Abstract
Pain management in rabbits can be difficult because they are adept at hiding pain and can be stressed by handling and restraint for injection. The use of opioid analgesics with prolonged durations of activity could alleviate pain, but associated adverse effects including gastrointestinal ileus, inappetence, and tissue reactions have been reported. In this study, we compared gross tissue reactions at the site of injection, food consumption, and fecal production after single injections of buprenorphine HCl (Bup; n = 7), sustained-release buprenorphine (BupSR; n = 8), and high-concentration buprenorphine (BupHC; n = 7) during the first 3 d after minor survival surgery. We also measured plasma concentrations of the parent drug, buprenorphine, and 3 metabolites (buprenorphine-3-glucuronide (B3G), norbuprenorphine-3β-glucuronide (N3G), and norbuprenorphine (NB)). Plasma levels of buprenorphine remained above the theoretical minimal analgesic concentration for 4 h for Bup and 42 h for BupHC. For BupSR, plasma levels of buprenorphine remained above the theoretical minimal analgesic concentration for approximately 77 h, starting 15 h after administration. For all 3 formulations, N3G was the most prominent metabolite in the blood. No injection site reactions were visible grossly in any rabbit. Relative to baseline measures and compared with controls (n = 8), food consumption was suppressed on days 1 through 3 in rabbits that received BupSR and on days 2 through 3 in those given BupHC. Feces production on day 3 was reduced to a greater extent in BupSR rabbits than control animals. Two rabbits in the BupHC group exhibited neurologic signs after drug administration. These adverse effects should be considered when choosing a long-lasting buprenorphine formulation to manage pain in rabbits.
Abbreviations: λz, terminal elimination rate constant; B3G, buprenorphine-3-glucuronide; Bup, buprenorphine HCl; BupHC, high-concentration buprenorphine; BupSR, sustained-release buprenorphine; N3G, norbuprenorphine-3β-glucuronide; NB, norbuprenorphine; r2, variability; t1/2λz: apparent terminal half-life; TMC, theoretical minimal concentration
Pain management in rabbits is a challenging and complex task. As a prey species, rabbits are adept at hiding pain and can be stressed by handling and restraint for injection.29 The use of drugs with prolonged durations can be beneficial in rabbits by reducing the number of restraint episodes and their potential associated stress. However, several adverse effects are associated with opioid use in rabbits, including gastrointestinal ileus, nausea, and anorexia, which can be life-threatening in this species.13,30 In addition, adverse effects reported in animals given either the sustained-release or high-concentration formulation of buprenorphine include tissue reaction and sterile abscesses at the site of injection.5,8,21,26 In humans, adverse effects of buprenorphine include respiratory depression, miosis, and mood changes.4
Buprenorphine is a partial µ agonist, δ and κ antagonist, opioid analgesic.1,4,8 This drug is commonly used to treat pain in rabbits and is typically administered via subcutaneous or intravenous injection.7,8 For veterinary use, buprenorphine is available as an FDA-approved injectable formulation, a compounded sustained-release formulation, and a high-concentration formulation that is FDA-labeled for use in cats. In the veterinary practice setting, dispensing a controlled substance and administration by clients may pose problems after the animal has left the clinic. Therefore, the use of a sustained-release formulation of buprenorphine would be beneficial to improve client compliance, prevent drug misuse, reduce patient stress, and provide longer-lasting analgesia for rabbits.
Previous pharmacokinetic studies in humans have identified 3 major metabolites of buprenorphine that contribute to analgesia: buprenorphine-3-glucuronide (B3G), norbuprenorphine-3β-glucuronide (N3G), and norbuprenorphine (NB).4 In humans, B3G has a high affinity for µ, δ, and nociception receptors but not for κ receptors.4 N3G has high affinity for κ and nociception receptors but not µ or δ receptors.4 NB has affinity for µ, δ, and κ receptors but not nociception receptors.4 Other than norbuprenorphine, metabolites of buprenorphine have not yet been delineated in animals. The current literature recommendation regarding the use of buprenorphine in rabbits is 0.01 to 0.05 mg/kg every 6 to 12 h.7 However, few publications examine the efficacy of buprenorphine in rabbits.7,8,10,14,32 Two previous studies of buprenorphine pharmacokinetics in rabbits15,20 used HPLC to evaluate buprenorphine and metabolites in blood after intravenous administration of buprenorphine.
The current study assessed the pharmacokinetics of buprenorphine and 3 metabolites (B3G, N3G, and NB) after the administration of 3 formulations of injectable buprenorphine administered subcutaneously in rabbits: buprenorphine HCl (Bup), sustained-release buprenorphine (BupSR), and high concentration buprenorphine (BupHC). We assessed the effects of Bup, BupSR, and BupHC on gastrointestinal motility, behavior, and local tissue reaction at the injection site. Our goal was to determine whether longer-lasting formulations of buprenorphine, such as BupSR and BupHC, are suitable for use in rabbits. We hypothesized that BupSR and BupHC administered subcutaneously to rabbits would maintain plasma drug concentrations above the therapeutic threshold for longer than 12 h with no difference in adverse effects compared with Bup. Our study was based on 3 main assumptions: 1) 0.1 ng/mL is an appropriate theoretical minimal concentration (TMC) for buprenorphine to achieve analgesia in rabbits: 2) the cat dose of high-concentration buprenorphine is appropriate for rabbits; and 3) the primary metabolites of buprenorphine in humans are the same in the rabbit species.
Materials and Methods
Animals.
Male New Zealand white rabbits (Oryctolagus cuniculus; n = 31; age, 13 to 15 wk; weight, 2.96 to 3.85 kg) were purchased from Charles River Labs (Wilmington, MA) and housed individually (Euro Rabbit, Allentown Caging Equipment, Allentown, NJ) in an AAALAC-accredited facility. The facility had a 12:12-h light:dark cycle, temperature of 70 ± 2 °F (21.1 ± 1.0 °C), and average humidity of 50% (range, 15% to 80%). Prior to shipping, all rabbits were vendor-verified to be free of reovirus, lymphocytic choriomeningitis virus, parainfluenza viruses types 1 and 5, rotavirus, rabbit hemorrhagic disease virus, Bordetella bronchiseptica, Helicobacter spp., Lawsonia spp., Pasteurella spp., Salmonella spp., Treponema spp., Tyzzer disease pathogen, CAR bacillus, Cheyetiella parasitovorax, Leporacarus gibbus, Psoroptes cuniculi, other ectoparasites, Passalurus ambiguus, other helminths, Eimeria spp., Eimeria stiedae, other intestinal protozoa, and Encephalitozoon cuniculi by the vendor prior to shipping. However, the vendor reported past positive results for Pasteurella aeruginosa in the room of origination. Rabbits were physically examined on arrival and allowed to acclimate for 7 d prior to the start of the study. Rabbits were fed a commercial pelleted rabbit diet (Laboratory Rabbit Diet High Fiber, Purina LabDiet, St Louis, MO) and provided acidified water in water bottles ad libitum throughout the project. Each rabbit received 1 handful of timothy hay daily. Paper cage pan liners were changed daily. All work conducted in this study was compliant with the Animal Welfare Act and the Guide for the Care and Use of Laboratory Animals.2,16 All study procedures were approved by the Tulane University IACUC.
Presurgical procedures.
Rabbits were randomly divided into 4 groups (Bup, n = 7; BupSR, n = 8; BupHC, n = 7; Control, n = 8). The study was performed with 9 cohorts in total and 3 or 4 animals per cohort, with each cohort always including a control rabbit. Equal group sizes were not possible because the cohort size was determined according to the time needed to perform sample collections throughout the 96-h timeline. One control rabbit was removed from all analyses due to delayed recovery from shipping stress during the acclimation period. On the day of surgery, each rabbit was weighed and premedicated with acepromazine (0.25 mg/kg [0.11mg/lb] SC; VetOne, Boise, ID). The control group weighed 3.3 ± 0.2 kg (mean ± 1 SD), Bup group was 3.2 ± 0.2 kg, BupSR group weighed 3.5 ± 0.1 kg, and the BupHC group was 3.6 ± 0.2 kg. Bup rabbits differ significantly from BupSR and from BupHC rabbits (P = 0.021 and P = 0.002, respectively). Control rabbits also differ significantly from the BupSR and BupHC rabbits (P = 0.025 and P = 0.002, respectively). At 5 min after acepromazine administration, a 24-gauge catheter was placed in a central ear artery, and a blood sample was obtained for CBC, clinical chemistry, and baseline pharmacokinetics analysis. The hair over the dorsum at the region of the lumbar vertebrae was clipped by using an electric shaver with a no. 40 blade, and the skin was wiped with isopropyl alcohol and allowed to air dry. A single dose of buprenorphine (either Bup, BupSR, or BupHC) was administered prior to anesthesia and the implantation of a central jugular catheter. For rabbits in treatment groups, buprenorphine HCl (0.05 mg/kg [0.02 mg/lb] SC; volume, 0.49 to 0.64 mL; Buprenex, Indivior, North Chesterfield, VA), sustained-release buprenorphine (0.15 mg/kg [0.07 mg/lb] SC; volume, 0.44 to 0.58 mL; Buprenorphine-SR, ZooPharm, Windsor, CO), or high-concentration buprenorphine (0.24 mg/kg [0.11 mg/lb] SC; volume, 0.39 to 0.51 mL; Simbadol, Zoetis, Parsippany, NJ) was administered into the subcutaneous tissue overlying the dorsal epaxial muscles in the area between the L1L3 vertebrae to the right of midline. An injection of sterile saline, equal in volume to the amount of drug given in the right side, was administered subcutaneously on the left side of midline. We used a permanent marker to identify where the injections were administered. In this study, we used the FDA-approved cat dose of the high-concentration formulation, 0.24 mg/kg, because an appropriate dose for rabbits is not available in the literature.9
Rabbits were anesthetized with ketamine (35 mg/kg [15.91 mg/lb] IM; Zetamine, VetOne) and xylazine (5 mg/kg [2.27 mg/lb] IM; XylaMed, VetOne) administered in the cranial or caudal thigh muscle. The surgical sites were shaved and prepped with povidone–iodine or chlorhexidine and alcohol. An ear vein catheter was placed in a lateral ear vein for the administration of lactated Ringer solution (10 mL/kg/h [4.55 mL/lb/h] IV; Hospira, Lake Forest, IL) throughout the surgical procedure.
Surgical procedures.
Animals were maintained on isoflurane (1% to 5% in oxygen; Fluriso, VetOne) delivered by mask throughout the surgical procedure. An approximately 2-in. ventral sagittal incision was made overlying either the left or right jugular vein on the neck. The jugular vein was isolated, and the adventitia was carefully dissected from the vessel. The vessel was occluded distally by using sterile silk suture (Ethicon, Somerville, NJ), and a small cut was made through 1/4 to 1/2 of the vessel wall circumference by using a sterile no. 11 blade. A jugular vein catheter (5-French, 30 cm long with female luer, SAI Infusion Technologies, Lake Villa, IL) was inserted approximately 3 to 10 cm into the jugular vein. Sterile silk suture was tied around the outside of the vessel snuggly, sufficient to prevent bleeding around the catheter without occluding the vessel. The catheter was flushed with sterile saline (Hospira) to ensure the catheter was patent and the silk ties were not occlusive. Hemostats were used to bluntly dissect through the subcutaneous tissue to the dorsolateral aspect of the neck. An approximately 0.5-in. incision was made over the dorsolateral aspect of the neck, and the catheter was exteriorized through the incision. Incisions were closed by using 3-0 polydioxanone (Ethicon) for muscle and subcutaneous tissue and 2-0 polydioxanone for the skin. A pocket made from elastic adhesive tape (Elastikon, Johnson and Johnson, New Brunswick, NJ) was sutured to the skin of each rabbit near the dorsolateral incision by using 2-0 polydioxanone. The catheter was checked for patency, and 0.3 mL of glycerol catheter-locking solution (SAI Infusion Technologies) was injected into the catheter. The catheter was coiled and placed in the tape pocket attached to the dorsolateral aspect of the neck. For additional security, cotton stockinette was placed around the cranial half of the rabbit's body, covering the tape pocket. All rabbits recovered in their home cages, and recovery was considered complete when they could maintain sternal recumbency.
Control rabbits.
Rabbits in the control group underwent the same procedures as other groups but did not undergo surgical catheter implantation. Control rabbits were anesthetized and prepped as described earlier and were maintained under isoflurane anesthesia for 64 min, on average. All control rabbits had tape pockets attached to the dorsolateral aspect of their necks and stockinet placed around the cranial half of the body, to protect the tape pocket. These steps ensured that personnel performing daily postoperative behavioral observations were blind to the animal's treatment group.
Blood collection.
Blood collection was performed at 0, 0.5, 1, 2, 4, 8, 12, 24, 36, 48, 60, 72, and 96 h after buprenorphine administration; control animals were sampled at 0 and 96 h only, and Bup-treated animals were not sampled at 60 and 72 h. All samples were analyzed for buprenorphine and metabolites (described later). In addition, samples obtained at 0 and 96 h underwent CBC and clinical chemistry analysis by an outside diagnostic laboratory (IDEXX, West Sacramento, CA) to determine health status and monitor any effects of the procedure or drugs. For time points that occurred prior to the jugular catheter implantation, blood was acquired from the central ear artery catheter; samples for the remaining time points were collected from the jugular catheter. A blood volume approximately 2 times the volume of the dead space was withdrawn through the jugular catheter and discarded prior to each sample collection. After sample collection, the jugular catheter was flushed with saline and refilled with the glycerol locking solution. The total volume of blood drawn from each rabbit did not exceed 1% of body weight over the 5-d period. Chemistry and CBC analyses required approximately 1.5 mL of blood, and bloodwork for pharmacokinetics required less than 3 mL of blood. The CBC panel included neutrophils, WBC, band neutrophils, RBC, Hgb, lymphocytes, nucleated RBC, Hct, monocytes, polychromasia, anisocytosis, Eosinophils, MCV, basophils, MCH, poikilocytosis, Heinz bodies, MCHC, metamyelocytes, platelet estimate, myelocytes, promyelocytes, and unclassified cells. The chemistry panel included ALP, AST, ALT, creatine kinase, albumin, bilirubin, total protein, globulin, BUN, creatinine, cholesterol, glucose, calcium, phosphorus, total CO2, chloride, potassium, sodium, hemolysis index, and lipemia index.
Daily observations.
Behavioral observations and quantitative measurements of food consumption and feces production were performed once daily for 2 d before the surgical procedure, as baseline measurements, and once daily during days 2 through 4 after the procedure. Daily observations were not performed on the day of surgery because the animals were recovering from anesthesia. A modified Rabbit Grimace Scale (Figure 1) was used to assess the pain and discomfort of each rabbit on days 2 through 4.17 Eye squinting, ear position, and movement in the cage were each used as an independent indicator of pain and discomfort. Three observers performed behavioral observations and were blind to the experimental groups. These observers were animal care technicians with experience in the rabbit species and were trained in the behavior and scoring system described above. The observations were recorded on individual sheets unavailable to the other observers. Quantitative measurements, including feces production and pellet food consumption, were used to assess gastrointestinal stasis according to previously published methods with modifications.13,30 Pellet food was added to a food hopper and weighed prior to attaching it to the cage as the baseline measurement prior to each 24-h recording period. The pellet food and food hopper were removed together and weighed on each subsequent day, with the difference from baseline being the amount of pelleted feces eaten. For feces measurements, the cage pan was removed, and a clean pan with an absorptive liner was replaced under the cage. The cage pan was removed at the subsequent 24-h recording period, and the feces were bagged and weighed after taring the bag. The person performing the quantitative observations (DDA) was not blind to the group to which the rabbits were assigned. All behavioral observations and measurements were collected at 24-h intervals, at approximately the same time each day, to ensure that time of day did not influence behavior, food consumption, or feces production.
Figure 1.
Modified Rabbit Grimace Scale used for behavioral observations. Total score ranges from 0 to 6.
Necropsy and histopathology.
Approximately 5 min prior to the final blood collection time point, the rabbits were anesthetized with ketamine–xylazine. After the final blood collection was performed, the rabbit was injected intravenously or intracardiac with a sodium pentobarbital–phenytoin euthanasia solution (exceeding 100 mg/kg [45.45 mg/lb]; Beuthanasia-D Special, Merck, Madison, NJ). A gross necropsy was performed, and tissue samples were collected when gross lesions were present. The skin and subcutis at the drug and control injection sites were collected and placed in 10% formalin. Each skin site was placed in its own formalin-containing specimen cup and labeled with a randomly generated number to ensure that the pathologist who evaluated the samples was blind to the treatment group.
Tissue processing and histopathology scoring.
Formalin-fixed skin samples were cut in perpendicular sections to generate 3 tissue sections from each sample. Sections were embedded in paraffin, cut in 5-µm sections, and stained with hematoxylin and eosin. Sections were examined by a board-certified veterinary pathologist (RVB) and graded semi-quantitatively for microscopic criteria of inflammation, including hemorrhage, edema, inflammatory infiltrate, and necrosis. Each criterion was scored on a scale from 0 to 4 as follows: 0, none; 1, minimal; 2, mild; 3, moderate; and 4, severe. After each criterion was scored individually, the scores were summated to generate an aggregate score of inflammation for each skin sample. Areas of inflammation were noted as being located in the superficial dermis, deep dermis, panniculus, or subcutis.
Analysis of buprenorphine and metabolites in rabbit plasma.
Standards, materials, and reagents.
All blood sample preparations and analyses for pharmacokinetics were performed at a veterinary diagnostic laboratory (TVMDL, College Station, TX). Untreated control rabbit plasma was obtained from Lampire Biologicals (no. 7326400, Pipersville, PA) and from control rabbits used in this study. Buprenorphine (B-902), norbuprenorphine glucuronide ([N3G] N-045), buprenorphine-3β-d-glucuronide ([B3G] B-902), norbuprenorphine ([NB] N-912), norbuprenorphine-D3 (N-920), and buprenorphine-D4 (B-901–1ML) reference materials were obtained from Cerilliant (Round Rock, TX). Reagent-grade water (reverse-osmosis-purified deionized water) was made inhouse by using a Purelab Ultra system (ELGA, High Wycombe, United Kingdom). All chemicals and reagents were ACS-grade and obtained from VWR Scientific (Radnor, PA).
Sample preparation.
To generate a calibration curve, samples of untreated rabbit plasma were spiked accordingly to produce concentrations of 0.05, 0.1, 0.25, 0.5, 1, 5, 10, 50, and 100 ng/mL. Samples were tested in aliquots of 500 µL, with 25 µL of a 1 ng/µL solution of norbuprenorphine-D3 and buprenorphine-D4 added to all samples as internal standards. Each sample was diluted with 1500 µL reverse-osmosis–treated H2O, making a final volume of 2 mL. Samples were vortexed and allowed to rest at room temperature for approximately 5 min prior to extraction.
Samples underwent solid phase extraction by using a CEREX48 Processor (SPEWare, Baldwin Park, CA). Water Wettable Polymer Solid Phase Extraction cartridges (no. 12-170418, SPEWare) were used. The cartridges were conditioned with 1 mL methanol and 1 mL reagent-grade water. Samples (15 µL) were added to cartridges and allowed to filter through at 1 to 2 mL/min. Cartridges were washed with 1 mL deionized water and dried at full pressure (80 psi) for approximately 5 min. Samples were eluted with 1 mL methanol and dried under nitrogen at 40 °C. Samples were reconstituted in 80 µL of 95:5 reagent-grade water:acetonitrile solution prior to analysis by LC–high-resolution accurate MS.
Instrument parameters.
Samples were analyzed by using a Thermo Q Exactive Plus Orbitrap LC/MS (Thermo Instruments, San Jose, CA) system. The analytes were separated by using an Eclipse Plus column (C18, 2.1 × 50 mm, 1.8 µm; no. 959757-902, Agilent Technologies, Santa Clara, CA). The mobile phases were composed of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The gradient began at 5% B and increased to 95% B over 8.0 min with a flow rate of 250 µL/min, held ratio from 8.0 to 8.6 min, then resumed 5% B until 9.3 min. LC–MS used a HESI ion source in positive ion mode and a mass resolution of 70,000, sheath gas flow rate of 48, auxiliary gas rate of 11, and sweep gas rate of 2 (all arbitrary units). The capillary temperature was 256 °C. NB and buprenorphine used a collision energy of 50 eV, N3G and B3G used a collision energy of 20 eV, and buprenorphine-D4 and norbuprenorphine-D3 used 15 eV. This assay used high-resolution accurate MS to 4 decimal places.
Pharmacokinetic analysis.
Noncompartmental analysis was performed by using industry-standard software (Phoenix 64 WinNonLin 8.0.0.3176, Certara, Princeton, NJ) to estimate various pharmacokinetic parameters of buprenorphine and 3 metabolites in plasma in each rabbit. The following parameters were estimated: Tmax, Cmax, apparent elimination half-life (t1/2, calculated as ln2/λz, with λz being the first-order rate constant associated with the terminal portion of the time–concentration curve as estimated by linear regression of time compared with log concentration [AUC0-last, calculated by the linear trapezoidal rule]), and AUC0-inf (calculated by adding the last observed concentration divided by λz to AUC0-last). For our experiment, we assumed TMC of the parent drug, buprenorphine, in the plasma to be 0.1 ng/mL. Any concentration above 0.1 ng/mL buprenorphine in blood has been suggested to have analgesic properties for rabbits.18,24
Statistical analysis.
Scores for behavior, CBC, clinical chemistry, food, and feces were analyzed across time points and between groups by using repeated-measures ANOVA, with posthoc tests and Bonferroni adjustments for identification of dyadic contrasts. Pearson correlation tests were used for analyses of the relationship between 2 continuous variables. The α value was set at 0.05 and a trend was identified if the P value was less than 0.10.
Results
Pharmacokinetics.
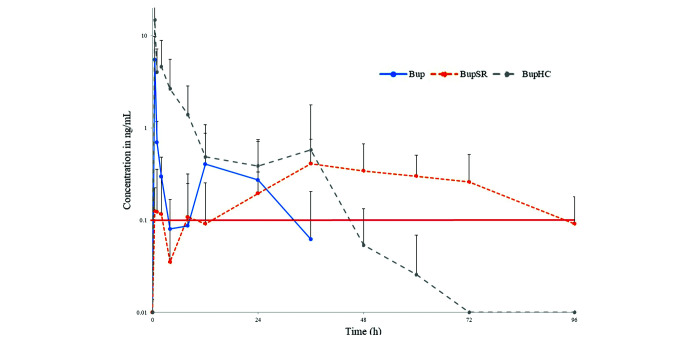
Figures 2, 3, 4 and 5 and Table 1 provide the results for buprenorphine pharmacokinetics. Regarding the parent drug concentration, Bup- and BupHC-treated rabbits had the same mean Tmax (i.e., 30 min). However, the mean Cmax in the BupHC-treated group (14.8 ng/mL) was approximately 2.69 times higher than that for the Bup-treated group (5.5 ng/mL). In Bup-treated animals, the average buprenorphine concentration remained above the TMC for analgesic effect from shortly after administration until just prior to 4 h after administration. The buprenorphine concentration then again rose above the TMC for analgesic efficacy from the 8-h time point to just before the 32-h time point. In the BupHC group, buprenorphine concentrations remained above the TMC for analgesic efficacy from shortly after the time of administration until prior to the 48-h postadministration time point. In contrast to those in the Bup- and BupHC-treated animals, buprenorphine concentrations in the BupSR-treated rabbits differed dramatically: mean Tmax was 39 h, and mean Cmax was 0.6 ng/mL. In the BupSR-treated rabbits, the average buprenorphine concentration remained above the TMC for analgesic effect from shortly after the 12-h postadministration time point until just prior to the 96-h time point.
Figure 2.
Mean blood plasma concentrations of the parent drug, buprenorphine, after a single injection of Bup at 0.05 mg/kg SC, BupSR at 0.15 mg/kg SC, or BupHC at 0.24 mg/kg SC. Red line indicates TMC of 0.1 ng/mL.
Figure 3.
Blood plasma concentrations of the parent drug, buprenorphine, and the 3 metabolites N3G, B3G, and NB after a single injection of Bup at 0.05 mg/kg SC.
Figure 4.
Blood plasma concentrations of the parent drug, buprenorphine, and the 3 metabolites N3G, B3G, and NB after a single injection of BupSR at 0.15 mg/kg SC.
Figure 5.
Blood plasma concentrations of the parent drug, buprenorphine, and the 3 metabolites N3G, B3G, and NB after a single injection of BupHC at 0.24 mg/kg SC.
Table 1.
Pharmacokinetic parameters (mean ± 1 SD) of buprenorphine (B; parent drug), NB, N3G, and B3G in rabbits after a single subcutaneous injection of either Bup at 0.05 mg/kg, BupSR at 0.15mg/kg, or BupHC at 0.24 mg/kg
| Bup |
BupSR |
BupHC |
||||||||||
| B | NB | N3G | B3G | B | NB | N3G | B3G | B | NB | N3G | B3G | |
| r2 | 0.6 ± 0.3 | 0.702 ± 0.167 | 0.370 ± 0.407 | 0.241 ± 0.323 | 0.571 ± 0.307 | 0.527 ± 0.396 | 0.044 ± 0.000 | 0.584 ± 0.399 | 0.863 ± 0.099 | 0.298 ± 0.175 | 0.856 ± 0.179 | 0.688 ± 0.334 |
| Tmax, h | 0.5 ± 0.0 | 3 ± 2 | 3 ± 3 | 3.9 ± 8.2 | 39 ± 26 | 18 ± 28 | 67 ± 32 | 57 ± 29 | 0.5 ± 0.0 | 28 ± 29 | 6 ± 4 | 1.4 ± 0.7 |
| Cmax, ng/mL | 5.5 ± 4.0 | 0.4 ± 0.1 | 20.5 ± 14.0 | 2.1 ± 1.1 | 0.6 ± 0.2 | 0.4 ± 0.3 | 20.0 ± 15.2 | 3.2 ± 3.7 | 14.8 ± 6.6 | 0.3 ± 0.1 | 54.4 ± 28.2 | 10.6 ± 10.0 |
| λz, /h | 0.278 ± 0.323 | 0.014 ± 0.013 | 0.020 ± 0.022 | 0.0111 ± 0.0065 | 0.038 ± 0.031 | 0.014 ± 0.013 | 0.006 ± 0.000 | 0.023 ± 0.022 | 0.099 ± 0.060 | 0.007 ± 0.003 | 0.038 ± 0.025 | 0.049 ± 0.040 |
| t1/2λz, h | 8.2 ± 9.1 | 89.2 ± 69.7 | 196.7 ± 256.0 | 91.1 ± 53.1 | 65.7 ± 92.4 | 217.1 ± 286.0 | 113.7 ± 0.0 | 92.0 ± 82.5 | 10.5 ± 6.6 | 119.4 ± 51.8 | 31.2 ± 26.3 | 29.4 ± 24.1 |
| AUC0-last, h × ng/mL | 9.9 ± 8.5 | 7.4 ± 2.8 | 221.5 ± 129.7 | 446.8 ± 459.3 | 22.2 ± 12.1 | 15.7 ± 11.5 | 721.3 ± 493.8 | 109.1 ± 114.1 | 44.0 ± 18.3 | 10.1 ± 4.2 | 1671.0 ± 771.6 | 122.0 ± 68.5 |
| AUC0-inf, h × ng/mL | 11.5 ± 11.0 | 16.1 ± 6.1 | 1188.4 ± 1443.0 | 48.6 ± 17.9 | 41.1 ± 40.3 | 69.2 ± 95.6 | 1089.5 ± 0.0 | 153.5 ± 60.7 | 44.7 ± 19.3 | 22.0 ± 10.8 | 2024.1 ± 791.7 | 148.2 ± 69.6 |
| AUC%extrapolated, % | 15.8 ± 19.3 | 50.2 ± 18.9 | 50.1 ± 31.8 | 57.9 ± 31.3 | 22.9 ± 27.3 | 48.6 ± 32.0 | 42.9 ± 0.0 | 40.8 ± 29.1 | 3.8 ± 4.1 | 56.9 ± 13.9 | 16.4 ± 18.2 | 16.0 ± 20.4 |
We also assessed the pharmacokinetics of 3 metabolites—NB, N3G, and B3G—for each of the 3 formulations of buprenorphine (Table 1, individual data not shown). N3G was the predominant metabolite for all 3 buprenorphine formulations.
Behavior.
Behavioral scores related to pain and discomfort did not differ among the 3 buprenorphine formulations or as compared with the control animals. In addition, there were no main effects of time or interaction effects between time and group.
Food consumption.
Food consumption was measured at 24-h intervals after surgery (Figure 6). All rabbits had a decrease in pellet food consumption after surgery compared with baseline, except for control animal D3; this rabbit was removed from all statistical analyses because it was the only animal to have a higher food consumption after surgery than during the baseline acclimation period. The lower food consumption during the baseline period was attributed to a delay in acclimation after shipment. When we compared baseline measures of food consumption prior to the day of surgery with that after surgery, food consumption was lower in BupSR subjects than in controls on days 2 and 3 (P < 0.001 for both). A lower intake was observed only on day 2 for the BupHC group (P < 0.001).
Figure 6.

Change in food consumption on days 1 through 3 after surgical procedure compared with baseline measurements.
Feces production.
Feces production was measured as an indicator of gastrointestinal motility at 24-h intervals after surgery. All rabbits had a decrease in feces production in the days after surgery, compared with baseline measurements. These data did not differ between treatment groups.
Tissue reaction at injection site.
No gross lesions were apparent in any tissue at necropsy. On histologic evaluation, all drug injection sites exhibited marked tissue reaction, compared with saline control sites. However, the amount of tissue reaction did not differ between treatment groups (data not shown). The specific area of the injection site reaction varied among superficial dermis, deep dermis, panniculus, and subcutis in all groups, including saline controls.
Other reactions.
Among the 7 rabbits in the BupHC group, 2 exhibited neurologic symptoms including depression, horizontal nystagmus, circling, and ataxia. One of the 2 rabbits, D19, was euthanized on day 3 due to the severity of the neurologic signs, necropsied, and its tissues examined by a veterinary pathologist (RVB). Lesions included severe pulmonary hemorrhage and vasculitis. In addition, this animal had unilateral segmental necrosis of the hippocampus. This lesion is caused by hypoxia–ischemia and is a known complication of seizures.
CBC and clinical chemistry analyses.
Blood for CBC and clinical chemistry analyses was collected at time 0, at the 48-h time point, and again at the terminal blood draw (96 h; Tables 2 and 3). Time 0 samples were inadvertently not collected from 2 Bup-treated animals and one control animal (animals D1, D2, and D3; Bup, BupHC, and control groups, respectively). Rabbit D19 (BupHC group) was euthanized prior to the 96-h time point due to neurologic conditions. No significant differences in CBC or chemistry parameters were found between treatment groups.
Table 2.
Serum chemistry values (mean ± standard deviation) for the 3 groups at the various time points
| Control |
Bup |
BupSR |
BupHC |
||||||||
| 0 h (n = 8) | 96 h (n = 9) | 0 h (n = 5) | 48 h (n = 7) | 96 h (n = 7) | 0 h (n = 8) | 48 h (n = 8) | 96 h (n = 8) | 0 h (n = 7) | 48 h (n = 7) | 96 h (n = 6) | |
| Glucose (mg/dL) | 135.9 ± 4.8 | 145.6 ± 16.4 | 136.6 ± 2.3 | 129.7 ± 6.0 | 128.0 ± 6.9 | 132.8 ± 7.1 | 130.3 ± 6.1 | 135.5 ± 7.5 | 137.0 ± 7.7 | 148.1 ± 40.2 | 151.8 ± 18.2 |
| BUN (mg/dL) | 15.5 ± 1.9 | 13.7 ± 1.3 | 13.6 ± 2.1 | 13.3 ± 4.5 | 13.0 ± 1.6 | 15.9 ± 1.9 | 16.0 ± 4 | 12.9 ± 2.9 | 15.4 ± 1.8 | 17.0 ± 3 | 13.5 ± 2.0 |
| Total protein (g/dL) | 5.2 ± 0.3 | 5.0 ± 0.4 | 5.0 ± 0.4 | 5.4 ± 0.2 | 5.2 ± 0.4 | 5.13 ± 0.2 | 5.5 ± 0.5 | 5.0 ± 0.3 | 5.1 ± 0.2 | 5.5 ± 0.4 | 5.1 ± 0.4 |
| Creatine kinase (U/L) | 378.5 ± 144.1 | 861.3 ± 307.8 | 418.0 ± 217.2 | 4317.1 ± 1442.3 | 1089.9 ± 776.2 | 464.0 ± 194.5 | 7206.3 ± 2723.8 | 931.8 ± 356.8 | 344.1 ± 82.1 | 5382.0 ± 2159.0 | 997.0 ± 817.9 |
| Albumin (g/dL) | 3.9 ± 0.2 | 3.6 ± 0.3 | 3.8 ± 0.3 | 3.9 ± 3.5 | 3.6 ± 0.2 | 3.8 ± 0.1 | 3.6 ± 0.3 | 3.4 ± 0.2 | 3.9 ±0.1 | 3.7 ± 0.3 | 3.5 ± 0.2 |
| Globulin (g/dL) | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.6 ± 0.1 | 1.9 ± 1.9 | 1.6 ± 0.2 | 1.4 ± 0.1 | 1.9 ± 0.2 | 1.6 ± 0.3 | 1.2 ± 0.2 | 1.8 ± 0.3 | 1.6 ± 0.3 |
| Total bilirubin (mg/dL) | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.1 | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.2 ± 0.2 | 0.2 ± 0.1 |
| ALP (U/L) | 81.5 ± 29.3 | 55.1 ± 16.9 | 140.2 ± 117.0 | 69.1 ± 58.8 | 57.0 ± 41.3 | 80.1 ± 35.1 | 45.5 ± 25.9 | 43.9 ± 24.2 | 81.9 ± 55.7 | 54.6 ± 27.0 | 47.8 ± 21.5 |
| ALT (U/L) | 23.2 ± 9.0 | 35.7 ± 13.8 | 19.0 ± 6.7 | 41.3 ± 15.0 | 28.0 ± 10.0 | 29.5 ± 10.7 | 69.6 ± 34.3 | 48.9 ± 28.2 | 40.9 ± 37.5 | 60.7 ± 22.0 | 39.0 ± 11.8 |
| AST (U/L) | 11.1 ± 3.4 | 14.4 ± 5.2 | 9.8 ± 2.0 | 26.0 ± 10.3 | 21.0 ± 7.7 | 12.3 ± 3.0 | 41.1 ± 20.2 | 15.1 ± 13.3 | 16.6 ± 7.3 | 38.9 ± 14.9 | 22.1 ± 11.5 |
| Cholesterol (mg/dL) | 35.6 ± 12.4 | 36.3 ± 9.9 | 30.8 ± 6.0 | 50.3 ± 12.7 | 52.3 ± 13.5 | 45.4 ± 41.1 | 57.4 ± 22.5 | 52.8 ± 14.9 | 34.6 ± 6.6 | 55.3 ± 23.7 | 51.2 ± 21.8 |
| Calcium (mg/dL) | 12.8 ± 0.5 | 12.7 ± 0.5 | 12.9 ± 0.5 | 11.6 ± 0.5 | 12.2 ± 0.4 | 13.0 ± 0.4 | 11.8 ± 0.4 | 12.2 ± 0.6 | 11.3 ± 4.8 | 11.8 ± 0.7 | 12.2 ± 0.5 |
| Phosphorus (mg/dL) | 4.5 ± 0.7 | 4.2 ± 0.8 | 4.3 ± 0.4 | 4.3 ± 0.3 | 5.0 ± 0.4 | 4.5 ± 0.5 | 4.4 ± 0.8 | 4.5 ± 0.5 | 4.4 ± 0.7 | 4.4 ± 0.9 | 4.6 ± 0.4 |
| Sodium (mmol/L) | 140.0 ± 1.3 | 141.4 ± 2.3 | 140.0 ± 2.4 | 137.3 ± 2.9 | 139.7 ± 1.1 | 139.5 ± 0.9 | 139.6 ± 4.3 | 140.9 ± 2.0 | 139.4 ± 0.5 | 141.6 ± 2.4 | 140.8 ± 3.0 |
| Potassium (mmol/L) | 4.5 ± 0.1 | 4.5 ± 0.3 | 4.4 ± 0.3 | 5.3 ± 0.8 | 5.0 ± 0.7 | 4.6 ± 0.4 | 5.4 ± 1.1 | 4.6 ± 0.5 | 5.1 ± 2.2 | 5.0 ± 1.1 | 4.9 ± 0.9 |
| Chloride (mmol/L) | 100.8 ± 2.4 | 104.6 ± 2.7 | 103.2 ± 1.3 | 103.1 ± 2.3 | 102.9 ± 1.5 | 101.2 ± 2.8 | 103.4 ± 3.8 | 105.4 ± 2.0 | 100.4 ± 2.5 | 104.1 ± 1.7 | 101.8 ± 2.3 |
| Creatinine (mg/gL) | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.6 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 |
| BUN/Creatinine | 18.9 ± 2.5 | 18.3 ±2.9 | 22.2 ± 4.1 | 18.8 ± 4.3 | 18.7 ± 2.4 | 20.4 ± 2.5 | 19.4 ± 4.0 | 15.9 ± 3.0 | 20.8 ± 2.3 | 19.2 ± 2.6 | 16.7 ± 3.2 |
Table 3.
Hematologic values (mean ± standard deviation) for the 3 groups at the different time points
| Control |
Bup |
BupSR |
BupHC |
||||||||
| 0 h (n = 8) | 96 h (n = 9) | 0 h (n = 5) | 48 h (n = 7) | 96 h (n = 7) | 0 h (n = 8) | 48 h (n = 8) | 96 h (n = 8) | 0 h (n = 7) | 48 h (n = 7) | 96 h (n = 6) | |
| RBC (×106/µL) | 6.4 ± 0.5 | 6.0 ± 0.8 | 6.0 ± 0.3 | 4.7 ± 0.2 | 4.8 ± 0.6 | 6.3 ± 0.5 | 5.1 ± 0.5 | 4.8 ± 0.4 | 6.0 ± 0.3 | 5.1 ± 0.2 | 4.5 ± 0.4 |
| Hgb (g/dL) | 13.5 ± 1.2 | 12.6 ± 1.7 | 13.0 ± 0.8 | 10.2 ± 0.5 | 10.4 ± 1.2 | 13.1 ± 0.9 | 10.6 ± 0.8 | 10.0 ± 0.7 | 12.7 ± 0.7 | 10.6 ± 0.6 | 9.6 ± 0.7 |
| Hct (%) | 41.0 ± 3.8 | 39.6 ± 4.5 | 39.6 ± 2.2 | 33.7 ± 2.8 | 33.5 ± 2.8 | 40.1 ± 2.8 | 33.7 ± 3.3 | 33.4 ± 2.9 | 38.7 ± 2.3 | 33.6 ± 2.3 | 31.5 ± 2.5 |
| MCV (fL) | 63.9 ± 1.5 | 67.1 ± 4.6 | 66.4 ± 3.6 | 71.6 ± 6.5 | 69.7 ± 5.4 | 63.4 ± 2.3 | 66.9 ± 4.3 | 68.9 ±4.0 | 64.4 ± 2.9 | 66.3 ± 3.3 | 69.5 ± 4.4 |
| MCH (pg) | 21.1 ± 0.4 | 21.3 ± 0.8 | 21.8 ± 1.0 | 21.6 ± 0.9 | 21.5 ± 1.0 | 20.7 ± 0.8 | 21.0 ± 0.6 | 20.7 ± 0.7 | 21.1 ± 0.8 | 20.9 ± 0.7 | 21.1 ± 0.8 |
| MCHC (g/dL) | 33.1 ± 0.5 | 31.8 ± 1.3 | 32.9 ± 0.7 | 30.4 ± 3.2 | 30.9 ± 1.4 | 32.7 ± 0.4 | 31.5 ± 1.5 | 30.4 ± 2.4 | 32.8 ± 0.3 | 31.6 ± 1.3 | 30.4 ± 1.0 |
| WBC (×103/uL) | 8.6 ± 2.2 | 9.3 ± 1.1 | 8.3 ± 0.8 | 9.6 ± 1.7 | 8.9 ± 1.5 | 8.2 ± 1.4 | 9.0 ± 1.5 | 8.9 ± 1.7 | 7.1 ± 1.0 | 7.2 ± 1.3 | 7.8 ± 1.6 |
| Neutrophils (%) | 22.0 ± 8.9 | 32.2 ± 13.0 | 37.4 ± 9.4 | 35.7 ± 5.5 | 32.4 ± 10.5 | 27.1 ± 10.3 | 42.7 ± 8.9 | 32.1 ± 12.4 | 24.0 ± 8.3 | 36.6 ± 9.1 | 31.8 ± 5.1 |
| Lymphocytes (%) | 69.1 ± 12.3 | 61.3 ± 11.5 | 53.1 ± 5.9 | 55.2 ± 6.5 | 58.1 ± 12.6 | 65.3 ± 9.8 | 47.6 ± 9.8 | 60.2 ± 15.4 | 65.9 ± 10.7 | 52.2 ± 9.8 | 55.4 ± 6.5 |
| Monocytes (%) | 4.8 ± 2.8 | 3.2 ± 2.0 | 5.3 ± 2.8 | 6.0 ± 1.9 | 6.7 ± 1.5 | 4.2 ± 2.9 | 6.1 ± 1.2 | 5.0 ± 4.3 | 6.1 ± 2.7 | 7.6 ± 1.4 | 8.7 ± 3.3 |
| Eosinophils (%) | 1.7 ± 1.2 | 1.3 ± 1.3 | 1.2 ± 1.2 | 0.9 ± 0.5 | 1.3 ± 0.6 | 1.0 ± 0.7 | 1.2 ± 0.6 | 1.5 ± 1.6 | 1.1 ± 0.9 | 0.8 ± 0.2 | 1.5 ± 0.5 |
| Basophils (%) | 2.5 ± 0.9 | 2.0 ± 1.2 | 3.1 ± 2.4 | 2.2 ± 1.8 | 1.6 ± 1.4 | 2.5 ± 1.6 | 2.5 ± 1.0 | 1.2 ± 1.2 | 2.9 ± 1.6 | 2.8 ± 1.5 | 2.7 ± 1.1 |
Discussion
We assessed 3 formulations of buprenorphine for the purpose of finding a suitable option that provided few adverse effects and prolonged analgesia for rabbits. A reported adverse effect of long-acting buprenorphine formulations is tissue reaction at the site of injection, causing sterile abscesses.8,21 In addition, these formulations can be associated with the adverse effects of gastrointestinal ileus, nausea, and anorexia.12,30 Another potential constraint associated with compounded drugs such as BupSR is that some states impose restrictions on the purchase of compounded controlled substances, making it difficult to acquire these drugs. The Office of Laboratory Animal Welfare, the agency that enforces the PHS Policy on Humane Care and Use of Laboratory Animals, recommends the use of FDA-approved drugs rather than compounded drugs whenever they are available.22 The long-acting buprenorphine drug that was used in this study is FDA-approved for use in cats every 24 h for as long as 3 d after surgery. This drug has not yet been approved for use in rabbits, and no dosing regimen has been identified in this species. Therefore, we used the recommended cat dose for this study. We also assessed the active metabolites of buprenorphine that have been identified to have analgesic effect in humans.4
The TMC of buprenorphine has been reported as 0.1 ng/mL in rhesus macaques (Macaca mulatta) to more 2 ng/mL in domestic cats, with a wide range between species.6,8,18,21,24,28 In a previous study using a dermal buprenorphine patch in rabbits, the effective plasma TMC was observed to be 0.1 to 0.5 ng/mL.24 In a study presented at the Annual Meeting for the Society of Toxicology in 2013, the author suggested that 0.1 ng/mL was an effective buprenorphine concentration for analgesia.18 Therefore, in our experiments, we assumed that the TMC for analgesia of the parent drug, buprenorphine, was 0.1 ng/mL.
The first objective of this study was to assess the plasma concentrations and pharmacokinetics of buprenorphine after administration of each of the formulations. After administration of Bup, 2 peaks in mean buprenorphine concentration were observed (at 0.5 and 12 h timepoints) and may represent enterohepatic recycling of the drug, as previously reported in rats given buprenorphine.23 Some of our rabbits might have experienced reduced analgesia between 4 and 8 h after drug administration because mean plasma concentrations were below the TMC for analgesia; however, we did not observe any behaviors indicative of pain. Nevertheless, rabbits are stoic in nature, and they may hide low levels of pain.28 In addition, the depressant effect of buprenorphine may have masked behavioral manifestations of pain. Alternatively, the concentration of buprenorphine required for analgesia may be lower in rabbits than in other species, or buprenorphine metabolites may play a role in the analgesic effect.
The recommended dose of BupSR in rabbits is 0.10 to 0.15 mg/kg SC and is reported to last for 96 h after administration.8,18,33 In our study, we administered BupSR at the highest reported dose: 0.15 mg/kg. After administration of BupSR, the mean plasma concentrations of buprenorphine reached TMC at approximately hour 14 and remained above the therapeutic threshold through approximately 96 h after administration. All assumptions of the duration above TMC are extrapolated from the graphs because we assume that the concentrations do not differ wildly when the trend of measured concentrations is up or down. The manufacturer reports that BupSR administered at 0.1 mg/kg starts acting within 30 min and lasts for approximately 96 h after dosing.18 Possible explanations for the differences in the onset of action reported by the manufacturer and found in our study include matrix variability between lots of the compounded product and species-associated differences in absorption of the drug from the administered formulation. Our results suggest that BupSR provides long-lasting analgesia for more than 3 d in rabbits but that this formulation may not be appropriate for immediate pain relief in this species and that the drug may have to be initially administered on the day before surgery.
The high concentration formulation labeled for cats has a recommended dose of 0.24 mg/kg SC administered 1 h prior to surgery and then once daily for a maximum of 3 d to achieve a total of 72 h of pain control in cats.25 After the administration of BupHC in our current study, mean plasma concentrations of buprenorphine in rabbits stayed above the 0.1-ng/mL therapeutic threshold for approximately 42 h. Although BupHC appears to have potential as a long-lasting analgesic drug for rabbits, 2 of the 7 animals in this group experienced neurologic side effects. Both rabbits developed horizontal nystagmus, ataxia, head tilt, and circling. One rabbit recovered by day 3 of the experiment and had no other neurologic signs, but the other (D19) was severely depressed at the beginning of day 2. This rabbit was euthanized at the 48-h time point due to the severity of its neurologic symptoms. Prior to anesthesia and catheter implantation, this rabbit had a generalized seizure and showed opisthotonos for approximately 10 s. The rabbit then exhibited a postictal phase, when it sat quietly for a few minutes and appeared to recover. We decided to continue with anesthesia and catheter placement given that the rabbit made a full recovery after the seizure. The rabbit recovered uneventfully from anesthesia and appeared bright, alert, and responsive during the next day. However, on the morning of the second day after surgery, the animal was noted to be depressed to obtunded and staring into space. When stimulated, the rabbit became alert and moved around for approximately 15 s before returning to the previous depressed or obtunded state. The rabbit had bilateral pupillary light response and was positive for the dazzle reflex in both eyes; he did not have any visible proprioceptive defects. No gross lesions of organs or brain were identified during gross necropsy, but cut sections revealed unilateral segmental necrosis of the hippocampus. This lesion is caused by hypoxia–ischemia and is a known complication of seizures. The neuronal necrosis is presumed to have occurred from the initial seizure; however, we cannot exclude pulmonary hemorrhage as a contributing factor to the neuronal necrosis. Full µ-agonist opioids such as fentanyl and morphine have previously been reported cause neuronal degeneration in the hippocampus of rats,3,12,19 but similar findings have not been reported in rabbits. Ultimately, the role that opioid administration played in the neuronal necrosis in this rabbit could not be definitively determined. Why 2 of the 7 animals in this group experienced similar neurologic symptoms after administration of high-concentration buprenorphine remains unclear at this time.
We also assessed the pharmacokinetics of the 3 active metabolites of buprenorphine—N3G, B3G, and NB—after administration of each of the 3 formulations. The potential for analgesic activity of these metabolites complicates the dosing of buprenorphine. Relying only on the plasma concentrations of the parent drug to evaluate the analgesic effect of buprenorphine may not be adequate. However, we did not assess the metabolites individually for analgesic effect. Because plasma concentrations in some rabbits that received BupSR were below theoretical analgesic concentrations but unaccompanied by signs of pain, we assume that the metabolites contributed to analgesia; a previous study in humans suggested that NB and B3G have 1/4 of the analgesic potency of buprenorphine whereas N3G has the smallest effect of the metabolites.4 The failure of our modified rabbit grimace scoring to detect differences in the groups may be due to the stoic nature of rabbits or may indicate that due to the minimally invasive nature of the surgery, the painful stimulus was not sufficiently severe to result in behavioral changes. The Rabbit Grimace Scale was originally designed to assess fully conscious animals and may be unsuitable for animals receiving opioid analgesics. Although several of our rabbits scored a 2 in a single category for either ears, eyes, or movement, the mean scores were calculated as 1, which has no clinical significance.
Gastrointestinal adverse effects were assessed by measuring body weight, food consumption, and feces production. As expected, most animals lost weight between the time of surgery and the end of the experiment (the 96-h time point), with the exception of one control and one Bup rabbit, which gained 0.03% and 0.04%, respectively, of their body weight over the course of the experiment. There was a trend toward significance in weight loss for BupSR and BupHC groups compared with controls (P < 0.10). This result may reflect the extended amount of time that the drug was present in the plasma, because buprenorphine is known to cause anorexia.13,30 Both the BupSR and BupHC groups had significant but transient decreases in food consumption compared with controls. BupSR animals had a decrease in food consumption on days 1 through 3, and the BupHC group had a decrease in food consumption on days 2 and 3. Opioid administration is known to decrease gastrointestinal movement and anorexia and may be the reason for the reduced food consumption.13 Another reason for less food consumption in rabbits is pain or stress.30 In addition, day 2 showed a trend toward decreased food consumption in BupSR compared with Bup rabbits (P < 0.08). However, due to the findings in our behavioral assessment, we associate the transient anorexia with opioid administration and not with pain or distress. Food consumption was not significantly reduced in either the control or Bup groups compared with those given the longer-lasting buprenorphine drugs. Moreover, because buprenorphine concentrations in Bup were low by the end of day 1, we would expect that these rabbits may have experienced more pain than either the BupSR or BupHC groups.
Feces production on day 3 was significantly reduced in BupSR rabbits relative to control animals and in the BupSR group compared with Bup rabbits. This finding is not surprising, given the reduced food consumption seen in the BupSR animals on days 1 through 3.
Injection site reactions associated with subcutaneous administration of BupSR have been previously reported.5,6,8,21 In our study, rabbits that received an injection of buprenorphine, regardless of formulation, had no visible tissue reactions at the 96-h time point but all had microscopic inflammation present on histology. The site of inflammation varied among superficial dermis, deep dermis, panniculus, and subcutis. Inflammation severity did not differ significantly between treatment groups, but each treatment group had significantly more inflammation in the drug injection site as compared with the saline control site. No gross signs of inflammation, such as redness or abscessation, were noted during this study. Therefore, the amount of inflammation noted on histology in our study was not clinically significant, and neither BupSR nor BupHC posed an increased risk for injection site reaction as compared with the administration of Bup.
Additional evaluations for adverse effects included CBC and blood chemistry analyses. Due to a limitation of the study design, an arterial blood sample was collected at time 0, whereas venous blood collections were performed at the later time points. Slight differences in results could reflect the site of sample collection. Control rabbits were assessed at the 0- and 96-h time points, whereas all rabbits in the experimental drug groups were assessed at the 0-, 48-, and 96-h time points. Most of the hematologic parameters were within normal limits.11,27 Many of the rabbits had a mild microcytic anemia at the 48- and 96-h time points, most likely due to the amount of blood drawn from the rabbits over the experimental period, even though the amount of blood withdrawn during the week remained below 1% of body weight. At time point 0, one rabbit in the BupHC group had a potassium level that exceeded 10 mmol/L and calcium that exceeded 0.4 mg/dL calcium; both of these parameters were normal at the 48 and 96-h time point. The results for the 0-h blood draw were most likely lab errors, because these values are incompatible with life. In addition, one rabbit had a low WBC count at time 0 but had normal results at both the 48- and 96-h time points; this spurious initial value may be due to a stress response to restraint, despite no indication of overt stress during restraint for blood collection at this time point. Furthermore, several rabbits had elevated hemolysis scores at the during the 48 and 96-h time points, perhaps due to stress or collection technique or both. A potential sequala of hemolysis is falsely high creatine kinase levels.31 All rabbits had elevated creatine kinase levels at the 48-h time point but levels were to normal to mildly elevated by the 96-h time point. Comparing hemolysis with creatine kinase levels did not reveal any statistically significant findings. Another explanation for elevated creatine kinase concentrations is tissue damage associated with the surgical implantation of the jugular catheter.31 Lastly, phosphorus levels at the 48- and 96-h time points typically were lower than normal, perhaps reflecting decreased dietary intake.31 Even though our animals apparently had reduced food intake, the statistical analysis did not show any significant relationship between food intake and phosphorus level. A more precise method of measuring food intake may have supported a significant relationship.
In conclusion, we found significant differences among the 3 buprenorphine formulations in the onset and duration of concentrations of drug thought to be analgesic. Our hypothesis that BupSR and BupHC would result in plasma concentrations above TMC for longer than 12 h was upheld. Despite the lack of clinically significant reactions at the injection sites, abnormal CBC or chemistry results, and absence of behavioral signs of pain with all 3 formulations, we did see adverse neurologic effects in 2 BupHC rabbits. Bup and BupSR appear to have analgesic properties that can be useful in rabbits in either the private practice or research settings. BupSR may be effective over an extended amount of time, requiring less handling and thus less stress to the animal. BupHC, although initially promising in light of its efficacy in cats, may not be an appropriate analgesic in rabbits at the studied dose. Our data suggest that the metabolites of buprenorphine should be considered when determining effective analgesic doses in rabbits. A deeper understanding of the direct effects of drug metabolites and of the combined effects of metabolites and parent drug is needed. In addition, more research is needed to determine the optimal method of pain scoring in rabbits that receive opioid analgesics. More experiments are necessary to determine differences in the effects of these drugs in female and aged New Zealand white rabbits. Overall, analgesic effect, analgesic duration, and adverse effects should all be considered when selecting an appropriate analgesic drug.
Acknowledgments
We thank the entire DCM staff at Tulane University for their assistance, support of the project, and care of the animals used for the project.
References
- 1.Andaluz A, Moll X, Abellán R, Ventura R, Carbó M, Fresno L, García F. 2009. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet J 181:299–304. 10.1016/j.tvjl.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Animal Welfare Regulations. 2013. 9 CFR § 3.129. [Google Scholar]
- 3.Atici S, Cinel L, Cinel I, Doruk N, Aktekin M, Akca A, Camdeviren H, Oral U. 2004. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int J Neurosci 114:1001–1011. 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- 4.Brown SM, Holtzman M, Kim T, Kharasch ED. 2011. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology 115:1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cary CD, Lukovsky-Akhsanov NL, Gallardo-Romero NF, Tansey CM, Ostergaard SD, Taylor J, Willie D, Morgan CN, Powell N, Lathrop GW. 2017. Pharmacokinetic profiles of meloxicam and sustained-release buprenorphine in prairie dogs (Cynomys ludovicianus). J Am Assoc Lab Anim Sci 56:160–165. [PMC free article] [PubMed] [Google Scholar]
- 6.Clark TS, Clark DD, Hoyt RF., Jr 2014. Pharmacokinetic comparison of sustained-release and standard buprenorphine in mice. J Am Assoc Lab Anim Sci 53:387–391. [PMC free article] [PubMed] [Google Scholar]
- 7.Coulter CA, Flecknell PA, Leach MC, Richardson CA. 2011. Reported analgesic administration to rabbits undergoing experimental surgical procedures. BMC Vet Res 7:1–6. 10.1186/1746-6148-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiVincenti L, Jr, Meirelles LA, Westcott RA. 2016. Safety and clinical effectiveness of a compounded sustained-release formulation of buprenorphine for postoperative analgesia in New Zealand white rabbits. J Am Vet Med Assoc 248:795–801. 10.2460/javma.248.7.795. [DOI] [PubMed] [Google Scholar]
- 9.Doodnaught GM, Monteiro BP, Benito J, Edge D, Beaudry F, Pelligand L, Steagall P. 2017. Pharmacokinetic and pharmacodynamic modelling after subcutaneous, intravenous and buccal administration of a high-concentration formulation of buprenorphine in conscious cats. PloS One 12:1–16. 10.1371/journal.pone.0176443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flecknell P, Liles J. 1990. Assessment of the analgesic action of opioid agonist-antagonists in the rabbit. Anaesthesia 17:24–29. 10.1111/j.1467-2995.1990.tb00384.x [DOI] [Google Scholar]
- 11.Fox JG, Anderson LC, Otto GM, Pritchett-Corning KR, Whary MT, editors. 2015. Laboratory animal medicine. Boston (MA): Elsevier. 10.1016/B978-0-12-409527-4.00001-8 [DOI] [Google Scholar]
- 12.Fujikawa DG. 2005. Prolonged seizures and cellular injury: understanding the connection. Epilepsy Behav 7:S3–S11. 10.1016/j.yebeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 13.Goldschlager GB, Gillespie VL, Palme R, Baxter MG. 2013. Effects of multimodal analgesia with lowdose buprenorphine and meloxicam on fecal glucocorticoid metabolites after surgery in New Zealand white rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 52:571–576. [PMC free article] [PubMed] [Google Scholar]
- 14.Hedenqvist P, Trbakovic A, Thor A, Ley C, Ekman S, Jensen-Waern M. 2016. Carprofen neither reduces postoperative facial expression scores in rabbits treated with buprenorphine nor alters long term bone formation after maxillary sinus grafting. Res Vet Sci 107:123–131. 10.1016/j.rvsc.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Ho S-T, Wang J-J, Ho W, Hu OY-P. 1991. Determination of buprenorphine by high-performance liquid chromatography with fluorescence detection: application to human and rabbit pharmacokinetic studies. J Chromatogr 570:339–350. 10.1016/0378-4347(91)80537-M. [DOI] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed. Washington (DC): National Academies Press. [Google Scholar]
- 17.Keating SC, Thomas AA, Flecknell PA, Leach MC. 2012. Evaluation of EMLA cream for preventing pain during tattooing of rabbits: changes in physiological, behavioural and facial expression responses. PLoS One 7:1–11. 10.1371/journal.pone.0044437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koetzner L, Stokes W, Lance W, Wnorowski G, South N, Boulet J. 2013. Development of sustained release buprenorphine for use as an improved analgesic in toxicology studies: assessment of formulation pharmacokinetics. Presented at the 52nd annual meeting and ToxExpo. San Antonio, Texas, 10–14 March 2013. [Cited 14 December 2017]. Available at: http://www.toxicology.org/pubs/docs/Prog/2013Program.pdf [Google Scholar]
- 19.Kofke WA, Garman RH, Stiller RL, Rose ME, Garman R. 1996. Opioid neurotoxicity: fentanyl dose-response effects in rats. Anesth Analg 83:1298–1306. 10.1213/00000539-199612000-00029. [DOI] [PubMed] [Google Scholar]
- 20.Liu S-Y, Liu K-S, Kuei C-H, Tzeng J-I, Ho S-T, Wang J-J. 2005. Simultaneous determination of buprenorphine and its prodrug, buprenorphine propionate, by high-performance liquid chromatography with fluorescence detection: application to pharmacokinetic studies in rabbits. J Chromatogr B Analyt Technol Biomed Life Sci 818:233–239. 10.1016/j.jchromb.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 21.Nunamaker EA, Halliday LC, Moody DE, Fang WB, Lindeblad M, Fortman JD. 2013. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 52:48–56. [PMC free article] [PubMed] [Google Scholar]
- 22.Office of Laboratory Animal Welfare. [Internet]. 2015. Public Health Service policy on humane care and use of laboratory animals. [Cited 21 August 2017]. Available at: https://olaw.nih.gov/policies-laws/phs-policy.htm
- 23.Ohtani M, Kotaki H, Sawada Y, Iga T. 1995. Comparative analysis of buprenorphine—and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 272:505–510. [PubMed] [Google Scholar]
- 24.Park I, Kim D, Song J, In CH, Jeong S-W, Lee SH, Min B, Lee D, Kim S-O. 2008. Buprederm, a new transdermal delivery system of buprenorphine: pharmacokinetic, efficacy and skin irritancy studies. Pharm Res 25:1052–1062. 10.1007/s11095-007-9470-6. [DOI] [PubMed] [Google Scholar]
- 25.Robertson SA. [Internet]. 2015. Zoetis: Simbadol (buprenorphine injection) Technical Monograph. [Cited 24 June 2020]. Available at: https://www.zoetisus.com/products/cats/simbadol/pdf/simbadoltechnicalmonograph.pdf
- 26.Smith BJ, Wegenast DJ, Hansen RJ, Hess AM, Kendall LV. 2016. Pharmacokinetics and paw withdrawal pressure in female guinea pigs (Cavia porcellus) treated with sustained-release buprenorphine and buprenorphine hydrochloride. J Am Assoc Lab Anim Sci 55:789–793. [PMC free article] [PubMed] [Google Scholar]
- 27.Suckow MA, Stevens KA, Wilson RP, editors. 2012. The laboratory rabbit, guinea pig, hamster, and other rodents. San Diego (CA): Elsevier. [Google Scholar]
- 28.Taylor PM, Luangdilok CH, Sear JW. 2015. Pharmacokinetic and pharmacodynamic evaluation of high doses of buprenorphine delivered via high-concentration formulations in cats. J Feline Med Surg 18:290–302. 10.1177/1098612X15581206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turner PV, Chen CH, Taylor MW. 2006. Pharmacokinetics of meloxicam in rabbits after single and repeat oral dosing. Comp Med 56:63–67. [PubMed] [Google Scholar]
- 30.Weaver LA, Blaze CA, Linder DE, Andrutis KA, Karas AZ. 2010. A model for clinical evaluation of perioperative analgesia in rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 49:845–851. [PMC free article] [PubMed] [Google Scholar]
- 31.Willard MD, Tvedten H. 2012. Small animal clinical diagnosis by laboratory methods. St Louis (MO): Elsevier. [Google Scholar]
- 32.Wootton R, Cross G, Wood S, West CD. 1988. An analgesiometry system for use in rabbits with some preliminary data on the effects of buprenorphine and lofentanil. Lab Anim 22:217–222. 10.1258/002367788780746395. [DOI] [PubMed] [Google Scholar]
- 33.Zanetti A. [Internet]. 2017. Safety and pharmacokinetic profiles of different buprenorphine SR doses in a rabbit model. [Cited 24 September 2017]. Available at: www.labroots.com/webinar/safety-pharmacokinetic-profiles-buprenorphine-sr-doses-rabbit-model.