Abstract
Regulators of chromatin dynamics and transcription are increasingly implicated in the aetiology of neurodevelopmental disorders. Haploinsufficiency of EHMT1, encoding a histone methyltransferase, is associated with several neurodevelopmental disorders, including Kleefstra syndrome, developmental delay and autism spectrum disorder. Using a mouse model of Ehmt1 haploinsufficiency (Ehmt1D6Cre/+), we examined a number of brain and behavioural endophenotypes of relevance to neurodevelopmental disorders. Specifically, we show that Ehmt1D6Cre/+ mice have deficits in information processing, evidenced by abnormal sensory-motor gating, a complete absence of object recognition memory, and a reduced magnitude of auditory evoked potentials in both paired-pulse inhibition and mismatch negativity. The electrophysiological experiments show that differences in magnitude response to auditory stimulus were associated with marked reductions in total and evoked beta- and gamma-band oscillatory activity, as well as significant reductions in phase synchronisation. The pattern of electrophysiological deficits in Ehmt1D6Cre/+ matches those seen in control mice following administration of the selective NMDA-R antagonist, ketamine. This, coupled with reduction of Grin1 mRNA expression in Ehmt1D6Cre/+ hippocampus, suggests that Ehmt1 haploinsufficiency may lead to disruption in NMDA-R. Taken together, these data indicate that reduced Ehmt1 dosage during forebrain development leads to abnormal circuitry formation, which in turn results in profound information processing deficits. Such information processing deficits are likely paramount to our understanding of the cognitive and neurological dysfunctions shared across the neurodevelopmental disorders associated with EHMT1 haploinsufficiency.
Keywords: Neurodevelopmental disorders, mouse model, startle and prepulse inhibition, auditory event–related potentials, NMDA-R hypofunction
Introduction
Post-translational modifiers of histone proteins influence chromatin dynamics and transcriptional regulation throughout development and are essential for the highly choreographed processes of lineage commitment and differentiation during neurodevelopment (Hirabayashi and Gotoh, 2010; Tyssowski et al., 2014). Perhaps unsurprisingly, exome sequencing studies and pathway analyses of genome wide association studies implicate genes encoding chromatin and transcriptional regulators in the aetiology of autism spectrum disorders (De Rubeis et al., 2014; Yuen et al., 2017), schizophrenia (Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015) and severe developmental disorders (Deciphering Developmental Disorders Study, 2017; Singh et al., 2016). One such gene, implicated in several neurodevelopmental and neuropsychiatric disorders (Talkowski et al., 2012), is EHMT1, which encodes the histone H3 lysine 9 mono- and di-methyltransferase G9a-like protein (GLP).
Haploinsufficiency of EHMT1 is the primary cause of the 9q34 subtelomeric-deletion syndrome, also known as Kleefstra syndrome (Kleefstra et al., 2005, 2006), a condition associated with intellectual disabilities, epilepsy, childhood hypotonia, facial dysmorphism, microcephaly, delays in reaching developmental milestones and behavioural problems such as aggressive outbursts and hypoactivity. Furthermore, analysis of copy number variants (CNVs) (Cooper et al., 2011) and a large exome sequencing study (Deciphering Developmental Disorders Study, 2017) have linked de novo mutations affecting EHMT1 to severe developmental delay more generally. Finally, CNVs spanning EHMT1 have also been associated with autism spectrum disorder (O’Roak et al., 2012) and schizophrenia (Kirov et al., 2012).
The importance of Ehmt1 in brain function is supported by data from animal models that demonstrate a range of behaviour changes reminiscent of neurodevelopmental disorders including exploration and/or anxiety phenotypes (Balemans et al., 2010; Schaefer et al., 2009), and abnormal learning and memory (Balemans et al., 2013; Benevento et al., 2017; Iacono et al., 2018; Kramer et al., 2011). More recently, studies using rodent neuronal cultures and ex vivo slices (Benevento et al., 2016), and human induced pluripotent stem cell (iPSCs) (Frega et al., 2019), have shown that appropriate expression of EHMT1 is required for the correct establishment and function neuronal networks. In human iPSCs, this neuronal network dysfunction is driven by the abnormal expression of GRIN1 expression and enhanced NMDA-R signalling (Frega et al., 2019).
Here, we explore endophenotypes of relevance to psychiatric problems seen in those carrying mutation of EHMT1, using a mouse model of Ehmt1 haploinsufficiency (Ehmt1D6Cre/+ mice). In order to reduce anatomical complexity and allow a more precise focus on the impact of Ehmt1 haploinsufficiency during development, on later behavioural and neurophysiological parameters, we generated a forebrain-specific Ehmt1 knockout mouse. Cre recombination was driven under the D6 promoter of the Dach1 gene limiting Ehmt1 heterozygous deletion to the forebrain, starting at embryonic stage 10.5 in the cortical vesicles (Van Den Bout et al., 2002; Machon et al., 2002). Specifically, we find deficits in sensory motor-gating and novel object recognition (NOR), and decreased anxiety. We then build upon recent in vitro and ex vivo evidence of abnormal neuronal networks and show a reduction in the magnitude of auditory evoked potentials in paired-pulse inhibition and mismatch negativity (MMN), providing in vivo electrophysiological evidence of an impairment in information processing in the Ehmt1D6Cre/+ mice. Gene expression data and pharmacological manipulation support the general idea that abnormal NMDA-R signalling in Ehmt1D6Cre/+ adult mouse contributing to the sensory-motor gating and information processing deficits.
Materials and methods
Animals
All procedures were conducted in accordance with the requirements of the UK Animals (Scientific Procedures) Act 1986, with additional ethical approval at Cardiff University.
In order to generate experimental cohorts, Ehmt1fl/fl (Schaefer et al., 2009) male studs were paired in trios with one homozygous females carrying two copies of the Tg(Dach1-cre)1Krs/Kctt Cre transgene, maintained on a F1(C57BL/6J x CBA/Ca) background; and one wild-type F1(C57BL/6J x CBA/Ca) female. The Ehmt1D6Cre/+ mouse model was used in order to limit the effects of the deletion to the forebrain and hippocampus only and confounding effects of the non-CNS phenotypes, such as obesity (Balemans et al., 2010). Experimental cohorts were reared together and then weaned into mixed cages (2–5 per cage) of Ehmt1D6cre/+ (experimental line) and Ehmt1fl/+ mice (control line). All experimental subjects were male mice, and aged between 4 and 6 months during behavioural testing. A subset of the behavioural cohort was subsequently used in the electrophysiology experiments (7–8 months). Animals were housed 12-h-light/12-h-dark (lights on at 7 a.m.), and standard laboratory diet and water were available ad libitum throughout testing. Experimenters were blind to the genotype of animals during behavioural testing.
Behaviour
All animals were initially subject to sensory-motor gating testing (Ehmt1fl/+, n = 25; Ehmt1D6cre/+ mice, n = 31). A subset of these was then subsequently tested on the rotor-rod (Ehmt1fl/+, n = 9; Ehmt1D6cre/+ mice, n = 18) and then elevated plus maze (EPM) and open field (OF). The remainder of the animals were subsequently tested on the NOR memory test (Ehmt1fl/+ mice, n = 16; Ehmt1D6cre/+ mice, n = 13).
Sensory-motor gating
Acoustic startle response (ASR) and prepulse inhibition (PPI) of the startle response were monitored using an SR-Lab apparatus (San Diego Instruments, San Diego, USA) modified for use in mice, according to published methods (McNamara et al., 2016). Briefly, animals were placed in a Perspex tube (internal diameter of 35 mm) and white noise stimuli were presented via a speaker. The amount of movement relayed as their startle response was measured as a piezoelectric measure converted to arbitrary startle units. The measurement used was the maximum startle (Vmax). Due to the effect of weight on this reflex movement measurement, all data were normalised for individual body weight. Pulse-alone trials consisted of a 40-ms 120-dB startle stimulus and a prepulse trial consisted of a 20 ms prepulse at 4, 8, or 16 dB above background and a 40-ms 120-dB startle stimulus, 70 ms after the prepulse. The stimuli were presented in a pseudorandom manner every 15 s. Whole body startle responses were recorded as the average startle during a 65-ms window timed from the onset of the startle pulse.
Percentage PPI score for each trial was calculated: (%PPI = 100 × (ASRstartle pulse alone − ASRprepulse + startle pulse)/ASRstartle pulse alone).
Rotarod testing
A rotarod task (Ugo Basile, Italy) was used to assess motor learning and co-ordination. This consisted of a rotating rod 30 mm in diameter, with five separated chambers 57 mm in width, with a rod elevation of 160 mm. Motor learning was assessed across six rotarod sessions; one morning session and one evening session, on three consecutive days. The rod speed accelerated incrementally from 5–50 r/min across the 300 s session. The main measure during training was latency to first fall. However, if the mice fell, they were continuously replaced on the rotating rod, until the full 300 s-session was over in order to prevent any confounds from arising from overall differences in time spent on the rotarod across sessions. In a separate test session on day four, the mice were given one 300 sec session at 10, 20, 30, 40, and 50 r/min consecutively in one morning session in order to assess motor coordination. Again, the latency to first fall was recorded for each animal at each speed.
NOR memory
The NOR test arena was a square 30 cm × 30 cm with 30 cm high, white Perspex walls. Four different, non-displaceable objects were used. All objects were white and selected for their equal appeal and available in triplicate to avoid the use of olfactory cues. In the habituation phase, 24 h prior to the task, each subject was allowed to explore the arena for 10 min in the absence of objects. In the acquisition phase, the subject was returned to the arena containing two identical sample objects (A, A’) and given 10 min to explore. After a retention phase, consisting of 15 or 90 min, the subject was returned to the arena with two objects, one identical to the sample and the other novel (A, B). During both the familiarisation and test phases, objects were located adjacent corners of the arena. The location of the novel object was counterbalanced. To prevent coercion to explore the objects, the subject was released in a third corner. Objects were cleaned with 70% ethanol wipes between sessions and object selection was randomised.
The main measure used was the Recognition Index (RI), indicating whether the animal investigated the novel object more than chance. This was calculated by the percentage of time spent investigating the novel object relative to the total object investigation (RI = TN/(TN + TF) × 100). An RI significantly above chance or 50% indicates recognition of novelty and an RI equal to or below 50% indicates either no preference for the novelty or no recognition of novelty. Other parameters recorded were overall time spent with each object, and frequency of visits to the zones containing an object. Data were collected in 1-min time bins across the 10-min session by a camera linked to a computer with EthoVision Observer software (Noldus, Nottingham, UK).
Electrophysiology
Adult male Ehmt1D6Cre/+ mice (n = 7) and Ehmt1fl/+ control cage-mates (n = 7) at 6–7 months of age were anesthetised with 2% isoflurane for stereotaxic electrode implantation. The electrode configuration used two bilateral frontal electrodes, one monopolar and one bipolar (2.7 mm anterior, 1.5 mm lateral, 1.2-deep relative to bregma); two bilateral hippocampal electrodes, one monopolar and one bipolar (2.7 mm posterior, 3 mm lateral, 2.2 mm deep relative to bregma); and one bipolar electrode in the auditory cortex (2.7 mm posterior, 4 mm lateral, 1.1-deep relative to bregma) as has been previously reported (Ehrlichman et al., 2008; Siegel et al., 2003) (for further details see Supplementary Figure 1). Due to animal loss, a subset of these was used in the MMN study; Ehmt1D6Cre/+ mice (n = 5) and Ehmt1fl/+ control cage-mates (n = 5).
Event-related potentials (ERPs) were obtained by averaging electroencephalography (EEG) traces centred at times 0 and 500 ms to 0 μV, respectively. For each trial, power was calculated using a complex Morlet wavelets w(t, f0) (Kronland-Martinet et al., 1987). The script used can be found at https://www.physics.lancs.ac.uk/research/nbmphysics/diats/tfr/. The wavelets have a Gaussian shape in the time domain (SD σt) and in the frequency domain (SD σt) around its central frequency ƒ0: w(t, f0) = A * exp(−t2/2 σt2) * exp(2iπf0t) with σf = 1/πσt. The wavelet family we used was defined by f0/σf = 1, with f0 ranging from 0.5 to 100 Hz in logarithmically distributed frequency steps (for full details, see Materials and Methods in Supplementary Information).
Auditory stimuli were generated using Spike2, version 7.2, and a Power1401 interface (CED, Cambridge, UK). Auditory stimulus was delivered with speakers positioned directly in front of each recording cage. Each mouse received an auditory, paired-pulse session and two sessions of MMN.
Paired pulse
Following Halene et al. (2009), each mouse received an auditory, paired-pulse session in which a single tone was presented at 1500 Hz and 90 dB (S1) followed by a 500-ms intra-trial interval and a second tone at 1500 Hz and 90 dB (S2). The tones were sinusoidal and 10 ms in duration. The inter-pulse interval between the two tones was 10 s. Each mouse received 1250 paired-pulse trials per recording session.
Mismatch negativity
The mice also received two sessions of the MMN protocol. Similar to Ehrlichman et al. (2008), the mice received 24 ‘standard’ tones at 90 dB and 1500 Hz and one ‘deviant’ tone at 90 dB and 2000 Hz. All tones were sinusoidal and 10 ms in duration and the intra-pulse interval between the 25 tones was 500 ms, while the inter-trial interval was 5 s. Each mouse was recorded for 360 trials in each of two sessions. In one session, 10 mg/kg of ketamine was administered, and in the second session, an equal volume of saline was administered. The dosage of ketamine was chosen based on previous work (Ehrlichman et al., 2008; Siegel et al., 2003). The within group design was counterbalanced by genotype and the order in which ketamine or saline sessions were administered. All recordings took place 5 min after intraperitoneal injections of either 10 mg/kg ketamine or the volume equivalent dose of saline. The waveform channels were filtered between 1 and 500 Hz.
Gene expression
RNA was extracted from macrodissected hippocampi using the RNeasy micro kit (Qiagen) following the manufacturer instructions. A 96-well RT2 Custom Profiler PCR Array (CAPM12608, Qiagen, Manchester, UK) for mice was used. The custom genes list was generated based on GLP targets identified in the literature and tissue-specific relevance. For the qPCR array 1 μg of total RNA was used and manufacturer instructions were followed, for cDNA synthesis – RT2 First Strand Kit (Qiagen) and for the RT-PCR reaction – RT2 SYBR Green ROX qPCR Matermix (Qiagen). The average Ct values across the three housekeeping genes B2m, B-actin and Gapdh were used as endogenous controls. ΔCt values were generated by normalising to the geometric mean of the Ct values for these three housekeeping genes. All individual reactions were carried out in triplicate. Real-time qPCR data were visualised using the ΔΔCt method (Livak and Schmittgen, 2001).
Statistics
All data were analysed using SPSS 20 (SPSS, Armonk, NJ, USA). The statistical differences between groups were analysed using independent samples t-tests, ANOVAs, or where appropriate Repeated Measures ANOVA (RP-ANOVAs). The main between-subject factor was GENOTYPE (Ehmt1fl/+ controls or Ehmt1D6Cre/+). The following within-subject factors were also analysed: TRIAL (Startle trial); PREPULSE (4, 8 or 16 dB); SESSION and SPEED (rotarod); PULSE (standard or deviant in the MMN). To check for normal distribution, Mauchly’s test of sphericity of the covariance matrix or Levene’s test for equality of variances were applied. The more conservative Huynh–Feldt corrections, with adjusted degrees of freedom are reported in cases in which test assumptions were violated. Non-parametric analyses were as follows. For the NOR task, the binomial distribution one-sample Kolmogorov–Smirnov (KS) test was applied to determine whether average RIs or SIs were significantly above chance (above 50%). For analysis of N1 amplitude, Wilcoxon rank-sum test was used. Statistical tests on the electrophysiological time-frequency data were performed using the permutation method with <1000 iterations (Westfall and Young, 1993) (see Materials and Methods in Supplementary Information for full details). For all comparisons, alpha value was set at p < 0.05.
Results
Impaired sensory-motor gating in Ehmt1D6Cre/+ mouse
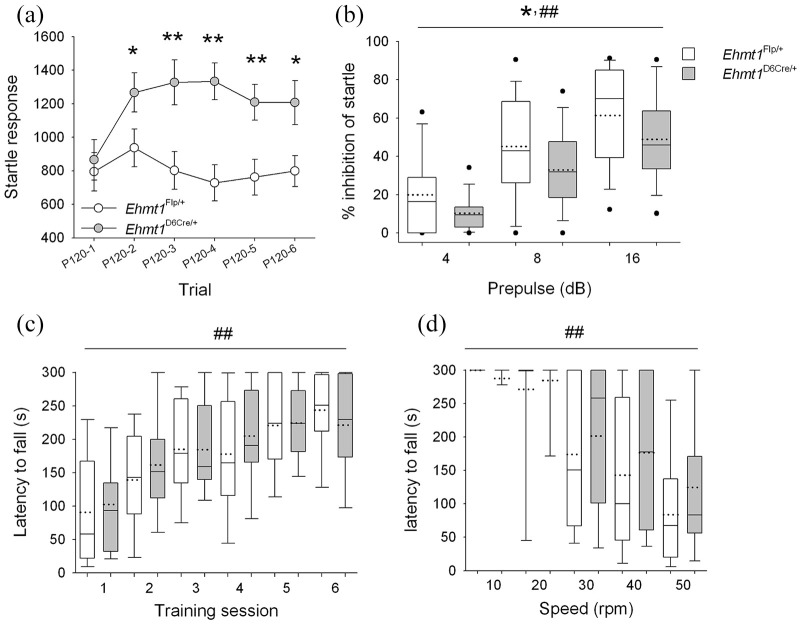
The ASR and PPI of this response, were used to examine sensory-motor function in the Ehmt1D6Cre/+ mice. Over the course of six consecutive auditory pulse (120 dB) startle trials, Ehmt1D6Cre/+ mice had, on average, twice the startle response compared to Ehmt1fl/+ mice (Figure 1(a); ANOVA, main effect of GENOTYPE, F1,53 = 6.86, p = 0.01). A significant interaction between GENOTYPE and TRIAL (F3.87,201.0 = 2.48, p = 0.03) indicated different patterns of startle reactivity and habituation relative to Ehmt1fl/+ mice. Post hoc analysis revealed that there was an equivalent startle response to the initial trial between genotypes (p = 0.68), but on average Ehmt1D6Cre/+ mice showed a significantly enhanced startle response in all consecutive trials (trial 2, p = 0.05; trial 3, p = 0.01; trial 4, p = 0.001; trial 5, p = 0.01; and trial 6, p = 0.02).
Figure 1.
Sensory-motor gating, and motor learning and coordination. (a) The startle response after six consecutive 120-dB pulse stimulations show Ehmt1D6Cre/+ mice (n = 31) had a significantly higher overall maximum startle response in all trials. (b) Prepulse inhibition of the startle response generally increased with the increasing volume of the prepulse as expected. However, Ehmt1D6Cre/+ displayed a 20%–40% reduction in PPI of startle relative to Ehmt1Fl/+ mice (n = 25). (c) Both Ehmt1Fl/+ (n = 9) and Ehmt1D6Cre/+ mice (n = 18) improved motor ability on the rotarod as evidenced by an increase in latency to fall across 6 training sessions. There was no also difference in genotype during training. (d) In a probe test, where rotarod speed accelerated throughout the session, there was a general decrease in the latency to fall as the speed increased, but again no difference between genotypes. Data are mean ± SEM, or box plots showing median (solid line), mean (dotted line) and 10th, 25th, 75th and 90th percentiles. * represents main effect of GENOTYPE p < 0.05; ## represents main effect of within-subject factor (TRIAL, PREPULSE, SESSION or SPEED).
As expected, increasing the prepulse volume led to a linear increase in the inhibition of the startle in both groups (Figure 1(b); ANOVA, main effect of PREPULSE, F1.49,80.35 = 73.26, p = 0.001). However, there was a 20%–40% PPI reduction in the Ehmt1D6Cre/+ mice relative to Ehmt1fl/+ cage-mates (Figure 1(b); ANOVA main effect of GENOTYPE, F1,54 = 5.54, p = 0.022), suggesting that, in addition to an enhanced startle response, the mutant mice were also impaired in the normal PPI response
Normal motor function in the Ehmt1D6Cre/+ mouse
Altered sensory-motor gating in Ehmt1D6Cre/+ mice was not due to any gross deficits in motoric competence. Training on the rotarod test indicated normal learning with repeated sessions, with latency to fall reducing across training sessions (Figure 1(c); ANOVA, main effect of SESSION, F5,130 = 21.33, p < 0.001), but there was no difference in GENOTYPE (F1,26 = 0.12, p = 0.73) and no interaction between training SESSION and GENOTYPE (F5,130 = 0.67, p = 0.65). Moreover, in an accelerating rotarod probe test of motor coordination, latency to fall decreased as the speed increased (Figure 1(d); ANOVA, main effect of SPEED, F4,104 = 38.38, p < 0.001), but there was no difference between GENOTYPE (F1, 26 = 0.67, p = 0.42) or interaction between SPEED and GENOTYPE (F4,104 = 0.65, p = 0.63).
Reduced anxiety in the Ehmt1D6Cre/+ mouse
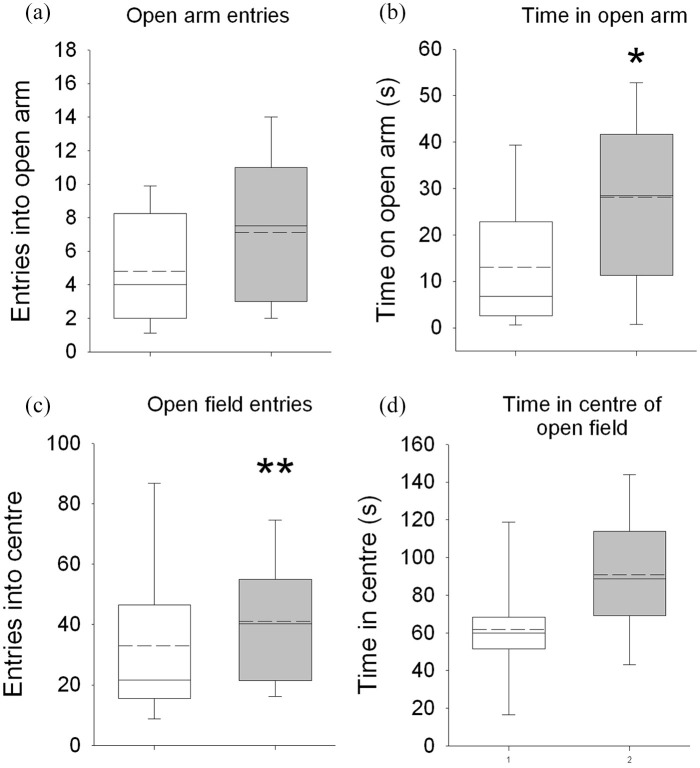
A number of measures in the elevated plus maze and open field tests indicated that Ehmt1D6Cre/+ mice have a reduced anxiety phenotypes. On the EPM (Figure 2(a) and (b)), Ehmt1D6Cre/+ mice spent on average twice as long as Ehmt1Fl/+ cage-mates on the open-arm (t26 = −2.08, p = 0.04) and made on average 40% more entries into the open arm of EPM more than controls, although this did not reach statistical significance (t26 = −1.52, p = 0.14). On the OF test (Figure 2(c) and (d)), Ehmt1D6Cre/+ mice made on average 25% more entries in the inner zone of the OF (t26 = −3.21, p = 0.004) and spent 50% more time in the inner zone than Ehmt1Fl/+ mice, although this did not reach statistical significance (t26 = −1.86, p = 0.07).
Figure 2.
Behaviour in the elevated plus maze and open field. On the EPM, Ehmt1D6Cre/+ mice (n = 17) showed a pattern of behaviour consistent with reduced anxiety relative to Ehmt1Fl/+ mice (n = 9), including increased open arm entries (a) and increased time on time on the open arm (b), although only the latter was statistically different from controls. Convergent evidence of a reduced anxiety phenotype was seen in the OF test. Here, Ehmt1D6Cre/+ mice made significantly more entries into the centre of the OF (c) and spent more time in the inner zone (d), although this did not reach significance. Box plots showing median (solid line), mean (dashed line) and 10th, 25th, 75th and 90th percentiles. * represents main effect of GENOTYPE p < 0.05; ** p < 0.01.
Impaired NOR in Ehmt1D6Cre/+ mouse
The NOR task takes advantage of the preference of rodents to attend to new objects in their environment as a means for testing declarative memory (Antunes and Biala, 2012). Here, two inter-trial intervals were used: half of the cohort was examined following a 15-min delay between the initial object exposure and the test trial, and half the cohort was examined after a 90-min delay. In the test phase, as expected the Ehmt1fl/+ control mice explored the novel object significantly more than chance (50%) in both the 15-min and 90-min retention trials (Figure 3; Kolmogorov–Smirnov test (KS), 15 min, 60%, p = 0. 02; 90 min retention trial, 67%, p = 0.002). However, exploration of the novel object by Ehmt1D6Cre/+ mice was not significantly above chance, at either 15-min (52%, p = 0.97) or 90-min retention trial (50%, p = 0.44).
Figure 3.
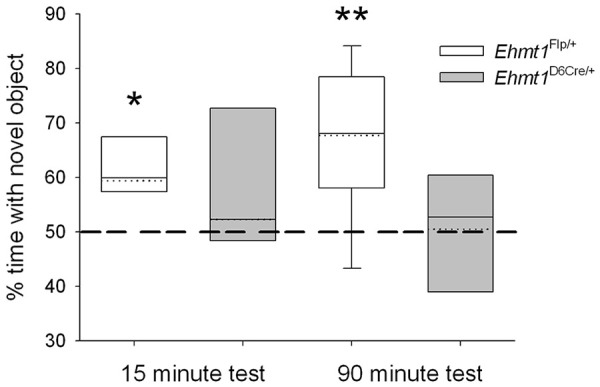
Novel object recognition. In both the 15-min and 90-min retention tests of the NOR task Ehmt1fl/+ control mice (n = 16) explored the novel object more than chance. However, exploration of the novel object by Ehmt1D6Cre/+ mice (n = 13) was not significantly above chance, for either the 15- or 90-min retention trials indicating a deficit in declarative memory. Data are box plots showing median (solid line), mean (dotted line) and 10th, 25th, and 75th and 90th percentiles (dots represent 5th and 95th percentile). * represents main effect of GENOTYPE p < .05; ** p < .01.
This difference at test was not due to differences during the habituation phase, as indicated by overall exploration time of the object that was replaced by a novel object at test (Ehmt1fl/+ mean = 114 s, ± SEM = 15; Ehmt1D6Cre/+ mean = 113 s ± SEM = 12; ANOVA, no main effect of GENOTYPE, F1,28 = 0.031, p = 0.86).
Electrophysiological measurements of paired-pulse auditory evoked potentials
In order to gain an insight into the neural changes underpinning the deficits in sensory-motor gating seen in Ehmt1D6Cre/+ mice, we used electrophysiological methods. A subset of the behavioural cohort was then subject to EEG recording to measure auditory evoked potentials (AEPs). AEPs are voltage fluctuations time-locked to an auditory event used for brain dysfunction clinical diagnosis (Luck et al., 2011). We used a paired-pulse paradigm in which two pulses were delivered back-to-back with a 500-ms interval between the first stimulus (S1) and the second stimulus (S2) (Figure 4(a)). The grand average waveforms show a stereotypic maximal positive deflection (P1) and maximal negative deflection (N1) (Figure 4(b1)). Ehmt1D6Cre/+ mice had a nearly twofold lower N1 amplitude response after the S1 (Figure 4(b1)) but not after S2 (Figure 4(b2)). In addition, Ehmt1D6Cre/+ mice had a significant reduction in the S2/S1 ratio for the N1 component (Wilcoxon, rank sum = 64, p = 0.018), what is considered an electrophysiological measurement of sensory gating (Figure 4(c)). During peak activation of the paired-pulse experiment we observed an increase in total power across high-frequency bands from 10–100 Hz in both groups of mice (Figure 4(d1) and (d2)). The difference time-frequency plot and the permutation test between Ehmt1D6Cre/+ and Ehmt1fl/+ control mice however, revealed that the distributed peak that occurred approximately 40 ms after the S1 pulse, a time corresponding with the N1, was significantly greater in the beta (13–30 Hz) and gamma (30–70 Hz) frequency bands in the Ehmt1fl/+ control mice (Figure 4(g1) and (g2)). The late (>200 ms after the S1 pulse) decrease in the delta frequency band (~4 Hz) prominent in Ehmt1fl/+ (Figure 4(d1)–(g1)) was not different from Ehmt1D6Cre/+ (Figure 4(g2)).
Figure 4.
AEP paired-pulse measurements. (a) Schematic showing the paired-pulse stimulus paradigm. (b1) Grand average ERP waveform after pulse 1 (S1) in Ehmt1D6Cre/+ (red) and Ehmt1fl/+ (black). The P1 and N1 components are indicated on the waveform. (b2) The waveforms for pulse 2 (S2); (c) N1 amplitude for Ehmt1D6Cre/+ (n = 7) and Ehmt1fl/+ (n = 7) mice. (d1 and d2) Normalised total power (in time-frequency plot) of both S1 (time 0) and S2 (time 500 ms) for Ehmt1fl/+ (d1) and Ehmt1D6Cre/+ (d2). Note the early increase in the beta/gamma range (10–100 Hz) and the late decrease in the delta range (~4 Hz) and the reduction of both components in the Ehmt1D6Cre/+. (e1 and e2) Evoked power time-frequency plots for the same data set; (f1 and f2) phase locking factor for the same data set; (g1) difference of total power between Ehmt1fl/+ and Ehmt1D6Cre/+; (g2) Statistical significance heat map based on permutation tests of total power are indicated by the colour scale (p < 0.05); (h1) difference of evoked power between Ehmt1fl/+ and Ehmt1D6Cre/+ mice; and (h2) statistical significance between evoked power of Ehmt1fl/+ and Ehmt1D6Cre/+ mice (spurious spots above 50 Hz are due to occasional 50 Hz noise contamination in some of the recordings). Data shown as mean ± SEM.
Measurements of evoked power, EEG power which is phase-locked with the event onset across trials, demonstrated increases in the delta (~4 Hz), beta (13–30 Hz) and low gamma (here ~40 Hz) band responses in both groups of mice approximately ~30–50 ms after the S1 (Figure 4(e1) and (e2)). Again, permutation tests revealed a reduction in evoked power in Ehmt1D6Cre/+ mice after both the S1 and ~40 ms after the S2 pulse (Figure 4(h1) and (h2)). In complement, we measured the phase locking factor (PLF), which provide a measurement of trial-to-trial reliability across the frequency domain (Goffin et al., 2014). To extract the PLF, magnitude information is transformed to 1 and averaged so that the phase angle with respect to event onset is extracted (Roach and Mathalon, 2008). Values between zero and one are obtained, in which one reflects perfect synchrony of phase angles across trials. Ehmt1D6Cre/+ mice did not show PLF values above .17 at any point, while control mice show nearly twofold PLF synchrony (+.25) at ~40 ms post-S1 pulse, between ~20 and 40 Hz. Overall Ehmt1D6Cre/+ mice demonstrated reduced PLF (Figure 4(f1) and (f2)).
Electrophysiological measurements of MMN AEPs
The MMN response is elicited when a qualitative feature of the stimulus does not match the pattern in a previous series (Light and Braff, 2005) (Figure 5(a)). One core feature of MMN is the importance of NMDA receptor function. For instance, non-competitive NMDA-R antagonists, like ketamine and the selective antagonist of the NR2B NMDA subunit, CP-101,606 reduce MMN amplitude (Sivarao et al., 2014), the level of which predicts the magnitude of psychotic experiences in response to these drugs (Umbricht et al., 2002). Therefore, in order to probe this function further here, mice were given either saline or 10 mg/kg of ketamine prior to the MMN test.
Figure 5.
AEP mismatch negativity phenotypes and NMDA mRNA expression. (a) Mismatch negativity stimulus. (b) Grand average ERP waveforms (shaded areas) after the saline or ketamine conditions in the in Ehmt1fl/+ (n = 5) and Ehmt1D6Cre/+ (n = 5), for the deviant (red) and standard (black) tone for each condition. (c1 and c2) Time-frequency plot of evoked power for the standard and deviant pulse in Ehmt1fl/+ mice after saline injection. (c3 and c4) Time-frequency plot of evoked power for the standard and deviant pulse in Ehmt1D6Cre/+ mice following saline injection. (d) N1 amplitude peak amplitude corresponding to each condition. (e1) Permutation test showing heat map of p-values for the difference of distribution between deviant tone maps of saline treated Ehmt1fl/+ (c2) and Ehmt1D6Cre/+ (c4). (e2) Permutation test showing heat map of p-values for the difference of distribution between deviant tone maps of ketamine treated Ehmt1fl/+ (c2) and Ehmt1D6Cre/+ (c4). (f) NMDA-R subunit mRNA expression in hippocampal samples (Ehmt1fl/+ n = 4, Ehmt1D6Cre/+ n = 8). Data shown are mean ± SEM. * represents main effect of GENOTYPE p < 0.05.
In saline-treated animals, there was a difference between Ehmt1D6Cre/+ and Ehmt1fl/+ mice in response to the deviant pulse (Figure 5(c)–(d)) as indicated by analysis of the N1 amplitude that showed an interaction between GENOTYPE and PULSE (repeated measures ANOVA, F1,8 = 12.70, p = 0.007). In the standard pulse condition Ehmt1fl/+ mice, amplitude corresponded to an increase in ~10–40 Hz evoked potential approximately 30–50 ms post-stimulus (Figure 5(c1)–(c2)). In the deviant pulse condition, Ehmt1fl/+ mice not only show an increase in ~10–40 Hz evoked potential, but also one with greater peak latency, at 40–70 ms post-stimulus (Figure 5(c2)). In contrast, Ehmt1D6Cre/+ mice treated with saline showed no pattern of change in the amplitude response for either standard or deviant pulses.
However, following 10 mg/kg of ketamine administration, the difference in the N1 amplitude between the standard and deviant pulse was absent for both genotypes (Figure 5(b) and (d)), as indicated by a lack of interaction between GENOTYPE and PULSE (repeated measures ANOVA, F1,7 = 0.011, p = 0.921). Further analysis underlined this finding. Permutation tests after saline administration for the deviant-only condition demonstrates a difference between Ehmt1fl/+ controls and Ehmt1D6Cre/+ wavelet transforms, at 30 Hz between 50 and 80 ms post-stimulus (Figure 5(e1)), driven by the higher amplitude response in the Ehmt1fl/+ control mice. In contrast, after ketamine administration, the difference in deviant response between Ehmt1fl/+ controls and Ehmt1D6Cre/+ mice was abolished (Figure 5(e2)).
Altered NMDA-R expression in Ehmt1D6cre/+ mutant mice
In light of similarity in basal Ehmt1D6Cre/+ electrophysiological phenotype and the electrophysiological phenotype in Ehmt1fl/+ control mice following ketamine administration, we assessed the NMDA system in Ehmt1D6Cre/+ mice. RT qPCR was used to examine the mRNA levels of NMDA-R subunits Grin1, Grin2a, Grin2b and Grin2c in the adult hippocampus. Expression of Grin1, the gene encoding NMDA NR1 subunit, was significantly reduced by 40% on average in Ehmt1D6cre/+ mice (Figure 5(f); t = −3.07, p = 0.014). No difference was seen in expression of other NMDA-R subunits examined (Figure 5(f) Grin2a, t = −1.02, p = 0.331; Grin2b, t = −0.814, p = 0.435; or Grin2c, t = −0.71, p = 0.498). Furthermore, there were no genotype differences in AMPA-R subunit gene expression examined (see Supplementary Figure 4).
Discussion
Recent studies in human genomics strongly implicate a number of genes found to regulate chromatin dynamics in the aetiology of neurodevelopmental disorders from developmental delay (Singh et al., 2016), to autism spectrum disorders (De Rubeis et al., 2014; Yuen et al., 2017), and schizophrenia (Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015). EHMT1 is one such gene, associated with both neurodevelopmental and neuropsychiatric disorders. Here, we examined behavioural and electrophysiological correlates of information processing in a mouse model of Ehmt1 haploinsufficiency, specifically in the forebrain. We found that Ehmt1D6Cre/+ mice have sensorimotor and auditory gating deficits, reduced anxiety, and learning and memory deficits, in the absence of generic deficits in motor function. Ehmt1D6Cre/+ mice also showed a number of abnormalities in electrophysiological measurements including a reduced magnitude of AEPs after paired-pulse inhibition and MMN tasks, reduced evoked and total power in high-frequency bands, and reduce PLF. Overall, these data indicate that Ehmt1D6Cre/+ mice show deficits in sensory motor gating and information process, possibly related to abnormal NMDA-R functioning.
Ehmt1D6Cre/+ mice displayed decreased anxiety in a number of measures across a two separate tests, namely, the EPM and OF. Although these findings in our model were not confounded by impaired motor competence as indicated by normal behaviour and learning on the rotarod test, not all measures on both the EPM and OF reached statistical significance. It may be that a more robust assessment of anxiety in this model would be achieved using a unified score from a number of separate measures (Harrison et al., 2020). Generally, however, these findings of decreased anxiety are consistent with previous studies of Ehmt1 haploinsufficient models (Balemans et al., 2010; Iacono et al., 2018), although other models, specifically a CamKII-driven full Ehmt1 deletion shows decreased anxiety (Schaefer et al., 2009). This indicates that the relationship between Ehmt1 function and anxiety behaviour probably depends on extent, timing and location of the genetic lesion.
EHMT1 is a risk gene associated with a number of neurodevelopmental and psychiatric disorders (Talkowski et al., 2012), thus it was important for this work to focus not only on translational phenotypes but those that overlap traditional diagnostic boundaries. Several prominent features shared by these disorders are associated with deficits in attention and gating, or filtering out intrusive stimuli (Belmonte et al., 2004; Javitt, 2009; Orekhova et al., 2008; Perry et al., 2007). Behaviourally, the Ehmt1D6Cre/+ mice showed evidence of this in an acoustic startle test. Consistent with previous findings in other Ehmt1 haploinsufficiency models (Balemans et al., 2013; Iacono et al., 2018), sensory motor gating deficits were manifest by both a greatly enhanced ASR and a decreased PPI of startle.
Information processing deficits are another common phenotype associated with EHMT1 risk populations. Accordingly, Ehmt1D6Cre/+ mice were examined on their performance in the NOR task, a paradigm where an animal’s ability to remember a previously encountered object is indicated by an increased willingness to explore the novel object over the familiar object. The Ehmt1D6Cre/+ mice showed no evidence of NOR, even with only a 15-min interval after habituation. Importantly, this was not due to reduced interest in exploration or exposure to the familiar object, as time spent in exploration was equivalent across Ehmt1fl/+ control and Ehmt1D6Cre/+ mutant mice. Similar to the acoustic startle and PPI phenotype, deficits in a NOR paradigm have been seen in other Ehmt1+/− mice (Balemans et al., 2013; Iacono et al., 2018). This convergence across models and laboratories suggests that both sensory motor-gating and information processing deficits are key behavioural features of Ehmt1 haploinsufficiency.
Given the robustness of behavioural deficits in sensory motor-gating and information processing seen in models of Ehmt1 haploinsufficiency, we explored this further by examining electrophysiological measures of these domains. Ehmt1D6Cre/+ had reduced N1 amplitude in response to stimulus 1 (S1). Furthermore, Ehmt1D6Cre/+ mice had a significant reduction in the S2/S1 ratio for the N1 component, an electrophysiological correlate of sensory gating.
Evidence of information processing deficits in the Ehmt1D6Cre/+ mice was provided by the reduced response in a MMN paradigm. The decreased amplitude responses in these AEP measurements meanwhile corresponded to significant reductions in total and evoked power in high-frequency bands. Such reductions may be particularly insightful, as numerous studies report disruptions in gamma-band activity across neurodevelopment (Kwon et al., 1999; Orekhova et al., 2008), neuropsychiatric (Tatard-Leitman et al., 2015) and neurodegenerative diseases (Iaccarino et al., 2016). Whether reduced gamma activity is actually directly associated with disease pathologies across these patient populations remains largely unknown. Recently, however, reductions in gamma-band activity were found to precede the onset of plaque formations and cognitive decline in a mouse model of Alzheimer’s disease. Meanwhile, the stimulation of fast-spiking interneurons in the gamma frequency range (~40 Hz), a way to boost gamma-band activity (Cardin et al., 2009), led to the reduction in the plaque forming amyloid isoforms and attenuated plaque load in ageing mice (Iaccarino et al., 2016). In a more functional assessment, the induction of long-term potentiation using high-frequency stimulation (~100 Hz) was found to restore spine density and long-term memory in early stages of the disease in mice (Roy et al., 2016). While these findings are specific to Alzheimer’s disease, they also confirm an important link between gamma synchrony and cognitive function exists (Fries, 2015). Furthermore, we may find disruptions in high-frequency oscillation patterns tightly correspond with the degree of cognitive impairment and range of pathologies across EHMT1 risk populations.
The decreases in high-frequency activity coupled with reduced Grin1 mRNA are suggestive of overall disruptions in local connectivity and may hint at more global imbalances in excitation/inhibition (E/I). Such findings do corroborate previous reports showing abnormalities in neural development and connectivity in Ehmt1+/− mouse models (Balemans et al., 2013, 2014; Benevento et al., 2017), and that Ehmt1-mediated H3K9me2 levels dynamically regulate synaptic scaling, thus playing a direct role in the fine balance between excitation and inhibition at the level of individual neurons (Benevento et al., 2016). And indeed, many of the Ehmt1D6Cre/+ mice phenotypes are markedly similar to Grin1 mouse mutants (Furuse et al., 2010). The Grin1neo−/− mice and the Grin1Rgsc174 heterozygous mice both show increased stereotypy (Mohn et al., 1999) and deficits in sensorimotor gating (Duncan et al., 2004). Mice with even subtle reductions in the NR1 receptor are found to have decreases in MMN (Featherstone et al., 2015), gamma-band disruptions and reduction in E/I balance (Gandal et al., 2012). Nevertheless, it is important to note that the studies presented here represent a change in mRNA and not protein levels and, moreover, only provide a correlative link between the electrophysiological deficits seen in Ehmt1D6Cre/+ mice and Grin1 reduction. Interestingly however, a recent study has demonstrated increased Grin1 expression and NMDA-R hyperactivity in iPSCs derived from Kleefstra syndrome patients (Frega et al., 2019). Despite the discrepancies, together these data suggest that future investigation of Ehmt1 haploinsufficiency may benefit from further examination of the relationship with the NMDA system.
In summary, Ehmt1D6Cre/+ forebrain-specific haploinsufficiency produced deficits in sensory-gating and information processing. Behavioural evidence from explicit tests of sensorimotor gating and findings from a learning and memory task, suggest that Ehmt1D6Cre/+ mice do not attend to or process information in order to inform appropriate behavioural responses. Neural correlates of these abnormalities were further demonstrated using electrophysiological studies, which indicated deficits related to disruptions in local connectivity and NMDA function. Taken together these data suggest that Ehmt1 haploinsufficiency leads to abnormal circuit formation and behavioural abnormalities that likely underpin deficits seen across the broad spectrum of neurodevelopmental and neuropsychiatric disorders with which EHMT1 is associated.
Supplemental Material
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_EPOCH for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_NOR for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_qPCR for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_Rotarod for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_Startle_and_PPI for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
Supplemental material, Davis_et_al_Suppl_Info_Revision for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
Footnotes
Author contributions: B.A.D., F.D. and A.R.I. contributed to the conceptualisation; B.A.D., F.D., C.O’.R. and M.A. contributed to the data curation; B.A.D., F.D. and A.R.I. contributed to the formal analysis; B.A.D. and A.R.I. contributed to the funding acquisition; B.A.D. and F.D. contributed to the investigation methodology; B.A.D., F.D. and A.R.I. contributed to the project administration; V.C. and A.R.I. contributed to the resources; B.A.D. and F.D. contributed to the software; A.J.H., V.C. and A.R.I. contributed to the supervision; B.A.D. and F.D. contributed to the validation; F.D., M.A. and A.R.I. contributed to the visualisation; B.A.D. and F.D. contributed to the writing the original draft; and B.A.D., F.D. and A.R.I. contributed to the writing the revision and editing.
Data availability: Much of the data presented in this paper are provided as supplementary files. Any materials that are not, will be made available by the authors on request where possible.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was funded by a Wellcome Trust Integrative Neuroscience PhD Grant (WT093765MA) to B.A.D., A.J.H. and A.R.I. V.C. was supported by a Wellcome Trust Programme Grant (91882). A.R.I. and A.J.H. are members of the MRC Centre for Neuropsychiatric Genetics and Genomics (G0801418).
ORCID iDs: François David  https://orcid.org/0000-0003-1993-8931
https://orcid.org/0000-0003-1993-8931
Anthony R Isles  https://orcid.org/0000-0002-7587-5712
https://orcid.org/0000-0002-7587-5712
Supplemental material: Supplemental material for this article is available online.
References
- Antunes M, Biala G. (2012) The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cognitive Processing 13(2): 93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balemans MC, Ansar M, Oudakker AR, et al. (2014) Reduced Euchromatin histone methyltransferase 1 causes developmental delay, hypotonia, and cranial abnormalities associated with increased bone gene expression in Kleefstra syndrome mice. Developmental Biology 386(2): 395–407. [DOI] [PubMed] [Google Scholar]
- Balemans MC, Huibers MM, Eikelenboom NW, et al. (2010) Reduced exploration, increased anxiety, and altered social behavior: Autistic-like features of euchromatin histone methyltransferase 1 heterozygous knockout mice. Behavioural Brain Research 208(1): 47–55. [DOI] [PubMed] [Google Scholar]
- Balemans MC, Kasri NN, Kopanitsa MV, et al. (2013) Hippocampal dysfunction in the Euchromatin histone methyltransferase 1 heterozygous knockout mouse model for Kleefstra syndrome. Human Molecular Genetics 22(5): 852–866. [DOI] [PubMed] [Google Scholar]
- Belmonte MK, Cook EH, Jr, Anderson GM, et al. (2004) Autism as a disorder of neural information processing: Directions for research and targets for therapy. Molecular Psychiatry 9(7): 646–663. [DOI] [PubMed] [Google Scholar]
- Benevento M, Iacono G, Selten M, et al. (2016) Histone methylation by the Kleefstra syndrome protein EHMT1 mediates homeostatic synaptic scaling. Neuron 91(2): 341–355. [DOI] [PubMed] [Google Scholar]
- Benevento M, Oomen CA, Horner AE, et al. (2017) Haploinsufficiency of EHMT1 improves pattern separation and increases hippocampal cell proliferation. Scientific Reports 7: 40284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin JA, Carlen M, Meletis K, et al. (2009) Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 459(7247): 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper GM, Coe BP, Girirajan S, et al. (2011) A copy number variation morbidity map of developmental delay. Nature Genetics 43(9): 838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deciphering Developmental Disorders Study (2017) Prevalence and architecture of de novo mutations in developmental disorders. Nature 519(7542): 223–228. [Google Scholar]
- De Rubeis S, He X, Goldberg AP, et al. (2014) Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515(7526): 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan GE, Moy SS, Perez A, et al. (2004) Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behavioural Brain Research 153(2): 507–519. [DOI] [PubMed] [Google Scholar]
- Ehrlichman RS, Maxwell CR, Majumdar S, et al. (2008) Deviance-elicited changes in event-related potentials are attenuated by ketamine in mice. Journal of Cognitive Neuroscience 20(8): 1403–1414. [DOI] [PubMed] [Google Scholar]
- Featherstone RE, Shin R, Kogan JH, et al. (2015) Mice with subtle reduction of NMDA NR1 receptor subunit expression have a selective decrease in mismatch negativity: Implications for schizophrenia prodromal population. Neurobiology of Disease 73: 289–295. [DOI] [PubMed] [Google Scholar]
- Frega M, Linda K, Keller JM, et al. (2019) Neuronal network dysfunction in a human model for Kleefstra syndrome mediated by enhanced NMDAR signaling. bioRxiv 585596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fries P. (2015) Rhythms for cognition: Communication through coherence. Neuron 88(1): 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse T, Wada Y, Hattori K, et al. (2010) Phenotypic characterization of a new Grin1 mutant mouse generated by ENU mutagenesis. European Journal of Neuroscience 31(7): 1281–1291. [DOI] [PubMed] [Google Scholar]
- Gandal MJ, Sisti J, Klook K, et al. (2012) GABAB-mediated rescue of altered excitatory-inhibitory balance, gamma synchrony and behavioral deficits following constitutive NMDAR-hypofunction. Translational Psychiatry 2(7): e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffin D, Brodkin ES, Blendy JA, et al. (2014) Cellular origins of auditory event-related potential deficits in Rett syndrome. Nature Neuroscience 17(6): 804–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halene TB, Ehrlichman RS, Liang Y, et al. (2009) Assessment of NMDA receptor NR1 subunit hypofunction in mice as a model for schizophrenia. Genes, Brain and Behavior 8(7): 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison DJ, Creeth HDJ, Tyson HR, et al. (2020). Unified behavioral scoring for preclinical models. Frontiers in Neuroscience 14: 313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirabayashi Y, Gotoh Y. (2010) Epigenetic control of neural precursor cell fate during development. Nature Reviews Neuroscience 11(6): 377–388. [DOI] [PubMed] [Google Scholar]
- Iaccarino HF, Singer AC, Martorell AJ, et al. (2016) Gamma frequency entrainment attenuates amyloid load and modifies microglia. Nature 540(7632): 230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono G, Dubos A, Meziane H, et al. (2018) Increased H3K9 methylation and impaired expression of Protocadherins are associated with the cognitive dysfunctions of the Kleefstra syndrome. Nucleic Acids Research 46(10): 4950–4965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javitt DC. (2009) Sensory processing in schizophrenia: Neither simple nor intact. Schizophrenia Bulletin 35(6): 1059–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirov G, Pocklington AJ, Holmans P, et al. (2012) De novo CNV analysis implicates specific abnormalities of postsynaptic signalling complexes in the pathogenesis of schizophrenia. Molecular Psychiatry 17(2): 142–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, Brunner HG, Amiel J, et al. (2006) Loss-of-function mutations in euchromatin histone methyl transferase 1 (EHMT1) cause the 9q34 subtelomeric deletion syndrome. American Journal of Human Genetics 79(2): 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleefstra T, Smidt M, Banning MJ, et al. (2005) Disruption of the gene Euchromatin Histone Methyl Transferase1 (Eu-HMTase1) is associated with the 9q34 subtelomeric deletion syndrome. Journal of Medical Genetics 42(4): 299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JM, Kochinke K, Oortveld MA, et al. (2011) Epigenetic regulation of learning and memory by Drosophila EHMT/G9a. PLOS Biology 9(1): e1000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronland-Martinet R, Morlet J, Grossmann A. (1987) Analysis of sound patterns through wavelet transforms. International Journal of Pattern Recognition and Artificial Intelligence 1(2): 273–302. [Google Scholar]
- Kwon JS, O’donnell BF, Wallenstein GV, et al. (1999) Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Archives of General Psychiatry 56(11): 1001–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light GA, Braff DL. (2005) Stability of mismatch negativity deficits and their relationship to functional impairments in chronic schizophrenia. American Journal of Psychiatry 162(9): 1741–1743. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25(4): 402–408. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Mathalon DH, O’donnell BF, et al. (2011) A roadmap for the development and validation of event-related potential biomarkers in schizophrenia research. Biological Psychiatry 70(1): 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machon O, Van Den Bout CJ, Backman M, et al. (2002) Forebrain-specific promoter/enhancer D6 derived from the mouse Dach1 gene controls expression in neural stem cells. Neuroscience 112(4): 951–966. [DOI] [PubMed] [Google Scholar]
- McNamara GI, Davis BA, Dwyer DM, et al. (2016) Behavioural abnormalities in a novel mouse model for Silver Russell Syndrome. Human Molecular Genetics 25(24): 5407–5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohn AR, Gainetdinov RR, Caron MG, et al. (1999) Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell 98(4): 427–436. [DOI] [PubMed] [Google Scholar]
- Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium (2015) Psychiatric genome-wide association study analyses implicate neuronal, immune and histone pathways. Nature Neuroscience 18(2): 199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orekhova EV, Stroganova TA, Prokofyev AO, et al. (2008) Sensory gating in young children with autism: Relation to age, IQ, and EEG gamma oscillations. Neuroscience Letters 434(2): 218–223. [DOI] [PubMed] [Google Scholar]
- O’Roak BJ, Vives L, Girirajan S, et al. (2012) Sporadic autism exomes reveal a highly interconnected protein network of de novo mutations. Nature 485(7397): 246–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry W, Minassian A, Lopez B, et al. (2007) Sensorimotor gating deficits in adults with autism. Biological Psychiatry 61(4): 482–486. [DOI] [PubMed] [Google Scholar]
- Roach BJ, Mathalon DH. (2008) Event-related EEG time-frequency analysis: An overview of measures and an analysis of early gamma band phase locking in schizophrenia. Schizophrenia Bulletin 34(5): 907–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Arons A, Mitchell TI, et al. (2016) Memory retrieval by activating engram cells in mouse models of early Alzheimer’s disease. Nature 531(7595): 508–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Sampath SC, Intrator A, et al. (2009) Control of cognition and adaptive behavior by the GLP/G9a epigenetic suppressor complex. Neuron 64(5): 678–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel SJ, Connolly P, Liang Y, et al. (2003) Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology 28(4): 675–682. [DOI] [PubMed] [Google Scholar]
- Singh T, Kurki MI, Curtis D, et al. (2016) Rare loss-of-function variants in SETD1A are associated with schizophrenia and developmental disorders. Nature Neuroscience 19(4): 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarao DV, Chen P, Yang Y, et al. (2014) NR2B antagonist CP-101,606 abolishes pitch-mediated deviance detection in awake rats. Frontiers in Psychiatry 5: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkowski ME, Rosenfeld JA, Blumenthal I, et al. (2012) Sequencing chromosomal abnormalities reveals neurodevelopmental loci that confer risk across diagnostic boundaries. Cell 149(3): 525–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatard-Leitman VM, Jutzeler CR, Suh J, et al. (2015) Pyramidal cell selective ablation of N-methyl-D-aspartate receptor 1 causes increase in cellular and network excitability. Biological Psychiatry 77(6): 556–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyssowski K, Kishi Y, Gotoh Y. (2014) Chromatin regulators of neural development. Neuroscience 264: 4–16. [DOI] [PubMed] [Google Scholar]
- Umbricht D, Koller R, Vollenweider FX, et al. (2002) Mismatch negativity predicts psychotic experiences induced by NMDA receptor antagonist in healthy volunteers. Biological Psychiatry 51(5): 400–406. [DOI] [PubMed] [Google Scholar]
- Van Den Bout CJ, Machon O, Rosok O, et al. (2002) The mouse enhancer element D6 directs Cre recombinase activity in the neocortex and the hippocampus. Mechanisms of Development 110(1–2): 179–182. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Young SS. (1993) Resampling-based Multiple Testing: Examples and Methods for P-value Adjustment. New York: Wiley. [Google Scholar]
- Yuen RKC, Merico D, Bookman M, et al. (2017) Whole genome sequencing resource identifies 18 new candidate genes for autism spectrum disorder. Nature Neuroscience 20(4): 602–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_EPOCH for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_NOR for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_qPCR for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_Rotarod for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
This article is distributed under the terms of the Creative Commons Attribution 4.0 License (http://www.creativecommons.org/licenses/by/4.0/) which permits any use, reproduction and distribution of the work without further permission provided the original work is attributed as specified on the SAGE and Open Access pages (https://us.sagepub.com/en-us/nam/open-access-at-sage).
Supplemental material, Davis_et_al_Startle_and_PPI for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances
Supplemental material, Davis_et_al_Suppl_Info_Revision for Impairments in sensory-motor gating and information processing in a mouse model of Ehmt1 haploinsufficiency by Brittany A Davis, François David, Ciara O’Regan, Manal A Adam, Adrian J Harwood, Vincenzo Crunelli and Anthony R Isles in Brain and Neuroscience Advances