Abstract
In humans, most of our new memories are in some way or another related to what we have already experienced. However, in memory research, especially in non-human animal research, subjects are often mostly naïve to the world. But we know that previous knowledge will change how memories are processed and which brain areas are critical at which time point. Each process from encoding, consolidation, to memory retrieval will be affected. Here, we summarise previous knowledge effects on the neurobiology of memory in both humans and non-human animals, with a special focus on schemas – associative network structures. Furthermore, we propose a new theory on how there may be a continuous gradient from naïve to expert, which would modulate the importance and role of brain areas, such as the hippocampus and prefrontal cortex.
Keywords: Schema, memory consolidation, hippocampus, cortex, prefrontal cortex, previous knowledge
Introduction
Once we have reached adulthood, we rarely learn new information in isolation. Instead, most of what we experience will fit into what we know in some way or another. However, in most non-human animal research, the subjects are naïve to the world and have had very little experiences in life. This is in harsh contrast to the adult human we are trying to model. This discrepancy is quite surprising since we do know that previous experience influences how new memories are processed (Bartlett, 1932; Harlow, 1949).
In general, our memories tend to be stronger either when the encoded material ‘fits’ into our previous knowledge or when the information is completely novel (Fernández and Morris, 2018; Van Kesteren et al., 2012). However, the mechanism underlying how these memories become long lasting is thought to be different for each case (for review, please see Duszkiewicz et al., 2019). Very novel experiences will lead to increased neuronal firing within the locus coeruleus, which releases dopamine into the hippocampus and strengthens the memory trace within this brain area (Genzel et al., 2017; Takeuchi et al., 2016). In contrast, if the new experience fits into what we already know, increased memory reactivations that occur later during non-rapid eye movement (NonREM) sleep lead to the consolidation of the hippocampal memory trace to the cortex (Genzel et al., 2014, 2017; McNamara et al., 2014). In humans, the very novel side of the spectrum is most likely quite rare once we reached adulthood. In contrast, memory research in non-human animals will rarely be in the context of much pre-existing knowledge.
However, the mechanistic complexity does not stop there. Even within the realm of updating information that is easily incorporated into pre-existing knowledge, there seems to be a gradient. The more something fits into your previous knowledge, the faster it can be incorporated into that pre-existing network. It has been shown that schemas – associated network structures that encode knowledge – lead to the acceleration of the systems consolidation process and thus, consolidation from the hippocampus to the cortex occurs in days rather than weeks (Tse et al., 2007). And again it is important to point out that memory research in humans will range across the whole spectrum of some to extensive previous knowledge, while research in non-human animals involving any or even extensive previous knowledge is currently incredibly rare.
The concept of previous knowledge, more specifically, the idea of memory schemas, is relatively old within human research (Bartlett, 1932). However, these psychological concepts only began to be studied using animal models in 2007 (Tse et al., 2007). This seminal study by Tse and colleagues led to a renewed interest in the concept in both animal and human research, with a special focus on understanding the neurobiology of previous knowledge and memory schemas.
In this review, we will highlight different findings related to how previous knowledge affects memory consolidation in both humans and animals and discuss the possible roles for both hippocampus and cortical areas.
Previous knowledge and schemas
The term schema was defined in 1932 by Bartlett (1932) as an active organisation of past reactions or experiences, which would always be operating during a well-adapted organic response. In a more current definition by Fernández and Morris (2018), a schema is a
framework of acquired knowledge, skills or attitudes implemented within a network of connected neurons in which the memory traces of associated information have been stored that, when activated, can alter the manner in which new information is processed, including memory encoding, consolidation and retrieval.
Van Kesteren et al. (2012), regarding human research, define a schema as a pre-existing network of interconnected neocortical representations that affect the processing of new information.
In their review, Ghosh and Gilboa (2014) define schema as an associative network structure, which is based on multiple similar experiences but lacks unit detail and is adaptable. As such, it expedites long-term memory at both encoding and retrieval levels. Furthermore, schemas are sensitive to chronological relationships, hierarchical organisation, cross-connectivity and embedded response options (see Table 1).
Table 1.
Schema definitions from other reviews.
| Authors | Definitions |
|---|---|
| Fernández and Morris (2018) | - Framework of acquired knowledge, skills or
attitudes - Network of connected neurons - Memory traces of associated information - When activated, can affect the processes of memory encoding, consolidation and retrieval |
| Van Kesteren et al. (2012) | - Pre-existing network - Interconnected neocortical representations - Affects the processing of new information |
| Ghosh and Gilboa (2014) | - Associative network structure - Based on multiple experiences - Lacks unit detail - Adaptable - Expedites long-term memory - Hierarchical organisation, cross-connectivity and chronological relationships - Embedded response options |
| Bartlett (1932) | - Active organisation of past
experiences - Always active during an organic response |
Since we will summarise human and rodent studies in this review, it is important to understand that in humans, most of what is learned is rarely completely novel once adulthood is reached; the many years of experience have created knowledge structures over time that can be harnessed for new learning. In contrast, in laboratory rodents, which start their lives in a non-natural, simplified environment of a home cage, are only subjected to what the experimenters may expose them to; their prior knowledge is very limited. Thus, by default, most human and non-human animal memory research will differ in the amount of previous knowledge. It can be incredibly difficult to accumulate enough previous knowledge in rodents for it to count as a schema as defined by the above authors. Overall, in recent years, many rodent memory studies referred to schemas, despite the fact that they would not fulfil the above-mentioned criteria but instead only comprise very little previous knowledge. Furthermore, many of the features described above are very difficult to test in rodents, in which, for example, unit detail is almost impossible to assess since we cannot ask the rodent in which level of detail memories are present. Instead, simple responses, such as digging, swimming or path finding, are measured, which can be explained by different types of memory quality or memory process.
Previous knowledge will affect learning in many different ways and many sub-characterisations have been described in human psychology, such as expert knowledge (Bellezza and Buck, 1988), schema (Bartlett, 1932), scripts (Schank and Abelson, 1977) and learning sets (Harlow, 1949). These different types of previous knowledge are hard, if not impossible, to differentiate in non-human animal research, but we attempt to summarise memory effects across species in this review, we will refrain from using such specific terms and instead try to refer to previous knowledge as an overall concept.
Finally, previous knowledge can influence learning through different processes, such as curiosity (Gruber Matthias et al., 2014), attention (Kruschke, 2006) and many others, but again since these are hard to discriminate in non-human animals and would also reach beyond the scope of this review, we will refrain from discussing them here (for other reviews, see Gottlieb and Oudeyer, 2018).
Previous knowledge studies in humans
Tabula rasa or ‘blank slate’ refers to the concept that an individual is born without innate mental content and that we thus have to gain all knowledge through experience. Knowledge build-up throughout life requires coordinated activity between the hippocampus and medial prefrontal cortex (mPFC; Sweegers et al., 2014) and is thought to facilitate the processing of new information, possibly by providing a structure into which the incoming information can be easily integrated.
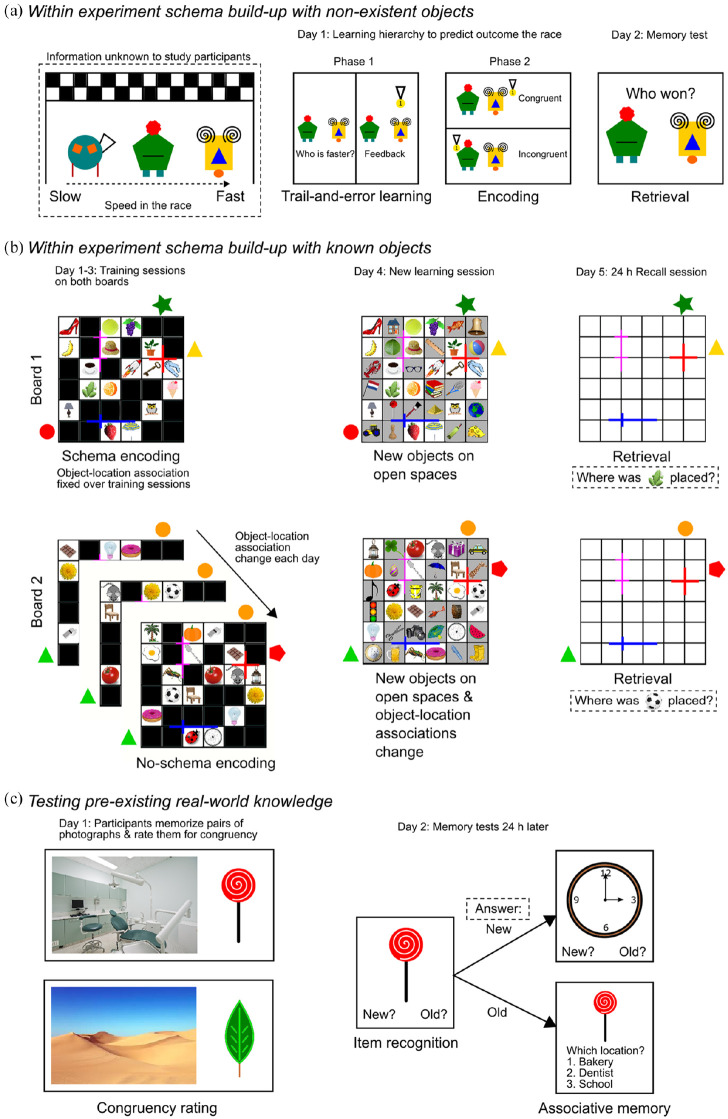
During the last decade, a variety of experiments measuring varying levels of prior knowledge (i.e. from newly learned information within the experiment up to testing common, pre-existing world knowledge) have been investigated using human participants. These have tested, for instance, hierarchical rules about non-existent objects (Brod et al., 2015) (see Figure 1(a)), newly learned visual–spatial layouts (van Buuren et al., 2014) (see Figure 1(b)) similar to experiments performed in rodents (Tse et al., 2007, 2011), rule-like associations between known objects (Preston and Eichenbaum, 2013; Zeithamova et al., 2012) and pre-existing real-world knowledge (Van Kesteren et al., 2014) (see Figure 1(c)) to determine how new information is integrated into the existing knowledge network during the different stages of memory formation (i.e. encoding, consolidation and retrieval).
Figure 1.
Examples human schema studies.
Overview of representative examples from human schema studies ranging from using intra-experimental new schema build-up of non-existent objects to testing pre-existing real-world knowledge. (a) Study design from Brod et al. (2015) in which participants acquired a new schema within the experiment through trail-and-error learning about the outcome of a race between two ‘fribbles’ (i.e. non-existing objects). After learning the hierarchy, participants learned both congruent (i.e. winner according to hierarchy) and incongruent (i.e. winner does not fit the hierarchy) pairs during the following encoding phase on which they were tested during the retrieval phase the next day. (b) Study design from Van Buuren et al. (2014) in which participants over multiple days had to learn associations between known objects and their location on both a schema board (i.e. object locations were the same on each encoding day) and no-schema board (i.e. object locations changed during each encoding day) with the help of both intra- and extra-board cues. On the last encoding day (Day 4), open spaces on both boards were filled with new objects. In addition, the objects on the no-schema board changed location again. On the retrieval day, participants were presented with an empty (schema/no-schema) board and had to retrieve the location of one of the objects. (c) Study design from Van Kesteren et al. (2013) in which participants had to memorise pairs of photographs portraying one known object and one real-world scene which were either congruent (i.e. they co-occur in the real world) or incongruent (i.e. they do not co-occur in the real world). The next day, the participants were tested on their item recognition followed by an associative memory task.
Though humans provide a wider range of cognitive skills than other animals, which can be used to investigate previous knowledge, it is more difficult to look at the dependency of particular brain regions as human studies are mostly observational (e.g. functional magnetic resonance imaging (fMRI)), though advances in interventional studies (e.g. transcranial magnetic stimulation (TMS)) are being made (Berkers et al., 2017; Bovy et al., 2020). This is in contrast to rodent studies, in which the effect of temporary and state-dependent inactivation (i.e. during encoding/consolidation/retrieval) on schema can be examined in addition to the effects of lesions in specific brain regions. Moreover, most human studies use pre-experimental knowledge, and test item and non-spatial associative memory, while rodent studies use previous knowledge learned during the experiment related to, for example, item-location associations within a complex spatial layout. This means that, to date, previous knowledge studies in humans and other animals differ substantially. In the following sections, we will look at the effects of previous knowledge on the different stages of memory formation and updating in humans, and since humans research is quite extensive on the general topic of previous knowledge and can differentiate between different types of previous knowledge, we will focus on schema studies specifically and summarise the results of many different studies.
Effects of previous knowledge on encoding in humans
The encoding of new information can form both the basis for a new schema as well as adjusting or adding to an existing schema. Most human studies examine encoding of new information within pre-existing knowledge, so we will summarise here the effect of schema on encoding of new information in humans.
When incoming information can be directly linked to a pre-existing schema, the mPFC appears to be the main cortical node responsible for memory encoding (Van Kesteren et al., 2010a, 2013), which it does through both strengthening of cortico-cortical functional connections and at the same time, by inhibiting the hippocampus (Van Kesteren et al., 2013). The involvement of the mPFC in schema memory processing is furthermore supported by the results from studies using TMS during schema encoding (Berkers et al., 2017; Bovy et al., 2020) and a study using the Deese–Roediger–McDermott (DRM) paradigm (Roediger and McDermott, 1995) in patients with ventromedial mPFC (vmPFC) lesions (Warren et al., 2014). However, hippocampal activity is increased and the hippocampus–mPFC connection is strengthened when incoming information is novel, and therefore inconsistent with existing schemas (Bein et al., 2014; Van Kesteren et al., 2013). This may be a strategy used to prevent new but inconsistent information interfering with the existing structured knowledge representations stored within the neocortical network.
The degree of hippocampal involvement during the encoding of new information seems to not only depend on the novelty of the new information but also on the complexity of the schema in which the new information is to be encoded. Van Buuren et al. (2014), for instance, show that the hippocampus is involved when new information in a visual–spatial layout is to be integrated into a newly learned, and thus still simple, schema. Similarly, Van Kesteren et al. (2018) show hippocampal involvement in translating previous spatial knowledge (i.e. a newly learned spatial schema) into new goal-directed behaviour. However, Coutanche and Thompson-Schill (2014, 2015) show that fast mapping (i.e. the new to-be-encoded item is presented in the context of a known, real-world item during learning) can completely bypass the hippocampus, likely due to the complexity of the existing schema (but for controversy, also see Cooper et al., 2019). Furthermore, while the encoding of schema-related events appears to be resilient to pre-encoding sleep loss, encoding of unrelated events is not (Alberca-Reina et al., 2014). Alberca-Reina et al. (2014) suggest that sleep loss-related encoding impairment of schema-inconsistent information is likely due to the fact that these memories require a higher level of hippocampal engagement.
In conclusion, although hippocampal–mPFC connectivity is reduced during the encoding of schema-related associations in humans, this type of encoding is not completely independent of hippocampal involvement. Thus far, the amount of hippocampal involvement during encoding seems to mainly depend on the novelty or familiarity of to-be-encoded information and the complexity of the existing schema. This means that, if the new information fits within an existing knowledge structure, more cortico-cortical connections are involved than in the case when the new information does not fit as well.
Effects of previous knowledge on consolidation in humans
Newly encoded memories need to be consolidated offline (e.g. during sleep) and integrated within existing knowledge structures to be able to persist over long time periods. One idea is that memory reactivations during sleep enable the updating of the cortex (long-term memory) by the hippocampus (short-term memory buffer) through coordinated neuronal activity and/or reactivations (Marr, 1970, 1971). Here, we summarise studies that examined the effect of schema on memory consolidation in humans.
In general, consolidation requires context-guided retrieval of previously acquired memories to facilitate the integration of the new memories within pre-existing knowledge. Integration of new information, and thus, modification of the existing schema structure, is a continuous process. In the end though, it is necessary to reach an equilibrium that adapts schemas to be consistent with the external reality (Preston and Eichenbaum, 2013). Only then will schemas be able to support novel inferences between indirectly related events (e.g. if A is linked to B and B linked to C, then A is also linked to C) and thus generalise towards new situations. Similar to encoding, the involvement and extent of involvement thereof, of certain brain regions during consolidation of new information, depend on how consistent this information is with already existing schemas or whether a new schema needs to be formed. For instance, hippocampal–prefrontal interactions are sustained during the resting period following schema formation, whereas these interactions are fewer when the newly encoded information fits within an existing schema (Van Kesteren et al., 2010a). In addition, offline reactivations of neuronal patterns in these brain regions, originally active during encoding, seem to facilitate the consolidation of newly formed schemas (Preston and Eichenbaum, 2013). Similar offline hippocampal–cortical interactions have also been found during rest following an associative encoding task (Tambini et al., 2010).
Depending on the amount of previous knowledge, and thus, the extent of the existing schema, the schema effect can arise immediately after encoding, as is the case for associative memories, or can only be seen after consolidation, as is the case for visual item recognition (i.e. a task that involves the hippocampus during encoding (Van Kesteren et al., 2013)). Moreover, consolidation of schema-consistent information is resilient to sleep loss and to any kind of information interference after learning, while consolidation of schema-inconsistent information is quite vulnerable to both post-learning sleep loss and interference (Alberca-Reina et al., 2014). The need for sleep-mediated consolidation seems to, therefore, depend on the type of learning and might thus be related to how well the newly acquired memory was integrated into the existing schema during encoding (Himmer et al., 2017). However, if sleep is present, it may still contribute to the consolidation of schema-related items. Hennies et al. (2016) taught subjects a new schema over a 2-week period and showed that sleep-spindle density was correlated with decreased hippocampal activity at test for new schema-related items in contrast to non-schema items learned the day before.
Overall, the current evidence seems to point towards the need of persistent, functionally relevant hippocampal-neocortical crosstalk during consolidation (Van Kesteren et al., 2010a, 2014) to form a new schema or update a freshly learned schema, while adding information to a longer, pre-existing schema seems to depend less on this interaction (Van Kesteren et al., 2010a).
Effects of previous knowledge on retrieval in humans
After offline consolidation, new information is integrated within the previous knowledge structure and is thus ready to be retrieved in the following day(s). In the following section, we summarise current knowledge regarding the effect of schema on memory retrieval in humans.
Whether a memory is properly retrieved after consolidation depends not only on whether the retrieval context provides enough information to recreate the encoding context but also on whether this context and the associated memory forms one unit (Brod et al., 2013). This means that the information present during the retrieval context needs to trigger the recombination of the representations of both the consolidated memory and the encoding context of that memory. However, it is not clear where in the brain the recombination of these neocortical representations might take place. Wagner et al. (2015) shed some light on this matter by showing that the angular gyrus plays an important role in converging distributed representations of the rule-based schema components into one coherent memory representation. This converging role fits with the proposed other functions of the angular gyrus, namely involvement in cortical binding of information (Shimamura, 2011) and the representation of memory content during successful retrieval (Kuhl and Chun, 2014). Van der Linden et al. (2017) go even further and propose that for a visual schema-associated memory task, the schema information itself might to be stored in the angular gyrus. The mPFC seems to play a role in biasing retrieval towards schema-consistent memories (Ghosh and Gilboa, 2014; Preston and Eichenbaum, 2013), even for recently acquired schemas and when the time between encoding and retrieval is very short (Brod et al., 2015).
Retrieval of inconsistent information, however, seems to rely on the lateral PFC through interaction with the striatum (suggested by Scimeca and Badre (2012), shown in Brod et al. (2015)). Neither Van Kesteren et al. (2010b), Brod et al. (2015) nor Van Buuren et al. (2014) found that hippocampal activity was reduced during the retrieval of both schema-consistent and schema-inconsistent memories. In addition, Brod et al. (2015) show that the connectivity between the mPFC and hippocampus was not enhanced for the retrieval of incongruent compared to congruent information. In fact, the left hippocampus was involved in successful memory retrieval for both schema-consistent and schema-inconsistent memories, without significant difference between the two (Brod et al., 2015).
Interestingly, Prull (2015) shows an age difference in retrieval but not in the encoding of schema-inconsistent memories. Moreover, schema effects appear to be more extreme in older adults and may be able to alleviate age-related deficits in memory (review in Umanath and Marsh, 2014). This seems to also be true for procedural memory, as Muller et al. (2016) showed that prior motor experience does not only increase procedural learning but also has a protective effect against age-related decline for the consolidation of novel but related manual movements. In contrast, Badham and Maylor (2015, 2016) show that schemas can also have a negative impact on memory performance in older adults.
In summary, the hippocampus may not be necessary for retrieval but, when accessible (i.e. not lesioned or actively suppressed as possible in other animals), may still contribute to the retrieval of schema-related information. This means that even after consolidation, the full expression of schemas may depend on (a perhaps low level of) continual hippocampal–prefrontal cortex interaction, possibly through a constant cycle of memory updating during retrieval (reviewed in Preston and Eichenbaum, 2013). However, due to the fact that human studies are mostly restricted to observational methods like fMRI, instead of being able to utilise, for instance, brain lesions to study the necessity of specific brain regions in the retrieval of (schema) memories, it will be difficult to obtain a definitive answer on the exact involvement of specific brain regions during schema retrieval.
Summary previous knowledge studies in humans
A variety of schema types, including motor schemas in athletes versus non-athletes (Pereira et al., 2013), word schemas (Takashima et al., 2014), cultural schemas (Porubanova et al., 2014), music or tonal schemas (Vuvan et al., 2014), have been described in humans over the past years. Overall, human neuroimaging studies converge with rodent studies in showing that the hippocampus and neocortex are complementary learning systems that interact during schema formation, consolidation and retrieval. However, the extent to which each brain region is involved depends on the to-be-encoded or remembered information as well as the extent of the existing schema. Furthermore, the range of the schema effect seems to depend on the task, type of memory, how much time has passed since learning (i.e. whether consolidation has taken place or not) and the extent to which the existing schema can be harnessed. As most of the above-mentioned studies test consolidation and retrieval of schemas over relative short time scales (i.e. shortly after learning), future research should investigate the specific roles of the prefrontal cortex and hippocampus in these processes over time. Because examination of schema neurobiology in humans is mostly bound to observational studies (e.g. fMRI), we will need to rely on interventional studies (e.g. lesions of particular brain regions) in rodents to extend our knowledge on the exact role of each brain region involved in schema formation, consolidation and retrieval.
Previous knowledge studies in non-human animals
By observing fluctuations of voltage or metabolites in human participants using fMRI, positron emission tomography (PET) and other methods while performing schema-related tasks, we can study dynamic interactions between brain areas. Research has suggested that during studies, involving previous knowledge-based tasks, the hippocampus is critically involved during encoding if it is a highly novel event, and less if the event fits into an already established extensive previous knowledge, such that a gradient of hippocampal involvement is inversely proportional to the complexity of the existing knowledge network.
Using animal research in combination with an ever-developing range of tools will allow us to take a step closer to understanding the neurobiological mechanisms involved in memory. From electrophysiological and imaging recordings to pharmacological and genetic manipulations, a combination of these tools with specific and complex behavioural protocols provides us with the power to measure and target specific neural types or areas and thus moves from observational to interventional methods that allow us to draw conclusions on causality and mechanisms.
Behaviourally, designing a task to evaluate previous knowledge or even schema specifically at a rodent level requires creative thinking since we cannot rely on pre-existing world knowledge when working with laboratory animals. In a way, the advantage of having perfect control over the experience an animal has actually makes developing previous knowledge paradigms more difficult in rodents that do not have ‘real-world’ knowledge, which we can harness. Having such tasks gives the chance to further understand the anatomical connectivity and synaptic properties involved in the dynamics of schema formation and updating.
As mentioned before, a schema was defined by Ghosh and Gilboa (2014), as an associative network structure, which should be based on multiple similar experiences, with a lack of unit detail and adaptable. As such, it facilitates long-term memory at both encoding and retrieval levels. However, the term schema in rodent studies has been used loosely for different concepts, which we will highlight next in this review. Many of the studies cited below would not fulfil the strict definitions of a schema, thus especially in this part highlighting non-human animal research, we will refer to previous knowledge instead of schema. Furthermore, to emphasise the amount of previous knowledge present in each study, we will describe the articles and procedures more in detail than the previous human examples. The presence of a previous knowledge facilitates encoding of new congruent elements as well as expediting retrieval, for which its structure is not static, but constantly developing and updating with experience. It quickly identifies similar patterns or situations anticipating outcomes, giving the chance to make the best choice based on experience.
But where can we find a previous knowledge structure? These memory representations and multimodal associations are thought to be found distributed along the neocortex in the mPFC, orbitofrontal cortex (Orb), retrosplenial cortex (RSC) and anterior cingulate cortex (ACC). These structures are involved in making an act of judgement/decision-making based on previous experiences. They are richly interconnected, giving them the power to judge, predict and learn (Skelin et al., 2019).
The ACC is connected to several cortical structures and the limbic system and is involved in problem-solving, making choices, anticipation and motivations. The RSC lies close to the hippocampus and visual areas and is involved with imagining future events, episodic memory and navigation. The Orb receives inputs from visual, taste, olfactory and somatosensory areas, as well as from the amygdala and is involved in correcting behaviours related to reward or punishment. The prelimbic cortex (PrL) integrates a diverse range of information to perform a behavioural response and is involved in goal-directed behaviours, attention and working memory (Aston-Jones and Cohen, 2005; Frankland and Bontempi, 2005; Mao et al., 2018).
Paired-associates schema in rodents
Even though the concept of previous knowledge and schema in psychology has been known since the 1930s (Bartlett, 1932), seven decades later the interest in this memory process reemerged. The seminal study by Tse et al. (2007) introduced the concept of schema to neurobiology and opened up the possibilities of understanding the molecular mechanisms involved. To date, different interventional approaches have been used in the same task (i.e. the paired-associates (PA) task), and in this section, we will explore the current findings in more detail.
In the PA task (Tse et al., 2007), rats have to learn a map of six flavour-place associations, or PA, in a large event arena (1.5 × 1.5 m2). One training session consists of six trials; at the start of each trial, rats are given a flavoured pellet, and during this trial, only one sandwell is rewarded with more of the same flavour pellets. The location remains the same throughout the experiment. For the second trial, a different flavour pellet is associated with a different sandwell location, and so on (see Figure 2(a), middle). Rats have to learn associations with flavours and spatial locations as elements, the map of the event arena and its sandwell locations as a relational network (Figure 2(a), left).
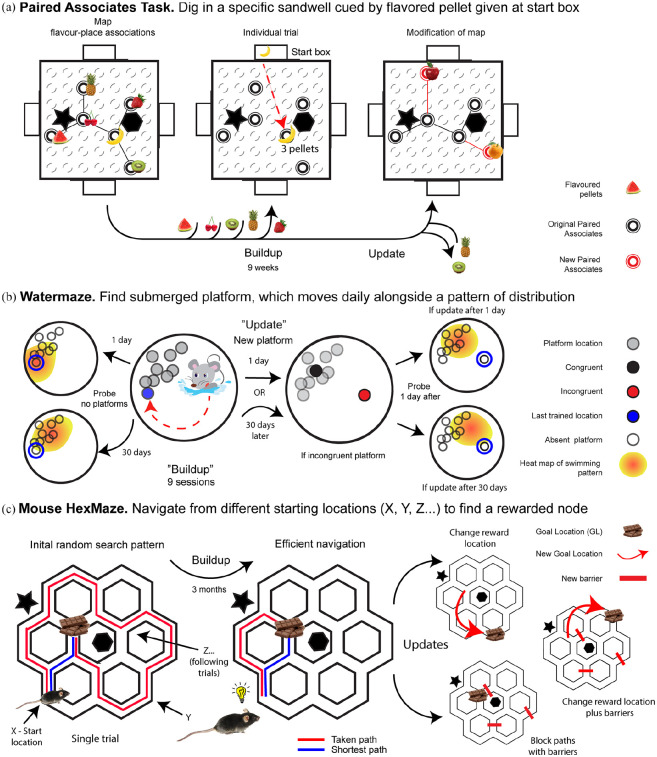
Figure 2.
Examples rodent schema studies.
Schematic overview of rodent paradigms. (a) PA task. Event arena contains a 7 × 7 grid of potential sandwell locations from which a map of six sandwells associated to flavours is formed over time, as shown on the left arena. There are four start boxes around the maze, and intra- as well as extra-maze cues (star and hexagon). In a single trial, the animal is given a flavoured pellet in the start box, as shown in the central arena, for example, a banana pellet, and the rat has to dig in one out of six sandwells for more banana pellets (and repeated for the remaining five flavours). This is repeated during 3 months in a period denominated build-up, where animals increase their performance over time, indexed by digging time in the correct sandwell and performing fewer errors when choosing the correct sandwell to dig in first. After this time, an update to the flavour-place associations is made, seen as a change of two flavours in new locations, presented in the red symbols on the maze on the right. As discussed in the main text, this update can be learned within a single exposure. (Tse et al., 2007). (b) Water maze. Mice need to find a submerged platform within the circular pool, each day, four times a week. The platform location changes every day, and they were drawn from a statistical predefined distribution in space (grey circles). Animals were probed with no platforms present, 1 or 30 days after the end of their training or ‘build-up’. Swimming patterns were translated into a heatmap of average dwell time, as shown on the left of the figure. Animals that were tested 1 day after the build-up showed preference for the last presented platform, whereas those that were tested 30 days later showed preference for the overall distribution of the platforms. On the right: as an update, a new platform is introduced, which could be placed in a consistent position, as shown in the black filled circle, or in an inconsistent position, as shown in the red filled circle. This update can happen either 1 or 30 days after the original build-up training. If the inconsistent position is shown 1 day after the build-up, a probe trial conducted a day later shows that the search pattern is more inclined towards the overall platform distribution. If this update happens 30 days after the build-up, a probe trial conducted a day later shows a search pattern between the original distribution and the new platform (Richards et al., 2014). (c) Mouse HexMaze. Animals navigate a big maze to find a rewarded location (GL). On the left, the red trace shows a trial where a random path is taken by a naïve animal, until it reaches the chocolate reward. In one training session, the mouse performs several trials, always from different start locations (X, Y, Z . . .). Performance is calculated by comparing the path taken to the shortest possible path (blue trace). The build-up of the task consists of 3 months, and overtime, the navigation improves. Later updates are introduced, where barriers can be added, the reward can be moved, or both. As discussed in the text, these updates can be learned in just one session. Intra- and extra-maze cues (star and hexagon) aid navigation in the maze. (Alonso et al., 2020)
Hippocampal lesions (Tse et al., 2007) and pharmacological studies (Hasan et al., 2019) show that the hippocampus is critical for the initial learning of the task, which takes place gradually over several sessions. Similarly, if ACC was inhibited (by lidocaine or demyelination) (Hasan et al., 2019), animals could not learn the task, suggesting that initial learning of a potential schema is dependent on both the hippocampus and cortico-cortical interactions to stabilise the memories, with the hippocampus being more important at an early stage.
Since adaptability is a core feature of schemas, 9 weeks into training the original PA map was updated by replacing two of the original PAs with new flavours in nearby locations (Figure 2(a), right). If by then rats had built a schema of the PAs, learning new associations should be faster. Indeed, exposure to a single trial of the new PAs was enough for them to recall the updates 24 h later (Tse et al., 2007). For the memory to persist, hippocampal dopamine plays a critical role at the time of encoding, as shown by Dopamine DA1/DA5 receptor antagonists given at the time of encoding (Bethus et al., 2010); rats could correctly remember if they were tested 30 min after exposure to new PAs but not 24 h later.
Tse et al. (2007) further investigated hippocampal dependency at the stage of updating information. A group of rats was given hippocampal lesions either 3 or 48 h following exposure to the new associations, revealing a gradient where 3 h after encoding the memory still depended on the hippocampus, but 48 h later it did not. Later, when rats with hippocampal lesions were exposed to either new PAs or a completely new map, they could not learn the new associations, showing that it was not the task itself that had to be learned, but the associations within a relational network (Tse et al., 2007).
If retrieval of new information after a certain time point is no longer dependent on hippocampus, the memory should then rely on extrahippocampal structures. Measuring immediate early gene (IEG) expression allows to evaluate which brain regions are experiencing synaptic changes. Brains from experienced rats were extracted 80 min after being exposed to either the six original PAs, two new associations and four original PAs or a totally new map with six new PAs (Tse et al., 2011). A test minutes before brain extraction showed that, for the original and updated PAs conditions, animals could recall the associations correctly, but not for the completely new map. While IEG expression is always present, immunohistochemistry against Arc and Zif68 showed that when updating the two new PAs into the schema, there was a greater upregulation of IEG expression during encoding in cortical areas PrL (Tse et al., 2011), ACC (Wang et al., 2012), RSC and hippocampal region CA1 (Tse et al., 2011). In contrast, when rats had to learn a completely new map, brain regions that showed an increase in IEG activation were limited to CA1. In the same study, pharmacological manipulation of the PrL during the encoding of new PAs revealed that both synaptic transmission and N-methyl-D-aspartate receptor (NMDAR)-mediated synaptic plasticity were required in the PrL for successful encoding. In addition, Wang et al. (2012) used pharmacological disruption of the ACC and found that NMDAR-mediated plasticity was necessary in this region for the encoding of new PAs into a pre-existing schema. Both studies indicate that parallel encoding is occurring in both the hippocampus and prefrontal cortex (PrL and ACC).
However, Lesburgueres et al. (2011) showed that there is simultaneous IEG activation in the hippocampus and neocortex even when there is no previous knowledge present, which means the emphasis should be that with existing schema, the cortical tagging is larger in magnitude (Tse et al., 2011). In Lesburgueres’ et al. (2011) study, rats performed the social transmission of food preference paradigm, where animals learned by smelling another rat’s breath whether food was safe to eat and were then tested 30 days later. Increase in IEG activation in orbitofrontal cortex was observed at encoding and inhibition of orbitofrontal cortex by NMDAR antagonists on the day of encoding resulted in no memory persistence, showing that an early ‘tagging’ of cortical networks is crucial for the formation and maintenance of memories even without previous knowledge. A similar finding with increased IEGs expression after encoding in the prefrontal cortex was also shown in the water maze paradigm (Genzel et al., 2017).
The increase in IEGs goes hand in hand with cellular processes, such as synaptic transmission for which α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) and NMDA receptors are directly involved. These receptors can be inhibited in the PrL and the ACC at different stages of schema acquisition (Tse et al., 2011; Wang et al., 2012). AMPA receptors are associated with general synaptic transmission, and they were needed for encoding and retrieval of the new PAs. NMDA receptors are associated with plasticity and long-term potentiation, and they were found to be critical for memory encoding but not retrieval (Tse et al., 2011; Wang et al., 2012). Furthermore, offspring from rats exposed to dioxins, which inhibit gene expression and NMDA expression in the prefrontal cortex, could not learn the PAs task at all (Kakeyama et al., 2014).
In summary, the PA task opened the gates for understanding the molecular mechanisms underlying how previous knowledge affects learning and memory. Initial encoding of a potential schema is dependent both on hippocampal and cortical areas, such as ACC and PrL, but once a schema is formed, retrieval is rapidly independent of the hippocampus. Encoding of new information that fits within existing previous knowledge is dependent on an intact hippocampus, dopamine transmission around the time of encoding (necessary for memories to persist), and both NMDA and AMPA receptors, which are involved in long-term potentiation and general transmission. Retrieval of information from schemas is not hippocampus-dependent if at least 48 h have passed since encoding.
Other tasks testing previous knowledge in non-human animals
Efforts have been made to develop other tasks utilising schemas; in this section, we discuss that these are mostly related to cumulative experience and represent general effects of previous knowledge and not schemas per se.
Schemas are based on multiple episodes and should not be detailed (Ghosh and Gilboa, 2014). With this in mind, a task was developed for mice to identify a pattern over time; however, many other schema prerequisites were not included. In a water maze-based task (Figure 2(b), centre-left), mice had to find a hidden platform below the water surface in a fixed place over four trials in 1 day, but the position of the platform changed slightly from 1 day to the next over nine training days, drawn from a statistically predefined distribution in space. Animals were later tested 1 day or 30 days after with no platform present (Richards et al., 2014). Mice tested at 30 days expressed a strong correspondence between search strategy and overall platform distribution compared to those tested 1 day later. The 1-day group search pattern was more accurate with respect to the actual positions of the platforms with the final platform position dominating, while the 30-day group’s search strategy was centred in the mean position of the platform distribution (Figure 2(b), left). This suggests that, in presence of a long time period between encoding and retrieval, search patterns are driven by cumulative experience rather than specific events.
A schema needs to be adaptable and to test this, in a variation of the same protocol, after the training of the distributed platforms, which we can call a ‘build-up’, an update was introduced either 1 or 30 days after the build-up. During the update, the platform was placed either in a congruent or an incongruent position (Figure 2(b), centre-right), congruent being within the mean distribution of previous daily locations and incongruent being far. Focusing in the incongruent platform update, a probe was done 1 day after, and the group where they had the update after 30 days had higher prediction error than the 1-day group, seen as a change in the search strategy (Figure 2(b), right). This strategy switch in the 30-day group was not seen when the mPFC was inhibited before being exposed to the last platform, suggesting that the role of the mPFC in rapid consolidation may be limited to the learning of new incongruent information (Richards et al., 2014).
Another way of evaluating cumulative experience is shown in the object space task (Genzel et al., 2019), where rodents are exposed to a pattern of four possible object locations in an open field throughout the week, with one location that is stable across days, while the others are shifted between the three other possibilities. Based on the natural tendency of rodents to explore novelty in the presence of familiarity, exploration time of the object placed on the stable location should decrease over time. Across 20 trials in 1 week, a semantic-like memory is expressed with an extracted pattern of locations, which then guides their behaviour towards exploring the object that was not in the stable location. However, 1 week is not long enough to suppose schema formation or semantic memories per se, thus this task should rather be seen as simple, previous knowledge-based task (Genzel et al., 2019).
A simpler form of previous knowledge can also just be the pre-exposure to the spatial environment in which learning should occur. Genzel et al. (2017) contrasted two different behaviours that can lead to memory persistence: post-training novelty and post-training sleep (Duszkiewicz et al., 2019). Post-training novelty should lead, through synaptic tagging and capture mechanisms, to increased hippocampal cellular consolidation. In contrast, sleep allows for memory reactivations and thus systems consolidation and integration into cortical networks (Duszkiewicz et al., 2019). Rats were taught two platform locations in the water maze, one of which was followed by sleep, while the other was followed by novelty exposure combined with sleep deprivation. In the probe trial, 1 week later, rats remembered both platform locations but spent more time at the platform location followed by novelty. However, if animals were pre-exposed to the spatial layout and cues before training (with a dry-land inlay), this difference was abolished and now only having sleep after learning was sufficient for a strong long-term memory (Genzel et al., 2017) perhaps due to the possibility of harnessing pre-existing cortical memory networks, even though in this case they were clearly not complex schema representations.
Categorisation of objects groups similar elements together, and this process can be seen as a form of semantic memory. A set of tests evaluating the categorisation of objects in mice (Creighton et al., 2019) showed that mice could recognise the categories of objects. In a sample phase, mice were presented with two objects of the same category, and during a test phase, they were presented with two novel objects, one belonging to the category presented during the sample phase and another unrelated object. Mice could recognise the familiar category over a short delay but not over a long one (30 min versus 1 h). If animals were pre-exposed to the category, they could discriminate the familiar object after long delays. This effect was lost under scopolamine (acetylcholine antagonist) if it was systemically administered before the test phase, which was expected since acetylcholine plays a role in memory and perception (Creighton et al., 2019).
Previous knowledge that facilitates encoding and retrieval on its own is not sufficient for the classification of ‘schema’. By performing tasks with common features, certain features can be drawn from them and can facilitate ‘learning how to learn’ or learning set (Harlow, 1949). For example, training rodents in two similar tasks, the original water maze, where throughout 5 days animals need to find a stable platform, and the delayed matching-to-place task, where the platform changes each day, throughout 26 training days (Ocampo et al., 2018). A commonality between these tasks is a circular water pool and a platform that needs to be found. If either of the two tasks is trained first, the second benefits from what was learned in the former. For early learning, the entire hippocampus is necessary, but the second task was not dependent on hippocampal region CA1, which is the main output pathway from hippocampus to neocortex (Ocampo et al., 2018).
Other studies refer to hippocampal schemas, without taking into consideration the enhancement of long-term memory, which is crucial in the definition of the schema effect, thus, perhaps, while using the term, these do not test schemas as defined in the human literature. McKenzie et al. (2013) trained rats to find a water reward in circular maze, cued by a LED signal, and after these were learned, in a course of 6 days, they had to learn new reward locations that were spatially defined. By analysis of electrophysiological recordings in the CA1 region, they could show that by adding the non-cued reward sites gradually, hippocampal representations from the cued learning were modified to add the non-cued learning (McKenzie et al., 2013). However, because long-term memory was never assessed, this study is most likely to be classified as an initially naïve learning where a potential schema is still hippocampus-dependent.
Similarly, Dragoi and Tonegawa (2013) wanted to study place-firing of cells in naïve animals, wild-types and CA3-NMDA receptor knockouts. In this paradigm, mice were put on a linear track for two sessions on Day 1 (novel), and similarly on Day 2 (familiar), but on this second day, the linear track was transformed to an L track by the addition of a perpendicular linear track (novel feature). By Day 3, the L track would be familiar, after which a novel linear track was introduced for two sessions. Place-cell firing stability was determined, and in the first novel-feature condition, place-cell stability was reduced in both the control and knockout (KO) condition, and stability increased with experience. On the novel track at Day 3, only the KO mice had reduced place-cell stability, suggesting that NMDA receptors in the CA3 area are necessary when novel situations arise and not when there is a previous knowledge network (Dragoi and Tonegawa, 2013). And here again, while the term schema is used, it rather represents a very simple form of previous knowledge or experience.
These studies have all been performed in rodents; however, a recent study has used macaques. In this study (Baraduc et al., 2019), macaques were presented with both a familiar and a novel virtual maze sharing a common ‘schema’ (spatial map), yet differing in surface features, in which macaques had to search for food. Food locations were defined in relation to landmarks. During learning, a proportion of hippocampal neurons had firing rates modulated by task-related information in the novel maze, which matched that of the familiar maze in a manner, suggesting that these neurons abstracted spatial elements from the environment and encode space in a representation of a potential schema (Baraduc et al., 2019).
Through repeated experiences, naïve animals can subtract patterns and categories which guide behaviour and facilitate ‘learning how to learn efficiently’ (i.e. learning set). Learning sets transform the strategy of adapting by trial-and-error to a reasoning-like strategy, involving hypothesis and insights. The time frame of most studies reviewed in this section was between 1 and 6 weeks, and in some cases, the long-term memory was tested once, but additional tests were not performed. Thus in most cases above, it is hard to assess if they would fulfil the strict criteria of schema, instead most studies were testing the effect of previous knowledge in the simplest form. An intact hippocampus appears to be essential for memory persistence in the presence of novel situations, slowly disengaging as cumulative experiences start forming a relational cortical network.
The HexMaze for mice
In the effort to establish a different task to test for previous knowledge in rodents, the HexMaze was developed (Alonso et al., 2020). It is based on multiple episodes, is adaptable and has the same cognitive load throughout.
In the HexMaze, mice learn to navigate a large gangway maze (Figure 2(c)), where a chocolate-flavoured reward can be found in one of the 24 nodes. The goal location stays stable for several sessions. In a training day, the animal is placed repeatedly over many trials in different random nodes within the maze, from which the animal should navigate towards the food (Figure 2(c), left). The previous knowledge in this case is the map that they need to navigate using the environmental cues as reference points. And how this previous knowledge affects new learning is tested by changing the goal location and measuring how quickly animals can adapt their behaviour to this new information. Performance is measured by the length of their navigational paths, as in the number of nodes the animal visited, in relation to the shortest path possible.
Initially, animals run around the maze, exploring randomly until they find the reward. This is the case in the first sessions in the maze, as well as each time a new goal location is introduced. As experience in the maze increases, so does performance level, as animals slowly learn to recognise their position based on the cues placed around the maze and choosing more efficient routes to the reward (Figure 2(c), middle).
Similar to the PA task (Tse et al., 2007), the task consists of a build-up phase of 3 months, during which the location of the food changes every 7 to 5 sessions. This build-up phase is followed by a phase of updates (Figure 2(c) right), where a change is introduced weekly. These changes could be a new goal location, adding a barrier, or both. Each training session consists of 30-min period during which the animal performs several trials (20–35). Due to the design of the task, different types of previous knowledge can be tested in this paradigm.
In the first 3 weeks of the build-up, during which the location of the reward stays stable, a gradual increase in the performance was seen in each session, as mice gained experience in navigating the maze. If the goal location changed, performance initially dropped to the same level as when the animal was first introduced to the task, but by the second session following this goal location change, the overall performance was better compared to the second session of the previous goal location (Alonso et al., 2020). However, in this build-up phase, long-term memory (48 h) still took multiple sessions to develop. Because there were several trials per session, the first trial served as a test for long-term memory, while the overall performance indicated working memory in addition to efficient navigation through the maze. While the overall performance increased during the second session of the second goal location, this was not the case for the first trial.
During the updates, changes to the maze were made weekly. By the first session of the first update, the performance was already significantly better than the first session of the build-up. Furthermore, performance continued to improve throughout the week. This performance gain from build-up to updates to sessions throughout the update is reminiscent of a learning set (Harlow, 1949). However, since the updates are of three different kinds, changing goal location, adding a barrier, or both, it can be shown that the rate of learning differs depending on the amount of overlapping information. For example, adding a barrier would be the easiest condition to learn, since the goal location remains the same, while if both location and barrier change, the conflicting information is greater. This was evident by a drop in performance on the first trial of the first session following an update when the goal location changed. However, by the following session, performance improved at the same level for all conditions, showing that one session was enough for the memory update and long-term memory (Alonso et al., 2020).
In addition, we found that the build-up phase was not dependent on amount of training, but rather on time, that is, by training mice three times a week versus twice a week. The increase in performance depended on the amount of time that had passed since the beginning of the experiment and not in how many times they were trained per week (Alonso et al., 2020).
With a flexible task like this one, the effects of previous knowledge on memory, encoding, updating and retrieval can be evaluated independently. Currently, we are also developing a HexMaze for rats (4 × 9 m2), in which the same spatial structure will be used but four times the size.
Summary previous knowledge studies in non-human animals
Clever behavioural tasks allow us to understand how previous knowledge affects learning in subjects with no ‘real-world’ knowledge. From the water maze to the PA task, they fulfil all or some but usually not all of the criteria that makes a schema: an associative structure, based on multiple episodes, not detailed and dynamic (Ghosh and Gilboa, 2014). Different paradigms focus on different memory levels, from naïve learning and prediction error based on cumulative experience to long-term memory persistence and in rare cases, the schema effect.
We have had a closer look at the gradient in which the hippocampus and neocortex depend on each other to form and sustain long-lasting memories, mainly due to the advantage of interventional techniques, such as lesions, pharmacology and invasive recording methods (e.g. electrophysiology), that allow us to try and decipher how individual cells communicate with each other.
To support coherent long-lasting memories, there must be a developing dynamic between the hippocampus and the neocortex. The hippocampus is essential for acquiring novel experiences, both at an early stage of memory build-up and during the updating of memory structures. Simultaneous synaptic activity, seen as IEG activation, is critical at both the hippocampus and neocortex during encoding. The period of time during which a new event depends on the hippocampus diminishes with the amount of the previous knowledge of that experience, and once a schema is present, system consolidation is greatly accelerated.
Theories on the role of the hippocampus and prefrontal cortex in memory
Classic systems consolidation theory states that memories are initially encoded in a whole-brain network, but only the hippocampus trace is sufficient for retrieval (Frankland and Bontempi, 2005). But over time (weeks/months/years), cortical connections become reinforced in offline consolidation processes so that later on they are sufficient for retrieval (McClelland et al., 1995; Squire et al., 2015). The transformation theory expanded on this concept and suggested that this type of systems consolidation would also lead to a change in the type or quality of memory: from hippocampal episodic or event memories to abstracted, gist-like memories in the cortex (Moscovitch et al., 2016; Nadel and Moscovitch, 1997). However, both theories had not yet proposed a special role for the prefrontal cortex or considered previous knowledge in any significant manner until recently (McClelland, 2013). In light of many recent findings, various new theories on how both the prefrontal cortex and the hippocampus could play a role in memory have emerged. In this next section, we will highlight these different theories.
The schema-linked interactions between medial prefrontal and medial temporal regions (SLIMM) theory is based on human schema results and proposes that the mPFC functions as resonance detector to recognise information that fits into pre-existing networks. Once activated, the mPFC then suppresses hippocampal activity during memory encoding, which would not occur when the information is very novel and does not fit into what we know (Van Kesteren et al., 2012). Thus, the former would be immediately encoded into the cortex, while the later would be encoded in the hippocampus.
In contrast, Eichenbaum (2017) suggested that the hippocampus organises memories within the context in which they are experienced and the mPFC would be relevant to retrieving the context-appropriate memories. During encoding, the context cues would first be fed from the hippocampus to the mPFC and this information would be fed back to the hippocampus during retrieval to bias the hippocampal network to the appropriate context.
A slightly different view is that the hippocampus creates a rapid binding and encoding of all events as they occur, as an automatic, day-to-day recorder (Wang and Morris, 2009), serving as an index or pointer to information coded in the cortex (Buzsáki and Tingley, 2018; Skelin et al., 2019). However, most of these impressions would not last but fade away overnight. Memories that would be tagged as salient would be consolidated to the cortex, and the prefrontal cortex would take over the binding function of the hippocampus for memories that are related to established cortical networks (schemas) (Genzel and Battaglia, 2017; Squire et al., 2015; Wang and Morris, 2009).
Some more recent theories move beyond the concept of the hippocampus as a ‘memory’ area. Barry and Maguire (2019) highlight the fact that most evidence for the hippocampus being involved in memory comes from naïve animals and only looking at very short time scales. They argue that with the rapid turnover of synapses (average life of 10 days) in the hippocampus, a memory would not last very long there. Instead, the role of the hippocampus in memory would be defined by the process occurring within. More specifically, they propose that the hippocampus is critical for scene construction, that is, creating our inner movie (Barry and Maguire, 2019). Thus, the hippocampus would reconstruct remote memories in the absence of the original trace by assembling consolidated neocortical elements into a spatially coherent scene. This would be facilitated by the mPFC. Evidence for this idea comes from patients with hippocampal lesions, in which imagining the future – a task that requires scene reconstruction – is just as affected as the recall of episodic memories. The scene construction theory proposes that the hippocampus continuously constructs and anticipates scene representations beyond our immediate sensorium. In this context, a scene is a naturalistic 3D spatially coherent representation of the world typically populated by objects and viewed from an egocentric perspective. Scenes represent the fundamental components of unfolding mental events, whether recalling autobiographical memories, navigating through environments, forecasting plausible futures, or creating novel scenarios, all domains in which hippocampal-damaged patients are impaired (Barry and Maguire, 2019).
Another recent proposition on the role of the hippocampus also emphasised the general properties of the hippocampus. Instead of coding for space and time, both components of episodic memories, the hippocampus would be a general sequence generator (Buzsáki and Tingley, 2018). And whatever information is fed into the system – the ‘what’s’ – would be coded in the cortex and mapped onto content-free pointers in the hippocampus. Thus, activating the hippocampal sequence would lead to the retrieval of the sequence of experience. Furthermore, in the hippocampus, self-organised activation during offline states would be constrained by existing attractor manifolds, or maps, and may be biased towards particular mapped locations by salient experience, which would result in the appearance of experience-specific replay (Swanson et al., 2020). Similarly, the impact of sharp-wave-ripple-associated reactivation on downstream regions, which would function as second readers, would not be a simple transfer of hippocampal representational content. Rather, the response of downstream regions would depend on a transformation function, defined by both the feedforward and local circuit architecture, as well as the ‘listening state’ of the downstream region (Swanson et al., 2020).
In sum, the concept of schema, as well as other more recent findings in memory research, has induced a plethora of new theories on what the mPFC and hippocampus does mechanistically in memory. Most of these theories move beyond the idea that memories are simply ‘stored’ in the hippocampus and then ‘transferred’ to the cortex, and instead consider which physiological mechanisms or processes the hippocampus is involved in.
From naïve to expert: a new theory of previous knowledge
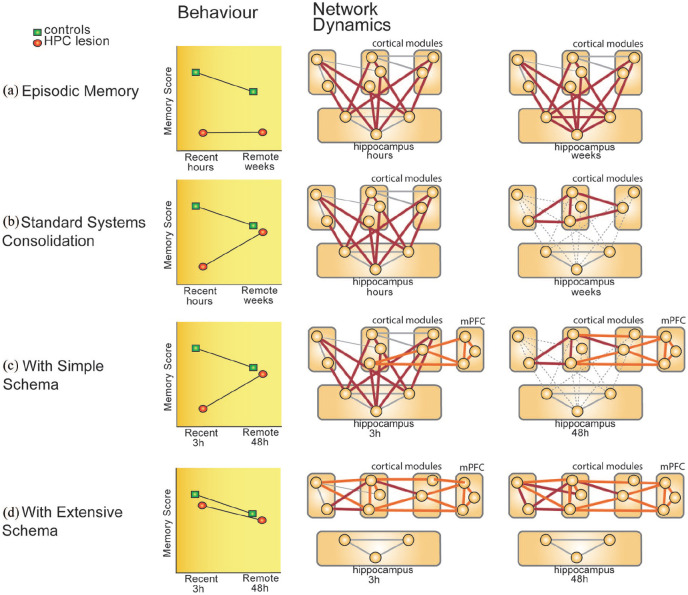
While the theories mentioned above do consider how previous knowledge influences how we encode and consolidate memories, it is often seen as ‘either-or’ phenomena. Instead, the amount of previous knowledge tested should be considered as a gradient, which can range from none in naïve situations to very extensive, as is often the case in much human cognition. In the following section, we propose such a gradient schema theory and how it would influence which brain areas are needed during encoding, consolidation and retrieval (Figure 3).
Figure 3.
From naïve to expert: a new schema theory.
How critical the hippocampus is for memory encoding and retrieval would depend on the type of memory and how much experience encoded in cortical networks can be harnessed. (a) For very novel and unique events that will be retained in the form of episodic memories, the hippocampus would always be involved. (b) New memories that are consolidated to abstracted, gist-like memories, the hippocampus would be involved during encoding and hippocampal independency at retrieval would take weeks to years. These types of memories are described in standard systems consolidation theory. (c) In contrast, if new memories are congruent with pre-existing knowledge, but this knowledge is still quite new and forms a more simple schema, the same gradient of hippocampal involvement during encoding and hippocampal independency during retrieval is seen but now sped up. Memories can be hippocampal-independent after a few days, perhaps with sleep as a crucial factor during the consolidation period. (d) Finally, if new memories are congruent to large, extensive schemas, the hippocampus can already be bypassed during encoding and memories directly stored in cortical networks.
Most animal research on memory would be placed on one side of this gradient, with new memories only able to rely on very little (if any) previous knowledge that animals had acquired during, for example, habituation or shaping periods in training. How unique the new event or experience is would then influence how these memories are consolidated and the outcome of this process (Duszkiewicz et al., 2019).
Very unique, emotionally arousing experiences would lead to increased initial cellular consolidation in the hippocampus resulting in a longer-lasting hippocampal memory trace for these event memories (Figure 3(a)) (Duszkiewicz et al., 2019). The hub-like anatomical position of the hippocampus would allow it to orchestrate a wide range of cortical and subcortical networks during memory retrieval and thus link more detailed aspects of a given experience that are represented in distributed neocortical modules (Skelin et al., 2019). In this way, activity in the hippocampus can trigger the reactivation of neocortical patterns resulting in the retrieval of a memory in more detail and together with the scene reconstruction properties, would thus always be necessary for the retrieval of episodic, detailed memories (Barry and Maguire, 2019; Moscovitch et al., 2016; Skelin et al., 2019). These types of memories would be very rare in adult humans, due to the amount of previous knowledge influencing everything that is newly learned.
New memories that can rely only on very little previous knowledge but are not as unique or emotionally arousing as the memories mentioned above would be consolidated to the cortex over time. However, in this process, they would lose their episodic detail and instead only salient information would be retained in a gist-like quality (Moscovitch et al., 2016). These types of memories would depend on hippocampal activation during retrieval for weeks to months and only consolidate very slowly to cortical networks (Figure 3(b)). Most current animal memory research would be operating on this level.
In an intermediate phase, some previous knowledge encoded in cortical networks is present, which can already be retrieved without the hippocampus. In this intermediate phase, updating of these cortical networks would still need the activity of the hippocampus during encoding, as well as following offline periods of sleep, to enable a slow updating of the cortical networks. However, this updating would be more rapid (e.g. days instead of weeks) since less cortical changes are needed than in the naïve animal (Figure 3(c)). The paradigms used by Tse et al. (2007) and Van Buuren et al. (2014) are examples of this case.
On the other end of the spectrum, when a lot of previous knowledge is present in a more complex cortical network, the hippocampus would not even be needed or at least needed much less for the update process. This phenomenon of rapid, cortical consolidation can be seen in ‘fast mapping’, when new information is presented in the context of previous known information (Figure 3(d)). Studies harnessing real-world knowledge as schema would be operating at this level. Overall, most naturalistic human learning would be represented either on this or the previous level of the gradient.
In sum, here we propose a new memory theory in which the extent of previous knowledge influences the extent to which the hippocampus is involved in encoding, consolidation and retrieval. Overall, both levels would remain a continuous gradient with more extensive previous cortical networks leading to less dependence of the memory on the hippocampus and a faster shift from hippocampus to cortex as necessary memory structures (Genzel, 2020).
What does the hippocampus do?
Since the famous hippocampal lesioned patient H.M., the hippocampus has been viewed as the brain area associated with memory. The subsequent discovery of place cells in this brain structure initially supported the idea of the hippocampus being a critical memory brain area especially for spatial memories. However, more recent findings do not really fit into this concept and have made many researchers rethink what and how the hippocampus really contributes to memory. In this section, we will propose how the hippocampus could contribute to memory.
One of the first and still most influential ideas on hippocampal function was proposed by David Marr (1970, 1971). He proposed that the hippocampus would be the ‘fast learner’ and with its increased plasticity would store memories as they occur. This ‘fast learner’ would act as an intermediate buffer and during offline periods, especially during sleep, would slowly update the ‘slow learner’ (cortex) through memory reactivations. Wilson and McNaughton (1994) and many others later showed evidence for these memory reactivations, supporting this idea.
However, some recent findings have made us question if the hippocampus really ‘contains’ or stores memories. For example, it has been shown that place cells, that were thought be invariant encoders of space, contain much more information that just location and in certain task situations will encode for other elements, such as time elapsed and not space. Furthermore, Tanaka et al. (2018) combined classic, electrophysiological place-cell recordings with the engram tagging technique (Josselyn and Tonegawa, 2020) and showed that, surprisingly, it was not classic place cells that showed plasticity related changes in the form of IEG expression. These, and other findings, have led researchers to propose new theories of hippocampal function, as either a sequence generator (Buzsáki and Tingley, 2018; Swanson et al., 2020) or to enable scene reconstruction (Barry and Maguire, 2019) as covered in the previous section.
We would propose that the hippocampus would have multiple functions that could differ between encoding and recall. During encoding, it would serve as the ‘fast learner’ and intermediate memory buffer. However, this function would usually be very short-lived. In the adult animal or human, with abundant real-world previous knowledge acquired during childhood and adolescence, this buffer function would only be needed for hours and last mainly for the first few nights after the experience. After a night of sleep with reactivations playing the new memories into the cortex reinforcing those connections, the hippocampal memory trace would quickly disappear due to synaptic renormalisation during sleep (see also Navarro-Lobato and Genzel, 2019 for more detail). The hippocampus would be ideal for this role as memory buffer, due to its ability to generate sequences that can be used to quickly map on associations between different ‘what’s’ that can be fed to ‘secondary readers’ downstream (Buzsáki and Tingley, 2018; Swanson et al., 2020).
During memory retrieval, the scene reconstruction properties of the hippocampus would become more important (Barry and Maguire, 2019). Perhaps the reason that episodic memories are ‘dependent’ on the hippocampus, that is, to be able to experience memory retrieval as a vivid memory re-experience, you need the scene reconstruction properties. However, as mentioned above, every night during sleep the hippocampal trace would be renormalised and thus this trace would disappear perhaps not completely but mostly over time. With most memories in the real world, this would occur in a few nights and with more salient memories a longer time may be required.
When an animal is totally naïve to an experience and cannot harness cortical, previous-knowledge networks, the hippocampal trace would be necessary for a longer time period but still undergo deterioration over time. The dopamine signal from the locus coeruleus would facilitate this hippocampal persistence of memory trace (Duszkiewicz et al., 2019).
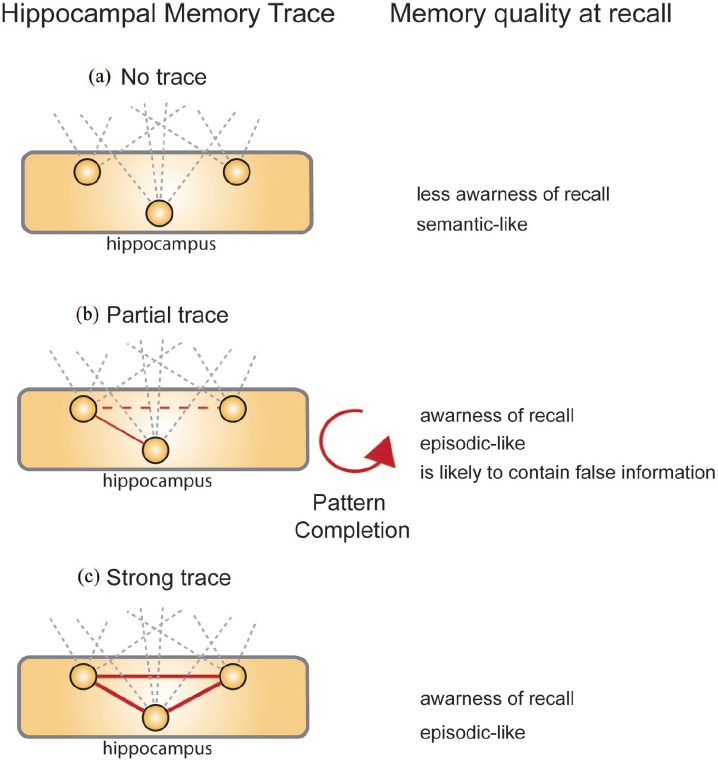
Thus, when recalling a memory, the type of retrieval experience would depend on how much of the hippocampal memory trace is left. If no hippocampal memory trace is left, the hippocampus would have more difficulty in reconstructing the scene. Consequently, you would retrieve a sense of familiarity without explicit experience of recalling a memory (i.e. remembering the event of learning); this would be the case for classic semantic memories (Figure 4(a)). On the other side of the gradient, if the hippocampal memory trace is still mostly intact, scene reconstruction would be faithful to the original experience and retrieval would come in the form of correct, episodic recall (Figure 4(c)). You would become aware of the memory and the past event and thus have more direct recollection of the encoding event instead of just a sense of familiarity. The most interesting case would be when a partial but not complete trace is present in the hippocampus (Figure 4(b)). Then the pattern completion properties of the hippocampus would come into play and the hippocampus would still try to reconstruct the scene for episodic-like recall. However, in absence of the complete, original trace, the memories would become increasingly vulnerable to inaccuracy and distortion, as often can be observed in ‘flashbulb’ memories of unique events (Barry and Maguire, 2019).
Figure 4.
Possible hippocampal function.
How would the hippocampus be involved in memory retrieval? (a) If no hippocampal memory trace is left, memory retrieval would be fully dependent on cortical networks. This would result in less awareness of recall, that is, more a sense of familiarity not explicit recall and a classic semantic memory. (b) If a partial trace is left in the hippocampus, the properties of this brain area would lead to pattern completion therein. Thus, more awareness at recall and episodic-like quality but the memory would also have a higher likelihood of including false information. (c) Finally, if the hippocampus would still contain a strong, complete trace, it would contribute to awareness of recall with episodic-like quality that in this case is still faithful to the original experience.
One implication of this hypothesis is that semantic memories (and thus perhaps statistical regularities) would be recalled more faithfully if no or less of a hippocampal trace is present, in contrast to if a partial trace is present that could generate memory distortions. And since the hippocampal memory trace would decay over time, it would follow that semantic memories would be expressed better after longer time periods.
In sum, we propose that the hippocampus is not simply a brain area for storing memories. Instead, its computational properties as a sequence generator, pattern completer and scene reconstructor can explain its involvement in memory encoding, consolidation and retrieval.
Conclusion
Most of what we learn can be put in context of what we already know. In this review, we have summarised the existing research on neurobiology of previous knowledge, especially in relation to schemas even though in non-human animal research it can be hard to define if a schema in contrast to simpler forms of previous knowledge is truly present. Based on these findings, we proposed a novel theory on how involvement of brain areas can shift from hippocampus to cortex depending on the level and amount of previous knowledge. When going from naïve to expert, the hippocampus loses its critical function during encoding, and cortical areas become more independent.
Building up such cortical networks will usually occur early in the lifespan of a human or any other animal. Perhaps this would also relate to why our cortical networks are more plastic when we are younger and once past adolescence most learning would occur within the context of accumulated world-knowledge decreased cortical plasticity in the adult is likely important to protect our pre-existing knowledge and avoid catastrophic interference when learning something new.
While much has been learned since the concept of previous knowledge and schemas was brought up as a concept in the 1930s (Bartlett, 1932) and then picked up in neurobiology in 2007 (Tse et al., 2007), many open questions remain. For example, how exactly is previous knowledge in the cortex updated? What is the importance of sleep and therein reactivations occurring? Furthermore, we currently focus on hippocampus and cortex as memory structures, but how do other brain areas contribute? The nucleus reuniens has been shown to be critical for long-term memory persistence (Barker and Warburton, 2018; Ferraris et al., 2018; Mei et al., 2018; Troyner et al., 2018; Wagner et al., 2019), but could it be critical for schema updating as well? And how do different connections between the hippocampus and prefrontal cortex contribute to this process? There is a direct connection not only from ventral hippocampus (Jay and Witter, 1991) but also indirect pathways through reuniens (Barker and Warburton, 2018; Ferraris et al., 2018; Mei et al., 2018; Troyner et al., 2018; Wagner et al., 2019) and mPFC (Olafsdottir et al., 2017), but which pathways relate to which role remains unclear.
It is critical for memory researchers to consider the amount of previous knowledge and especially for studies done on non-human animals to venture more into this domain. We are trying to understand human cognition, which rarely does not harness world knowledge. Furthermore, when it comes to real-life application of our results, we have to consider implications on education in the young with less previous knowledge as well as adults who have more (Ruiter et al., 2012; Van Kesteren et al., 2014). For example, we could show in the HexMaze that the time since first exposure is more critical for build-up of previous knowledge than the amount of training an animal has received (Alonso et al., 2020). This stands in opposition to the current preferred practice of ‘cramming’ right before an exam as seen in many high school and university students. Instead, students should space out their learning over longer time periods, if they want to create long-term knowledge instead of just a short-term memory.
Footnotes
Author contributions: All authors wrote the first draft, made the figures, and edited the final version of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Lisa Genzel  https://orcid.org/0000-0001-9537-7959
https://orcid.org/0000-0001-9537-7959
References
- Alberca-Reina E, Cantero JL, Atienza M. (2014) Semantic congruence reverses effects of sleep restriction on associative encoding. Neurobiology of Learning and Memory 110: 27–34. [DOI] [PubMed] [Google Scholar]
- Alonso A, Bokeria L, van der Meij J, et al. (2020) The HexMaze: A previous knowledge and schema task for mice. Biorxiv. Available at: https://www.biorxiv.org/content/10.1101/441048v2 [DOI] [PMC free article] [PubMed]
- Aston-Jones G, Cohen JD. (2005) An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience 28: 403–450. [DOI] [PubMed] [Google Scholar]
- Badham SP, Maylor EA. (2015) What you know can influence what you are going to know (especially for older adults). Psychonomic Bulletin & Review 22: 141–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badham SP, Maylor EA. (2016) Antimnemonic effects of schemas in young and older adults. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 23(1): 78–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraduc P, Duhamel JR, Wirth S. (2019) Schema cells in the macaque hippocampus. Science 363(6427): 635–639. [DOI] [PubMed] [Google Scholar]
- Barker GRI, Warburton EC. (2018) A critical role for the nucleus reuniens in long-term, but not short-term associative recognition memory formation. The Journal of Neuroscience 38(13): 3208–3217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry DN, Maguire EA. (2019) Remote memory and the hippocampus: A constructive critique. Trends in Cognitive Sciences 23(2): 128–142. [DOI] [PubMed] [Google Scholar]
- Bartlett F. (1932) Remembering: A Study in Experimental and Social Psychology. Cambridge: Cambridge University Press. [Google Scholar]
- Bein O, Reggev N, Maril A. (2014) Prior knowledge influences on hippocampus and medial prefrontal cortex interactions in subsequent memory. Neuropsychologia 64: 320–330. [DOI] [PubMed] [Google Scholar]
- Bellezza FS, Buck DK. (1988) Expert knowledge as mnemonic cues. Applied Cognitive Psychology 2(2): 147–162. [Google Scholar]
- Berkers RM, van der Linden M, de Almeida RF, et al. (2017) Transient medial prefrontal perturbation reduces false memory formation. Cortex 88: 42–52. [DOI] [PubMed] [Google Scholar]
- Bethus I, Tse D, Morris RG. (2010) Dopamine and memory: Modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. The Journal of Neuroscience 30(5): 1610–1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovy L, Berkers R, Pottkamper JCM, et al. (2020) Transcranial magnetic stimulation of the medial prefrontal cortex decreases emotional memory schemas. Cerebral Cortex 30(6): 3608–3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brod G, Lindenberger U, Werkle-Bergner M, et al. (2015) Differences in the neural signature of remembering schema-congruent and schema-incongruent events. NeuroImage 117: 358–366. [DOI] [PubMed] [Google Scholar]
- Brod G, Werkle-Bergner M, Shing YL. (2013) The influence of prior knowledge on memory: A developmental cognitive neuroscience perspective. Frontiers in Behavioral Neuroscience 7: 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G, Tingley D. (2018) Space and time: The hippocampus as a sequence generator. Trends in Cognitive Sciences 22(10): 853–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper E, Greve A, Henson RN. (2019) Little evidence for Fast Mapping (FM) in adults: A review and discussion. Cognitive Neuroscience 10(4): 196–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutanche MN, Thompson-Schill SL. (2014) Fast mapping rapidly integrates information into existing memory networks. Journal of Experimental Psychology: General 143(6): 2296–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutanche MN, Thompson-Schill SL. (2015) Rapid consolidation of new knowledge in adulthood via fast mapping. Trends in Cognitive Sciences 19(9): 486–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creighton SD, Collett HA, Zonneveld PM, et al. (2019) Development of an ‘object category recognition’ task for mice: Involvement of muscarinic acetylcholine receptors. Behavioral Neuroscience 133(5): 527–536. [DOI] [PubMed] [Google Scholar]
- Dragoi G, Tonegawa S. (2013) Development of schemas revealed by prior experience and NMDA receptor knock-out. eLife 2: e01326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duszkiewicz AJ, McNamara CG, Takeuchi T, et al. (2019) Novelty and dopaminergic modulation of memory persistence: A tale of two systems. Trends in Neurosciences 42(2): 102–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenbaum H. (2017) Prefrontal-hippocampal interactions in episodic memory. Nature Reviews Neuroscience 18(9): 547–558. [DOI] [PubMed] [Google Scholar]
- Fernández G, Morris RGM. (2018) Memory, novelty and prior knowledge. Trends in Neurosciences 41(10): 654–659. [DOI] [PubMed] [Google Scholar]
- Ferraris M, Ghestem A, Vicente AF, et al. (2018) The nucleus reuniens controls long-range hippocampo-prefrontal gamma synchronization during slow oscillations. The Journal of Neuroscience 38(12): 3026–3038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B. (2005) The organization of recent and remote memories. Nature Reviews Neuroscience 6(2): 119–130. [DOI] [PubMed] [Google Scholar]
- Genzel L. (2020) Memory and sleep: Brain networks, cell dynamics and global states. Current Opinion in Behavioral Sciences 32: 72–79. [Google Scholar]
- Genzel L, Battaglia FP. (2017) Cortico-hippocampal circuits for memory consolidation: The role of the prefrontal cortex. In: Axmacher N, Rasch B. (eds) Cognitive Neuroscience of Memory Consolidation. Cham: Springer International Publishing, pp. 265–281. [Google Scholar]
- Genzel L, Kroes MCW, Dresler M, et al. (2014) Light sleep versus slow wave sleep in memory consolidation: A question of global versus local processes? Trends in Neurosciences 37(1): 10–19. [DOI] [PubMed] [Google Scholar]
- Genzel L, Rossato JI, Jacobse J, et al. (2017) The Yin and Yang of memory consolidation: Hippocampal and neocortical. PLoS Biology 15(1): e2000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genzel L, Schut E, Schroder T, et al. (2019) The object space task shows cumulative memory expression in both mice and rats. PLoS Biology 17(6): e3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh VE, Gilboa A. (2014) What is a memory schema? A historical perspective on current neuroscience literature. Neuropsychologia 53: 104–114. [DOI] [PubMed] [Google Scholar]
- Gottlieb J, Oudeyer P-Y. (2018) Towards a neuroscience of active sampling and curiosity. Nature Reviews Neuroscience 19(12): 758–770. [DOI] [PubMed] [Google Scholar]
- Gruber Matthias J, Gelman Bernard D, Ranganath C. (2014) States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron 84(2): 486–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow HF. (1949) The formation of learning sets. Psychological Review 56(1): 51–65. [DOI] [PubMed] [Google Scholar]
- Hasan M, Kanna MS, Jun W, et al. (2019) Schema-like learning and memory consolidation acting through myelination. The FASEB Journal 33(11): 11758–11775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennies N, Lambon Ralph MA, Kempkes M, et al. (2016) Sleep spindle density predicts the effect of prior knowledge on memory consolidation. The Journal of Neuroscience 36(13): 3799–3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Himmer L, Muller E, Gais S, et al. (2017) Sleep-mediated memory consolidation depends on the level of integration at encoding. Neurobiology of Learning and Memory 137: 101–106. [DOI] [PubMed] [Google Scholar]
- Jay TM, Witter MP. (1991) Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. The Journal of Comparative Neurology 313(4): 574–586. [DOI] [PubMed] [Google Scholar]
- Josselyn SA, Tonegawa S. (2020) Memory engrams: Recalling the past and imagining the future. Science 367(6473): eaaw4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakeyama M, Endo T, Zhang Y, et al. (2014) Disruption of paired-associate learning in rat offspring perinatally exposed to dioxins. Archives of Toxicology 88(3): 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschke JK. (2006) Learned attention. In: Fifth international conference on development and learning, Bloomington, IN; CiteSeer; Available at: https://pdfs.semanticscholar.org/b0c8/4a4fb68d228713050f6b87fb7966c130503e.pdf [Google Scholar]
- Kuhl BA, Chun MM. (2014) Successful remembering elicits event-specific activity patterns in lateral parietal cortex. The Journal of Neuroscience 34(23): 8051–8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesburgueres E, Gobbo OL, Alaux-Cantin S, et al. (2011) Early tagging of cortical networks is required for the formation of enduring associative memory. Science 331(6019): 924–928. [DOI] [PubMed] [Google Scholar]
- McClelland JL. (2013) Incorporating rapid neocortical learning of new schema-consistent information into complementary learning systems theory. Journal of Experimental Psychology: General 142(4): 1190–1210. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O’Reilly RC. (1995) Why there are complementary learning systems in the hippocampus and neocortex: Insights from the successes and failures of connectionist models of learning and memory. Psychological Review 102(3): 419–457. [DOI] [PubMed] [Google Scholar]
- McKenzie S, Robinson NT, Herrera L, et al. (2013) Learning causes reorganization of neuronal firing patterns to represent related experiences within a hippocampal schema. The Journal of Neuroscience 33(25): 10243–10256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara CG, Tejero-Cantero A, Trouche S, et al. (2014) Dopaminergic neurons promote hippocampal reactivation and spatial memory persistence. Nature Neuroscience 17(12): 1658–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Neumann AR, Sun J, et al. (2018) Hippocampus-dependent emergence of spatial sequence coding in retrosplenial cortex. Proceedings of the National Academy of Sciences 115(31): 8015–8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr D. (1970) A theory for cerebral neocortex. Proceedings of the Royal Society B: Biological Sciences 176(1043): 161–234. [DOI] [PubMed] [Google Scholar]
- Marr D. (1971) Simple memory: A theory for archicortex. Philosophical Transactions of the Royal Society B: Biological Sciences 262(841): 23–81. [DOI] [PubMed] [Google Scholar]
- Mei H, Logothetis NK, Eschenko O. (2018) The activity of thalamic nucleus reuniens is critical for memory retrieval, but not essential for the early phase of ‘off-line’ consolidation. Learning & Memory 25(3): 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovitch M, Cabeza R, Winocur G, et al. (2016) Episodic memory and beyond: The hippocampus and neocortex in transformation. Annual Review of Psychology 67: 105–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller NC, Genzel L, Konrad BN, et al. (2016) Motor skills enhance procedural memory formation and protect against age-related decline. PLoS ONE 11(6): e0157770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. (1997) Memory consolidation, retrograde amnesia and the hippocampal complex. Current Opinion in Neurobiology 7(2): 217–227. [DOI] [PubMed] [Google Scholar]
- Navarro-Lobato I, Genzel L. (2019) The up and down of sleep: From molecules to electrophysiology. Neurobiology of Learning and Memory 160: 3–10. [DOI] [PubMed] [Google Scholar]
- Ocampo AC, Squire LR, Clark RE. (2018) The beneficial effect of prior experience on the acquisition of spatial memory in rats with CA1, but not large hippocampal lesions: A possible role for schema formation. Learning & Memory 25(3): 115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsdottir HF, Carpenter F, Barry C. (2017) Task demands predict a dynamic switch in the content of awake hippocampal replay. Neuron 96(4): 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira T, Abreu AM, Castro-Caldas A. (2013) Understanding task- and expertise-specific motor acquisition and motor memory formation and consolidation. Perceptual and Motor Skills 117(1): 1150–1171. [DOI] [PubMed] [Google Scholar]
- Porubanova M, Shaw DJ, McKay R, et al. (2014) Memory for expectation-violating concepts: The effects of agents and cultural familiarity. PLoS ONE 9(4): e90684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. (2013) Interplay of hippocampus and prefrontal cortex in memory. Current Biology 23(17): R764–R773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prull MW. (2015) Adult age differences in memory for schema-consistent and schema-inconsistent objects in a real-world setting. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition 22(6): 731–754. [DOI] [PubMed] [Google Scholar]
- Richards BA, Xia F, Santoro A, et al. (2014) Patterns across multiple memories are identified over time. Nature Neuroscience 17(7): 981–986. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. (1995) Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition 21(4): 803–814. [Google Scholar]
- Ruiter DJ, van Kesteren MT, Fernandez G. (2012) How to achieve synergy between medical education and cognitive neuroscience? An exercise on prior knowledge in understanding. Advances in Health Sciences Education: Theory and Practice 17(2): 225–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schank RC, Abelson RP. (1977) Scripts, Plans, Goals and Understanding: An Inquiry into Human Knowledge Structures. Hillsdale, NJ: Lawrence Erlbaum. [Google Scholar]
- Scimeca JM, Badre D. (2012) Striatal contributions to declarative memory retrieval. Neuron 75(3): 380–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP. (2011) Episodic retrieval and the cortical binding of relational activity. Cognitive, Affective, & Behavioral Neuroscience 11(3): 277–291. [DOI] [PubMed] [Google Scholar]
- Skelin I, Kilianski S, McNaughton BL. (2019) Hippocampal coupling with cortical and subcortical structures in the context of memory consolidation. Neurobiology of Learning and Memory 160: 21–31. [DOI] [PubMed] [Google Scholar]
- Squire LR, Genzel L, Wixted JT, et al. (2015) Memory consolidation. Cold Spring Harbor Perspectives in Biology 7: a021766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RA, Levenstein D, McClain K, et al. (2020) Variable specificity of memory trace reactivation during hippocampal sharp wave ripples. Current Opinion in Behavioral Sciences 32: 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweegers CC, Takashima A, Fernandez G, et al. (2014) Neural mechanisms supporting the extraction of general knowledge across episodic memories. NeuroImage 87: 138–146. [DOI] [PubMed] [Google Scholar]
- Takashima A, Bakker I, van Hell JG, et al. (2014) Richness of information about novel words influences how episodic and semantic memory networks interact during lexicalization. NeuroImage 84: 265–278. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Duszkiewicz AJ, Sonneborn A, et al. (2016) Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537(7620): 357–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A, Ketz N, Davachi L. (2010) Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65(2): 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka KZ, He H, Tomar A, et al. (2018) The hippocampal engram maps experience but not place. Science 361(6400): 392–397. [DOI] [PubMed] [Google Scholar]
- Troyner F, Bicca MA, Bertoglio LJ. (2018) Nucleus reuniens of the thalamus controls fear memory intensity, specificity and long-term maintenance during consolidation. Hippocampus 28(8): 602–616. [DOI] [PubMed] [Google Scholar]
- Tse D, Langston RF, Kakeyama M, et al. (2007) Schemas and memory consolidation. Science 316(5821): 76–82. [DOI] [PubMed] [Google Scholar]
- Tse D, Takeuchi T, Kakeyama M, et al. (2011) Schema-dependent gene activation and memory encoding in neocortex. Science 333(6044): 891–895. [DOI] [PubMed] [Google Scholar]
- Umanath S, Marsh EJ. (2014) Understanding how prior knowledge influences memory in older adults. Perspectives on Psychological Science 9(4): 408–426. [DOI] [PubMed] [Google Scholar]
- Van Buuren M, Kroes MC, Wagner IC, et al. (2014) Initial investigation of the effects of an experimentally learned schema on spatial associative memory in humans. The Journal of Neuroscience 34(50): 16662–16670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Linden M, Berkers R, Morris RGM, et al. (2017) Angular gyrus involvement at encoding and retrieval is associated with durable but less specific memories. The Journal of Neuroscience 37(39): 9474–9485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MTR, Beul SF, Takashima A, et al. (2013) Differential roles for medial prefrontal and medial temporal cortices in schema-dependent encoding: From congruent to incongruent. Neuropsychologia 51(12): 2352–2359. [DOI] [PubMed] [Google Scholar]
- Van Kesteren MTR, Brown TI, Wagner AD. (2018) Learned spatial schemas and prospective hippocampal activity support navigation after one-shot learning. Frontiers in Human Neuroscience 12: 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MTR, Fernandez G, Norris DG, et al. (2010. a) Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proceedings of the National Academy of Sciences 107(16): 7550–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MTR, Rijpkema M, Ruiter DJ, et al. (2010. b) Retrieval of associative information congruent with prior knowledge is related to increased medial prefrontal activity and connectivity. The Journal of Neuroscience 30(47): 15888–15894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kesteren MTR, Rijpkema M, Ruiter DJ, et al. (2014) Building on prior knowledge: Schema-dependent encoding processes relate to academic performance. Journal of Cognitive Neuroscience 26(10): 2250–2261. [DOI] [PubMed] [Google Scholar]
- Van Kesteren MTR, Ruiter DJ, Fernandez G, et al. (2012) How schema and novelty augment memory formation. Trends in Neurosciences 35(4): 211–219. [DOI] [PubMed] [Google Scholar]
- Vuvan DT, Podolak OM, Schmuckler MA. (2014) Memory for musical tones: The impact of tonality and the creation of false memories. Frontiers in Psychology 5: 582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner IC, van Buuren M, Fernandez G. (2019) Thalamo-cortical coupling during encoding and consolidation is linked to durable memory formation. NeuroImage 197: 80–92. [DOI] [PubMed] [Google Scholar]
- Wagner IC, van Buuren M, Kroes MC, et al. (2015) Schematic memory components converge within angular gyrus during retrieval. eLife 4: e09668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SH, Morris RGM. (2009) Hippocampal-neocortical interactions in memory formation, consolidation, and reconsolidation. Annual Review of Psychology 61: 49–79. [DOI] [PubMed] [Google Scholar]
- Wang SH, Tse D, Morris RG. (2012) Anterior cingulate cortex in schema assimilation and expression. Learning & Memory 19: 315–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren DE, Jones SH, Duff MC, et al. (2014) False recall is reduced by damage to the ventromedial prefrontal cortex: Implications for understanding the neural correlates of schematic memory. The Journal of Neuroscience 34(22): 7677–7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. (1994) Reactivation of hippocampal ensemle memories during sleep. Science 265(5172): 676–679. [DOI] [PubMed] [Google Scholar]
- Zeithamova D, Dominick AL, Preston AR. (2012) Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron 75(1): 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]