Abstract
Visual loss after spine surgery in the prone position is a disastrous postoperative complication because it is almost irreversible. Additionally, the optimal treatments and recommended professional guidelines for visual loss after spine surgery are deficient. A 43-year-old man developed visual loss after spine surgery in the prone position. Immediate ophthalmic consultation confirmed central retinal artery occlusion. Therefore, combined therapies were administered, including neurotrophy, anticoagulation, vasodilation, and adequate fluid infusion, followed by hyperbaric oxygen treatment. After active treatment, his visual acuity gradually recovered from 5 hours postoperatively and continued to improve thereafter. We reviewed the literature on postoperative visual loss with a focus on spine surgery in the prone position. Because the etiology of this complication is complex and has few effective treatments, the best method for its avoidance is to pay close attention to preventing it during surgery.
Keywords: Postoperative visual loss, visual acuity, ischemic optic neuropathy, retinal artery occlusion, prone position, spine surgery
Background
Visual loss after spine surgery in the prone position is a rare complication with an incidence of about 0.017% to 1.000%.1,2 Although rare, it is a potentially disastrous postoperative complication and may even be irreversible. Visual loss after spine surgery is attributable to various risk factors, including excessive blood loss, a long surgical duration, hypoxia, use of vasoconstrictors, hypotension, high venous pressure, high-volume fluid replacement, and poor head positioning.3 These risk factors might lead to ischemic optic neuropathy (ION), central retinal artery occlusion (CRAO), or retinal vein occlusion. Patients with carotid stenosis or occlusion are reportedly more vulnerable to this complication than those without stenosis.4 Although CRAO and ION might be the ordinal causes of postoperative visual loss (POVL),5 the exact pathophysiologic mechanism of POVL has not been determined.6 We herein describe a patient who developed transient unilateral visual loss after spine surgery in the prone position under general anesthesia (GA), although the patient lacked the above-mentioned recognized perioperative risk factors for POVL. Because of timely diagnosis and therapy, his visual acuity (VA) recovered soon after surgery. This patient was fortunate because of his very quick recovery, which differs from most reported cases. We also herein analyze the possible etiologies and effective treatments to help avoid this complication in future.
Case presentation
A 43-year-old man was admitted because of the development of repeated numbness and pain in the left leg with recent worsening of his symptoms. His weight was 78 kg, height was 170 cm, and body mass index was 26.99 kg/m2. His medical history was unremarkable. Physical examination revealed left-sided tenderness at L5 to S1 with radiation to the left hip. No other abnormalities were found, including VA abnormalities or cerebral infarction. Most of his laboratory examination findings were within the reference ranges. Magnetic resonance imaging indicated lumbar disc herniation from L5 to S1.
The patient was scheduled to undergo L5 to S1 percutaneous endoscopic lumbar discectomy under GA. Anesthesia was induced with intravenous administration of 2 mg midazolam, 50 mg propofol, 20 µg sufentanil, and 10 mg cisatracurium. He was intubated with a 7.0 reinforced endotracheal tube and placed in the prone position. GA was maintained with propofol at 4 mg/kg/h and oxygen at 3 L/min in conjunction with administration of 10 mg cisatracurium and 30 µg sufentanil during the operation. The mechanical ventilation protocol included an inspiratory:expiratory ratio of 1:2 and an inspired oxygen fraction of 1.0. The tidal volume and respiratory rate were regulated to maintain the end-tidal carbon dioxide partial pressure within the normal range.
The total duration of anesthesia was 150 minutes. During the operation, the total fluid input was 1600 mL crystalloids, the urine output was 100 mL, and the estimated blood loss was 10 mL. His noninvasive blood pressure and heart rate measurements were 105–143/52–81 mmHg and 55 to 92 beats/minute, respectively. He was extubated 5 minutes after the end of the operation without administration of medications.
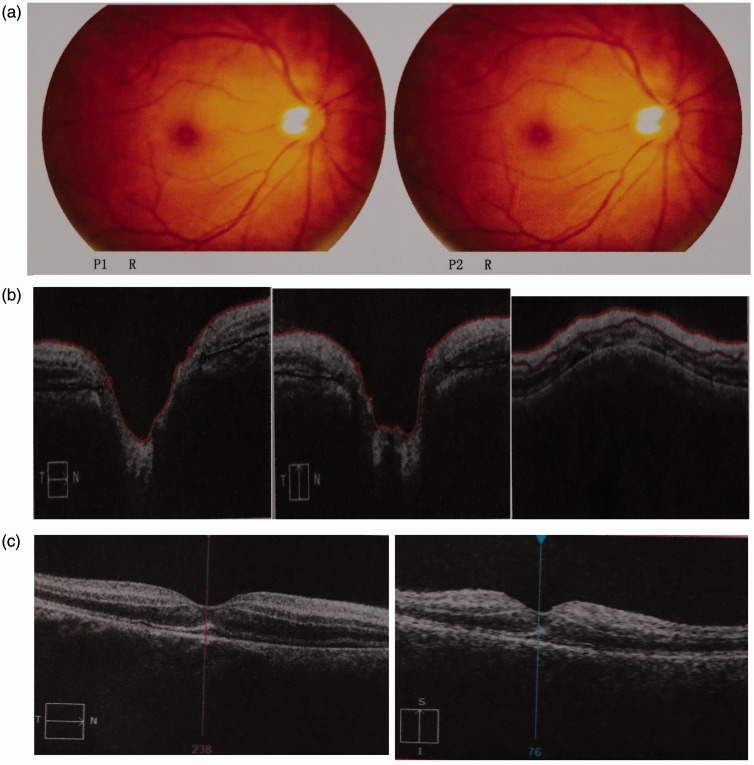
Twenty minutes postoperatively, on fully waking up from GA, he complained of no vision and light perception in his right eye without remarkable eye pain. The appearance and movement of both of his eyelids and bulbus oculi were normal, as were both pupils. An on-call team performed further ophthalmic and neurologic examinations at the bedside. The ophthalmic examination demonstrated that his right eye had no light perception. However, his left eyelid, bulbus oculus, and conjunctiva were normal, and his corneas and lenses were clear bilaterally. The diameter of both pupils was 2.5 mm. The right direct light pupillary reflex was absent, but the indirect light reflex was present; i.e., both pupils shrank when light was shone in the left eye, but they did not shrink when light was shone in the right eye. When light was alternately shone in both eyes, the right pupil enlarged and the left pupil shrank; thus, a relative afferent pupillary defect was elicited. The intraocular pressure was normal, and the patient therefore received no intraocular pressure-lowering medications. The right fundus examination revealed an attenuated retinal artery, pale optic disc, and cherry-red macula without obvious exudation or vitreous bleeding. Optical coherence tomography revealed an edematous retina (Figure 1(a)–(c)).
Figure 1.
Fundus photography of the right eye. (a) Fundus photography (P1 R and P2 R) showed thinning of the retinal artery with a pale optic disc and cherry-red macula. Optical coherence tomography of the right eye revealed an edematous retina. (b) Optic disc cube image and (c) macular cube image.
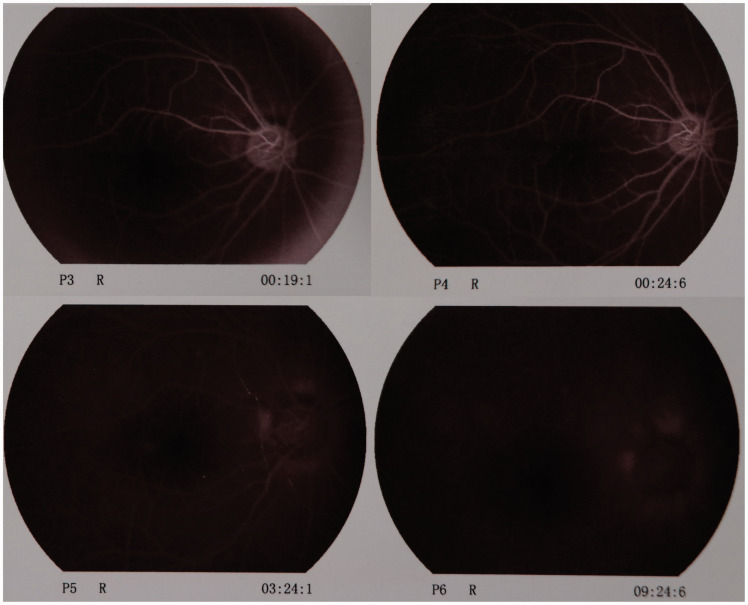
Because right CRAO was suspected, a peribulbar injection of 0.5 mg atropine was immediately administered. At the same time, 4100 IU low-molecular-weight heparin calcium was injected subcutaneously, and 30 mg papaverine was injected intramuscularly. The patient was also treated with high-flow oxygen inspiration and adequate fluid infusion. Five hours postoperatively, the VA of his affected eye recovered to counting fingers. Fundus examination revealed that the color of the retina had returned to normal with a pink macula, and the retinal artery was mostly normal. Fluorescein fundus angiography (FFA) showed that the arm-to-retina circulation time was prolonged and that the choroidal background fluorescence and retina artery were being slowly perfused; perfusion was still demonstrated after 24 s. In the later phase of FFA, a small amount of fluorescein leakage was present on the margin of the optic disc (Figure 2). The anticoagulation, vasodilation, and neurotrophy therapies were continued, followed by hyperbaric oxygen treatment the next day.
Figure 2.
Fundus fluorescein angiography of the right eye 7 hours after treatment. The arm-to-retina circulation time was prolonged and the choroidal background fluorescence and retina artery were slowly perfused; perfusion was even demonstrated after 24 seconds. In the later phase of fundus fluorescein angiography, slight fluorescein leakage was present on the margin of the optic disc.
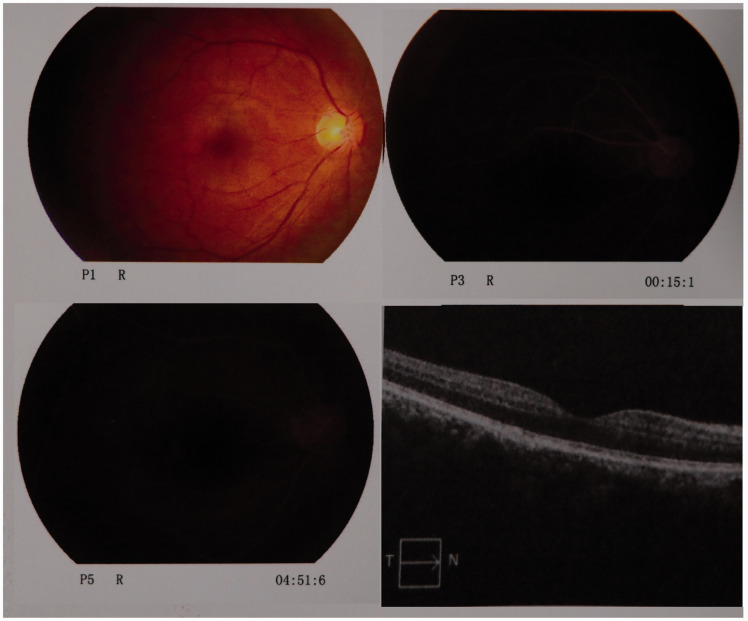
On the first postoperative day, ophthalmic examination showed light perception in the affected eye without amaurosis. The diameter of the right pupil was 6 mm with a sluggish papillary reaction because of the long-acting mydriatic. A bedside ophthalmic consultation on the second postoperative day showed that the VA of his right eye was 0.1 without a visual field defect, and that in his left eye was 0.5. The VA was measured using the decimal point method. Further fundus examination revealed a normal retina with a pink optic disc and macula. The retinal artery was slim. On the fifth postoperative day, the diameter of the affected eye pupil had shrunk to 4 mm with an obtuse light reaction. On the 11th postoperative day, he underwent another ophthalmic consultation. He reported slight blurring of vision in the right eye. The right and left VA was 0.3 and 0.6, respectively. The bilateral conjunctivae showed no obvious hyperemia or hydroncus. The bilateral corneas were clear, the diameters of the pupils were 3 mm, and the pupils were equal and round with acute reaction to light. No keratic precipitate or aqueous flare was present on either side. The bilateral lenses and vitreous were clear. Fundus examination showed that the margin of the yellowish-orange optic disc was distinct without expansion or deepening, and the macula was pink (Figure 3). The retinal artery:vein ratio was 1:3.
Figure 3.
Sixteen days after treatment, fundus photography showed a normal retina color with a pink macula, and the retina artery was mostly normal (P1 R). Fundus fluorescein angiography was performed (P3 R and P5 R). A macular region optical coherence tomography scan showed that the retina edema had faded (lower right).
Conclusion and literature review
Although POVL following spinal surgery in the prone position is rare, it is a potentially disastrous complication that may be irreversible and can seriously reduce patients’ quality of life. In 2016, Epstein3 reviewed 20 cases of blindness after spine surgery; unexpectedly, 8 patients had permanent POVL. Therefore, this complication must be given more attention. In 2012, Quraishi et al.7 described a patient who lost bilateral eyesight following lumbar surgery in the prone position; however, the patient’s VA recovered within 48 hours. That case might involve the shortest known period of visual loss. However, our patient’s VA recovered within 5 hours, and follow-up ophthalmic examination confirmed the VA of the affected eye recovered to 0.3. Therefore, this was a fortunate case with the most rapid recovery reported to date.
Visual loss after spine surgery under GA in the prone position has recently been gaining increasingly more recognition. In the United States, the incidence of this complication was the second highest among all cases of POVL after nonocular operations.8 ION is the most frequently cited cause of POVL under GA.9 No less than 89% of all cases of POVL are attributed to ION, which includes anterior ION and posterior ION depending on the location of the lesion.3,5 Because of our patient’s reduced light reflex and edematous retina, we suspected that CRAO had led to ION.
There are multiple risk factors for the development of POVL in the prone position. However, the patient in our case had none of these recognized or suspected risk factors, such as obesity, a prolonged operation time, or diabetes.3 During the operation, his hemodynamic parameters were tightly controlled and remained stable without great blood loss. Additionally, his head was secured with no pressure on the globe and positioned correctly in a neutral prone position without cervical rotation. Moreover, his head was maintained in a neutral forward position higher than his heart during the operation.
Our patient might have had other risk factors, such being a man. Male sex is a known risk factor for POVL.1,8 Additionally, he was in the prone position during surgery, which might have increased the direct pressure on the abdomen and obstructed the venous return to the heart. Obstruction of venous return increases the central venous pressure and thus further increases the intraocular pressure. This is another recognized risk factor for POVL.2,7 The patient lost about 10 mL of blood and 100 mL of urine, and his intraoperative fluid input was about 1600 mL of crystalloids. Although the fluid volume was not large, colloids were lacking. Intraoperative crystalloid overload contributes to optic nerve perfusion pressure reduction, which is also considered to result in POVL.10 The 1600-mL crystalloid volume might not be considered overload for a 78-kg man; we merely speculated that colloid shortage was a contributing factor.
However, 1600 mL of crystalloids is actually considered a shortage for this 78-kg man because he had fasted for >10 hours preoperatively. Such a shortage might cause hypercoagulability, which is a risk factor for CRAO.11 Therefore, various anticoagulation and vasodilation treatments and adequate fluid infusion were administered.
Although muscarinic agonists can produce intact blood vessel dilation in various species,12 this vasorelaxation only occurs in normal blood vessels. For abnormal blood vessels, muscarinic agonist might cause paradoxical vasoconstriction.13 Thus, based on previous clinical experience, the patient was administered an atropine peribulbar injection, which might have relieved the spasm of his retinal artery.14
The patient was also treated with high-flow oxygen inspiration immediately after returning to his ward because the treatment could be easily implemented by orthopedists. Additionally, hyperbaric oxygen was started on the first postoperative day following the suggestions of the ophthalmologist. Oxygen therapy shows beneficial effects for patients with CRAO, especially when 100% oxygen and hyperbaric oxygen are administrated; this treatment has been proven to significantly improve their VA.15,16 As an efficacious, well-tolerated therapy with few adverse effects, hyperbaric oxygen can be used to treat acute and subacute CRAO; however, it is also recommended as a therapeutic option in the emergency setting.17–19 We firmly believe that these combined therapies produced the good clinical outcome in our patient.
Although our patient had no obvious risk factors, he still developed transient unilateral POVL. He must have had some etiologies that we did not detect and that worked together to lead to POVL after spinal surgery in the prone position. Most cases of POVL are irreversible; our patient was very fortunate because his VA began to recover at 5 hours postoperatively. Further ophthalmic consultation confirmed that the VA of his affected eye was continuously improving. This is the most rapid recovery of POVL after spine surgery in the prone position reported in PubMed and PubMed Central to date. The immediate diagnosis and combined treatments were undoubtedly important to his favorable clinical prognosis. However, knowledge and recognition of these etiologies and risk factors is critical to avoid POVL after surgery in the prone position. The best method for avoiding POVL is to prevent it.20
This study had some limitations. First, although carotid stenosis is a reported risk factor for POVL, a preoperative cervical ultrasound examination was not performed to confirm whether carotid stenosis or plaques were present. If magnetic resonance imaging or computed tomography had been implemented when the patient complained of visual loss, direct evidence of CRAO would have been obtained. Another limitation is that the patient was not examined by FFA in a timely manner. FFA had to be delayed because of his complaint of dizziness. Although late FFA revealed slow retinal artery perfusion, the best chance to confirm vessel occlusion was lost. If FFA had been immediately performed, CRAO might have been observed directly. However, despite the lack of the above-mentioned examinations, the correct diagnosis of POVL was obtained by combining the patient’s complaint, ophthalmic signs, fundus examination findings, and optical coherence tomography results.
POVL must be considered to be a severe complication after surgery in the prone position. There are many perioperative risk factors for POVL; thus, preoperative counseling, strict intraoperative monitoring, correct patient positioning, stable hemodynamics, and postoperative follow-up are essential.
Authors’ contributions
Jun Xiong and Guiling Liang were the anesthesiologists responsible for this patient’s general anesthesia, and they wrote the initial draft of the manuscript. Jun Xiong and Guiling Liang contributed equally to this manuscript and are regarded as co-first authors. Liang Hu, Wei Chen, Jie Deng, and Jun Gu provided suggestions regarding this patient’s treatment. Guoyi Wang and Yushi Li were involved in literature search and critical revision of the draft for content. Yongxing Sun is the corresponding author. All authors read and approved the final manuscript.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Ethics approval and informed consent
The ethics committee of Sanbo Brain Hospital, Capital Medical University approved this study. Informed consent was obtained from the patient described in this report.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
ORCID iD
Yongxing Sun https://orcid.org/0000-0003-2107-1601
References
- 1.Nickels TJ, Manlapaz MR, Farag E. Perioperative visual loss after spine surgery. World J Orthop 2014; 5: 100–106. DOI:10.5312/wjo.v5.i2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamming D, Clarke S. Postoperative visual loss following prone spine surgery. Br J Anaesth 2005; 95: 257–260. DOI: 10.1093/bja/aei173. [DOI] [PubMed] [Google Scholar]
- 3.Epstein NE. Perioperative visual loss following prone spine surgery: a review. Surg Neurol Int 2016; 7: S347–S360. DOI: 10.4103/2152-7806.182550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Chung I, Choi DS, et al . Visual loss due to optic nerve infarction and central retinal artery occlusion after spine surgery in the prone position. Medicine (Baltimore) 2017; 96: e7379. DOI: 10.1097/MD.0000000000007379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakra D, Bala I, Pratap M. Unilateral postoperative visual loss due to central retinal artery occlusion following cervical spine surgery in prone position. Paediatr Anaesth 2007; 17: 805–808. DOI: 10.1111/j.1460-9592.2007.02222.x. [DOI] [PubMed] [Google Scholar]
- 6.Hassani V, Homaei MM, Shahbazi A, et al. Human erythropoietin effect in postoperative visual loss following spine surgery: A case report. Anesth Pain Med 2014; 4: e7291–e7294. DOI: 10.5812/aapm.7291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quraishi NA, Wolinsky JP, Gokaslan ZL. Transient bilateral post-operative visual loss in spine surgery. Eur Spine J 2012; 21: S495–S498. DOI: 10.1007/s00586-011-2117-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Drum M, Roth S. The prevalence of perioperative visual loss in the United States: a 10-year study from 1996 to 2005 of spine, orthopedic, cardiac, and general surgery. Anesth Analg 2009; 109: 1534–1545. DOI: 10.1213/ane.0b013e3181b0500b. [DOI] [PubMed] [Google Scholar]
- 9.Myers MA, Hamilton SR, Bogosian AJ, et al. Visual loss as a complication of spine surgery. A review of 37 cases. Spine (Phila Pa 1976) 1997; 22: 1325–1329. DOI: 10.1097/00007632-199706150-00009. [DOI] [PubMed] [Google Scholar]
- 10.Shifa J, Abebe W, Bekele N, et al. A case of bilateral visual loss after spinal cord surgery. Pan Afr Med J 2016; 23: 119. DOI: 10.11604/pamj.2016.23.119.8443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mac Grory B, Lavin P, Kirshner H, et al. Thrombolytic therapy for acute central retinal artery occlusion. Stroke 2020; 51: 687–695. DOI: 10.1161/STROKEAHA.119.027478. [DOI] [PubMed] [Google Scholar]
- 12.Chen N, Lv J, Bo L, et al. Muscarinic-mediated vasoconstriction in human, rat and sheep umbilical cords and related vasoconstriction mechanisms. BJOG 2015; 122: 1630–1639. DOI: 10.1111/1471-0528.13144. [DOI] [PubMed] [Google Scholar]
- 13.Qipshidze N, Metreveli N, Lominadze D, et al. Folic acid improves acetylcholine-induced vasoconstriction of coronary vessels isolated from hyperhomocysteinemic mice: an implication to coronary vasospasm. J Cell Physiol 2011; 226: 2712–2720. DOI: 10.1002/jcp.22621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin FY, Fu MS. Central retinal artery occlusion after uneventful glaucoma valve implantation surgery with retrobulbar anesthesia: a case report. Int J Ophthalmol 2019; 12: 1362–1365. DOI: 10.18240/ijo.2019.08.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu X, Chen S, Li S, et al. Oxygen therapy in patients with retinal artery occlusion: a meta-analysis. PLoS One 2018; 13: e0202154. DOI: 10.1371/journal.pone.0202154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olson EA, Lentz K. Central retinal artery occlusion: a literature review and the rationale for hyperbaric oxygen therapy. Mo Med 2016; 113: 53–57. [PMC free article] [PubMed] [Google Scholar]
- 17.Baqli BS, Cevik SG, Cevik MT. Effect of hyperbaric oxygen treatment in central retinal artery occlusion. Undersea Hyperb Med 2018; 45: 421–425. [PubMed] [Google Scholar]
- 18.Masters TC, Westgard BC, Hendriksen SM, et al. Case series of hyperbaric oxygen therapy for central retinal artery occlusion. Retin Cases Brief Rep 2019. DOI: 10.1097/ICB.0000000000000895. [DOI] [PubMed] [Google Scholar]
- 19.Youn TS, Lavin P, Patrylo M, et al. Current treatment of central retinal artery occlusion: a national survey. J Neurol 2018; 265: 330–335. DOI: 10.1007/s00415-017-8702-x. [DOI] [PubMed] [Google Scholar]
- 20.Epstein NE. How to avoid perioperative visual loss following prone spine surgery. Surg Neurol Int 2016; 7: S328–S330. DOI: 10.4103/2152-7806.182543. [DOI] [PMC free article] [PubMed] [Google Scholar]