Abstract
Background
Patients suffering from postoperative recurrent glioblastoma have an extremely unfavorable outcome because there are no proven therapeutic options. The median overall survival for those with relapsed glioblastoma after surgery is only 7.5 months.
Case presentation: Between March 2015 and October 2019, a 44-year-old female patient with recurrent glioblastoma was treated by our medical team. After several failed rounds of therapy, the patient was subsequently treated with the anti-programmed death (PD)-1 antibody nivolumab, anti-vascular endothelial growth factor (VEGF) antibody bevacizumab, and cytotoxic agent temozolomide.
Results
The patient showed a sustainable complete response to the regimen. To date, there have been no serious toxic side effects. As of October 2019 (the last follow-up), the patient has been in complete remission for 17 months since recurrence.
Conclusion
The experience of this complicated case indicates the possible application of immune checkpoint inhibitors, anti-angiogenesis agents, and cytotoxic reagents for recurrent glioblastoma. The administration of this three-agent regimen appears safe and effective. However, further clinical trials are warranted.
Keywords: Glioblastoma, nivolumab, bevacizumab, temozolomide, immunochemotherapy, anti-angiogenesis
Article Summary
Strengths and limitations:
This is the first report of a patient with recurrent WHO stage IV glioblastoma treated with nivolumab combined with bevacizumab and temozolomide.
This patient's outcome is encouraging, and the regimen appears safe and effective.
Clinical trials involving more patients with recurrent glioblastoma are warranted.
Introduction
Glioblastoma is the most common primary malignant tumor in the central nervous system. It originates from glial cells and accounts for more than 14.6% of all brain tumors.1 Glioblastoma is highly invasive, metastatic, and difficult to surgically resect. The median overall survival of patients with recurrence is reported to be only between 7.5 and 9 months.2,3 The standard treatment regimen for glioblastoma is surgery combined with radiotherapy and adjuvant temozolomide chemotherapy,4 but there are no widely-accepted treatment regimens for patients with recurrent glioblastoma. Angiogenesis plays an important role in the initiation and progression of tumors. Patients with malignant tumors often have an increased level of vascular endothelial growth factor (VEGF). Bevacizumab is a monoclonal antibody against human VEGF-A. Several recent clinical studies have indicated that bevacizumab administration increases the objective remission rate of patients with recurrent malignant glioma, but the median survival time remains less than 1 year.5–7 Additionally, anti-programmed cell death receptor-1 (PD-1) antibodies have shown remarkable efficacy in the treatment of non-small cell lung cancer, colorectal cancer, melanoma, and other tumors8 by enhancing cytotoxic T cell functions. Studies have shown that PD-1 and its ligand programmed cell death-ligand 1 (PD-L1) are overexpressed in a subset of patients with glioblastoma,9 but the application of PD-1 antibodies in glioma remains controversial.10–12 Here, we report the case of glioblastoma patient treated with nivolumab (anti-PD-1 antibody) combined with bevacizumab (anti-VEGF antibody) and temozolomide. The survival outcome of our patient was encouraging, and the regimen showed acceptable safety.
Patient
Patient and public involvement
A patient with recurrent glioblastoma after surgery was described in this report. Currently, there is no available therapeutic option for this patient.
Clinical presentation
A 44-year-old woman was admitted to Southwest Hospital at the end of March 2015 with a history of dizziness and headaches. Brain magnetic resonance imaging (MRI) scans indicated a space-occupying lesion in the right insular lobe. In May 2015, right frontotemporal craniotomy tumor resection was performed at Beijing Tiantan Hospital affiliated with Capital Medical University. Postoperative pathology and genetic examinations indicated glioblastoma with oligodendroglioma components involving the subarachnoid space [World Health Organization (WHO) stage IV] without 1p/19q co-deletion. Postoperative adjuvant radiotherapy [radiation dose: 6 mv-x gross tumor target volume (GTV) 63 Gy, clinical target volume (CTV) 59.4 Gy for 33 cycles] and 6-cycle temozolomide chemotherapy [200 mg/m2, days 1–5, every 28 days (q28d)] were given in Southwest Hospital starting on 7 August 2015. On 25 March 2018, a follow-up craniocerebral MRI showed the existence of abnormal enhancement shadows in the right frontal lobe and corpus callosum knee and body, suggesting tumor recurrence. The anti-PD-1 antibody nivolumab (OPDIVO®, manufactured by Bristol Myers Squibb Company, NY, USA) combined with bevacizumab (Avastin®, manufactured by Genentech, CA, USA) and temozolomide (Temodal®, manufactured by Schering-Plough Corporation, NJ, USA) was immediately given. The regimen was as follows: nivolumab 3 mg/kg, every 2 weeks (q2w); bevacizumab 7.5 mg/kg, q2w; and temozolomide 200 mg/m2, days 1 to 5, q28d, for a total of 10 cycles. Subsequently, bevacizumab and temozolomide were discontinued, but nivolumab continued to be administered. On 22 November 2018, we examined the expression of O6-methylguanine–DNA methyltransferase (MGMT) by polymerase chain reaction (PCR) amplicon Bisulfite genomic sequencing following a published protocol.13 The expression of PD-1 and PD-L1 was measured by immunochemistry in surgically resected samples according to a published method.14
Results
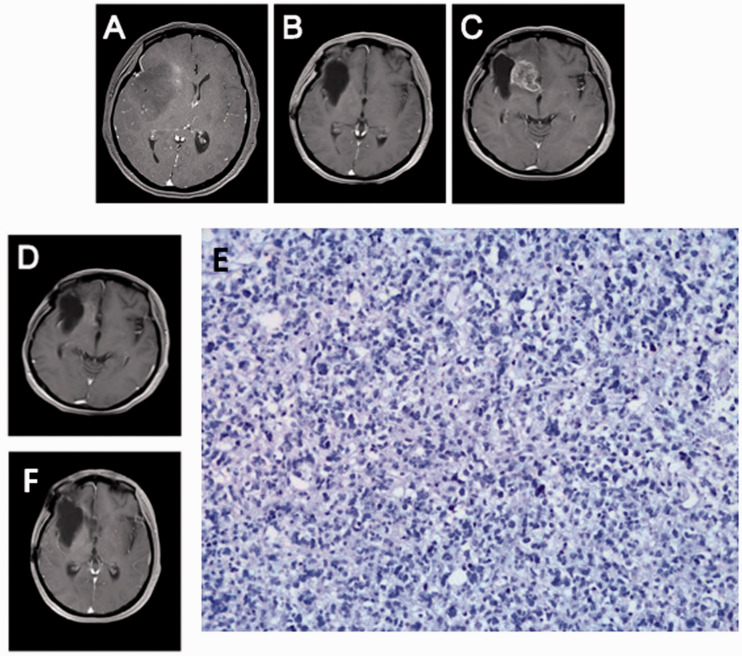
The treatment response was measured according to Response Evaluation Criteria in Solid Tumors version 1.1 every 2 months. The MRI images of the patient are shown in Figure 1. The right insular lobe space-occupying lesion was found on 2 May 2015 (Figure 1a), which led to the diagnosis of primary glioblastoma. After surgery and adjuvant radiochemotherapy, the tumor completely disappeared (Figure 1b). On 25 March 2018, tumor recurrence (Figure 1c, arrowhead) was detected on an MRI scan. Two months (31 May 2018) after treatment with nivolumab, bevacizumab, and temozolomide, the tumor was in complete remission (Figure 1d). The results of PCR amplicon Bisulfite genomic sequencing indicated a positive methylation status of the MGMT promoter. The expression of PD-1 or PD-L1 was negative (Figure 1e). On 24 August 2018, the patient was re-evaluated, and the cancer was still in complete remission (Figure 1f). The patient was last examined in the clinic on 17 October 2019 with no clinical evidence of relapse. The patient was then lost to follow-up. From the diagnosis of recurrence (March 2018), the overall survival was 19 months, whereas the period of complete remission was 17 months. No severe toxic effects were observed. Liver and kidney functions remained in the normal ranges. The muscle strength of the upper and lower limbs on the left was 5-, and that of the right limbs was normal. The secondary tumor was in complete remission for 17 months, and the patient responded well to the three-agent regimen.
Figure 1.
Representative images of the important clinical events in a 44-year-old female patient with recurrent glioblastoma. (a) Magnetic resonance imaging (MRI) scan at the initial diagnosis (2 May 2015). (b) MRI scan after surgery and adjuvant therapy (23 September 2015). (c) MRI scan at recurrence (25 March 2018). (d) MRI scan 2 months after triple-agent therapy with nivolumab, bevacizumab, and temozolomide (31 May 2018). (e) Immunohistochemistry staining indicated negative programmed death-ligand 1(PD-L1) expression in the primary tumor (200×). (f) MRI scan at the most recent visit (30 October 2019).
Discussion
According to the WHO classification, Grade III and IV gliomas are categorized as malignant, which are more aggressive than Grade I and II tumors.15,16 Patients with malignant glioma have a poor prognosis with a recurrence rate of up to 100%. Although primary glioblastoma can be treated with surgery and adjuvant chemoradiotherapy, there is no standard care for recurrent glioblastoma. Current therapeutic methods for recurrent glioblastoma mainly include reoperation, radiotherapy, chemotherapy, and other palliative treatment methods. As research progresses, the molecular subtyping of glioma has been applied clinically. A previous study showed that glioblastoma patients with IDH1-mutated tumors exhibit a better prognosis.17 In patients with anaplastic gliomas, those with ATRX loss and IDH mutations have a better prognosis.18 Among glioblastoma patients with MGMT promoter methylation, it has been reported that temozolomide prolongs their overall survival.19 In addition, patients with 1p/19q codeletions show longer survival after radiochemotherapy.20 Those with postoperative recurrence of malignant glioma do not benefit from surgical treatment and radiotherapy, leaving chemotherapy as a more feasible option. However, no widely-accepted chemotherapy regimen has been introduced for patients with recurrent glioblastoma after adjuvant therapy.21,22
Immune checkpoint inhibitors, such as anti-PD-1 antibodies, enhance the functions of cytotoxic T cells to execute tumor-killing effects.23 Anti-PD-1 strategies have been used in the treatment of many tumors, but their efficacy in recurrent glioblastoma patients remains controversial. For instance, in a phase 1 study12 of 40 patients with recurrent glioblastoma treated with nivolumab or nivolumab and ipilimumab, 11 individuals experienced a partial response or stable disease longer than 12 weeks, indicating that nivolumab-based regimens might be effective in a subset of patients with recurrent glioblastoma. In a single-institute retrospective study, patients with recurrent glioblastoma who had failed bevacizumab treatment were administered nivolumab.24 No patient demonstrated a therapeutic response to nivolumab, and no survival benefit was observed.24 Another retrospective11 study also indicated that nivolumab or pembrolizumab (anti-PD-L1 antibodies) did not show any survival benefit in patients with recurrent high-grade glioma, even when the patients were concurrently treated with bevacizumab. Although these studies failed to show a survival benefit in patients treated with anti-PD-L1/PD-1 and anti-angiogenic agents, it is worth noting the above three studies11,12,24 were uncontrolled studies with relatively small cohorts. Therefore, well-designed clinical trials with larger cohorts are warranted to test the efficacy of nivolumab in patients with recurrent glioblastoma.
Anti-VEGF molecular targeted therapy provides a new direction for different types of malignant tumors that cannot be surgically resected. In 2009, the US Federal Drug Administration (FDA) approved bevacizumab for the treatment of recurrent glioblastoma based on the increased response rates observed in two phase 2 studies (06-C-0064E trial25 and AVF3708 trial6). Several researchers26 have suggested that anti-angiogenic drugs can normalize tortuous and disorganized tumor vessels, thereby theoretically enhancing the function of anti-PD-1 antibodies. Presently, the combination of anti-angiogenic drugs and anti-PD-1 antibodies has been tested in clinical trials for many solid tumors, and promising results have been obtained. The combination of nivolumab and bevacizumab has been evaluated in patients with recurrent ovarian cancer.27 In this small-scale phase 2 trial,27 the objective response rate to nivolumab and bevacizumab was 28.9% in the entire cohort (40.0% in platinum-sensitive patients and 16.7% in platinum-resistant individuals), indicating that a fraction of patients might achieve a long-term benefit from the combination.
In the present case, after the failure of adjuvant chemoradiotherapy, we administered salvage chemotherapy with temozolomide combined with the immune checkpoint inhibitor nivolumab and anti-angiogenic bevacizumab. The recurrent patient responded to the regimen with complete remission for 17 months, which was longer than the median overall survival of 9.3 months from recurrence reported by a previous meta-analysis.28 In the clinical course, we did not find any positive markers for a good response to anti-PD-1 antibodies. However, the fact that the patient survived longer than previous reports suggests other biomarkers might be more practical to predict therapeutic responses. To the best of our knowledge, this is the first report of a recurrent glioblastoma patient treated with temozolomide, nivolumab, and bevacizumab. The survival of this patient appears to be most encouraging, suggesting that this regimen might be effective in a larger population, although clinical trials are warranted.
Targeted therapy for recurrent glioblastoma based on the understanding of molecular biology has gained significant attention from researchers.29 In addition to immune checkpoint inhibitors and anti-angiogenics, other strategies should also be considered, especially combinations with cytotoxic agents.
In conclusion, anti-PD1 antibodies combined with bevacizumab and chemotherapy can potentially be used in patients with recurrent glioblastoma. The combination of anti-angiogenic treatment and immunotherapy enhances the anti-tumor effects of anti-PD1 antibodies. Further clinical trials are required for the clinical application of this regimen in patients with recurrent glioblastoma.
Author contributions
Chen Can, Zuo Wenwei, Yang Pan, and Zhang Yanling took care of the patient. Chen Can prepared the first draft. Zhang Yanling served as the medical team leader and reviewed the draft.
Data availability statement
The data generated and/or analyzed in this case report are available upon request to the corresponding author.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics and consent for publication
The patient provided written informed consent for the publication of this case report.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Ostrom QT, Cioffi G, Gittleman H, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol 2019; 21: v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 1999; 17: 2572–2578. [DOI] [PubMed] [Google Scholar]
- 3.Cloughesy TF, Landolfi J, Vogelbaum MA, et al. Durable complete responses in some recurrent high-grade glioma patients treated with Toca 511 + Toca FC. Neuro Oncol 2018; 20: 1383–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.NCCN Guidelines Version 2. 2018. Central Nervous System Cancers. http://www.nccn.org.
- 5.Diaz RJ, Ali S, Qadir MG, et al. The role of bevacizumab in the treatment of glioblastoma. J Neurooncol 2017; 133: 455–467. [DOI] [PubMed] [Google Scholar]
- 6.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 2009; 27: 4733–4740. [DOI] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 2007; 25: 4722–4729. [DOI] [PubMed] [Google Scholar]
- 8.Alsaab HO, Sau S, Alzhrani R, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol 2017; 8: 561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonios JP, Soto H, Everson RG, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro Oncol 2017; 19: 796–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang X, Guo G, Guan H, et al. Challenges and potential of PD-1/PD-L1 checkpoint blockade immunotherapy for glioblastoma. J Exp Clin Cancer Res 2019; 38: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurz SC, Cabrera LP, Hastie D, et al. PD-1 inhibition has only limited clinical benefit in patients with recurrent high-grade glioma. Neurology 2018; 91: e1355–e1359. [DOI] [PubMed] [Google Scholar]
- 12.Omuro A, Vlahovic G, Lim M, et al. Nivolumab with or without ipilimumab in patients with recurrent glioblastoma: results from exploratory phase I cohorts of CheckMate 143. Neuro Oncol 2018; 20: 674–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Y, Tollefsbol TO. DNA methylation detection: bisulfite genomic sequencing analysis. Methods Mol Biol 2011; 791: 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ancevski Hunter K, Socinski MA, Villaruz LC. PD-L1 testing in guiding patient selection for PD-1/PD-L1 inhibitor therapy in lung cancer. Mol Diagn Ther 2018; 22: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol 2016; 131: 803–820. [DOI] [PubMed] [Google Scholar]
- 16.Wesseling P, Capper D. WHO 2016 Classification of Gliomas. Neuropathol Appl Neurobiol 2018; 44: 139–150. [DOI] [PubMed] [Google Scholar]
- 17.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 2010; 120: 707–718. [DOI] [PubMed] [Google Scholar]
- 18.Wiestler B, Capper D, Holland-Letz T, et al. ATRX loss refines the classification of anaplastic gliomas and identifies a subgroup of IDH mutant astrocytic tumors with better prognosis. Acta Neuropathol 2013; 126: 443–451. [DOI] [PubMed] [Google Scholar]
- 19.Weller M, Felsberg J, Hartmann C, et al. Molecular predictors of progression-free and overall survival in patients with newly diagnosed glioblastoma: a prospective translational study of the German Glioma Network. J Clin Oncol 2009; 27: 5743–5750. [DOI] [PubMed] [Google Scholar]
- 20.Wick W, Hartmann C, Engel C, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 2009; 27: 5874–5880. [DOI] [PubMed] [Google Scholar]
- 21.Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs 2016; 20: S2–S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campos B, Olsen LR, Urup T, et al. A comprehensive profile of recurrent glioblastoma. Oncogene 2016; 35: 5819–5825. [DOI] [PubMed] [Google Scholar]
- 23.Gao M, Wang T, Ji L, et al. Therapy with carboplatin and anti-PD-1 antibodies before surgery demonstrates sustainable anti-tumor effects for secondary cancers in mice with triple-negative breast cancer. Front Immunol 2020; 11: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chamberlain MC, Kim BT. Nivolumab for patients with recurrent glioblastoma progressing on bevacizumab: a retrospective case series. J Neurooncol 2017; 133: 561–569. [DOI] [PubMed] [Google Scholar]
- 25.Kreisl TN, Kim L, Moore K, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol 2009; 27: 740–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramjiawan RR, Griffioen AW, Duda DG. Anti-angiogenesis for cancer revisited: Is there a role for combinations with immunotherapy? Angiogenesis 2017; 20: 185–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu JF, Herold C, Gray KP, et al. Assessment of combined nivolumab and bevacizumab in relapsed ovarian cancer: a phase 2 clinical trial. JAMA Oncol 2019; 5: 1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong ET, Gautam S, Malchow C, et al. Bevacizumab for recurrent glioblastoma multiforme: a meta-analysis. J Natl Compr Canc Netw 2011; 9: 403–407. [DOI] [PubMed] [Google Scholar]
- 29.Bahmad HF, Mouhieddine TH, Chalhoub RM, et al. The Akt/mTOR pathway in cancer stem/progenitor cells is a potential therapeutic target for glioblastoma and neuroblastoma. Oncotarget 2018; 9: 33549–33561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated and/or analyzed in this case report are available upon request to the corresponding author.