of the zebrafish housing system. The water reservoir is located at the side of the shelf, the pump connects to the water supply line. The supply line supplies water to the 3 upper shelves that contain zebrafish tanks. Every shelf is supplied independently and can be individually controlled (on or off) by means of a valve (V). Water is supplied to individual tanks using water taps (T). The supply line also has a valve at the bottom end for maintenance flushing of the system, and at the upper end it continues into the drainage line. The drainage line receives water from the supply line and from the shelves containing fish tank and ends into the water reservoir tanks.
Figure 2.
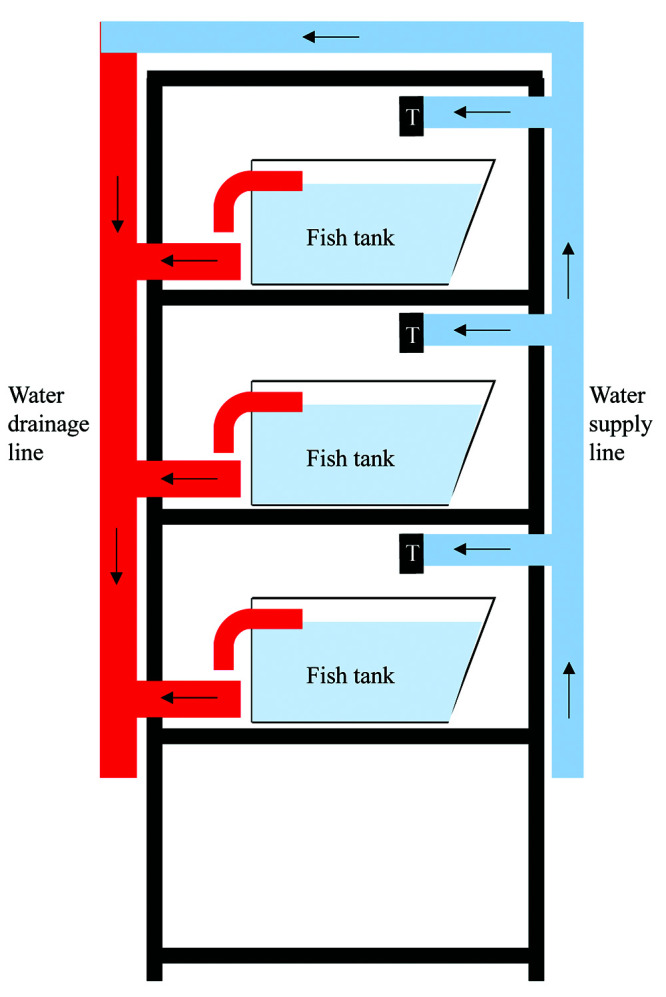
Side view of the zebrafish housing system. From the supply line, pipes supplying water to individual shelves containing zebrafish tanks, and can be individually controlled (on or off) by a tap (T). Excess water from the fish tank will then drain into the drainage line via an outlet. Water collected will then drain into the water reservoir.
Physical condition.
The room temperature was maintained between 26 to 28.5 °C. The lights in the laboratory were switched on from 0700 to 2100 daily and were regulated by an automatic timer.
Zebrafish rack and tank with lid.
The trapezoid-shaped zebrafish tanks were made from 5 mm thick transparent acrylic sheets (Figure 3 A). The size of each tank was 23 × 13 cm (L × W) (base), 28 × 13 cm (L × W) (top) with the height of 15 cm and can hold 6 L water for housing zebrafish. The acrylic sheet was cut using a plastic cutter and glued using marine-safe acrylic glue. The outlet of the tank was constructed by drilling a 20 mm hole on one side of the tank, to fit a 1/2” PVC fitting V-tank connector and a 1/2” PVC fitting equal elbow. This is the overflow outlet pipe which will drain excess water from the tank into the draining line located in front of the tanks. All the tanks were covered individually with removable lids made from 5 mm thick transparent acrylic sheets that measured 30 × 15 cm (L x W) with 2 15 mm holes that were used for feeding and water inlet (Figure 3 B).
Figure 3.

Zebrafish tank with lid. (A) Each zebrafish tank is made of 5 mm transparent acrylic sheet, gluing with marine-safe acrylic glue. Each can hold 6 L water, making each tank could house a total of 30 zebrafish (5 fish/L). The outlet of the tank is constructed by drilling a hole on one side of the tank, and fix with a PVC fitting V-tank connector and an equal elbow. A mesh is installed onto the outlet, preventing fish from reaching the drainage line. (B) Each tank received continuous flow of water from the supply line. Excess water will then flow into the drainage line through the outlet. (C) The tank lid contains 2 openings used for feeding and as water inlet.
The overall size of the rack was 184 cm × 36 cm × 94 cm (H × D × W). The rack was made of galvanized steel with a maximum load per shelf of 33 kg. The 3 upper shelves can hold a total of 12 zebrafish tanks. The lower shelf held the air pump, pipeline filters and UV sterilizer.
Assembly of the water reservoir and filtration system.
We used a single 100 L polyethylene tank as the water reservoir. Its dimensions are 53 × 35 cm (L × W) (base), 73 × 50 cm (L × W) (top), and a height of 38 cm. A submersible water pump was installed in the tank (Figure 4). A total of 30 bio balls (1 for 4 L of water) were placed in the water reservoir. Bio balls are spheres consisting of tiny tubes that provide a large surface area on which water-filtering microbes can grow. They are commonly used in fish tank and fishpond filters to transform potentially harmful ammonia into benign nitrates.
Figure 4.
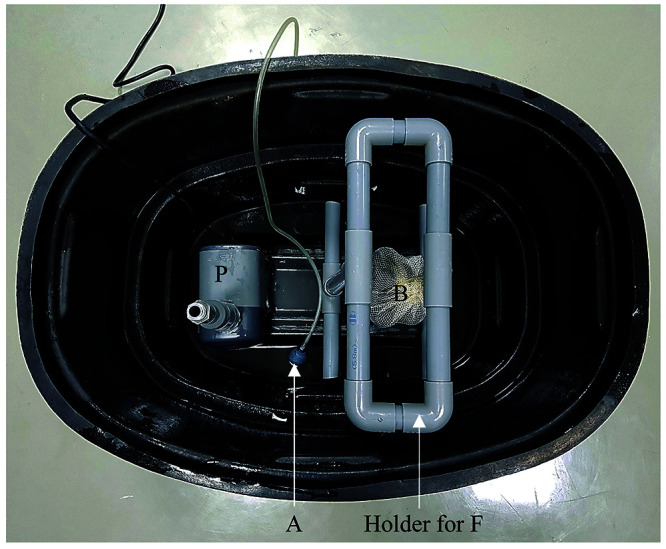
Water reservoir and its components. A 100 L polyethylene tank was used as the water reservoir. Submergible water pump (P), aeration tube (A), bio balls (B) and holder for trickle box filter system (F) are installed as components of the water reservoir tank.
An air-stone connected to an air pump via silicone tubing was placed in the water reservoir tank to continuously aerate the water. In addition, a submersible aquarium water heating rod was installed on the side of the reservoir to maintain the water temperature at 28 ± 1 °C.
The trickle box filter system was assembled using 5 mm acrylic sheets with marine-safe acrylic glue (Figure 5 A). Its dimensions were 40 × 13 × 10 cm (L×W x H). Once assembled, it was placed on the water reservoir to collect water from the draining line and filter it to the water reservoir (Figure 5 B). Bio rings were placed in each compartment of the box filter for denitrification purposes. The bio rings were then covered with cotton sponge filter in each compartment of the box filter.
Figure 5.
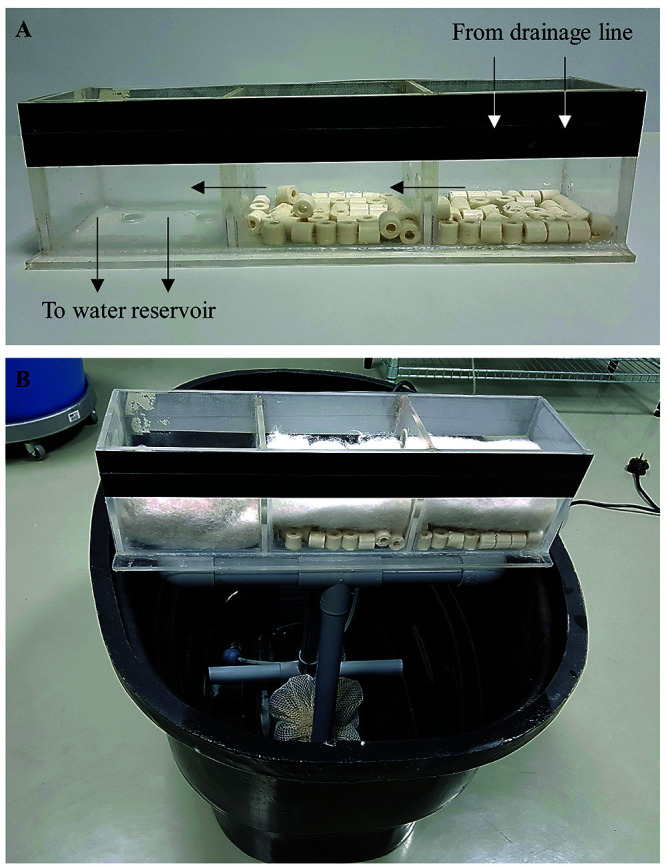
Trickle box filter system. (A) Assembled using 5 mm transparent acrylic sheet, the trickle box is placed on the water reservoir to collect water from the draining line and filter it before returning the water to the water reservoir. Each compartment contains ceramic rings and cotton sponge filter. Arrows shows the flow of water. (B) The trickle box filter system is placed on the water reservoir by sitting on a holder made with PVC fittings.
Pipeline filters and UV sterilizers were assembled with PVC pipes and fittings using PVC primer (Figure 6). It was then attached to the bottom-most shelf of the zebrafish rack using cable ties. The submerged water pump was connected to the pipeline filter using a short flexible tubing.
Figure 6.
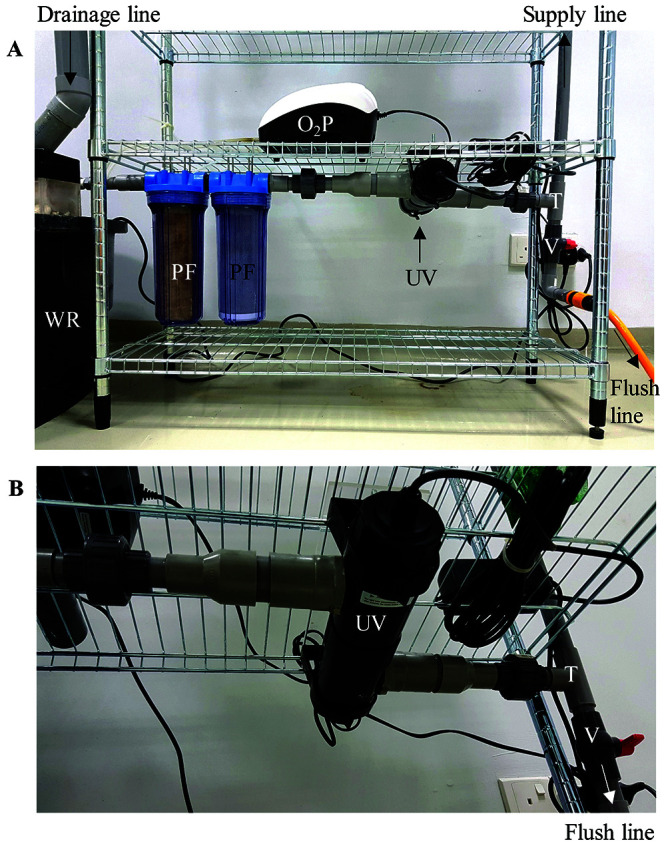
Filtration system and air pump of the zebrafish housing system. (A, B) The submergible water pump located in the water reservoir (WR) is connected to the 2 pipeline filters (PF) and the UV sterilizer (UV). An equal tee (T) is added next to the UV lamp and a valve (V) is added to the bottom of the equal tee to serve as a drain for maintenance flushing of the system. Air pump (O2P) provides continuous aeration in the water reservoir.
Assembly of the water supply line.
The vertically placed supply line provides water to the shelves that housed the zebrafish tanks. This line connected to the UV sterilizer via a 3/4” PVC fitting equal tee at the side of the rack. An on-off valve was added vertically to the bottom of the equal tee to serve as a draining line for maintenance flushing of the system. A 1/2” PVC pipe was horizontally aligned from the water supply line to provide water to each shelf that held zebrafish tanks, using a PVC reducing tee (3/4” to 1/2”) (Figure 7). On-off valves were also fitted to the 1/2” PVC pipe to allow for isolation of each shelf. This allowed the utilization of different shelves to meet the capacity needs of users. Water was supplied to the zebrafish tanks via individual 1/2” water tap, which was connected to the horizontally aligned 1/2” PVC pipe using 1/2” PVC fitting equal tee. On the top shelf, a horizontal 3/4” PVC pipe was used to connect the supply line to the draining line.
Figure 7.
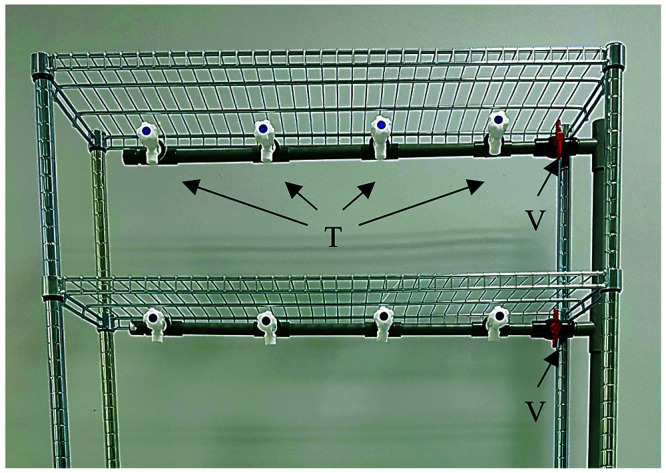
Supply line of the zebrafish housing system. The supply line supplies water to the 3 upper shelves which will house zebrafish tanks. Every shelf is supplied independently and can be individually controlled by means of a valve (V). Water inlet to individual tank is controlled using water tap (T).
Assembly of the water drainage line.
The drainage line used a vertical 1.5” PVC pipe to receive water from the supply line at the top of the zebrafish rack and drain lines from the zebrafish tanks of each shelf (Figure 8). Water from individual zebrafish tanks was collected using a 1.5” PVC equal tee. At the end of the draining line, water collected was expelled to the trickle box filter system over a cotton sponge filter and bio rings and then drained into the water reservoir.
Figure 8.
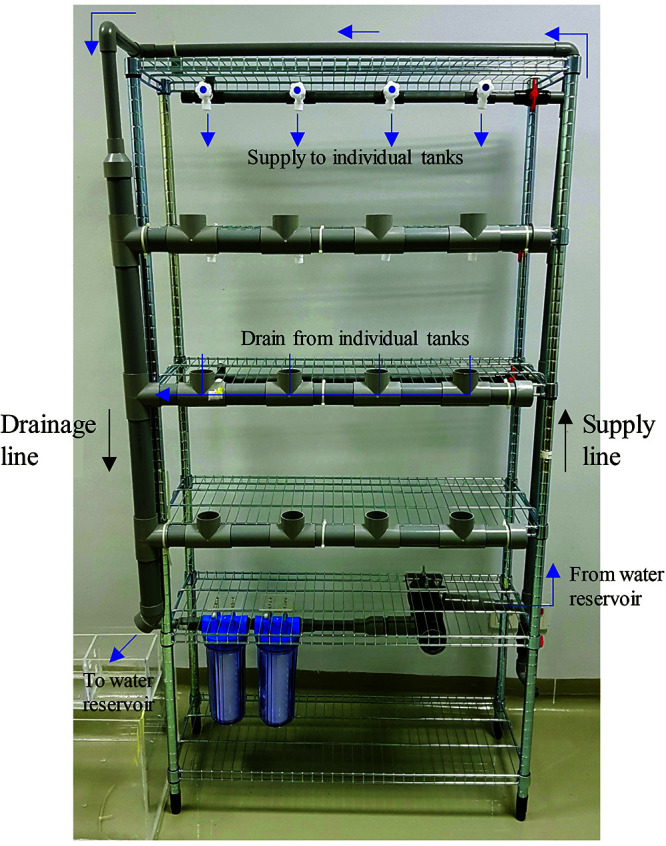
Draining line of the zebrafish housing system. The draining line uses a vertical PVC pipe to receive water from the supply line at the top of the zebrafish rack and drain lines from the zebrafish tanks of each shelf. It ends by draining water into the trickle box filter system of the water reservoir tanks.
Running the system.
Once all components of the zebrafish housing system were in place, a test run of the system was performed for one week to check for leakage. Aged water (that is, water left at room temperature for 48 h) was circulated through the system. The water was changed every day throughout the test run to eliminate any contaminants that may have been in the pipes. After 1 wk, a total of 60 adult zebrafish purchased from local aquarium were housed in 3 zebrafish tanks (20 fish per tank, placed on different shelves) for a month. Dry pellet feed (Adult zebrafish irradiated diet, Zeigler) was fed to the fish twice daily. During this period, water parameters were measured daily (temperature and pH), weekly (nitrite and nitrate levels), or monthly (ammonia and hardness levels).1,10 The pH and temperature of the system water were measured using a pH/temperature bench meter (Mi150, Milwaukee) while other parameters measured using a colorimetric kit (API freshwater test kits, Mars Fishcare).
Ethical approval.
Ethical approval was obtained from the Institutional Animal Ethics Committee prior to the study (ANAT/PP/2018/SEONG LIN/28-NOV./963-NOV.-2018-OCT.-2019).
Results
System functionality.
After the construction of the system, we performed a 1-wk test run with 12 zebrafish tanks on the rack to ensure system functionality. Water flowed into all the tanks from the water supply line and drained through the overflow outlet pipe into the draining line and back into the water reservoir. We did not detect any leakage or low water pressure during the test run. If leakage was detected, the modular nature of the system would allow the affected pipe to be removed and the system reassembled using the methodology described.
Water quality.
To further test the functionality of the system, 60 fish were housed in the system in 3 separate tanks for 1 month. All water parameters remained constant and were within the tolerated range throughout the housing period (Table 2). About half of the water in the water reservoir (≈ 40 L) was drained every week and replaced with aged tap water.
Table 2.
Water parameters measured and survival rate in the zebrafish housing system.
| Water parameters | Testing frequency* | Tolerated range* | Constructed system housing 60 adult zebrafish |
| pH | Daily | Stable, within 6.5–8.0 | 7.58 ± 0.03 |
| Temperature | Daily | Stable, within 24–29 °C | 28.14 ± 0.10 |
| Ammonia (NH3/ NH4+) | Weekly | < 0.1 mg/L | 0 mg/L |
| Nitrite (NO2−) | Weekly | < 0.3 mg/L | ≤ 0.25 mg/L |
| Nitrate (NO3−) | Weekly | < 25 mg/L, up to 200 mg/L | ≤ 20 mg/L |
| General hardness | Monthly | °dGH 3–8 | °dGH 5 |
| Carbonate hardness | Monthly | — | °dKH 3 |
| Survival rate | 91.67% (5 fish died during the first week of acclimatization) |
Zebrafish health.
Fish was monitored daily for any change in appearance (loss of pigmentation, skeletal deformities, presence of lesions or distinct weight loss) and behavior (lethargy, slow swimming or swimming near the bottom of the tank). A total of 5 fish died during the first week. Dead fish were removed upon detection. The remaining fish were in healthy condition.
Regular maintenance of system.
No visible signs of algae build-up in the zebrafish tanks were observed for 2 wk. However, a significant build-up of algae was detected in the tanks and the water reservoir during the fourth week. The tanks were cleaned by scrubbing under running water. Since the zebrafish tanks were not designed to self-clean, solid wastes (excess food debris and feces) tended to accumulate on the bottoms of the tanks. These solid wastes were siphoned out twice every week. Other maintenance of the system was summarized in Table 3.
Table 3.
Regular maintenance of the zebrafish housing system.
| Maintenance | Frequency |
| Check water pump in the water reservoir | Every week |
| Check air pump | Every week |
| Wash/replace cotton sponge filter in the trickle box filter system | Every 3 d |
| Replace pipeline filter fiber cartridge | Every week |
| Replace pipeline filter activated carbon cartridge | Every 2 wk |
| Replace UV sterilizer lamp | Every 9 mo |
Discussion
The zebrafish housing system constructed in this study is a recirculating aquaculture apparatus consisting of a water reservoir, water supply and drainage lines and equipped with a 3 stage filtration system. This system is designed to resemble most commercially available zebrafish housing systems.
To maintain water temperature, the constructed housing system was placed in a laboratory in which the room temperature was maintained between 26 to 28.5 °C.2 Furthermore, the system water was maintained at 28 ± 1 °C by using a submersible aquarium water heating rod. However, water temperatures between 22 to 31 °C are acceptable for successful zebrafish housing.18 The lighting in the laboratory was maintained at a 14:10 hr (light:dark) cycle.
Zebrafish health is dependent on the water quality. The system we constructed contains a 3-stage filtration process to provide clean recirculated water to the zebrafish. Water from the draining line will first flow into the first stage filtration process, consisting of a series of cotton sponge filters located in each compartment of the trickle box filter system to remove large particles. Water is then pumped from the water reservoir tank into 2 pipeline filters; a mechanical filter equipped with a 5-micron fiber cartridge to remove smaller particles, and an activated chemical carbon filter cartridge to adsorb volatile organic contaminants. Lastly, the water passed through a UV sterilizer to eliminate pathogens from the filtered water before reaching the supply line. These filters were checked and changed regularly to ensure their proper function.2 To further maintain the water quality, about half of the water in the water reservoir (≈ 40 L) was replaced every week with tap water, aged 48 h in an open tank to release chlorine.21
Maintaining a low concentration of ammonia metabolites by using biologic filters is particularly important in a recirculating system. Bio balls and ceramic rings were both used in the current setup. They are important because they provide a substrate with a large surface area for the growth of nitrifying bacteria.19 The use of continuous aeration in the water reservoir was necessary to ensure sufficient dissolved oxygen concentration in the water. The constant flow of this recycled water into individual zebrafish tanks provided enough oxygen; thus, aeration of individual tanks was not required.14
The constructed system can hold a total of 12 tanks. With the housing density of 5 zebrafish/L, the constructed system can hold up to 360 fish. We recommend housing adult zebrafish in tanks at a stocking density of 4 to 10 zebrafish/L to allow the animals to express their normal behavior and to avoid stress to the fish.1,15 However, a previous study suggested that housing density as high as 12 zebrafish/L does not cause sufficient stress to affect reproductive performance.4
The cost and housing capacity of open-design and commercially available zebrafish housing system is summarized in Table 4. The cost for the described setup was approximately 3,000 MYR (750 USD) and only 3 d were required to complete the whole system. The cost might vary depending on various factors such as the scale of the zebrafish laboratory setup, quality of materials used and labor cost. The construction and maintenance of zebrafish housing system in this setup is simple, and thus ideal for any small laboratory. Furthermore, we conserved water, which helps to lower the cost of maintenance.
Table 4.
Comparison of zebrafish housing system built.
| Author | Cost in MYR (USD) | Fish housing capacity |
| Kim and colleagues 2009 | ≈ 4,000 (1,000) | ≈ 800 |
| Paige and colleagues 2014 | ≈ 6,000 (1,500) | ≈ 300 |
| Nema and Bhargava 2017 | ≈ 2,800 (700) | ≈ 300 |
| Current setup | ≈ 3,000 (750) | ≈ 360 |
| Commercially available unit 1 (3 shelves stand-alone system) | ≈ 41,000 (10,000) | ≈ 750 |
| Commercially available unit 2 (3 shelves stand-alone system) | ≈ 81,000 (20,000) | ≈ 1800 |
The current setup still has room for improvement. Self-cleaning units could be installed to the zebrafish tanks to reduce the effort of regular maintenance. This could easily be done by modifying the pipe setup at the outlet of the tanks.12 The lights in the laboratory can also be set to gradually decrease and increase in light intensity, mimicking dusk and sunrise.1
In the future, a comparison of the performance of commercially available zebrafish housing system with our current setup would further validate our results. Also, continued monitoring of the health of fish housed in this system, including post-mortem evaluation, might reveal possible long-term harmful effects of the materials used to construct the system.
Conclusion
Constructing an open-design zebrafish housing system is easy because most materials are available in hardware and pet supply stores at a reasonable price. This system can be used to promote the use of zebrafish as a complementary or alternative animal model to the traditionally used rodent model.
Acknowledgments
The authors declare no conflicts of interest with respect to the research, authorship and publication of this article. This study was supported by Universiti Kebangsaan Malaysia (Research grant number: GGPM-2017-082). The authors thank Harizal Bin Nor Zakariar and Mohamad Faizul Bin Mohamad, Department of Anatomy, UKM for their technical assistance.
References
- 1.Aleström P, D'Angelo L, Midtlyng PJ, Schorderet DF, Schulte-Merker S, Sohm F, Warner S. 2019. Zebrafish: Housing and husbandry recommendations. Lab Anim 54:213–224. 10.1177/0023677219869037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Avdesh A, Chen M, Martin-Iverson MT, Mondal A, Ong D, Rainey-Smith S, Taddei K, Lardelli M, Groth DM, Verdile G, Martins RN. 2012. Regular care and maintenance of a zebrafish (Danio rerio) laboratory: an introduction. J Vis Exp 69:1–8. doi: 10.3791/4196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhargava Y. 2018. Open-design recirculating systems for zebrafish culture. Aquacult Eng 81:71–79. 10.1016/j.aquaeng.2018.03.004. [DOI] [Google Scholar]
- 4.Castranova D, Lawton A, Lawrence C, Baumann DP, Best J, Coscolla J, Doherty A, Ramos J, Hakkesteeg J, Wang C, Wilson C, Malley J, Weinstein BM. 2011. The effect of stocking densities on reproductive performance in laboratory zebrafish (Danio rerio). Zebrafish 8:141–146. 10.1089/zeb.2011.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clark TS, Pandolfo LM, Marshall CM, Mitra AK, Schech JM. 2018. Body condition scoring for adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 57:698–702. 10.30802/AALAS-JAALAS-18-000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. 2013. The zebrafish reference genome sequence and its relationship to the human genome. Nature 496:498–503. 10.1038/nature12111. Erratum in: Nature 2014 505:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalueff AV, Stewart AM, Gerlai R. 2014. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci 35:63–75. 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinth P, Mahesh G, Panwar Y. 2013. Mapping of zebrafish research: a global outlook. Zebrafish 10:510–517. 10.1089/zeb.2012.0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko SK, Chen X, Yoon J, Shin I. 2011. Zebrafish as a good vertebrate model for molecular imaging using fluorescent probes. Chem Soc Rev 40:2120–2130. 10.1039/c0cs00118j. [DOI] [PubMed] [Google Scholar]
- 10.Lawrence C, Mason T. 2012. Zebrafish housing systems: A review of basic operating principles and considerations for design and functionality. ILAR J 53:179–191. 10.1093/ilar.53.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee CJ, Tyler CR, Paull GC. 2020. Geographic range and natural distribution, p 41–56. Chapter 4. In: Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders GE, editors. The zebrafish in biomedical research: Biology, husbandry, diseases, and research applications. London: Elsevier. [Google Scholar]
- 12.Nema S, Bhargava Y. 2016. Designing and testing of self-cleaning recirculating zebrafish tanks. Zebrafish 13:369–373. 10.1089/zeb.2016.1250. [DOI] [PubMed] [Google Scholar]
- 13.Nema S, Bhargava Y. 2017. Open–RAC: Open-design, recirculating and auto-cleaning zebrafish maintenance system. Zebrafish 14:371–378. 10.1089/zeb.2016.1403. [DOI] [PubMed] [Google Scholar]
- 14.Paige C, Hill B, Canterbury J, Sweitzer S, Romero-Sandoval EA. 2014. Construction of an affordable and easy-to-build zebrafish facility. J Vis Exp 93:1–9. 10.3791/51989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlidis M, Digka N, Theodoridi A, Campo A, Barsakis K, Skouradakis G, Samaras A, Tsalafouta A. 2013. Husbandry of zebrafish, Danio rerio, and the cortisol stress response. Zebrafish 10:524–531. 10.1089/zeb.2012.0819. [DOI] [PubMed] [Google Scholar]
- 16.Santana S, Rico EP, Burgos JS. 2012. Can zebrafish be used as animal model to study Alzheimer's disease? Am J Neurodegener Dis 1:32–48. [PMC free article] [PubMed] [Google Scholar]
- 17.Simonetti RB, Marques LS, Streit DP, Oberst ES. 2015. Zebrafish (Danio rerio): The future of animal model in biomedical research. J FisheriesSciences.com 9:39–45. [Google Scholar]
- 18.Sfakianakis DG, Leris I, Laggis A, Kentouri M. 2011. The effect of rearing temperature on body shape and meristic characters in zebrafish (Danio rerio) juveniles. Environ Biol Fishes 92:197–205. 10.1007/s10641-011-9833-z. [DOI] [Google Scholar]
- 19.Suantika G, Pratiwi MI, Situmorang ML, Djohan YA, Muhammad H, Astuti DI. 2016. Ammonium removal by nitrifying bacteria biofilm on limestone and bioball substrate established in freshwater trickling biofilter. Poult Fish Wildl Sci 4:1–6. doi:10.4172/2375-446X.1000157. [Google Scholar]
- 20.Varga ZM. 2011. Aquaculture and husbandry at the Zebrafish International Resource Center, p 453–478. Chapter 24. In: Detrich HW, Westerfield M, Zon LI. editors. The zebrafish: Genetics, genomics and informatics, vol 104. San Diego (CA): Elsevier. [DOI] [PubMed] [Google Scholar]
- 21.Westerfield M. 2000. The zebrafish book: A guide for the laboratory use of zebrafish (Danio rerio), 4th ed Eugene (OR): University of Oregon Press. [Google Scholar]


