Abstract
Neurons derived from human induced pluripotent stem cells (hiPSC-derived neurons) offer novel opportunities for the development of preclinical models of human neurodegenerative diseases (NDDs). Recent advances in the past few years have increased substantially the potential of these techniques and have uncovered new challenges that the field is facing. Here, we outline and discuss challenges related to the functional characterization of hiPSC-derived neurons and propose ways to overcome current difficulties. In particular, the enormous variability among studies in the electrical properties of hiPSC-derived neurons and broad differences in cell maturation are factors that impair reproducibility. Furthermore, we discuss how the use of 3D brain organoids are of help in resolving some difficulties posed by 2D cultures. Finally, we elaborate on recent and future advances that may help to overcome the discussed challenges and speed-up progress in the field.
Keywords: hiPSC, cellular cultures, organoids, neurons, astrocytes, plasticity, patch-clamp, MEA
Introduction
The field of stem cell biology has expanded rapidly over the past few years, bringing exciting technical advances as well as new avenues for treatment of human neurodegenerative diseases (NDDs). Neurons derived from human induced pluripotent stem cells (hiPSC-derived neurons) offer novel opportunities for the development of preclinical models of human NDDs such as Alzheimer’s disease (Lee et al., 2016; Mungenast et al., 2016), Parkinson’s disease (Byers et al., 2012; Fernández-Santiago et al., 2015), Huntington’s disease (Nekrasov et al., 2016), epilepsy (Parent and Anderson, 2015), schizophrenia (Wen et al., 2014; Wright et al., 2014), amyotrophic lateral sclerosis (ALS) (Chestkov et al., 2014), and autism spectrum disorders (ASD) (Darville et al., 2016; Vitrac and Cloëz-Tayarani, 2018; Drakulic et al., 2020). Since hiPSC-derived neurons contain the genetic information of the patient, they represent an invaluable resource to study diseases with strong genetic component. In addition, they offer the possibility of studying disease development and cell function in living human neurons (Dolmetsch and Geschwind, 2011).
Intense research efforts devoted to the optimization of a methodology to obtain functional neurons from hiPSCs have yielded a vast diversity of hiPSC-derived neurons from many laboratories across the world (Rhee et al., 2019). This diversity of neuronal types comes at a price of a high variability and heterogeneity in neuronal electrical properties and developmental stage, hampering reproducibility (Volpato and Webber, 2020). In addition, the use of inconsistent and variable criteria to validate hiPSC-derived neurons and incomplete functional and physiological characterization (Xie et al., 2018) add further difficulties to obtain reproducible results. Imaging techniques showing the morphology of the cells, immunohistochemistry assays showing the expression of neuronal markers and gene expression analysis of hiPSCs are necessary but not enough to confirm a neuronal phenotype. Electrophysiological and imaging techniques must be combined to describe single-cell intrinsic electrical properties, connectivity, or network activity to better describe and validate the neuronal phenotype of hiPSCs and their degree of maturation. Therefore, as the field evolves, it is becoming evident the need for standardizing and unifying the functional characterization of hiPSC-derived neurons.
In this Perspective, we will outline and discuss several challenges regarding functional phenotyping of hiPSC-derived neurons in 2D and 3D cultures. We will also highlight the problem of the developmental stage of hiPSC-derived neurons and its relevance in disease modeling. Finally, we provide our views on how some of these challenges may be overcome based on new technologies and strategies that are being implemented nowadays.
The Challenge of Unifying Functional Measurements of hiPSC-Derived Neurons
Neurons are electrical entities, as such; electrophysiological methods represent the gold standard to measure functional properties from neurons cultured in a dish to in vivo behaving animals (Figures 1A,B). Currently, whole-cell patch-clamp recordings of hiPSC-derived neurons are routinely performed in many laboratories worldwide and provide accurate measurements of the intrinsic properties of these cells. Relevant parameters to study the degree of differentiation of hiPSC lines such as development of stable resting membrane potential, input resistance (Ri), membrane capacitance (Cm), and action potential characteristics can be studied measuring the voltage response of the cell to injected hyperpolarizing or depolarizing current pulses in current-clamp mode. In addition, sodium currents underlying action potential and their kinetics can be studied under voltage-clamp mode by applying depolarizing voltage steps. Furthermore, the specific firing pattern in response to 0.5–1 s long depolarizing current pulses is a widely used criterion to classify neuronal cell types. Further analysis of active properties can be expanded by measuring ionic currents under different voltage clamp protocols, to test for the presence of specific sets of functional ion channels. In addition, the analysis of neuronal connectivity within a culture can be performed using electrophysiological techniques such as voltage and current clamp recordings that allow for detection of postsynaptic currents and potentials. As neurons mature, they contact each other following random or specific patterns, giving rise to spontaneous network activity. Impaired network activity is a hallmark of several NDDs like schizophrenia or Alzheimer’s disease.
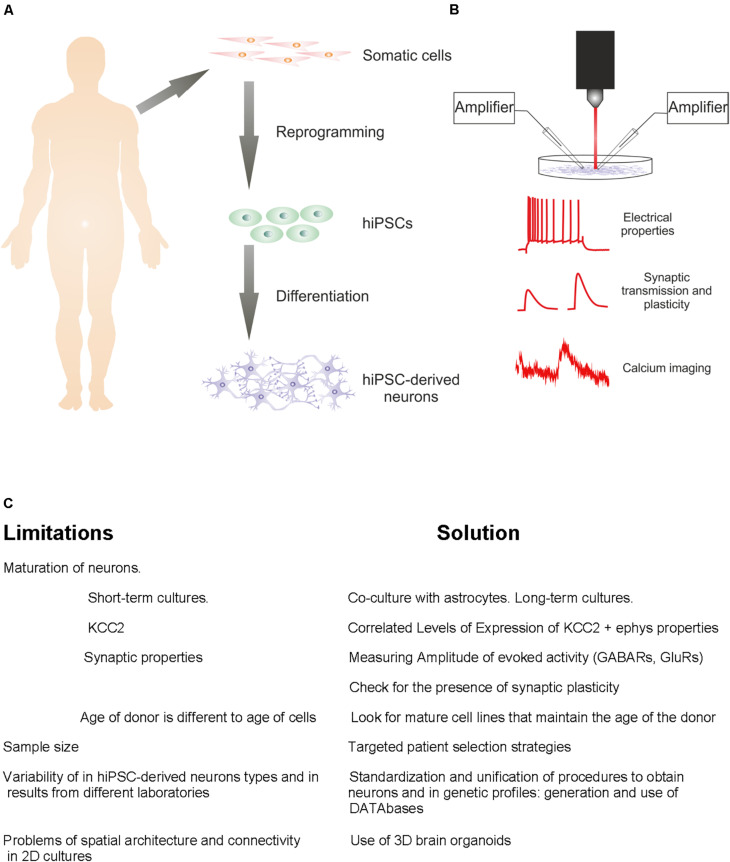
FIGURE 1.
From patient to the study of hiPSC-derived neurons. (A) Diagram of standard steps required to convert somatic cells from diseased or healthy individuals into hiPSC-derived neurons. (B) Representation of electrophysiological approaches for functional characterization of hiPSC-derived neuronal characteristics. (C) Challenges to be resolved and solutions.
Synaptic transmission requires the presence of functional neurotransmitter receptors. This can be measured by recording spontaneous postsynaptic currents with fast rise time and slow decay phase reflecting dynamics of presynaptic release and biophysical properties of postsynaptic receptors (Yang et al., 2011). Additionally, the pharmacological blockade of glutamate or GABA receptors during spontaneous or evoked activity can tease apart the amount of excitatory and inhibitory activity received by a single neuron (Yang et al., 2011; Espuny-Camacho et al., 2013; Liu et al., 2013; Belinsky et al., 2014; Prè et al., 2014; Wen et al., 2014; Kang et al., 2017; Xie et al., 2018; Prius-Mengual et al., 2019). Such approach also allows for the evaluation of the excitatory/inhibitory (E/I) ratio in cultured hiPSC-derived neurons. Balanced E/I ratio in cortical neurons is a key feature during information processing in mammalian neocortex (Shu et al., 2003; Haider et al., 2006; Yizhar et al., 2011; Xue et al., 2014). Furthermore, imbalanced E/I ratio has been found in multiple disorders such as epilepsy (Fritschy, 2008), fragile X syndrome (Gibson et al., 2008), autism (Nelson and Valakh, 2015) or schizophrenia (Selten et al., 2018). A fundamental property of synaptic transmission is the ability of neurons to modify the strength and efficacy of their synapses depending on intrinsic or extrinsic stimuli, a phenomenon called synaptic plasticity (commented in Mateos-Aparicio and Rodríguez-Moreno, 2019). The activity of synapses can be facilitated or depressed within different timescales, referred as short-term plasticity (lasting from milliseconds to minutes) and long-term plasticity (lasting from minutes to hours, days or weeks). Plasticity forms such as spike-timing dependent plasticity (STDP), an activity-dependent type of plasticity known to be important for circuit remodeling during development (Caporale and Dan, 2008; Markram et al., 2011), can be produced by distinct pre- and postsynaptic mechanisms (Rodríguez-Moreno and Paulsen, 2008; Banerjee et al., 2009, 2014; Rodríguez-Moreno et al., 2011, 2013; Andrade-Talavera et al., 2016) and vary their sign at distinct developmental stages (Hensch, 2004; Pérez-Rodríguez et al., 2019). Since STDP is a relevant mechanism during circuit formation that can be readily studied in neuronal cultures (Debanne et al., 2008), hiPSC-derived neuronal cultures offer an interesting platform to study synaptic plasticity in in vitro models of NDDs. Therefore, the analysis of synaptic transmission and plasticity must be a key step in modeling NDDs in vitro.
Extracellular recordings performed with multielectrode arrays (MEAs) can capture the spatiotemporal properties of overall synaptic transmission and plasticity in hiEPSC-derived neuronal cultures (Amin et al., 2016; Xu et al., 2017). This type of preparation can also provide important information about the frequency properties of network oscillatory activity in the culture, a main feature of network activity in the brain.
Patch-clamp or extracellular MEA recordings can be combined with Ca2+ imaging techniques to study Ca2+ regulation in hiPSC-derived neuronal cultures (Belinsky et al., 2014). At single cell level, membrane-impermeable fluorescent Ca2+ indicators can be loaded through the patch pipette and subcellular rises in cytosolic Ca2+ are recorded. This assay is useful to track the subcellular distribution, dynamics and properties of Ca2+ processes within a single hiPSC-derived neuron. As synaptic plasticity is dependent on Ca2+ levels (reviewed in Mateos-Aparicio and Rodríguez-Moreno, 2020), the role of Ca2+ in plasticity may also be determined. At the network level, multisite Ca2+ imaging can be performed by extracellular loading of membrane-permeable fluorescent Ca2+ indicators, which will be subsequently taken up by surrounding cells. Multisite Ca2+ imaging provides information about network Ca2+ oscillations and overall Ca2+ regulation in hiPSC-derived neuronal cultures. Recently, a novel strategy using all-optical electrophysiology has been successfully applied to characterize hiPSC-derived motor neurons in a model of ALS (Kiskinis et al., 2018). The main advantage of all-optical electrophysiology is that it captures action potential statistics and properties with higher throughput than patch clamp experiments. However, all-optical electrophysiology at the moment has lower resolution and higher noise level than patch clamp techniques, so this approach is less useful to study sub threshold events or absolute voltage values.
All methods listed above are useful for the characterization of functional properties of hiPSC-derived neurons. This is important in regard of NDDs, since most of them manifest impairment in single-cell or network activity derived from impaired functional properties. In fact, the analysis of calcium signals proved successful to discriminate schizophrenia from autism models (Grunwald et al., 2019). Since hiPSC-derived neurons are obtained from human patients, a disadvantage of using these cells is a high variability in results and availability of limited sample size at the same time, which on the other hand emphasizes the need for standardization of methods. In this regard, it has been suggested the implementation of patient selection strategies that would reduce heterogeneity. For example, in schizophrenia studies, following targeted patient selection strategies will reduce the number of non-specific phenotypes, maximizing the probability of finding causal pathways (Hoekstra et al., 2017). Also, variability of techniques in different studies makes hard to find a study applying a systematic analysis of functional properties of hiPSC-derived neurons in a culture. We suggest that efforts must be directed toward unifying and standardize techniques among studies so complete and consistent functional properties of different types of neurons can be described and compared depending on the specific needs. The creation of databases might be a step forward in this direction.
The Maturation Problem: Are hiPSC-Derived Neurons Comparable to Human Neurons From Adult Individuals?
It is clear that patient-specific iPSCs can differentiate into neurons which can be further developed to achieve electrophysiological mature properties (Xie et al., 2018). However, the criteria defining “functional maturity” are typically vaguely described and vary between studies, so it remains unclear whether functional hiPSC-derived neurons are functionally comparable to native neurons both from control individuals and patients. In general, in vitro disease modeling techniques use short-term cultures which fall short of producing really mature hiPSC-derived neurons. To overcome this limitation, new approaches such as co-culture with astrocytes (Tang et al., 2013), prolonged neural differentiation (Paavilainen et al., 2018), or long-term cultures (Odawara et al., 2016; Lam et al., 2017) have proven enhanced neural maturation. The developmental stage of cells is not trivial in NDDs modeling, for example, insights obtained using cultured hiPSC-derived cell lines of Alzheimer’s disease models, need to be taken with caution since Alzheimer’s disease typically develops over the age of 70 (Penney et al., 2020).
One open question is whether donor species or age affects the degree of maturation of cultured iPSC-derived neurons. IPSC-derived neurons from mice develop their electrophysiological properties faster than those from humans in vitro, however, when immature hiPSC-derived neurons are transplanted into living mouse brain, they follow the developmental program of the host species (Yin et al., 2019). In general, immature neurons and hiPSC-derived neurons show depolarized resting membrane potential (>−50 mV), high Ri, low Cm, and low amplitude, slow kinetics, and depolarized threshold action potentials. As the cell matures, passive and active properties resemble more to those measured in primary neuron cultures, for example resting membrane potential hyperpolarizes below −50 or −60 mV and Cm increases reflecting larger membrane surface, Ri also decreases <1 Gohm mainly due to increased membrane surface and higher density of ion channels, action potential kinetics become faster and regenerative allowing for repetitive firing, and amplitude increases (Yang et al., 2011; Xie et al., 2018). Furthermore, a reduction of AP half-width (<3 ms) is observed (Prè et al., 2014; Bardy et al., 2016; Gunhanlar et al., 2017; Fink and Levine, 2018). In addition, synaptic properties vary according to their functional maturation state. The analysis of spontaneous synaptic activity in hiPSC-derived neuronal cultures is also used as a tool for evaluating the maturational state of the network. As the in vitro network matures, measurements such as the frequency of spontaneous firing or synaptic responses increase their pharmacological sensitivity to agonists/antagonists of GABAA, AMPA, NMDA, and KA receptors (Odawara et al., 2016). Importantly, it has been recently argued that the analysis of miniature synaptic responses, commonly used as evidence for synaptic maturation, fails to predict synaptic maturity since its frequency does not correlate with the synchronicity of evoked transmission (Meijer et al., 2019). Therefore, in order to properly assess the degree of maturation of synaptic function, conclusions should be based on the analysis of evoked synaptic transmission rather than miniature synaptic events. In addition to this, the expression of synaptic plasticity, which is typically poorly evaluated in studies using hiPSC-derived neurons, must be more thoroughly assessed (Meijer et al., 2019).
The neuron-specific membrane K+-Cl– cotransporter 2 (KCC2) is the main responsible for setting low intracellular Cl– concentration during development. Upregulation of KCC2 expression during development is responsible for the switch from excitatory to inhibitory GABAergic actions (Kaila et al., 2014). Defects in KCC2 expression levels have been found in several disorders including epilepsy (Chen et al., 2017) or neuropathic pain (Coull et al., 2005). In general, KCC2 expression is linked to neuronal maturation (Rivera et al., 1999). In animal models such as rodents, KCC2 is expressed early before birth (Rivera et al., 1999), however, upregulation of KCC2 expression occurs during the first three postnatal weeks, starting by the time of birth, with a steep increase during the first postnatal week (Kovacs et al., 2014). Some studies in humans report a negligible KCC2 expression in perinatal human brain, following upregulation during the early postnatal period, reaching a plateau after the first year of life (Dzhala et al., 2005; Jansen et al., 2010). However, a systematic analysis of KCC2 expression in humans recently reported that, in the human neocortex, KCC2 expression begins during mid-fetal period and reaches mature levels during the first 6 postnatal months (Sedmak et al., 2016). Therefore, it seems that KCC2 upregulation takes longer in human brains compared to rodent brains.
These findings suggest that KCC2 expression must be taken into account when analyzing the maturation state of hiPSC-derived neurons. It could well be that KCC2 upregulation in human neurons in vitro occurs faster than in vivo, making maturational state of hiPSC-derived neurons more comparable to miPSC-derived neurons in vitro. Also, hiPSC-derived neurons from Rett syndrome patients showed significant deficits in KCC2 expression and therefore a delayed functional GABAergic switch from excitation to inhibition (Tang et al., 2016). Furthermore, overexpression of KCC2 in these neurons rescued functional GABA deficits, suggesting the restoration of KCC2 function as a strategy for potential treatment of Rett syndrome and perhaps other autistic-like disorders (Tang et al., 2016). In conclusion, the KCC2 expression pattern and temporal profile must be assessed together with the electrophysiological properties of hiPSC-derived neurons to properly analyze their functional maturation state in vitro (Figure 1C).
Toward the Brain in vitro: Functional Studies in Brain Organoids
The challenges of spatial architecture, connectivity and distribution present in conventional 2D cultures of hiPSC-derived neurons are being resolved with the rise of 3D brain organoids (Cakir et al., 2019; Qian et al., 2019). When hiPSC-derived neurons are grown in 3D aggregates instead of in direct contact with a flat surface, they are capable of self-organize and differentiate in organized, patterned structures that resemble the fetal human brain. From a structural point of view, brain organoids are much smaller than human cerebral cortex, but they recapitulate important aspects of human cortical architecture like neuronal layer patterning or neural lineage cell types. In light of the challenges discussed above in 2D hiPSC-derived neuron cultures, functional studies in 3D brain organoids by means of patch-clamp recordings showed a high variability and different maturational stages of cells, although this variability is constrained within a range of immature functional phenotypes. Experiments in more mature organoids, although still immature, revealed some degree of maturation, in the form of robust excitatory and inhibitory currents as well as trains of action potentials upon current injection (Paşca and Sloan, 2015), indicating certain degree of maturation over time. Extracellular multi-electrode recordings and photostimulation in organoids developed over more than 9 months have shown burst-like firing activity and relatively more mature functional properties (Quadrato et al., 2017). Overall, although hiPSC-derived neurons in brain organoids are able to mature over time (Qian et al., 2016), literature is consistent with the idea that neurons in 3D cultures are immature and comparable to neurons in embryonic stages (Paşca and Sloan, 2015; Qian et al., 2016, 2019; Birey et al., 2017). While these methods are excellent to model NDDs that show alterations during development, they fall short in diseases like Alzheimer’s disease in which alterations manifest much later in time. Recent developments, like the so-called assembloids, which result from the fusion of previously generated region-specific organoids, or from a mixture of cells of different lineages with biomaterials or cells with organization capabilities, offer the potential to develop more complex models of inter-regional interactions that can better approach network activity in different NDDs (Paşca, 2018).
Future Perspectives
We have discussed several challenges that the stem cell research field is facing in light of recent newly developed iPSC technologies like the use of hiPSC-derived neurons in 2D and 3D cultures. In the short-term future, it will be necessary to standardize and unify the plethora of hiPSC-derived neuron lines and their methods of obtention, functional properties, genetic profiles, pharmacology, among others. At this point, a new issue arises, the organization of the amount of information generated in different laboratories using different techniques. A way to solve this problem is the creation of databases containing morphological, genetic, protein expression, biophysical and electrophysiological properties, combined with differentiation protocols for the generation of specific hiPSC-derived neurons. In this regard, existing efforts in current neuroscience oriented to improve neuronal classification in animal models can be helpful to create similar platforms related to hiPSCs. In particular, examples of data sharing initiatives which may be of interest for the stem cell research field are the Allen Brain Institute Cell Types Database, which is producing massive quantities of data on neuronal cell types (Stockton and Santamaria, 2017), the Neurodata Without Borders consortium led by the Kavli Foundation with the goal of develop open-source data format of cellular neurophysiological data (Teeters et al., 2015), the Neuroelectro project which has the objective of compiling and organizing published data about electrical properties of neurons (Tripathy et al., 2014), or the ModelDB database which compiles and organizes published biophysical model of neurons (Hines et al., 2004). Such initiatives may serve as a model for the stem cell research field to share data and organize information for better reproducibility. Such databases can be analyzed by using machine-learning based methods to help identify which cell line is best to model a specific NDD, thereby saving time and improving reproducibility of results. Machine-learning techniques are already in use and have proven effective in improving reproducibility by helping automatic detection of reprogrammed iPSC colonies (Fan et al., 2017), for example.
In order to overcome the challenge of maturation of hiPSC-derived neurons, the creation of mature cell lines that maintain the age of the donor or methods for accelerated aging in 2D and 3D cultures are an exciting possibility that may provide a new paradigm in the field (Mertens et al., 2018; Traxler and Edenhofer, 2019). Finally, in the recent years the functional properties of living human neurons in brain slices have been elucidated. Human cortical neurons show remarkable properties and computational capabilities that differ significantly from those observed in rodents (Mansvelder et al., 2019; Gidon et al., 2020). For example, recently it was shown that human pyramidal neurons in the neocortex display previously unknown classes of dendritic action potentials, making their activity much more complex than previously thought (Gidon et al., 2020). It would be of great interest in the future to explore dendritic functional properties of hiPSC-derived neurons to compare with regular human neurons and confirm their similarities in the information processing.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. The work in our group was supported by the Spanish Ministerio de Economía y Competitividad (MINECO: Grant BFU2015-68655-P). PM-A was supported by a postdoctoral Juan de la Cierva-Formación Fellowship from the MINECO.
References
- Amin H., Maccione A., Marinaro F., Zordan S., Nieus T., Berdondini L. (2016). Electrical responses and spontaneous activity of human iPS-Derived neuronal networks characterized for 3-month Culture with 4096-Electrode arrays. Front. Neurosci. 10:121. 10.3389/fnins.2016.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade-Talavera Y., Duque-Feria P., Paulsen O., Rodriguez-Moreno A. (2016). Presynaptic spike timing-dependent long-term depression in the mouse hippocampus. Cereb. Cortex 26 3637–3654. 10.1093/cercor/bhw172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., González-Rueda A., Sampaio-Baptista C., Paulsen O., Rodríguez-Moreno A. (2014). Distinct mechanisms of spike timing-dependent LTD at vertical and horizontal inputs onto L2/3 pyramidal neurons in mouse barrel cortex. Physiol. Rep. 2:e00271. 10.1002/phy2.271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee A., Meredith R. M., Rodriguez-Moreno A., Mierau S. B., Auberson Y. P., Paulsen O. (2009). Double dissociation of spike timing-dependent potentiation and depression by subunit-preferring NMDA receptor antagonists in mouse barrel cortex. Cereb. Cortex 19 2959–2969. 10.1093/cercor/bhp067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardy C., Van Den Hurk M., Kakaradov B., Erwin J. A., Jaeger B. N., Hernandez R. V., et al. (2016). Predicting the functional states of human iPSC-derived neurons with single-cell RNA-seq and electrophysiology. Mol. Psychiatry 21 1573–1588. 10.1038/mp.2016.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belinsky G. S., Rich M. T., Sirois C. L., Short S. M., Pedrosa E., Lachman H. M., et al. (2014). Patch-clamp recordings and calcium imaging followed by single-cell PCR reveal the developmental profile of 13 genes in iPSC-derived human neurons. Stem Cell Res. 12 101–118. 10.1016/j.scr.2013.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birey F., Andersen J., Makinson C. D., Islam S., Wei W., Huber N., et al. (2017). Assembly of functionally integrated human forebrain spheroids. Nature 545 54–59. 10.1038/nature22330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., Lee H. L., Reijo Pera R. (2012). Modeling Parkinson’s disease using induced pluripotent stem cells. Curr. Neurol. Neurosci. Rep. 12 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir B., Xiang Y., Tanaka Y., Kural M. H., Parent M., Kang Y.-J., et al. (2019). Engineering of human brain organoids with a functional vascular-like system. Nat. Methods 16 1169–1175. 10.1038/s41592-019-0586-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporale N., Dan Y. (2008). Spike timing-dependent plasticity: a Hebbian learning rule. Annu. Rev. Neurosci. 31 25–46. 10.1146/annurev.neuro.31.060407.125639 [DOI] [PubMed] [Google Scholar]
- Chen L., Wan L., Wu Z., Ren W., Huang Y., Qian B., et al. (2017). KCC2 downregulation facilitates epileptic seizures. Sci. Rep. 7:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chestkov I. V., Vasilieva E. A., Illarioshkin S. N., Lagarkova M. A., Kiselev S. L. (2014). Patient-specific induced pluripotent stem cells for SOD1-associated amyotrophic lateral sclerosis pathogenesis studies. Acta Naturae 6 54–60. 10.32607/20758251-2014-6-1-54-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull J. A., Beggs S., Boudreau D., Boivin D., Tsuda M., Inoue K., et al. (2005). BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438 1017–1021. 10.1038/nature04223 [DOI] [PubMed] [Google Scholar]
- Darville H., Poulet A., Rodet-Amsellem F., Chatrousse L., Pernelle J., Boissart C., et al. (2016). Human pluripotent stem cell-derived cortical neurons for high throughput medication screening in autism: a proof of concept study in SHANK3 haploinsufficiency syndrome. EBIO Med. 9 293–305. 10.1016/j.ebiom.2016.05.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D., Boudkkazi S., Campanac E., Cudmore R. H., Giraud P., Fronzaroli-Molinieres L., et al. (2008). Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Prot. 3:1559. 10.1038/nprot.2008.147 [DOI] [PubMed] [Google Scholar]
- Dolmetsch R., Geschwind D. H. (2011). The human brain in a dish: the promise of iPSC-derived neurons. Cell 145 831–834. 10.1016/j.cell.2011.05.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakulic D., Djurovic S., Syed Y. A., Trattaro S., Caporale N., Falk A., et al. (2020). Copy number variantes (CNVs): a powerfull tool for iPSC-based modelling of ASD. Mol. Autism. 11:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzhala V. I., Talos D. M., Sdrulla D. A., Brumback A. C., Mathews G. C., Benke T. A., et al. (2005). NKCC1 transporter facilitates seizures in the developing brain. Nat. Med. 11 1205–1213. 10.1038/nm1301 [DOI] [PubMed] [Google Scholar]
- Espuny-Camacho I., Michelsen K. A., Gall D., Linaro D., Hasche A., Bonnefont J., et al. (2013). Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron 77 440–456. 10.1016/j.neuron.2012.12.011 [DOI] [PubMed] [Google Scholar]
- Fan K., Zhang S., Zhang Y., Lu J., Holcombe M., Zhang X. (2017). A machine learning assisted, label-free, non-invasive approach for somatic reprogramming in induced pluripotent stem cell colony formation detection and prediction. Sci. Rep. 7:13496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Santiago R., Carballo-Carbajal I., Castellano G., Torrent R., Richaud Y., Sánchez-Danés A., et al. (2015). Aberrant epigenome in iPSC-derived dopaminergic neurons from Parkinson’s disease patients. EMBO Mol. Med. 7 1529–1546. 10.15252/emmm.201505439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink J. J., Levine E. S. (2018). Uncovering true cellular phenotypes: using induced pluripotent stem cell-derived neurons to study early insults in neurodevelopmental disorders. Front. Neurol. 9:237. 10.3389/fneur.2018.00237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschy J.-M. (2008). Epilepsy, E/I balance and GABA(A) receptor plasticity. Front. Mol. Neurosci. 1:5. 10.3389/neuro.02.005.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. R., Bartley A. F., Hays S. A., Huber K. M. (2008). Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J. Neurophysiol. 100 2615–2626. 10.1152/jn.90752.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidon A., Zolnik T. A., Fidzinski P., Bolduan F., Papoutsi A., Poirazi P., et al. (2020). Dendritic action potentials and computation in human layer 2/3 cortical neurons. Science 367 83–87. 10.1126/science.aax6239 [DOI] [PubMed] [Google Scholar]
- Grunwald L.-M., Stock R., Haag K., Buckenmaier S., Eberle M.-C., Wildgruber D., et al. (2019). Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with schizophrenia and autism. Translat. Psychiatry 9:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunhanlar N., Shpak G., Van Der Kroeg M., Gouty-Colomer L. A., Munshi S. T., Lendemeijer B., et al. (2017). A simplified protocol for differentiation of electrophysiologically mature neuronal networks from human induced pluripotent stem cells. Mol. Psychiatry 23:1336. 10.1038/mp.2017.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B., Duque A., Hasenstaub A. R., Mccormick D. A. (2006). Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J. Neurosci. 26 4535–4545. 10.1523/jneurosci.5297-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensch T. K. (2004). Critical period regulation. Annu. Rev. Neurosci. 27 549–579. 10.1146/annurev.neuro.27.070203.144327 [DOI] [PubMed] [Google Scholar]
- Hines M. L., Morse T., Migliore M., Carnevale N. T., Shepherd G. M. (2004). ModelDB: a database to support computational neuroscience. J. Comp. Neurosci. 17 7–11. 10.1023/b:jcns.0000023869.22017.2e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra S. D., Stringer S., Heine V. M., Posthuma D. (2017). Genetically-Informed patient selection for iPSC studies of complex diseases may aid in reducing cellular heterogeneity. Front. Cellullar Neurosci. 11 164–164. 10.3389/fncel.2017.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen L. A., Peugh L. D., Roden W. H., Ojemann J. G. (2010). Impaired maturation of cortical GABA(A) receptor expression in pediatric epilepsy. Epilepsia 51 1456–1467. 10.1111/j.1528-1167.2009.02491.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaila K., Price T. J., Payne J. A., Puskarjov M., Voipio J. (2014). Cation-chloride cotransporters in neuronal development, plasticity and disease. Nat. Rev. Neurosci. 15 637–654. 10.1038/nrn3819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S., Chen X., Gong S., Yu P., Yau S., Su Z., et al. (2017). Characteristic analyses of a neural differentiation model from iPSC-derived neuron according to morphology, physiology, and global gene expression pattern. Sci. Rep. 7:12233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiskinis E., Kralj J. M., Zou P., Weinstein E. N., Zhang H., Tsioras K., et al. (2018). All-Optical electrophysiology for high-throughput functional characterization of a human iPSC-Derived motor neuron model of ALS. Stem Cell Rep. 10 1991–2004. 10.1016/j.stemcr.2018.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs K., Basu K., Rouiller I., Sik A. (2014). Regional differences in the expression of K(+)-Cl(-) 2 cotransporter in the developing rat cortex. Brain Struct. Funct. 219 527–538. 10.1007/s00429-013-0515-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam R. S., Töpfer F. M., Wood P. G., Busskamp V., Bamberg E. (2017). Functional maturation of human stem cell-derived neurons in long-term cultures. PLoS One 12:e0169506. 10.1371/journal.pone.0169506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H.-K., Velazquez Sanchez C., Chen M., Morin P. J., Wells J. M., Hanlon E. B., et al. (2016). Three dimensional human neuro-spheroid model of Alzheimer’s disease based on differentiated induced pluripotent stem cells. PLoS One 11:e0163072. 10.1371/journal.pone.0163072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Liu H., Sauvey C., Yao L., Zarnowska E. D., Zhang S. C. (2013). Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat. Protoc. 8 1670–1679. 10.1038/nprot.2013.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansvelder H. D., Verhoog M. B., Goriounova N. A. (2019). Synaptic plasticity in human cortical circuits: cellular mechanisms of learning and memory in the human brain? Curr. Opin. Neurobiol. 54 186–193. 10.1016/j.conb.2018.06.013 [DOI] [PubMed] [Google Scholar]
- Markram H., Gerstner W., Sjöström P. J. (2011). A history of spike-timing-dependent plasticity. Front. Synaptic Neurosci. 3:4. 10.3389/fnsyn.2011.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Aparicio P., Rodríguez-Moreno A. (2019). The impact of studying brain plasticity. Front. Cell Neurosci. 13:66. 10.3389/fncel.2019.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Aparicio P., Rodríguez-Moreno A. (2020). Calcium dynamics and synaptic plasticity. Adv. Exp. Med. Biol. 1131 965–984. 10.1007/978-3-030-12457-1_38 [DOI] [PubMed] [Google Scholar]
- Meijer M., Rehbach K., Brunner J. W., Classen J. A., Lammertse H. C. A., Van Linge L. A., et al. (2019). A single-cell model for synaptic transmission and plasticity in human iPSC-Derived neurons. Cell Rep. 27 2199–2211.e6. 10.1016/j.celrep.2019.04.058 [DOI] [PubMed] [Google Scholar]
- Mertens J., Reid D., Lau S., Kim Y., Gage F. H. (2018). Aging in a dish: iPSC-Derived and directly induced neurons for studying brain aging and age-related neurodegenerative diseases. Ann. Rev. Genet. 52 271–293. 10.1146/annurev-genet-120417-031534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mungenast A. E., Siegert S., Tsai L. H. (2016). Modeling Alzheimer’s disease with human induced pluripotent stem (iPS) cells. Mol. Cell. Neurosci. 73 13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekrasov E. D., Vigont V. A., Klyushnikov S. A., Lebedeva O. S., Vassina E. M., Bogomazova A. N., et al. (2016). Manifestation of Huntington’s disease pathology in human induced pluripotent stem cell-derived neurons. Mol. Neurodegener. 11:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. B., Valakh V. (2015). Excitatory/Inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87 684–698. 10.1016/j.neuron.2015.07.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odawara A., Katoh H., Matsuda N., Suzuki I. (2016). Physiological maturation and drug responses of human induced pluripotent stem cell-derived cortical neuronal networks in long-term culture. Sci. Rep. 6:26181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavilainen T., Pelkonen A., Mäkinen M. E. L., Peltola M., Huhtala H., Fayuk D., et al. (2018). Effect of prolonged differentiation on functional maturation of human pluripotent stem cell-derived neuronal cultures. Stem Cell Res. 27 151–161. 10.1016/j.scr.2018.01.018 [DOI] [PubMed] [Google Scholar]
- Parent J. M., Anderson S. A. (2015). Reprogramming patient-derived cells to study the epilepsies. Nat. Neurosci. 18:360. 10.1038/nn.3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca A. M., Sloan S. A. (2015). Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat. Methods 12 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paşca S. P. (2018). The rise of three-dimensional human brain cultures. Nature 553 437–445. 10.1038/nature25032 [DOI] [PubMed] [Google Scholar]
- Penney J., Ralvenius W. T., Tsai L.-H. (2020). Modeling Alzheimer’s disease with iPSC-derived brain cells. Mol. Psychiatry 25 148–167. 10.1038/s41380-019-0468-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Rodríguez M., Arroyo-García L. E., Prius-Mengual J., Andrade-Talavera Y., Armengol J. A., Pérez-Villegas E. M., et al. (2019). Adenosine receptor-mediated developmental loss of spike timing-dependent depression in the hippocampus. Cereb. Cortex 29 3266–3281. 10.1093/cercor/bhy194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prè D., Nestor M. W., Sproul A. A., Jacob S., Koppensteiner P., Chinchalongporn V., et al. (2014). A time course analysis of the electrophysiological properties of neurons differentiated from human induced pluripotent stem cells (iPSCs). PLoS One 9:e103418. 10.1371/journal.pone.0103418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prius-Mengual J., Pérez-Rodríguez M., Andrade-Talavera Y., Rodríguez-Moreno A. (2019). NMDAR containing GluN2C/2C/2D subunits mediate an increase in glutamate release at hippocampal CA3-CA1 dsynapses. Mol. Neurobiol. 56 1694–1706. 10.1007/s12035-018-1187-5 [DOI] [PubMed] [Google Scholar]
- Qian X., Nguyen H. N., Song M. M., Hadiono C., Ogden S. C., Hammack C., et al. (2016). Brain-region-specific organoids using mini-bioreactors for modeling ZIKV exposure. Cell 165 1238–1254. 10.1016/j.cell.2016.04.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Song H., Ming G.-L. (2019). Brain organoids: advances, applications and challenges. Development 146:dev166074. 10.1242/dev.166074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadrato G., Nguyen T., Macosko E. Z., Sherwood J. L., Min Yang S., Berger D. R., et al. (2017). Cell diversity and network dynamics in photosensitive human brain organoids. Nature 545 48–53. 10.1038/nature22047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee H. J., Shaib A. H., Rehbach K., Lee C., Seif P., Thomas C., et al. (2019). An autaptic culture system for standardized analyses of iPSC-derived human neurons. Cell Rep. 27 2212–2228.e7. 10.1016/j.celrep.2019.04.059 [DOI] [PubMed] [Google Scholar]
- Rivera C., Voipio J., Payne J. A., Ruusuvuori E., Lahtinen H., Lamsa K., et al. (1999). The K+/Cl- co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature 397 251–255. 10.1038/16697 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Moreno A., Gonzalez-Rueda A., Banerjee A., Upton A. L., Craig M. T., Paulsen O. (2013). Presynaptic self-depression at developing neocortical synapses. Neuron 77 35–42. 10.1016/j.neuron.2012.10.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A., Kohl M. M., Reeve R. E., Eaton T. R., Collins H. A., Anderson H. L., et al. (2011). Presynaptic induction and expression of timing-dependent long-term depression demonstrated by compartment-specific photoreease of a use-dependent NMDA receptor antagonist. J. Neurosci. 31 8564–8569. 10.1523/jneurosci.0274-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Moreno A., Paulsen O. (2008). Spike timing-dependent long-term depression requires presynaptic NMDA receptors. Nat. Neurosci. 11 744–745. 10.1038/nn.2125 [DOI] [PubMed] [Google Scholar]
- Sedmak G., Jovanov-Miloševiæ N., Puskarjov M., Ulamec M., Krušlin B., Kaila K., et al. (2016). Developmental expression patterns of KCC2 and functionally associated molecules in the human brain. Cereb. Cortex 26 4574–4589. 10.1093/cercor/bhv218 [DOI] [PubMed] [Google Scholar]
- Selten M., Van Bokhoven H., Nadif Kasri N. (2018). Inhibitory control of the excitatory/inhibitory balance in psychiatric disorders. F1000Research 7 23–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y., Hasenstaub A., Mccormick D. A. (2003). Turning on and off recurrent balanced cortical activity. Nature 423 288–293. 10.1038/nature01616 [DOI] [PubMed] [Google Scholar]
- Stockton D. B., Santamaria F. (2017). Integrating the allen brain institute cell types database into automated neuroscience workflow. Neuroinformatics 15 333–342. 10.1007/s12021-017-9337-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Kim J., Zhou L., Wengert E., Zhang L., Wu Z., et al. (2016). KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc. Natl. Acad. of Sci. U S A. 113 751–756. 10.1073/pnas.1524013113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X., Zhou L., Wagner A. M., Marchetto M. C., Muotri A. R., Gage F. H., et al. (2013). Astroglial cells regulate the developmental timeline of human neurons differentiated from induced pluripotent stem cells. Stem Cell Res. 11 743–757. 10.1016/j.scr.2013.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teeters J. L., Godfrey K., Young R., Dang C., Friedsam C., Wark B., et al. (2015). Neurodata without borders: creating a common data format for neurophysiology. Neuron 88 629–634. 10.1016/j.neuron.2015.10.025 [DOI] [PubMed] [Google Scholar]
- Traxler L., Edenhofer F. (2019). Next-generation disease modeling with direct conversion: a new path to old neurons. FEBS Lett. 593 3316–3337. 10.1002/1873-3468.13678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. J., Savitskaya J., Burton S. D., Urban N. N., Gerkin R. C. (2014). NeuroElectro: a window to the world’s neuron electrophysiology data. Front. Neuroinform. 8:40. 10.3389/fninf.2014.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitrac A., Cloëz-Tayarani I. (2018). Induced pluripotent stem cells as a tool to study brain circuits in autism-related disorders. Stem Cell Res. Ther. 9 226–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpato V., Webber C. (2020). Addressing variability in iPSC-derived models of human disease: guidelines to promote reproducibility. Dis. Mod. Mechan. 13:dmm042317. 10.1242/dmm.042317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z., Nguyen H. N., Guo Z., Lalli M. A., Wang X., Su Y., et al. (2014). Synaptic dysregulation in a human iPS cell model of mental disorders. Nature 515 414–418. 10.1038/nature13716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright R., Réthelyi J. M., Gage F. H. (2014). Enhancing induced pluripotent stem cell models of schizophrenia. JAMA Psychiatry 71 334–335. [DOI] [PubMed] [Google Scholar]
- Xie Y., Schutte R. J., Ng N. N., Ess K. C., Schwartz P. H., O’dowd D. K. (2018). Reproducible and efficient generation of functionally active neurons from human hiPSCs for preclinical disease modeling. Stem cell Res. 26 84–94. 10.1016/j.scr.2017.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X., Radulescu C. I., Utami K. H., Pouladi M. A. (2017). Obtaining multi-electrode array recordings from human induced pluripotent stem cell–derived neurons. Bio-protocol 7:e2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue M., Atallah B. V., Scanziani M. (2014). Equalizing excitation-inhibition ratios across visual cortical neurons. Nature 511 596–600. 10.1038/nature13321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N., Ng Y. H., Pang Z. P., Südhof T. C., Wernig M. (2011). Induced neuronal cells: how to make and define a neuron. Cell Stem Cell 9 517–525. 10.1016/j.stem.2011.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Xu J.-C., Cho G.-S., Kwon C., Dawson T. M., Dawson V. L. (2019). Neurons derived from human induced pluripotent stem cells integrate into rat brain circuits and maintain both excitatory and inhibitory synaptic activities. eNeuro 6:ENEURO.0148-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O., Fenno L. E., Prigge M., Schneider F., Davidson T. J., O’shea D. J., et al. (2011). Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477:171. 10.1038/nature10360 [DOI] [PMC free article] [PubMed] [Google Scholar]