Abstract
Mastocytosis is an accumulation of clonal mast cells within tissues and it is most commonly caused by an activating mutation in the KIT gene. In this study, we report a neonatal case who presented with diffuse cutaneous mastocytosis (CM) at birth. In China, nine other cases of neonatal-onset CM have been reported in the literature since 2006. In those cases, diffuse CM and urticaria pigmentosa were the main symptoms, and mutations in exon 17 at codon 816 in KIT were identified.
Keywords: Neonate, KIT gene, diffuse cutaneous mastocytosis, mast cell, serum tryptase, mastocytosis
Introduction
Mastocytosis is a heterogeneous group of disorders characterized by the proliferation and accumulation of mast cells in the skin and other organs, including hematopoietic organs.1 Generally, childhood-onset mastocytosis is characterized by cutaneous involvement, most commonly urticaria pigmentosa (UP, also called maculopapular mastocytosis), mastocytoma, and, less frequently, diffuse cutaneous mastocytosis (DCM).2 Mastocytosis is a clonal disease associated with mutations of the proto-oncogene KIT in both adult-onset and pediatric mastocytosis.3 A mutation of codon 816 (exon 17) in the KIT gene has been identified in 42% of children with mastocytosis, whereas mutations in exons 8 to 11 (i.e., outside exon 17) have been observed in 44% of cases.3,4
CM is a rare, severe variant that generally presents in the neonatal period, but the prognosis remains unclear. Here, we present a case of a neonate with characteristic cutaneous findings and discuss the clinical phenotype and genetic mutation identified. We also present a comprehensive review of all cases of neonatal cutaneous mastocytosis reported in China during the past 13 years.
Case report
The patient was a male infant who was the second child of healthy parents. He was born at 38 weeks of gestation with a birth weight of 3190 g via spontaneous vaginal delivery to a 31-year-old mother after an uncomplicated pregnancy. He has a 2-year-old healthy sister. The amniotic fluid was contaminated when the child was born, and the umbilical cord was wrapped around the neck. The Apgar score was 10 at 1 and 5 minutes. The neonate presented with firm, waxy, red-brown, indurated papules and plaques with discrete necrotic areas and few scattered vesicles on the skin of the scalp, face, trunk, extremities, palms, and soles at birth. Three hours after birth, he was transferred from a local hospital to our neonatal intensive care unit.
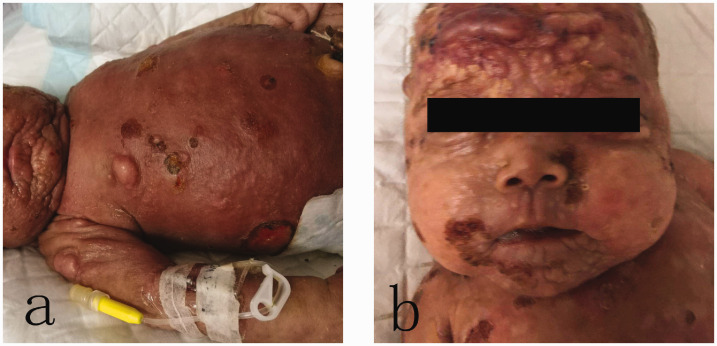
Physical examination revealed that the whole skin surface was covered with firm, waxy, red-brown, indurated papules and plaques, with discrete necrotic areas and few scattered vesicles (Figure 1). Hepatosplenomegaly was evident. The patient was given intensive care and skin care after admission. Antibiotics were administered for a suspected skin infection. After a clinical diagnosis of cutaneous mastocytosis, dexamethasone was given at 0.2 mg/kg per day for 1 week.
Figure 1.
(a) Indurated, red-brown, pachydermatous plaques scattered over the whole body. Note abdominal distension due to hepatosplenomegaly. (b) Diffuse nodules and blood blisters were scattered over the whole body at the second hospitalization 2 months after birth.
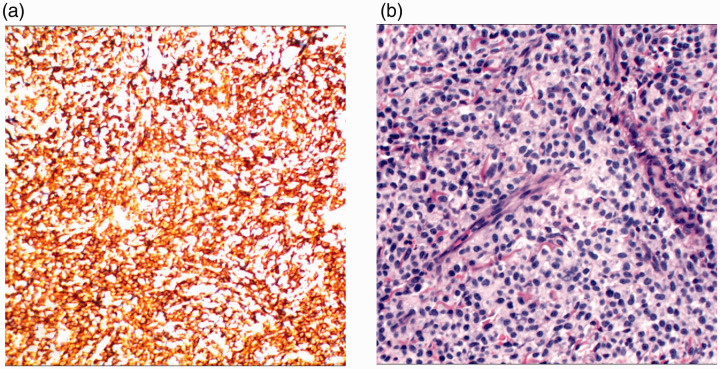
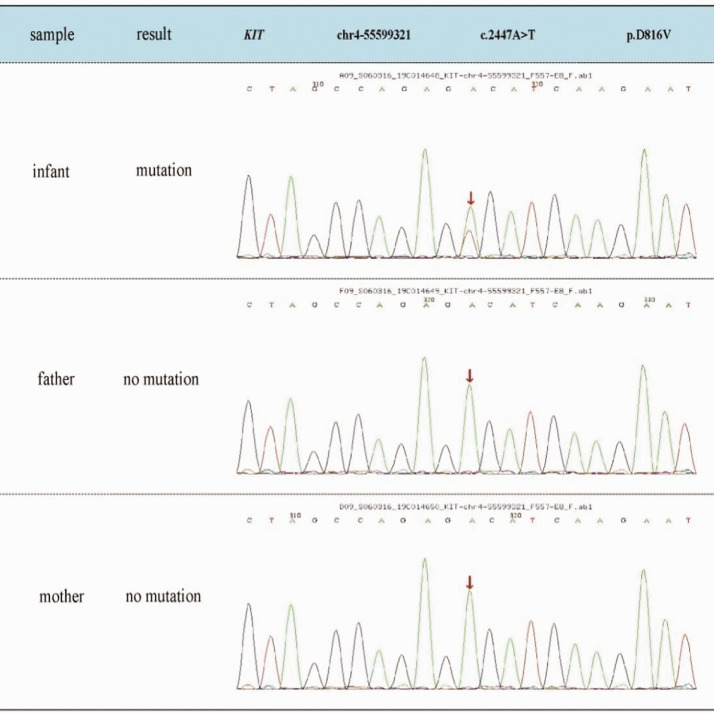
A laboratory work-up was carried out and results showed that bone marrow cytologic examination was negative and cerebrospinal fluid examination was normal. Color Doppler ultrasound of the liver and spleen confirmed hepatosplenomegaly and showed rough spots and enhanced echo. Cardiac color Doppler ultrasound showed that the patient had severe pulmonary hypertension, the arterial duct was not closed, and a sieve-type atrial septal defect was evident. Chest computed tomography (CT) indicated pneumonia with CT consolidation of the upper lobe part of the lungs and local emphysema-like changes. The upper lobe of the lungs and the bronchus below it could not be clearly observed by CT. Evaluation of a skin biopsy (Figure 2) indicated that mast cells were diffusely infiltrated in the whole layer of skin and dermis. Toluidine blue dyeing was positive. Immunohistochemistry showed CD45+, CD30+, CD34−, MPO−, CD43+, CD68+, CD117+, CD2+, BCL-2 individual+, and Ki-67 (3 to 5% +). With the parents’ consent, peripheral blood was drawn from the patient and the parents for genetic testing. The disease-related region p.D816V (2447A > T) in exon 17 of the KIT gene was sequenced in peripheral blood of the patient and his parents’ results were verified by Sanger sequencing. A mutation, p.D816V (2447A > T) was identified in exon 17 of the KIT gene (Figure 3). Sanger sequencing of the parents’ DNA showed no mutation in this region.
Figure 2.
(a) Immunohistochemical staining was positive for CD117/KIT. (b) Hematoxylin and eosin staining demonstrating sheets of mast cells within the dermis (400×) indicative of cutaneous mastocytosis.
Figure 3.
Sequence diagrams for the patient and parents. Genetic testing revealed that the patient had a heterozygous mutation in the KIT gene, c.2447A > T (p.D816V). No mutations of the KIT gene were found in the parents.
The patient’s first total hospital admission time was 30 days. During this time, he had a slight reduction in rash. Oral prednisone treatment continued after discharge. The patient was admitted again to the neonatal intensive care unit with main complaints of dyspnea, convulsions, and rash; he died 1 day later due to severe infection, respiratory failure, and circulatory failure.
Discussion
Mastocytosis results from the clonal proliferation of mast cells, which is caused by occasional somatic activation of mutations in the mast growth factor receptor c-Kit/CD117.5,6 KIT is a receptor for trypsin kinase, which is mainly responsible for mast cell growth, proliferation, and survival.7 Previous studies have suggested that mastocytosis in children is a benign and self-limiting disease, and analysis of the literature shows that in 67% of cases in children, the disease had partially or completely regressed. In the remaining one-third of patients, the condition may persist or progress. Mastocytosis has a varied clinical presentation, leading to different outcomes depending on the mast cell burden and the extent of tissue involvement.
Meni et al. reviewed 1747 cases published between 1950 and April 2014 to clarify the characteristics and course of pediatric mastocytosis.8 In that review, the authors first confirmed the reversal of the sex ratio in childhood disease compared with adults, with a preponderance of male patients in children (male:female ratio of 1.4:1). The review found that lesions occurred before the age of 2 years in 90% of cases, presenting as UP (75% of cases), mastocytoma (20%), or DCM (5%). The D816V mutation in the KIT gene was detected in 34% of 215 tested patients. Clinical regression (complete or partial) occurred in 67% of cases and stabilization occurred in 27%. However, 2.9% of patients died of the disease.8
In the past 13 years, 10 cases (including one pair of twins) of neonatal cutaneous mastocytosis, with a male:female ratio of 3:2, were reported in mainland China.9–16 Lesions presented as UP (1 case) or DCM (9 cases); mastocytoma was not observed (Table 1). Four patients were genetically tested. The clinical manifestations of the twins were DCM with the same genotypes; both had a mutation (NM-000222) in exon 17: c.2446-2447GA > TT (p.D816F). Another case was UP with genotype KIT-D816V (+). The child we reported had DCM and genotype NM-000222; exon 17; c.2447A > T (p.D816V) and died at 2 months of age. No studies have confirmed the relevance of the KIT mutation site to the outcome of mastocytosis.
Table 1.
Summary of clinical characteristics and laboratory results for neonatal mastocytosis cases reported during the past 13 years in China.
| Case (reference) | Year | Sex | Age | GA (weeks) | BW (kg) | Clinical symptoms | Gene mutation |
|---|---|---|---|---|---|---|---|
| 19 | 2006 | F | 25 days | Term | Unknown | Diffuse cutaneous mastocytosis | Not investigated |
| 210 | 2009 | M | 33 days | Term | 3.5 | Diffuse cutaneous mastocytosis | Not investigated |
| 316 | 2010 | F | 15 hours | Term | 3.1 | Diffuse cutaneous mastocytosis | Not investigated |
| 411 | 2010 | F | 3 hours | 40 | 3.25 | Diffuse cutaneous mastocytosis | Not investigated |
| 512 | 2012 | F | 4 days | Term | Unknown | Diffuse cutaneous mastocytosis | Not investigated |
| 613 | 2014 | M | 2 hours | Term | Unknown | Diffuse cutaneous mastocytosis | Not investigated |
| 7*14 | 2016 | F | 4 days | 36+1 | 2.6 | Erythema bullous nodule | Exon 17: 2446-2447 GA>TT (p.D816F) |
| 8*14 | 2016 | F | 4 days | 36+1 | 2.45 | Same as case 7 | Same as in case 7 |
| 915 | 2017 | M | 11 months | Term | Unknown | Urticaria pigmentosa | KIT, D816V (+) |
| 10 (current report) | 2019 | M | 3 hours | 38 | 3.19 | Diffuse cutaneous mastocytosis | Exon 17; c.2447A > T (p.D816V) |
GA, gestational age; BW, birth weight; F, female; M, male.
*Cases 7 and 8 are twins.
In adults, the KIT D816V allele load in peripheral blood is associated with serum tryptase level.17,18 Recent studies have shown that identifying and quantifying KIT D816V mutations in adult peripheral blood is not only of diagnostic importance19,20 but it can also determine mast cell burden, permit monitoring,21 and be used to assess the response to treatment.18
Carter et al.22 evaluated data on 65 children with mastocytosis, some of whom were shown to have systemic disease. They correlated KIT mutation status with clinical manifestations, serum tryptase levels, and bone marrow histopathology. In the study, they found no KIT D816V mutations (100% specificity) in peripheral blood of children with cutaneous disease. However, the KIT D816V mutation was present in peripheral blood of patients with systemic or likely systemic disease (sensitivity of 85.2%).22
Four children diagnosed with systemic mastocytosis by bone marrow biopsy had low mast cell burden, splenomegaly that subsided, and serum tryptase levels that decreased over time; the allele-specific quantitative PCR (ASqPCR) value was negative. These results are consistent with those of a previous study.23 The children no longer need daily medication, the skin lesions have gradually cleared, and serum tryptase level is significantly reduced, indicating that the mast cell burden is also decreasing. Therefore, if there is a clinical manifestation of organomegaly in children with cutaneous mastocytosis and a positive peripheral blood ASqPCR test, there is a high possibility of systemic disease. However, the onset of CM in children, especially in neonates, lacks long-term and large-scale follow-up studies to date. The study of prognostic-related factors of neonatal-onset CM will be the focus of future work.
DCM should be differentiated from maculopapular cutaneous mastocytosis (UP) and solitary cutaneous mastocytoma. DCM is characterized by the infiltration of most or all skin mast cells, rather than discrete lesions of other forms of skin mastocytosis. The skin thickness is typically increased, with color ranging from yellow-brown to red, and bullous eruptions may occur.24 Maculopapular cutaneous mastocytosis presents with pruritic, yellow-tan to reddish-brown macules/papules on the trunk and proximal extremities; in solitary cutaneous mastocytoma, pruritus is variable.
Many high-risk factors trigger DCM, including medications, foods, emotional stressors, temperature changes, dry skin, infections and trauma to lesions, lukewarm baths, and use of emollients.25 There is no cure for cutaneous mastocytosis. Treatment focuses on avoiding triggers and treating mast cell mediators. Both H1 and H2 antagonists can be used to treat flushing and itching in patients with DCM. Topical use of creams containing cromolyn sodium may also help relieve these symptoms.26 H2 antagonists and oral mast cell stabilizers (such as cromolyn sodium) can be used to reduce gastrointestinal symptoms associated with the disease. Symptoms of allergic reactions require administration of adrenaline.25 Oral corticosteroids can be used to treat severe skin lesions, but long-term use is not recommended. In our case, dexamethasone was given at 0.2 mg/kg per day for 1 week. Oral prednisone treatment continued after discharge for about 1 month. However, there are currently no dosing guidelines for the treatment of neonatal DCM. With the clarification of gene mutation sites by genetic testing, targeted therapy can be adopted for the mutation sites, and inhibiting the activation of the mutated KIT protein may become a new type of treatment.
In summary, neonatal mastocytosis is rare, making it difficult to diagnose and to determine whether a neonate has systemic disease. In addition to the skin manifestations of neonatal mastocytosis, other symptoms may be atypical. Combining the results of KIT mutation analysis, serum tryptase levels, and bone marrow biopsy, if necessary, may help us to determine whether a patient has systemic mastocytosis. It may be useful to correlate outcomes with KIT mutations to determine whether some types of mutations are linked to a better or worse prognosis in neonates with mastocytosis. Further studies should focus on this aspect in neonates to understand the pathophysiology of mastocytosis and define appropriate follow-up and treatment methods.
Author contributions
All authors read and approved the final manuscript as submitted and agreed to be accountable for both their own contributions and the accuracy and integrity of the work.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Ethics statement and informed consent
This study received ethical approved from the Research Ethics Boards of Qilu Children’s Hospital, Shandong University. Written informed consent for publication of clinical details and images was obtained from the parents of the patient.
ORCID iD
Xinjie Liu https://orcid.org/0000-0001-9911-2229
References
- 1.Castells M, Metcalfe DD, Escribano L. Diagnosis and treatment of cutaneous mastocytosis in children: practical recommendations. Am J Clin Dermatol 2011; 12: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akin C. Mastocytosis. Immunol Allergy Clin North Am 2014; 34: xvii–xviii. [DOI] [PubMed] [Google Scholar]
- 3.Bodemer C, Hermine O, Palmérini F, et al. Pediatric mastocytosis is a clonal disease associated with D816V and other activating c-KIT mutations. J Invest Dermatol 2010; 130: 804–815. [DOI] [PubMed] [Google Scholar]
- 4.Lanternier F, Cohen-Akenine A, Palmerini F, et al. Phenotypic and genotypic characteristics of mastocytosis according to the age of onset. PLoS One 2008; 3: e1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onnes MC, Tanno LK, Elberink JN. Mast cell clonal disorders: classification, diagnosis and management. Curr Treat Options Allergy 2016; 3: 453–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valent P. Diagnosis and management of mastocytosis: an emerging challenge in applied hematology. Hematology Am Soc Hematol Educ Program 2015; 2015: 98–105. [DOI] [PubMed] [Google Scholar]
- 7.Tremblay D, Carreau N, Kremyanskaya M, et al. Systemic mastocytosis: clinical update and future directions. Clin Lymphoma Myeloma Leuk 2015; 15: 728–738. [DOI] [PubMed] [Google Scholar]
- 8.Meni C, Bruneau J, Georgin-Lavialle S, et al. Paediatric mastocytosis: a systematic review of 1747 cases. Br J Dermatol 2015; 172: 642–651. [DOI] [PubMed] [Google Scholar]
- 9.Long YG, Liu K, Chen XX, et al. One case of neonatal diffuse cutaneous mastocytosis. J Clin Dermatol 2010; 39: 187–188. [Google Scholar]
- 10.Liang N, Zhao HL, Li SQ, et al. One case of neonatal cutaneous mastocytosis. J Clin Dermatol 2011; 40: 95–96. [Google Scholar]
- 11.Yin XJ, An YL, Zhang ZM, et al. One case of neonatal diffuse cutaneous mastocytosis. Chin J Neonatol 2010; 25: 114. [Google Scholar]
- 12.Deng RY, Chen L, Long YG, et al. One case of neonatal diffuse cutaneous mastocytosis. J Clin Dermatol 2015; 44: 248–249. [Google Scholar]
- 13.Chen DM, Xu JL, Wang RQ, et al. One case of neonatal diffuse cutaneous mastocytosis. Chin J Pediatr 2015; 53: 305–306. [Google Scholar]
- 14.Zhang HG, Liu Q, Ye Y, et al. Somatic mutations cause congenital diffuse cutaneous mastocytosis in identical twins. Chin J Pediatr 2017; 55: 706–707. [DOI] [PubMed] [Google Scholar]
- 15.Wang JC, Liu XY, Hu J, et al. A case of KIT-D816V positive infantile cutaneous mastocytosis. Chin J Dermatol 2017; 50: 135. [Google Scholar]
- 16.Li YM, Li N, Xu W, et al. One case of neonatal systemic mastocytosis. Chin J Contemp Pediatr 2011; 13: 848–849. [PubMed] [Google Scholar]
- 17.Kristensen T, Broesby-Olsen S, Vestergaard H, et al. Serum tryptase correlates with the KIT D816V mutation burden in adults with indolent systemic mastocytosis. Eur J Haematol 2013; 91: 106–111. [DOI] [PubMed] [Google Scholar]
- 18.Hoermann G, Gleixner KV, Dinu GE, et al. The KIT D816V allele burden predicts survival in patients with mastocytosis and correlates with the WHO type of the disease. Allergy 2014; 69: 810–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kristensen T, Vestergaard H, Bindslev-Jensen C, et al. Sensitive KIT D816V mutation analysis of blood as a diagnostic test in mastocytosis. Am J Hematol 2014; 89: 493–498. [DOI] [PubMed] [Google Scholar]
- 20.Jara-Acevedo M, Teodosio C, Sanchez-Munoz L, et al. Detection of the KIT D816V mutation in peripheral blood of systemic mastocytosis: diagnostic implications. Mod Pathol 2015; 28: 1138–1149. [DOI] [PubMed] [Google Scholar]
- 21.Erben P, Schwaab J, Metzgeroth G, et al. The KIT D816V expressed allele burden for diagnosis and disease monitoring of systemic mastocytosis. Ann Hematol 2014; 93: 81–88. [DOI] [PubMed] [Google Scholar]
- 22.Carter MC, Bai Y, Ruiz-Esteves KN, et al. Detection of KIT D816V in peripheral blood of children with manifestations of cutaneous mastocytosis suggests. Br J Haematol 2018; 183: 775–782. [DOI] [PubMed] [Google Scholar]
- 23.Carter MC, Clayton ST, Komarow HD, et al. Assessment of clinical findings, tryptase levels, and bone marrow histopathology in the management of pediatric mastocytosis. J Allergy Clin Immunol 2015; 136: e1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hartmann K, Henz BM. Mastocytosis: recent advances in defining the disease. Br J Dermatol 2001; 144: 682–695. [DOI] [PubMed] [Google Scholar]
- 25.Escribano L, Akin C, Castells M, et al. Mastocytosis: current concepts in diagnosis and treatment. Ann Hematol 2002; 81: 677–690. [DOI] [PubMed] [Google Scholar]
- 26.Matito A, Carter M. Cutaneous and systemic mastocytosis in children: a risk factor for anaphylaxis? Curr Allergy Asthma Rep 2015; 15: 22. [DOI] [PubMed] [Google Scholar]