Abstract
We investigated the clinical characteristics, prognostic factors, and post-recurrence prognostic factors of early- and late-recurrence patients for nasopharyngeal carcinoma (NPC) after definitive intensity-modulated radiation therapy (IMRT). This was a single-center retrospective analysis of patients in China from January 2010 to December 2015. The prognostic factors for overall survival (OS) and post-recurrence OS of early- and late-recurrence patients were identified using univariate and multivariate Cox regression analyses. Of the 9,468 patients included, 409 (4.3%), 325 (3.4%), and 182(1.9%) developed purely local recurrence, purely regional recurrence, and locoregional recurrence during follow-up, respectively. In the purely local recurrence group, 192 patients (46.9%) developed early local recurrence (ETR), and 217 patients (53.1%) developed late local recurrence (LTR). Of the 192 ETR patients, multivariate Cox regression analysis revealed that age and gender were independent risk factors of OS, and post-recurrence best supportive treatment (PRBST) was associated with poorer post-recurrence OS. Of the 217 LTR patients, the results revealed that baseline value of EBV-DNA was an independent risk factor for OS, while PRBST was associated with poorer post-recurrence OS. In the purely regional recurrence group, 183 patients (56.3%) developed early regional recurrence (ENR), and 142 patients (43.7%) developed late regional recurrence (LNR). Of the 183 ENR patients, multivariate Cox regression analysis revealed that alcohol abuse and TNM stage were independent risk factors of OS, while alcohol drinkers and PRBST were associated with poorer post-recurrence OS. Of the 142 LNR patients, PRBST was associated with poorer post-recurrence OS. In the locoregional recurrence group, 87 patients (47.8%) developed early locoregional recurrence (ELR), and 95 patients (52.2%) developed late locoregional recurrence (LLR). Of the 87 ELR patients, multivariate Cox regression analysis revealed that N stage and TNM stage were independent risk factors of OS, and N2/3 stage and PRBST were associated with poorer post-recurrence OS. Of the 95 LLR patients, the results revealed that T stage was an independent risk factor for OS, while T3/4 stage and PRBST were associated with poorer post-recurrence OS. Patients with LTR/LNR/LLR demonstrate significantly better OS compared with patients with ETR/ENR/ELR, Nevertheless, post-recurrence OS between patients with ETR/ENR/ELR and LTR/LNR/LLR was not significantly different.
Keywords: nasopharyngeal carcinoma, radiotherapy, prognosis, early locoregional recurrence, late locoregional recurrence
Introduction
Nasopharyngeal carcinoma (NPC), a malignant tumor that originates in the nasopharyngeal epithelium, is endemic in Southern China, Southeast Asia, North Africa, the Middle East, and Alaska (1, 2). As a result of its complex anatomical location and high radiosensitivity, radiotherapy with or without chemotherapy is the primary treatment modality for NPC (3, 4), and the application of IMRT has greatly improved locoregional control in NPC (5). However, the long-term prognosis remains unsatisfactory, given the high rate of locoregional recurrence of up to 5–10% in patients after definitive IMRT (6). This study focuses on the failure patterns of NPC except distant metastasis, which was separated clearly in three subgroups: (1) purely local recurrence (on the T site only), (2) purely regional recurrence (on the N site only), (3) locoregional recurrence (on the T and N sites simultaneously). Meanwhile, time to cancer recurrence differs in such patients, and the three subgroups were divided into ETR and LTR, ENR and LNR, and ELR and LLR, respectively, based on the time to recurrence after radiotherapy (7–9). To the best of our knowledge, research focusing on early and late recurrence in NPC patients remains rare and limited. Accordingly, we aimed to identify the clinical characteristics and prognostic factors of ETR and LTR, ENR and LNR, and ELR and LLR in a large cohort of patients with NPC who underwent long-term follow-up, providing data to clinicians for planning surveillance strategies.
Patients and Methods
Patient Selection
This study was performed according to the ethical principles of the Declaration of Helsinki, and the Sun Yat-sen University Cancer Center review board approved the study protocol. Written informed consent was obtained from all patients for their data to be used in clinical research without affecting their treatment options or violating their privacy. We retrospectively reviewed the records of all 9,468 patients with biopsy-proven NPC who had been treated with IMRT at our center between January 2010 and December 2015. All patients had completed a pretreatment evaluation including complete patient history, physical examination, hematology and biochemistry profiles, nasopharynx and neck magnetic resonance imaging (MRI), chest radiography, abdominal ultrasonography, and whole-body bone scan or positron emission tomography/computed tomography (PET/CT). All patients were restaged according to the 8th Union for International Cancer Control/American Joint Committee on Cancer staging system (10, 11). RT+Chemo was defined as treatment with both radiotherapy and chemotherapy, including induction chemotherapy and/or concurrent chemotherapy and/or adjuvant chemotherapy. Treatment options after recurrence were divided into four parts: salvage surgery, re-irradiation, chemotherapy, and best supportive treatment. During the study period, our institutional guidelines recommended no chemotherapy for stage I–IIA NPC, concurrent chemoradiation therapy for stage IIB NPC, and concurrent chemoradiation therapy with or without neoadjuvant/adjuvant chemotherapy for stage III to IVA–B NPC.
Follow-Up Schedule and Definition of ETR and LTR, ENR and LNR, and ELR and LLR
Patients attended follow-up visits every 3 months during the first 2 years, every 6 months during years 3–5, and annually thereafter or until death. Scheduled surveillance including fiberoptic endoscopy and head and neck CT/MRI scans was performed every 3 months during the first year and annually during years 2–5. Local recurrence was diagnosed by fiberoptic endoscopy and biopsy or nasopharynx and skull base CT/MRI scans. Regional recurrence was diagnosed by pathological examination with fine-needle aspiration or surgery or by radiology with neck CT/MRI scans. Additional tests were ordered whenever necessary. In this study, purely local recurrence was defined as recurrence on the T site only, which was divided into ETR and LTR according to time to NPC recurrence of ≤2 years and >2 years. Purely regional recurrence was defined as recurrence on the N site only, which was divided into ENR and LNR according to time to NPC recurrence of ≤2 years and >2 years, and locoregional recurrence was defined as recurrence on the T and N sites simultaneously, which was divided into ELR and LLR according to the time to NPC recurrence of ≤2 years and >2 years.
Statistical Analysis
The patients' clinical and pathological characteristics were summarized using frequencies and percentages for categorical covariates and medians and ranges for continuous covariates. The clinicopathological characteristics and treatment modalities among the patients with ETR and LTR, ENR and LNR, and ELR and LLR were compared using the chi-square test. The OS and post-recurrence OS were calculated with the Kaplan–Meier method, and differences between survival curves were assessed with the log-rank test. The prognostic factors of OS and post-recurrence OS of the patients with ETR and LTR, ENR and LNR, and ELR and LLR were evaluated using multivariate Cox regression analysis. P < 0.05 was considered significant. Statistical analyses were performed using SPSS version 23.0 (IBM).
Results
Comparison of Clinical Characteristics Between Patients With ETR and LTR, ENR and LNR, and ELR and LLR
Of the 9,468 patients included, 409 (4.3%) developed purely local recurrence, 325 (3.4%) developed purely regional recurrence, and 182 (1.9%) developed locoregional recurrence. Among the 409 patients with purely local recurrence, in whom the median time to recurrence was 25.4 months (range, 3.7–86.3 months), 207 patients (50.6%) died, 303 patients (74.1%) were male, 106 patients (25.9%) were female, and the median age was 47.0 years. At a median follow-up of 44.5 months (range, 9.9–104.9 months), 192 patients (46.9%) developed ETR, with a median time to recurrence of 15.4 months (range, 3.7–24.0 months); 217 patients (53.1%) developed LTR, with a median time to recurrence of 36.7 months (range, 24.1–86.3 months). After recurrence, 47 patients (11.5%) received BST, 19 patents (4.7%) were undergoing salvage surgery, 203 patients (49.6%) received re-irradiation, and 140 patients (34.2%) received chemotherapy. Among the 325 patients with purely regional recurrence, 114 patients (35.1%) died, 261 patients (80.3%) were male, 64 patients (19.7%) were female, and the median age was 45.0 years. At a median follow-up of 49.3 months (range, 7.9–111.0 months), 183 patients (56.3%) developed ENR, with a median time to recurrence of 14.5 months (range, 1.8–23.9 months), and 142 patients (43.7%) developed LNR, with a median time to recurrence of 37.5 months (range, 24.4–80.1 months). Among the 182 patients with locoregional recurrence, 88 patients (48.4%) died, 138 patients (75.8%) were male, 44 patients (24.2%) were female, and the median age was 44.0 years. At a median follow-up of 49.9 months (range, 6.9–101.8 months), 87 patients (47.8%) developed ELR, with a median time to recurrence of 14.70 months (range, 4.90–23.63 months), and 95 patients (52.2%) developed LLR, with a median time to recurrence of 34.63 months (range, 24.07–94.13 months). Tables 1–3 illustrate the comparisons of the baseline clinical characteristics between the patients with ETR and LTR, ENR and LNR, and ELR and LLR. The difference was significant in the baseline value of EBV-DNA between ENR and LNR groups (P = 0.009). No significant differences were found in the clinicopathological characteristics between ETR and LTR, ENR and LNR, and ELR and LLR.
Table 1.
Comparison of clinical characteristics of ETR and LTR patients in the purely local recurrence group.
| Characteristic | Total | ETR group | LTR group | P-value |
|---|---|---|---|---|
| (n = 409)% | (n = 192)% | (n = 217)% | ||
| Age (years) | 0.203 | |||
| ≤46 years | 199 (48.7) | 87 (45.3) | 112 (51.6) | |
| >46 years | 210 (51.3) | 105 (54.7) | 105 (48.4) | |
| Gender | 0.282 | |||
| Male | 303 (74.1) | 147 (76.6) | 156 (71.9) | |
| Female | 106 (25.9) | 45 (23.4) | 61 (28.1) | |
| Smoking status | 0.535 | |||
| Non-smoker | 243 (59.4) | 111 (57.8) | 132 (60.8) | |
| Smoker | 166 (40.6) | 81 (42.2) | 85 (39.2) | |
| Alcohol abuse | 0.804 | |||
| Non-drinker | 368 (90.0) | 172 (89.6) | 196 (90.3) | |
| Drinker | 41 (10.0) | 20 (10.4) | 21 (9.7) | |
| Tumor family history | 0.410 | |||
| No | 308 (75.3) | 141 (73.4) | 167 (77.0) | |
| Yes | 101 (24.7) | 51 (26.6) | 50 (23.0) | |
| Cranial nerve symptom | 0.741 | |||
| N0 | 356 (87.0) | 166 (86.5) | 190 (87.6) | |
| Yes | 53 (13.0) | 26 (13.5) | 27 (12.4) | |
| Baseline value of EBV-DNA | 0.212 | |||
| ≤2,000 | 189 (46.2) | 95 (49.5) | 94 (43.3) | |
| >2,000 | 220 (53.8) | 97 (50.5) | 123 (56.7) | |
| Histological type | 0.218 | |||
| WHO I/II | 18 (4.4) | 11 (5.7) | 7 (3.2) | |
| WHO III | 391 (95.6) | 181 (94.3) | 210 (96.8) | |
| T stage | 0.084 | |||
| 1/2 | 60 (14.7) | 22 (11.5) | 38 (17.5) | |
| 3/4 | 349 (85.3) | 170 (88.5) | 179 (82.5) | |
| N stage | 0.258 | |||
| 0/1 | 274 (67.0) | 134 (69.8) | 140 (64.5) | |
| 2/3 | 135 (33.0) | 58 (30.2) | 77 (35.5) | |
| TNM stage | 0.416 | |||
| I/II | 46 (11.2) | 19 (9.9) | 27 (12.4) | |
| III/IV | 363 (88.8) | 173 (90.1) | 190 (87.6) | |
| Induction chemotherapy | 0.927 | |||
| No | 195 (47.7) | 92 (47.9) | 103 (47.5) | |
| Yes | 214 (52.3) | 100 (52.1) | 252 (52.5) | |
| Concurrent chemotherapy | 0.171 | |||
| No | 86 (21.0) | 46 (24.0) | 40 (18.4) | |
| Yes | 323 (79.0) | 146 (76.0) | 17781.6) | |
| Adjuvant chemotherapy | 0.404 | |||
| No | 388 (94.9) | 184 (95.8) | 204 (94.0) | |
| Yes | 21 (5.1) | 8 (4.2) | 13 (6.0) | |
| Post-recurrence treatment options | 0.073 | |||
| BST | 47 (11.5) | 26 (13.5) | 21 (9.7) | |
| Salvage surgery | 19 (4.7) | 8 (4.2) | 11 (5.1) | |
| Re-irradiation | 203 (49.6) | 83 (43.2) | 120 (55.3) | |
| Chemotherapy | 140 (34.2) | 75 (39.1) | 65 (29.9) | |
Table 3.
Comparison of clinical characteristics of ELR and LLR patients in the locoregional recurrence group.
| Characteristic | Total | ELR group | LLR group | P-value |
|---|---|---|---|---|
| (n = 182)% | (n = 87)% | (n = 95)% | ||
| Age (years) | 0.088 | |||
| ≤46 years | 101 (55.5) | 54 (62.1) | 47 (49.5) | |
| >46 years | 81 (44.5) | 33 (37.9) | 48 (50.5) | |
| Gender | 0.162 | |||
| Male | 138 (75.8) | 70 (80.5) | 68 (71.6) | |
| Female | 44 (24.2) | 17 (19.5) | 27 (28.4) | |
| Smoking status | 0.870 | |||
| Non-smoker | 112 (61.5) | 53 (60.9) | 59 (62.1) | |
| Smoker | 70 (38.5) | 34 (39.1) | 36 (37.9) | |
| Alcohol abuse | 0.569 | |||
| Non-drinker | 154 (84.6) | 75 (86.2) | 79 (83.2) | |
| Drinker | 28 (15.4) | 12 (13.8) | 16 (16.8) | |
| Tumor family history | 0.603 | |||
| No | 137 (75.3) | 67 (77.0) | 70 (73.7) | |
| Yes | 45 (24.7) | 20 (23.0) | 25 (26.3) | |
| Cranial nerve symptom | 0.427 | |||
| N0 | 172 (94.5) | 81 (93.1) | 91 (95.8) | |
| Yes | 10 (5.5) | 6 (6.9) | 4 (4.2) | |
| Baseline value of EBV-DNA | 0.728 | |||
| ≤2,000 | 63 (34.6) | 29 (33.3) | 34 (35.8) | |
| >2,000 | 119 (65.4) | 58 (66.7) | 61 (64.2) | |
| Histological type | 0.246 | |||
| WHO I/II | 10 (5.5) | 3 (3.4) | 7 (7.4) | |
| WHO III | 172 (94.5) | 84 (96.6) | 88 (92.6) | |
| T stage | 0.552 | |||
| 1/2 | 39 (21.4) | 17 (19.5) | 22 (23.2) | |
| 3/4 | 143 (78.6) | 70 (80.5) | 73 (76.8) | |
| N stage | 0.316 | |||
| 0/1 | 97 (53.3) | 43 (49.4) | 54 (56.8) | |
| 2/3 | 85 (46.7) | 44 (50.6) | 41 (43.2) | |
| TNM stage | 0.826 | |||
| I/II | 22 (12.1) | 11 (12.6) | 11 (11.6) | |
| III/IV | 160 (87.9) | 76 (87.4) | 84 (88.4) | |
| Induction chemotherapy | 0.399 | |||
| No | 77 (42.3) | 34 (39.1) | 43 (45.3) | |
| Yes | 105 (57.7) | 53 (60.9) | 52 (54.7) | |
| Concurrent chemotherapy | 0.203 | |||
| No | 29 (15.9) | 17 (19.5) | 12 (12.6) | |
| Yes | 153 (84.1) | 70 (80.5) | 83 (87.4) | |
| Adjuvant chemotherapy | 0.065 | |||
| No | 173 (95.1) | 80 (92.0) | 93 (97.9) | |
| Yes | 9 (4.9) | 7 (8.0) | 2 (2.1) | |
| Post-recurrence treatment options | 0.415 | |||
| BST | 9 (4.9) | 5 (5.7) | 4 (4.2) | |
| Salvage surgery | 43 (23.6) | 22 (25.3) | 21 (22.1) | |
| Re-irradiation | 70 (38.5) | 28 (32.2) | 42 (44.2) | |
| Chemotherapy | 60 (33.0) | 32 (36.8) | 28 (29.5) | |
Table 2.
Comparison of clinical characteristics of ENR and LNR patients in the purely regional recurrence group.
| Characteristic | Total | ENR group | LNR group | P-value |
|---|---|---|---|---|
| (n = 325)% | (n = 183)% | (n = 142)% | ||
| Age (years) | 0.094 | |||
| ≤46 years | 175 (53.8) | 106 (57.9) | 69 (48.6) | |
| >46 years | 150 (46.2) | 77 (42.1) | 73 (51.4) | |
| Gender | 0.567 | |||
| Male | 261 (80.3) | 149 (81.4) | 112 (78.9) | |
| Female | 64 (19.7) | 34 (18.6) | 30 (21.1) | |
| Smoking status | 0.165 | |||
| Non-smoker | 192 (59.1) | 102 (55.7) | 90 (63.4) | |
| Smoker | 133 (40.9) | 81 (44.3) | 52 (36.6) | |
| Alcohol abuse | 0.420 | |||
| Non-drinker | 266 (81.8) | 147 (80.3) | 119 (83.8) | |
| Drinker | 59 (18.2) | 36 (19.7) | 23 (16.2) | |
| Tumor family history | 0.796 | |||
| No | 229 (70.5) | 130 (71.0) | 99 (69.7) | |
| Yes | 96 (29.5) | 53 (29.0) | 43 (30.3) | |
| Cranial nerve symptom | 0.936 | |||
| N0 | 304 (93.5) | 171 (93.4) | 133 (93.7) | |
| Yes | 21 (6.5) | 12 (6.6) | 9 (6.3) | |
| Baseline value of EBV-DNA | 0.009 | |||
| ≤2,000 | 84 (25.8) | 37 (20.2) | 47 (33.1) | |
| >2,000 | 241 (74.2) | 146 (79.8) | 95 (66.9) | |
| Histological type | 0.183 | |||
| WHO I/II | 12 (3.7) | 9 (4.9) | 3 (2.1) | |
| WHO III | 313 (96.3) | 174 (95.1) | 139 (97.9) | |
| T stage | 0.337 | |||
| 1/2 | 117 (36.0) | 70 (38.3) | 47 (33.1) | |
| 3/4 | 208 (64.0) | 113 (61.7) | 95 (66.9) | |
| N stage | 0.157 | |||
| 0/1 | 139 (42.8) | 72 (39.3) | 67 (47.2) | |
| 2/3 | 186 (57.2) | 111 (60.7) | 75 (52.8) | |
| TNM stage | 0.606 | |||
| I/II | 59 (18.2) | 35 (19.1) | 24 (16.9) | |
| III/IV | 266 (81.8) | 148 (80.9) | 118 (83.1) | |
| Induction chemotherapy | 0.077 | |||
| No | 111 (34.2) | 55 (30.1) | 56 (39.4) | |
| Yes | 214 (65.8) | 128 (69.9) | 86 (60.6) | |
| Concurrent chemotherapy | 0.625 | |||
| No | 47 (14.5) | 28 (15.3) | 19 (13.4) | |
| Yes | 278 (85.5) | 155 (84.7) | 123 (86.6) | |
| Adjuvant chemotherapy | 0.113 | |||
| No | 295 (90.8) | 162 (88.5) | 133 (93.7) | |
| Yes | 30 (9.2) | 21 (11.5) | 9 (6.3) | |
| Post-recurrence treatment options | 0.689 | |||
| BST | 17 (5.2) | 9 (4.9) | 8 (5.6) | |
| Salvage surgery | 162 (49.9) | 94 (51.4) | 68 (47.9) | |
| Re-irradiation | 64 (19.7) | 32 (17.5) | 32 (22.5) | |
| Chemotherapy | 82 (25.2) | 48 (26.2) | 34 (24.0) | |
Prognostic Factors Associated With OS
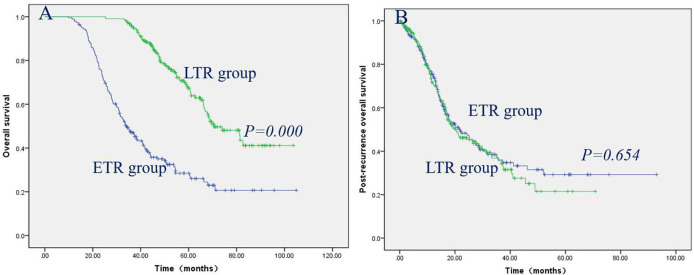
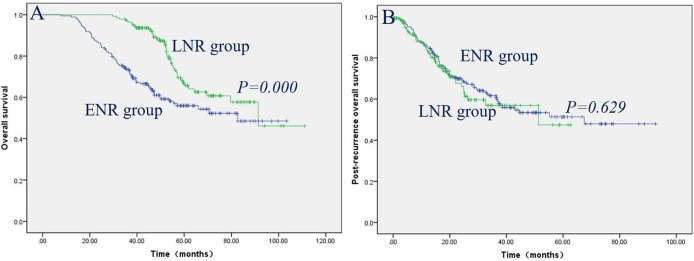
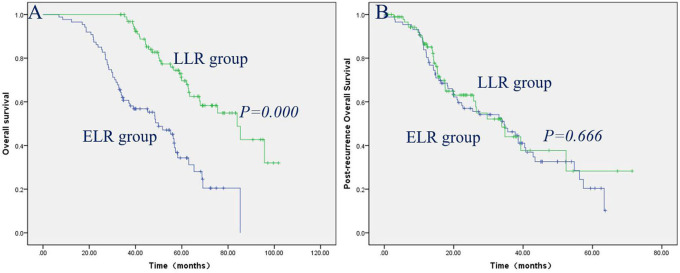
The prognostic factors contributing to long-term OS in ETR and LTR, ENR and LNR, and ELR and LLR were investigated using univariate and multivariate analyses (Tables 4–6). The effects of clinical factors on the OS with ETR group were evaluated. Age > 46 years and male gender were significantly associated with poorer OS. Cox regression modeling predicted that age [hazard ratio [HR], 1.645; 95% confidence interval [CI], 1.153–2.346; P = 0.006], gender (HR, 0.589; 95% CI, 0.377–0.920; P = 0.020) were independent risk factors of OS. Of the 217 patients with LTR, a baseline value of EBV-DNA > 2,000 was significantly associated with poorer OS. Cox regression modeling identified the baseline value of EBV-DNA (HR, 1.817; 95% CI, 1.115–2.962; P = 0.017) as an independent risk factor of OS. The effects of clinical factors on the OS with ENR group were evaluated. Alcohol drinking and TNM stage III/IV were significantly associated with poorer OS. Cox regression modeling predicted that alcohol abuse [hazard ratio [HR], 3.070; 95% confidence interval [CI], 1.551–6.076; P = 0.001], TNM stage (HR, 2.394; 95% CI, 1.178–4.864; P = 0.016) were independent risk factors of OS. Of the 142 patients with LNR, no clinical characteristics were significantly associated with OS. The effects of clinical factors on the OS with ELR group were also evaluated. Cox regression modeling predicted that N stage [hazard ratio [HR], 2.391; 95% confidence interval [CI], 1.328–4.271; P = 0.004], TNM stage (HR, 1.874; 95% CI, 1.248–2.812; P = 0.002) were independent risk factors of OS. Of the 95 patients with LLR, Cox regression modeling identified T stage (HR, 3.675; 95% CI, 1.241–10.882; P = 0.019) as an independent risk factor of OS. The patients with LTR/LNR/LLR demonstrated significantly better OS than the patients with ETR/ENR/ELR (Figures 1A, 2A, 3A), with a median OS of 33.1 months (range, 9.9–104.9 months)/53.0 months (range, 25.2–103.7 months), 44.1 months (range, 7.9–103.3 months)/53.6 months (range, 29.6–111.0 months), and 39.10 months (range, 6.90–85.27 months)/58.9 months (range, 33.6–101.8 months), respectively.
Table 4.
Univariate and multivariate analysis of prognostic factors of ETR and LTR in the purely local recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age (years) | 0.005 | 0.006 | 0.888 | NS | ||
| ≤46 years | Reference | |||||
| >46 years | 1.645 (1.153–2.346) | / | ||||
| Gender | 0.008 | 0.020 | 0.179 | NS | ||
| Male | Reference | |||||
| Female | 0.589 (0.377–0.920) | / | ||||
| Smoking status | 0.293 | NS | 0.264 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.211 | NS | 0.043 | NS | ||
| Non-drinker | ||||||
| Drinker | / | / | ||||
| Tumor family history | 0.883 | NS | 0.079 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.025 | NS | 0.836 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.381 | NS | 0.015 | 0.017 | ||
| ≤2000 | Reference | |||||
| >2000 | / | 1.817 (1.115–2.962) | ||||
| Histological type | 0.257 | NS | 0.399 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.110 | NS | 0.184 | NS | ||
| 1/2 | ||||||
| 3/4 | / | / | ||||
| N stage | 0.074 | NS | 0.150 | NS | ||
| 0/1 | ||||||
| 2/3 | / | / | ||||
| TNM stage | 0.069 | NS | 0.479 | NS | ||
| I/II | ||||||
| III/IV | / | |||||
| RT+/Chemo | 0.316 | NS | 0.182 | NS | ||
| RT alone | ||||||
| RT+Chemo | / | / | ||||
Table 6.
Univariate and multivariate analysis of prognostic factors of ELR and LLR in the locoregional recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age(years) | 0.069 | NS | 0.063 | NS | ||
| ≤46 years | ||||||
| >46 years | / | / | ||||
| Gender | 0.837 | NS | 0.101 | NS | ||
| Male | ||||||
| Female | / | / | ||||
| Smoking status | 0.245 | NS | 0.483 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.194 | NS | 0.817 | NS | ||
| Non-drinker | ||||||
| Drinker | / | / | ||||
| Tumor family history | 0.106 | NS | 0.095 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.139 | NS | 0.771 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.110 | NS | 0.600 | NS | ||
| ≤2,000 | ||||||
| >2,000 | / | / | ||||
| Histological type | 0.646 | NS | 0.392 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.557 | NS | 0.020 | 0.019 | ||
| 1/2 | Reference | |||||
| 3/4 | / | 3.675 (1.241–10.882) | ||||
| N stage | 0.026 | 0.004 | 0.966 | NS | ||
| 0/1 | Reference | |||||
| 2/3 | 2.391 (1.328–4.271) | / | ||||
| TNM stage | 0.363 | 0.002 | 0.047 | NS | ||
| I/II | Reference | |||||
| III/IV | 1.874 (1.248–2.812) | / | ||||
| RT+/Chemo | 0.765 | NS | 0.403 | NS | ||
| RT alone | ||||||
| RT+Chemo | / | / | ||||
Figure 1.
Patients with LTR had significantly better OS than patients with ETR (A), while post-recurrence OS did not reach significance between the patients with LTR and ETR (B).
Figure 2.
Patients with LNR had significantly better OS than patients with ENR (A), while post-recurrence OS did not reach significance between the patients with LNR and ENR (B).
Figure 3.
Patients with LLR had significantly better OS than patients with ELR (A), while post-recurrence OS did not reach significance between the patients with LLR and ELR (B).
Table 5.
Univariate and multivariate analysis of prognostic factors of ENR and LNR in the purely regional recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age (years) | 0.437 | NS | 0.997 | NS | ||
| ≤46 years | ||||||
| >46 years | / | / | ||||
| Gender | 0.749 | NS | 0.885 | NS | ||
| Male | ||||||
| Female | / | / | ||||
| Smoking status | 0.264 | NS | 0.705 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.032 | 0.001 | 0.970 | NS | ||
| Non-drinker | Reference | |||||
| Drinker | 3.070 (1.551–6.076) | / | ||||
| Tumor family history | 0.599 | NS | 0.796 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.035 | NS | 0.315 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.090 | NS | 0.323 | / | NS | |
| ≤2,000 | ||||||
| >2,000 | / | / | ||||
| Histological type | 0.979 | NS | 0.234 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.353 | NS | 0.580 | NS | ||
| 1/2 | ||||||
| 3/4 | / | / | ||||
| N stage | 0.048 | NS | 0.192 | NS | ||
| 0/1 | ||||||
| 2/3 | / | / | ||||
| TNM stage | 0.037 | 0.016 | 0.494 | NS | ||
| I/II | Reference | |||||
| III/IV | 2.394 (1.178–4.864) | / | ||||
| RT+/Chemo | 0.823 | NS | 0.740 | NS | ||
| RT alone | ||||||
| RT+Chemo | / | / | ||||
Prognostic Factors Associated With Post-recurrence OS
The clinical factors and treatment modalities of post-recurrence OS in ETR and LTR, ENR and LNR, and ELR and LLR were elevated by univariate and multivariate analyses (Tables 7–9). Of the 192 patients with ETR, PRBST was significantly associated with poorer OS. Of the 217 patients with LTR patients, Cox regression modeling identified post-recurrence treatment options (P = 0.000) was an independent risk factor of post-recurrence OS. Of the 183 patients with ENR, Cox regression modeling predicted that alcohol abuse (HR, 3.750; 95% CI, 1.909–7.367; P = 0.000) and post-recurrence treatment options (P = 0.000) were independent risk factors of post-recurrence OS. Of the 142 patients with LNR patients, Cox regression modeling predicted post-recurrence treatment options (P = 0.000) was an independent risk factor of post-recurrence OS. Of the 87 patients with ELR, Cox regression modeling predicted that N stage (HR, 2.216; 95% CI, 1.225−4.009; P = 0.008) and post-recurrence treatment options (P = 0.000) were independent risk factors of post-recurrence OS. Of the 95 patients with LLR patients, Cox regression modeling predicted that T stage (HR, 4.111; 95% CI, 1.337–12.635; P = 0.014) and post-recurrence treatment options (P = 0.000) were independent risk factors of post-recurrence OS. Post-recurrence OS was not significantly different between ETR and LTR, ENR and LNR, and ELR and LLR groups (Figures 1B, 2B, 3B), with a median post-recurrence OS of 16.2 months (range, 0–93.0 months) and 12.2 months (range, 0.2–69.1 months), 28.1 months (range, 0.5–92.6 months) and 15.9 months (range 0–62.6 months), and 22.6 months (range, 0–63.7 months) and 15.3 months (range, 0.6–71.4 months), respectively.
Table 7.
Univariate and multivariate analysis of post-recurrence prognostic factors of ETR and LTR in the purely local recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age (years) | 0.013 | NS | 0.144 | NS | ||
| ≤46 years | ||||||
| >46 years | / | / | ||||
| Gender | 0.014 | NS | 0.299 | NS | ||
| Male | ||||||
| Female | / | / | ||||
| Smoking status | 0.386 | NS | 0.262 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.296 | NS | 0.038 | NS | ||
| Non-drinker | ||||||
| Drinker | / | / | ||||
| Tumor family history | 0.786 | NS | 0.077 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.061 | / | NS | 0.994 | NS | |
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.299 | NS | 0.055 | NS | ||
| ≤2,000 | ||||||
| >2,000 | / | / | ||||
| Histological type | 0.296 | NS | 0.794 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.124 | NS | 0.297 | NS | ||
| 1/2 | ||||||
| 3/4 | / | / | ||||
| N stage | 0.095 | NS | 0.486 | NS | ||
| 0/1 | ||||||
| 2/3 | / | / | ||||
| TNM stage | 0.095 | NS | 0.669 | NS | ||
| I/II | ||||||
| III/IV | / | |||||
| Post-recurrence treatment options | 0.000 | 0.000 | 0.007 | / | 0.019 | |
| BST | Reference | Reference | ||||
| Salvage surgery | 0.162 (0.065–0.407) | 0.983 (0.359–2.691) | ||||
| Re-irradiation | 0.226 (0.135–0.378) | 0.369 (0.184–0.739) | ||||
| Chemotherapy | 0.302 (0.184–0.497) | 0.554 (0.270–1.138) | ||||
Table 9.
Univariate and multivariate analysis of post–recurrence prognostic factors of ELR and LLR in the locoregional recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age (years) | 0.069 | NS | 0.296 | NS | ||
| ≤46 years | ||||||
| >46 years | / | / | ||||
| Gender | 0.982 | NS | 0.076 | NS | ||
| Male | ||||||
| Female | / | / | ||||
| Smoking status | 0.284 | NS | 0.055 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.255 | NS | 0.489 | NS | ||
| Non-drinker | ||||||
| Drinker | / | / | ||||
| Tumor family history | 0.144 | NS | 0.075 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.050 | NS | 0.563 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.140 | NS | 0.963 | NS | ||
| ≤2,000 | ||||||
| >2,000 | / | / | ||||
| Histological type | 0.741 | NS | 0.056 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.387 | / | NS | 0.006 | 0.014 | |
| 1/2 | Reference | |||||
| 3/4 | 4.111 (1.337–12.635) | |||||
| N stage | 0.020 | 0.008 | 0.472 | / | NS | |
| 0/1 | Reference | |||||
| 2/3 | 2.216 (1.225–4.009) | |||||
| TNM stage | 0.252 | NS | 0.028 | NS | ||
| I/II | ||||||
| III/IV | / | / | ||||
| Post-recurrence treatment options | 0.002 | 0.007 | 0.000 | 0.000 | ||
| BST | Reference | Reference | ||||
| Salvage surgery | 0.238 (0.073–0.770) | 0.277 (0.028–1.749) | ||||
| Re-irradiation | 0.282 (0.090–0.885) | 0.565 (0.071–1.476) | ||||
| Chemotherapy | 0.666 (0.227–1.952) | 0.736 (0.343–1.284) | ||||
Table 8.
Univariate and multivariate analysis of post-recurrence prognostic factors of ENR and LNR in the purely regional recurrence group.
| Characteristic | ETR group | LTR group | ||||
|---|---|---|---|---|---|---|
| Univariate P-value HR (95% CI) | Multivariate P-value | Univariate P-value HR (95% CI) | Multivariate P-value | |||
| Age (years) | 0.462 | NS | 0.847 | NS | ||
| ≤46 years | ||||||
| >46 years | / | / | ||||
| Gender | 0.804 | NS | 0.670 | NS | ||
| Male | ||||||
| Female | / | / | ||||
| Smoking status | 0.208 | NS | 0.715 | NS | ||
| Non-smoker | ||||||
| Smoker | / | / | ||||
| Alcohol abuse | 0.045 | 0.000 | 0.717 | NS | ||
| Non-drinker | Reference | |||||
| Drinker | 3.750 (1.909–7.367) | / | ||||
| Tumor family history | 0.539 | NS | 0.858 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Cranial nerve symptom | 0.029 | NS | 0.373 | NS | ||
| No | ||||||
| Yes | / | / | ||||
| Baseline value of EBV-DNA | 0.138 | NS | 0.377 | NS | ||
| ≤2,000 | ||||||
| >2,000 | / | / | ||||
| Histological type | 0.910 | NS | 0.209 | NS | ||
| WHO I/II | ||||||
| WHO III | / | / | ||||
| T stage | 0.327 | NS | 0.910 | NS | ||
| 1/2 | ||||||
| 3/4 | / | / | ||||
| N stage | 0.070 | NS | 0.362 | NS | ||
| 0/1 | ||||||
| 2/3 | / | / | ||||
| TNM stage | 0.041 | NS | 0.790 | NS | ||
| I/II | ||||||
| III/IV | / | / | ||||
| Post-recurrence treatment options | 0.000 | 0.000 | 0.025 | 0.031 | ||
| BST | Reference | Reference | ||||
| Salvage surgery | 0.100 (0.042–0.238) | 0.200 (0.065–0.619) | ||||
| Re-irradiation | 0.159 (0.058–0.433) | 0.266 (0.080–0.886) | ||||
| Chemotherapy | 0.687 (0.309–1.528) | 0.328 (0.100–1.071) | ||||
Discussion
Here, we investigated the clinical characteristics and prognostic factors predicting OS and post-recurrence OS in NPC patients with ETR and LTR, ENR and LNR, and ELR and LLR. In this retrospective study, 409 (4.3%) developed purely local recurrence, 325 (3.4%) developed purely regional recurrence, and 182 (1.9%) developed locoregional recurrence, which is similar to the results of previous studies from other centers in China (12, 13); 192 patients (46.9%) developed early ETR, and 217 patients (53.1%) developed LTR, 183 patients (56.3%) developed ENR, and 142 patients (43.7%) developed LNR, while 87 patients (47.8%) developed ELR, and 95 patients (52.2%) developed LLR, which suggests that the incidence of early and late recurrence is nearly the same. The patients with LTR/LNR/LLR demonstrated significantly better OS than the patients with ETR/ENR/ELR, which is consistent with previous studies on renal cell carcinoma and intrahepatic cholangiocarcinoma (14, 15), while post-recurrence OS did not reach significance between the ETR and LTR, ENR and LNR, and ELR and LLR groups, which suggests that post-recurrence OS does not depend on the time of recurrence.
Multivariate Cox regression analysis revealed that age and gender were independent risk factors for OS with ETR, and the baseline value of EBV-DNA was an independent risk factor for OS with LTR; alcohol abuse and TNM stage were independent risk factors for OS with ENR, and no clinical characteristics were associated with OS with LTR, and N stage and TNM stage were independent risk factors for OS with ELR; and T stage was an independent risk factor for OS with LLR. In addition, multivariate Cox regression analysis revealed that post-recurrence treatment option was an independent risk factor of post-recurrence OS with ETR and LTR, while alcohol abuse and post-recurrence treatment option were independent risk factors of post-recurrence OS with ENR, and PRBST was associated with poorer post-recurrence OS with LNR. Meanwhile, N stage and post-recurrence treatment options were independent risk factors for post-recurrence OS with ELR, and T stage and post-recurrence treatment options were independent risk factors for post-recurrence OS with LLR. It has been suggested that patients with early initial T stage have a more favorable prognosis (16), which is consistent with the LLR patients in the present study. Post-recurrence treatment options including salvage surgery, re-irradiation, and chemotherapy are very important for NPC recurrence patients, which was shown that post-recurrence treatment options mentioned above have a better prognosis compared with PRBST.
There are various hypotheses for the occurrence of early and late recurrence. A probable hypothesis is the discrepancy of NPC tumor cell radiosensitivity. Recent studies have shown that apoptosis, DNA damage repair, a hypoxic microenvironment, and autophagy can be involved in regulating radiotherapy resistance (17–19), which results in the difference in early and late recurrence. A study from Japan shed light on the biological impact of DNA methylation status as a predictive biomarker of early recurrence in ovarian cancers (20). It has been suggested that the tumor dormancy-reactivation hypothesis might be applicable to NPC (21, 22). Furthermore, surgery for removing breast tumors may lead to the appearance of growth factors in the circulation in response to surgical wounding, which may terminate the dormancy of the tumor foci and result in accelerated recurrence (7, 23). Although the main treatment of NPC is radiotherapy instead of surgery, it may also give rise to the appearance and an increase of growth factors to result in the occurrence of recurrence. Therefore, it is reasonable to speculate that there might be intrinsic biological differences between patients with early and late recurrence, and this warrants further studies.
The definitions of ETR and LTR, ENR and LNR, and ELR and LLR were applied with 2 years as the cut-off point, which have also been proposed and proven in recent studies (8, 9, 24). Hence, the frequency and intensity of follow-up should be strengthened at the initial 2 years. However, the application of 2 years for differentiating early recurrence from late recurrence remains controversial, and several studies have used 5 years as another major demarcation point (14, 25, 26). We applied 5 years for differentiating early recurrence from late recurrence and we found that there are very few cases of early recurrence; the number imbalance between the two groups is likely to lead to statistical problems.
There are two new features in the present study that differ from previous reports. First, this study figured out the prognostic factors of OS and post-recurrence OS in NPC patients with ETR and LTR, ENR and LNR, and ELR and LLR in detail. Second, this study has a large sample size with long median-time follow-up, which might help oncologists predict patients' prognosis and design individualized follow-up strategies. Although our study yielded some unique results, certain limitations should be noted. First, this is a retrospective single-center study, which has inherent biases. Second, the study merely explores the impact of baseline clinical characteristics on post-recurrence OS rather than post-recurrence clinical characteristics, which are principal elements of post-recurrence OS. Third, some information was lacking because of the long follow-up duration. However, we believe that the present results are noteworthy and reliable because this is the only such large-cohort study to date.
Conclusions
The present study shows that age and gender were independent risk factors of OS with ETR, and the baseline value of EBV-DNA was an independent risk factor of OS with LTR. Alcohol abuse and TNM stage were independent risk factors for OS with ENR, while N stage and TNM stage were independent risk factors of OS with ELR; and T stage was an independent risk factor for OS with LLR patients. In addition, post-recurrence treatment option was an independent risk factor of post-recurrence OS with ETR and LTR. Alcohol abuse and post-recurrence treatment option were independent risk factors of post-recurrence OS with ENR, and PRBST was associated with poorer post-recurrence OS with LNR. Meanwhile, N stage and post-recurrence treatment options were independent risk factors for post-recurrence OS with ELR, and T stage and post-recurrence treatment options were independent risk factors for post-recurrence OS with LLR. Patients with LTR/LNR/LLR demonstrate significantly better OS compared with patients with ETR/ENR/ELR, whereas post-recurrence OS is not significantly different between patients with ETR/ENR/ELR and LTR/LNR/LLR. Further studies are warranted to confirm our results.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.
Ethics Statement
This study was performed according to the ethical principles of the Declaration of Helsinki, and the Sun Yat-sen University Cancer Center review board approved the study protocol. Written informed consent was obtained from all patients for their data to be used in clinical research without affecting their treatment options or violating their privacy.
Author Contributions
FL, F-PC, Y-PC, and G-QZ conceived and designed the study. YC, X-JH, X-DH, and Z-QZ contributed cases data collection. W-HZ, XL, and YS analyzed the data. FL, F-PC, and G-QZ wrote the paper. All authors read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The chief acknowledgment is to the subjects who provided information for this study and the research staff.
Glossary
Abbreviations
- NPC
nasopharyngeal carcinoma
- IMRT
intensity-modulated radiation therapy
- ETR
early purely local recurrence
- LTR
late purely local recurrence
- ENR
early purely regional recurrence
- LNR
late purely regional recurrence
- ELR
early locoregional recurrence
- LLR
late locoregional recurrence
- OS
overall survival
- WHO
World Health Organization
- TNM
tumor-node-metastasis
- MRI
magnetic resonance imaging
- PET/CT
positron-emission tomography/computed tomography
- UICC/AJCC
Union for International Cancer Control/American Joint Committee on Cancer
- EBV
Epstein-Barr virus
- HR
hazard ratio
- CI
Confidence interval
- RT
radiotherapy
- Chemo
chemotherapy
- PRBST
Post-recurrence best supportive treatment.
Footnotes
Funding. This study was supported by grants from the Chinese postdoctoral fund (No. 2019M653214).
References
- 1.Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet. (2016) 387:1012–24. 10.1016/S0140-6736(15)00055-0 [DOI] [PubMed] [Google Scholar]
- 2.Wei KR, Zheng RS, Zhang SW, Liang ZH, Ou ZX, Chen WQ. Nasopharyngeal carcinoma incidence and mortality in China in 2010. Chin. J. Cancer. (2014) 33:381–7. 10.5732/cjc.014.10086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan AT. Nasopharyngeal carcinoma. Ann Oncol. (2010) 21 (Suppl. 7): vii308–12. 10.1093/annonc/mdq277 [DOI] [PubMed] [Google Scholar]
- 4.Lee AW, Lin JC, Ng WT. Current management of nasopharyngeal cancer. Semin. Radiat. Oncol. (2012) 22:233–44. 10.1016/j.semradonc.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 5.Lai SZ, Li WF, Chen L, Luo W, Chen YY, Liu LZ, et al. How does intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy influence the treatment results in nasopharyngeal carcinoma patients? Int. J. Radiat. Oncol. Biol. Phys. (2011) 80:661–8. 10.1016/j.ijrobp.2010.03.024 [DOI] [PubMed] [Google Scholar]
- 6.Lee AW, Ma BB, Ng WT, Chan AT. Management of nasopharyngeal carcinoma: current practice and future perspective. J Clin Oncol. (2015) 33:3356–64. 10.1200/JCO.2015.60.9347 [DOI] [PubMed] [Google Scholar]
- 7.Retsky M, Demicheli R, Hrushesky WJ. Does surgery induce angiogenesis in breast cancer? Indirect evidence from relapse pattern and mammography paradox. Int J Surg. (2005) 3:179–87. 10.1016/j.ijsu.2005.08.002 [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Hatta W, Shimosegawa T, Takizawa K, Oyama T, Kawata N, et al. Different risk factors between early and late cancer recurrences in patients without additional surgery after noncurative endoscopic submucosal dissection for early gastric cancer. Gastrointest Endosc. (2019) 89:950–60. 10.1016/j.gie.2018.11.015 [DOI] [PubMed] [Google Scholar]
- 9.Mortensen MS, Lauritsen J, Kier MG, Bandak M, Appelt AL, Agerbæk M, et al. Late relapses in stage I testicular cancer patients on surveillance. Eur Urol. (2016) 70:365–71. 10.1016/j.eururo.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 10.Tang LL, Chen YP, Mao YP, Wang ZX, Guo R, Chen L, et al. Validation of the 8th edition of the UICC/AJCC staging system for nasopharyngeal carcinoma from endemic areas in the intensity-modulated radiotherapy era. J Natl Compr Canc Netw. (2017) 15:913–9. 10.6004/jnccn.2017.0121 [DOI] [PubMed] [Google Scholar]
- 11.OuYang PY, Xiao Y, You KY, Zhang LN, Lan XW, Zhang XM, et al. Validation and comparison of the 7th and 8th edition of AJCC staging systems for non-metastatic nasopharyngeal carcinoma, and proposed staging systems from Hong Kong, Guangzhou, and Guangxi. Oral Oncol. (2017) 72:65–72. 10.1016/j.oraloncology.2017.07.011 [DOI] [PubMed] [Google Scholar]
- 12.Wu LR, Liu YT, Jiang N, Fan YX, Wen J, Huang SF, et al. Ten-year survival outcomes for patients with nasopharyngeal carcinoma receiving intensity-modulated radiotherapy: an analysis of 614 patients from a single center. Oral Oncol. (2017) 69:26–32. 10.1016/j.oraloncology.2017.03.015 [DOI] [PubMed] [Google Scholar]
- 13.Yi JL, Gao L, Huang XD, Li SY, Luo JW, Cai WM, et al. Nasopharyngeal carcinoma treated by radical radiotherapy alone: ten-year experience of a single institution. Int J Radiat Oncol Biol Phys. (2006) 65: 161–8. 10.1016/j.ijrobp.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 14.Kroeger N, Choueiri TK, Lee JL, Bjarnason GA, Knox JJ, MacKenzie MJ, et al. Survival outcome and treatment response of patients with late relapse from renal cell carcinoma in the era of targeted therapy. Eur Urol. (2014) 65:1086–92. 10.1016/j.eururo.2013.07.031 [DOI] [PubMed] [Google Scholar]
- 15.Wang C, Pang S, Si-Ma H, Yang N, Zhang H, Fu Y, et al. Specific risk factors contributing to early and late recurrences of intrahepatic cholangiocarcinoma after curative resection. World J Surg Oncol. (2019) 17:2. 10.1186/s12957-018-1540-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yu KH, Leung SF, Tung SY, Zee B, Chua DT, Sze WM, et al. Survival outcome of patients with nasopharyngeal carcinoma with first local failure: a study by the Hong Kong Nasopharyngeal Carcinoma Study Group. Head Neck. (2005) 27:397–405. 10.1002/hed.20161 [DOI] [PubMed] [Google Scholar]
- 17.Lu ZX, Ma XQ, Yang LF, Wang ZL, Zeng L, Li ZJ, et al. DNAzymes targeted to EBV-encoded latent membrane protein-1 induce apoptosis and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer Lett. (2008) 265:226–38. 10.1016/j.canlet.2008.02.019 [DOI] [PubMed] [Google Scholar]
- 18.Zhai X, Yang Y, Wan J, Zhu R, Wu Y. Inhibition of LDH-A by oxamate induces G2/M arrest, apoptosis and increases radiosensitivity in nasopharyngeal carcinoma cells. Oncol Rep. (2013) 30:2983–91. 10.3892/or.2013.2735 [DOI] [PubMed] [Google Scholar]
- 19.Yang S, Chen J, Guo Y, Lin H, Zhang Z, Feng G, et al. Identification of prognostic biomarkers for response to radiotherapy by DNA microarray in nasopharyngeal carcinoma patients. Int J Oncol. (2012) 40:1590–600. 10.3892/ijo.2012.1341 [DOI] [PubMed] [Google Scholar]
- 20.Mase S, Shinjo K, Totani H, Katsushima K, Arakawa A, Takahashi S, et al. ZNF671 DNA methylation as a molecular predictor for the early recurrence of serous ovarian cancer. Cancer Sci. (2019) 110:1105–16. 10.1111/cas.13936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naumov GN, Townson JL, MacDonald IC, Wilson SM, Bramwell VH, Groom AC, et al. Ineffectiveness of doxorubicin treatment on solitary dormant mammary carcinoma cells or late-developing metastases. Breast Cancer Res Treat. (2003) 82:199–206. 10.1023/B:BREA.0000004377.12288.3c [DOI] [PubMed] [Google Scholar]
- 22.Braun S, Kentenich C, Janni W, Hepp F, de Waal J, Willgeroth F, et al. Lack of effect of adjuvant chemotherapy on the elimination of single dormant tumor cells in bone marrow of high-risk breast cancer patients. J Clin Oncol. (2000) 18:80–6. 10.1200/JCO.2000.18.1.80 [DOI] [PubMed] [Google Scholar]
- 23.Tagliabue E, Agresti R, Carcangiu ML, Ghirelli C, Morelli D, Campiglio M, et al. Role of HER2 in wound-induced breast carcinoma proliferation. Lancet. (2003) 362:527–33. 10.1016/S0140-6736(03)14112-8 [DOI] [PubMed] [Google Scholar]
- 24.Calderaro J, Petitprez F, Becht E, Laurent A, Hirsch TZ, Rousseau B, et al. Intra-tumoral tertiary lymphoid structures are associated with a low risk of early recurrence of hepatocellular carcinoma. J Hepatol. (2019) 70:58–65. 10.1016/j.jhep.2018.09.003 [DOI] [PubMed] [Google Scholar]
- 25.Lee JH, Kim HI, Kim MG, Ha TK, Jung MS, Kwon SJ. Recurrence of gastric cancer in patients who are disease-free for more than 5 years after primary resection. Surgery. (2016) 159:1090–8. 10.1016/j.surg.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Ichiyanagi O, Naito S, Ito H, Kabasawa T, Narisawa T, Kanno H, et al. Levels of 4EBP1/eIF4E activation in renal cell carcinoma could differentially predict its early and late recurrence. Clin Genitourin Cancer. (2018) 16:e1029–58. 10.1016/j.clgc.2018.06.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding author.