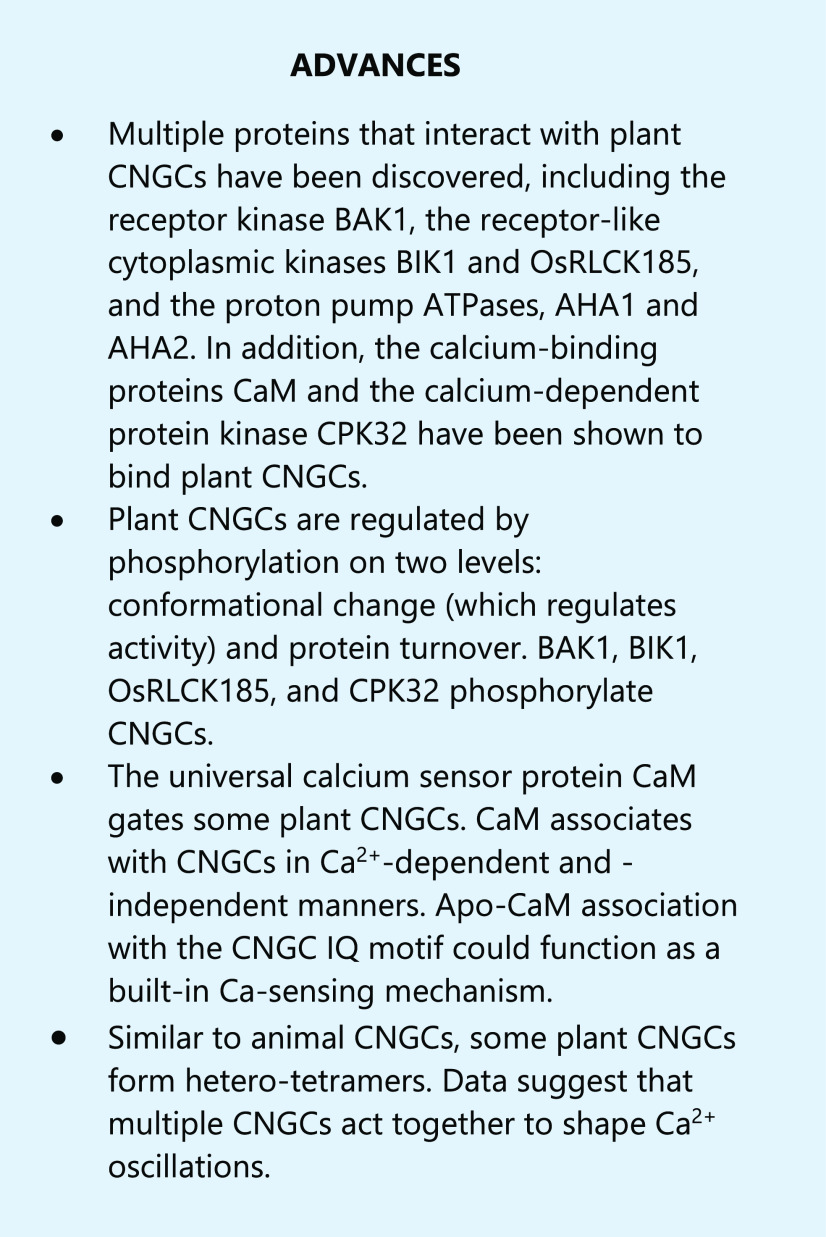
Abstract
Recent advances of plant cyclic nucleotide-gated channels give new insight into their molecular functions focusing on regulation, subunit assembly, and phosphorylation.
Calcium (Ca2+) signaling is crucial for all aspects of plant physiology, including defense, abiotic stress responses, and development; recent research has elucidated the role of plant cyclic-nucleotide–gated channels (CNGCs) in Ca2+ signaling and downstream processes. CNGCs belong to the superfamily of voltage-gated ion channels. Like voltage-gated K+ channels, animal CNGCs, and hyperpolarization and cyclic nucleotide-regulated channels, plant CNGCs are tetrameric and have six transmembrane domains, with a cytosolic N-terminal (NT) and C-terminal (CT) region per subunit (Jegla et al., 2018). The first plant CNGC isoforms were identified as calmodulin (CaM)-binding proteins in 1998 (Köhler and Neuhaus, 1998; Schuurink et al., 1998); over the past five years, pioneering work has established CNGCs as Ca2+-permeable channels involved in Ca2+ oscillations and possibly receptor-mediated signaling.
The spatio-temporal variation in cytosolic Ca2+ concentrations affects a wide range of cellular responses (Webb et al., 1996). For example, Ca2+ flux across the plasma membrane is an early signaling step in establishing symbiosis and immunity (Zipfel and Oldroyd, 2017). Moreover, Ca2+ affects many developmental processes: repetitive spiking or oscillations in cytosolic Ca2+ concentration entrain circadian rhythms, underlie polar expansion of root hairs and pollen tubes, occur in response to the application of auxin to elongating root cells, and control stomatal movements in response to CO2 and abscisic acid (Felle, 1988; McAinsh et al., 1995; Holdaway-Clarke et al., 1997; Allen et al., 2001; Love et al., 2004; Monshausen et al., 2008). The production of Ca2+ oscillations requires positive and negative feedback regulation, and theoretical modeling of Ca2+ oscillations in plants has been successfully applied to some model systems (Martins et al., 2013; Liu et al., 2019). However, understanding Ca2+ dynamics on the molecular and quantitative levels in plants has been hampered by lack of knowledge about the molecular nature and regulation of the channels that allow Ca2+ entry.
In this Update, we summarize recent advances in physiological, biochemical, and electrophysiological characterization of CNGCs, giving new insight into the molecular functions and regulation of plant CNGCs, focusing on subunit assembly, phosphorylation, and CaM binding.
ARE CNGCS TRULY “CYCLIC-NUCLEOTIDE–GATED” CHANNELS?
Progress in understanding the assembly, activation, and regulation of plant CNGCs has been slow. This may be due in part to the pronounced differences to their animal counterparts: In contrast to early assumptions that CNGCs were non-selectively permeable to cations (Talke et al., 2003), new research shows that several CNGCs conduct Ca2+ but often do not allow K+ to cross. Table 1 summarizes our current knowledge about the regulation by cyclic nucleotide monophosphates (cNMPs) and CaM of distinct CNGC subunits expressed in heterologous expression systems, such as Xenopus laevis oocytes and human embryonic kidney (HEK) cells.
Table 1. Comparison of CNGCs expressed in HEK293 cells or X. laevis oocytes.
| Subunit | Currents | Expression in X. laevis oocytes | Currents | Expression in HEK293 cells | ||||
|---|---|---|---|---|---|---|---|---|
| cNMPs | CaM | Reference | cNMPs | CaM | Reference | |||
| CNGC1 | K | db-cAMP activates | — | Leng et al., 2002 | K and Na | db-cAMP activates | — | Hua et al., 2003a |
| CNGC2 | K, no Na | db-cAMP or db-cGMP activates | — | Leng et al., 1999; 2002 | K, no Na | db-cAMP activates | — | Leng et al., 2002; Hua et al., 2003a |
| No | Not added | CaM7 has no effect | Tian et al., 2019 | K | cAMP is present | Ca2+-CaM4 inhibits | Hua et al., 2003b | |
| CNGC2 and 4 | Ca2+ | Not added | CaM7 inhibits | Tian et al., 2019 | — | — | — | — |
| CNGC4 | K or Na | cAMP or cGMP activates | — | Balagué et al., 2003 | — | — | — | — |
| No | Not added | CaM7 has no effect | Tian et al., 2019 | — | — | — | — | |
| CNGC7 | No | — | CaM2 has no effect | Pan et al., 2019 | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC8 | No | — | CaM2 has no effect | Pan et al., 2019 | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC9 | — | — | — | — | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC10 | — | — | — | — | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC11 | No | db-cAMP and 8Br-cAMP have no effect | CaM1 and CaM6 have no effect | Zhang et al., 2019 | — | — | — | — |
| CNGC12 | Ca2+ | db-cAMP and 8Br-cAMP have no effect | (apo)-CaM1 activates; CaM6 has no effect | Zhang et al., 2019 | — | — | — | — |
| CNGC14 | Ca2+ | — | — | Zhang et al., 2017 | — | — | — | — |
| Ca2+ | — | CaM2.2 has no effect; CaM7 inhibits | Zeb et al., 2020 | — | — | — | — | |
| CNGC16 | — | — | — | — | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC18 | Ca2+, but no K or Na | — | CaM2 has no effect | Pan et al., 2019 | Ca2+ | cAMP or cGMP activates | — | Gao et al., 2016 |
| CNGC18 and 7 or 8 | No/tiny | db-cAMP is present | CPK32 activates | Zhou et al., 2014 | — | — | — | — |
| No | — | CaM2 activates | Pan et al., 2019 | — | — | — | — | |
| CNGC19 | Ca2+ | — | Yu et al., 2019 | — | — | — | — | |
| Ca2+, but no K or Na | — | db-cAMP activates | Meena et al., 2019 | — | — | — | — | |
| CNGC20 | Ca2+ | — | — | Yu et al., 2019 | — | — | — | — |
| LjBRUSH | No/tiny | 8Br-cAMP is present | — | Chiasson et al., 2017 | — | — | — | — |
| Ljbrush mutant | Ca2+, no K | 8Br-cAMP is present | — | Chiasson et al., 2017 | — | — | — | — |
| OsCNGC9 | — | — | — | — | Ca2+, no K | — | — | Wang et al., 2019 |
| OsCNGC13 | — | — | — | — | Ca2+ | — | — | Xu et al., 2017 |
Controversial findings regarding the requirement of elevated cyclic adenosine monophosphate (cAMP) or cyclic guanosine monophosphate (cGMP) levels indicate that our current assumptions about CNGC gating may require major revision in the near future. As an example, Arabidopsis (Arabidopsis thaliana) CNGC2 was among the first cloned family members (Köhler and Neuhaus 1998). Initial electrophysiological characterization in X. laevis oocytes suggested that CNGC2 is a voltage-dependent K+-permeable channel activated by cAMP or cGMP (Table 1; Leng et al., 1999, 2002). In comparison, CNGC4, which together with CNGC2 forms subgroup B of clade IV of Arabidopsis CNGCs (Mäser et al., 2001), was reported to encode a voltage-independent cAMP- and cGMP-gated channel (Balagué et al., 2003). Recent work suggests that at least in X. laevis oocytes, both channels work as a hyperpolarization-activated calcium-permeable channel in a heteromeric assembly, without requirement for the elevation of cNMPs levels (Tian et al., 2019).
Similarly, patch-clamp recordings on plasma membranes of plant cell protoplasts have detected cNMP-dependent stimulation of hyperpolarization-activated Ca2+-permeable channels, and these could in some cases be attributed to distinct CNGC isoforms, as in case of e.g. CNGC5 and CNGC6 in Arabidopsis guard cells (Wang et al., 2013). However, depending on the expression system and experimental condition, there seems to be no absolute requirement for the elevation of cNMP levels above the resting state (Table 1), and cNMP affinities and binding dynamics have in most cases not been well studied. The usage of genetically encoded reporters for cAMP or cGMP (Isner and Maathuis, 2013; Jiang et al., 2017) and precise biochemical analysis may provide more definite answers regarding the physiological importance of cNMPs to activate CNGCs in vivo. In light of recent advances toward the characterization of phosphorylation and CaM binding as gating agents or modifiers, the regulation by cNMPs will have to be re-evaluated. cNMPs might act only on a subset of CNGC subunits, they may act as a cofactor rather than a true trigger for ligand-activation, or they may modify the voltage-dependence or affinities toward other regulators. In addition, several recent reports showed that the universal calcium sensor protein CaM plays a more complex and significant role in regulating CNGCs than previously thought (see below for details). Therefore, despite the significant progress in recent years, the permeability, functional regulation, and nature of ligands of plant CNGCs still need further studies.
REGULATION OF CNGC ACTIVATION AND TURNOVER BY PHOSPHORYLATION
In animals, phosphorylation is one way to regulate CNG and HCN channels (Kaupp and Seifert, 2002; Herrmann et al., 2015). For example, the vertebrate CNGCs CNGA1 and CNGB2 function as hetero-tetrameric channels in rod photoreceptors and the phosphorylation status of Tyr residues in these channels controls their activity (Molokanova et al., 2003). Likewise, Tyr phosphorylation alters the gating of the HCN4 pacemaker channel (Li et al., 2008). Early pharmacological studies showed that protein kinase inhibitors prevent the activity of hyperpolarization-dependent calcium channels in plant cells (Köhler and Blatt 2002; Stoelzle et al., 2003), indicating that protein phosphorylation plays a critical role in stimulus-specific Ca2+ signaling.
In recent years, regulation via direct phosphorylation by Ca2+-dependent protein kinases (CDPKs/CPKs), has been documented for a number of plant ion channels, including the K+ channel KAT1, SLOW ANION CHANNEL-ASSOCIATED1, and TWO-PORE CHANNEL1 (Geiger et al., 2009; Maierhofer et al., 2014; Ronzier et al., 2014; Kintzer and Stroud, 2016; Bender et al., 2018). In an extensive survey of CPK substrates in Arabidopsis, Curran et al. (2011) identified CNGC6, CNGC7, CNGC9, and CNGC18 as potential targets of CPK1, CPK10, or CPK34. So far, a specific CPK–CNGC interaction has only been shown for the kinase domain of CPK32 and CNGC18 by yeast two-hybrid assays (Y2H) and Förster resonance energy transfer analysis (Table 2; Zhou et al., 2014). Coexpression of the constitutively active form of CPK32 in X. laevis oocytes strongly enhanced CNGC18 channel activity, although actual phosphorylation was not shown and the phosphorylation sites in CNGC18 were not identified (Zhou et al., 2014). Positive regulation of CNGCs by CDPKs opens the possibility that an initial Ca2+ influx may precede activation of CNGCs by CDPKs. In this scenario, CNGCs may amplify or modify a Ca2+ response initiated by a different channel or from an internal calcium store, because some CDPK activation requires elevated Ca2+concentration. So far, negative regulation of CNGC activity by CDPKs has not been reported, but is possible. In any case, this notion supports the idea that CNGCs are part of larger protein complexes that include other channels, pumps, and decoders such as CDPKs, or are localized in proximity to these players. This notion is discussed in detail later in this article (Fig. 1)
Table 2. Known CNGC interactions with other proteins.
TEVC, Two-electrode voltage clamp; BiFC, bimolecular fluorescence complementation; CoIP, co-immunoprecipitation; nd, not determined.
| Clade | Isoform | Interactor | Position | Effect | Observation | Technique | Reference |
|---|---|---|---|---|---|---|---|
| I | CNGC12 | CaM | NT CaM-BD | De-activate | Deletion causes cell death | Transient expression | DeFalco et al., 2016 |
| CNGC11/12 | CaM1 | IQ domain | Activate | Mutation abolishes cell death | TEVC (X. laevis) | Zhang et al., 2019 | |
| Apo-CaM1 | IQ domain | Activate | DeFalco et al., 2016 | ||||
| II | CNGC6 | CaM2,3,5,7 | IQ domain | Negative | Plasma membrane Ca2+ conductance after heat shock | Whole-cell voltage patch clamping of protoplasts | Niu et al., 2020 |
| III | CNGC14 | CaM7 | CT | Negative | Inhibition of Ca2+ influx | TEVC (X. laevis) | Zeb et al., 2020 |
| OsCNGC9 | OsRLCK185 | CT | Positive | Phosphorylation | In vitro phosphorylation assay | Wang et al., 2019 | |
| Increased [Ca2+]cyt | Ca2+ imaging in HEK cells | ||||||
| CNGC18 | CNGC7/8 | CT | Negative | Inhibition of Ca2+ influx | TEVC (X. laevis) | Pan et al., 2019 | |
| Apo-CaM2 | IQ domain | Positive | Non-Ca2+ binding; CaM activates | TEVC (Xenopus laevis) | Zhou et al., 2014 | ||
| Ca2+ CaM2 | Negative | Release of Ca2+ CaM2 | Microscale thermophoresis | Meng et al., 2020 | |||
| CPK32 | Activate | Increased Ca2+ influx | TEVC (X. laevis) | ||||
| MLO5/9 | Increased pollen tube width | In vivo | |||||
| Pull-down, split-ubiquitin Y2H | |||||||
| MtCNGC15a, b, c | MtDMI1 | NT | Neutral | Simultaneous activation | Y2H, BiFC | Charpentier et al., 2016 | |
| CNGC15 | DMI1 | BiFC | Leitão et al., 2019 | ||||
| CNGC17 | BAK1 | nd | nd | Impaired PSK response in knockout | Split-ubiquitin Y2H | Ladwig et al., 2015 | |
| AHA1, AHA2 | nd | nd | FLIM | ||||
| IVa | CNGC19 | BAK1/SERK4 | CT | nd | Phosphorylation leads to turnover | CoIP | Yu et al., 2019 |
| CaM2, 3, 6, 7 | CT | ND | Y2H, BiFC | Meena et al., 2019 | |||
| CNGC20 | BAK1/SERK4 | CT | Negative | Phosphorylation leads to turnover | Mass spectrometry | Yu et al., 2019 | |
| CaM2 | IQ domain | nd | Y2H, BiFC | Fischer et al., 2013 | |||
| IVb | CNGC2 | BIK1 | IQ domain | ? | ? | CoIP, but no phosphorylation | Tian et al., 2019 |
| CaM7 | Negative | Inhibition of Ca2+ influx | TEVC (X. laevis) | Tian et al., 2019 | |||
| CNGC4 | BIK1 | CT | Activate | Phosphorylation | Patch-clamp of protoplasts | Tian et al., 2019 | |
| CaM7 | (and NT?) | Negative | Inhibition of Ca2+ influx | Mass spectrometry | Chin et al., 2013; Tian et al., 2019 | ||
| CNGC2 | IQ domain | Positive | Required for activity | TEVC (X. laevis) | |||
| nd | TEVC (X. laevis) |
Figure 1.
Model of a CNGC-containing signal complex (nanodomain/channelosome, shown in darker gray). A heterotetrameric CNGC channel is part of a sensing receptor complex containing PRRs, their clients (e.g. BIK1, RESPIRATORY BURST OXIDASE HOMOLOG D, RLCK), various pumps (e.g. proton ATPase and Ca2+ pumps), and decoders (e.g. CPKs and CaM). The formation of such a signal complex can be permanent or temporal upon recognition of specific stimuli (transient signaling complex) and the combination of specific players can contribute to generate precise spatiotemporal Ca2+ signals. Recruitment of CNGCs in a specific signaling complex may be achieved by MLO proteins. Phosphorylation plays significant roles to activate CNGCs or induce their turnover by E3 ubiquitin ligases and the 26s proteasome. V, Vesicle.
Considering the plasma membrane localization of most plant CNGCs, receptor kinases, or receptor-like kinases (RLKs) are likely candidates for the kinases that phosphorylate CNGCs. Indeed, Ladwig et al. (2015) reported that CNGC17 binds to the Arabidopsis H+-ATPases (AHA), AHA1 and AHA2, as well as to BRASSINOSTEROID INSENSITIVE1-ASSOCIATED RECEPTOR KINASE1 (BAK1). BAK1 is a Leu-rich repeat RLK (LRR-RLK), which can associate with various pattern recognition receptors (PRRs) as a coreceptor, forming functional receptor complexes that regulate a wide variety of physiological responses from growth to immunity (Kim and Wang, 2010; Ranf, 2017; Liang and Zhou, 2018). The growth-regulating phytosulfokine (PSK) receptor PSKR1, another LRR-RLK superfamily member, also binds to AHA1, AHA2, and BAK1, suggesting that CNGC17, PSKR1, BAK1, and AHAs may form a protein nanocluster to initiate downstream signals (Fig. 1; Ladwig et al., 2015). In addition, these interaction data suggest that BAK1 or other LRR-RLKs phosphorylate plant CNGCs.
In 2019, three studies revealed that LRR-RLKs and related kinases phosphorylate CNGCs, and examined the physiological relevance of this phosphorylation (Tian et al., 2019; Wang et al., 2019; Yu et al., 2019):
Tian et al. (2019) reported the relevance of CNGC phosphorylation in the recognition of pathogen/microbe-associated molecular patterns (PAMPs/MAMPs) in Arabidopsis. An increase of the cytosolic Ca2+ concentration ([Ca2+]cyt) is essential for the oxidative burst after recognition of PAMPs/MAMPs such as the bacterial elicitor peptide flagellin22 (flg22) or fungal chitin (Seybold et al., 2014; Kadota et al., 2015). Upon flg22 recognition by the PRR kinase FLAGELLIN SENSING2 (FLS2), a receptor complex consisting of FLS2, BAK1, and BOTRYTIS-INDUCED KINASE1 (BIK1) forms, leading to transphosphorylation of these kinases and release of BIK1, which activates downstream signaling (Couto and Zipfel, 2016). A well-studied downstream target of BIK1 is the membrane-localized NADP oxidase (NADPH), RESPIRATORY BURST OXIDASE HOMOLOG D, which is responsible for the oxidative burst during PAMP-triggered immunity (PTI; Li et al., 2014; Kadota et al., 2015). Tian et al. (2019) reported that CNGC4, but not CNGC2, also interacts with BIK1, and is phosphorylated at the CT cytosolic domain upon flg22 recognition via FLS2 (Fig. 1). BIK1 is a cytoplasmic kinase that is a central component of PRR complexes with components such as FLS2, EF-TU RECEPTOR, PERCEPTION OF THE ARABIDOPSIS DANGER SIGNAL PEPTIDES, and CHITIN ELICITOR RECEPTOR KINASE1 (Couto and Zipfel, 2016). CNGC2 and CNGC4 interact and form a functional heteromeric channel, which is inhibited in the presence of CaM (Chin et al., 2013; Tian et al., 2019). BIK1 can activate this CNGC2-CNGC4 heteromeric channel in the presence of the inhibitory CaM, possibly via phosphorylation of CNGC4, and was therefore suggested to induce CNGC2-CNGC4-mediated Ca2+ influx in response to PAMP recognition. Tian et al. (2019) showed CNGC2 (also known as DEFENSE NO DEATH1 [DND1]) and CNGC4 (also known as DND2/HYPERSENSITIVE RESPONSE-LIKE LESION MIMIC1) are positive regulators of PTI only under specific calcium concentrations (i.e. 1.5 mm [Ca2+]ext), as their null mutants showed reduced PTI under this condition, but behaved like wild type under lower calcium concentrations (i.e. 0.1 mm [Ca2+]ext). They reported that sufficient [Ca2+]ext is essential to activate calcium-dependent PTI. However, both cngc2 and cngc4 single null mutants are hypersensitive to calcium and have pleiotropic phenotypes (Yu et al., 1998; Clough et al., 2000; Chan et al., 2003; Wang et al., 2017). Furthermore, cngc2 (dnd1) mutants experience Ca2+ stress under normal Ca2+ levels (Chan et al., 2008), raising the possibility that they cannot respond normally to many triggers, including PAMPs. Therefore, future studies should clarify which channels mediate the Ca2+ response under low Ca2+ supply in cngc2 (dnd1) mutants and whether the compromised PTI in these mutants is a result or independent of other pleiotropic phenotypes.
In another recent study, Wang et al. (2019) examined the role of CNGC phosphorylation in rice (Oryza sativa) PTI and programmed cell death (PCD). The null mutants for CNGC2 (dnd1) and CNGC4 (dnd2/hypersensitive response-like lesion mimic1) show complex and contradictory phenotypes such as autoimmune phenotypes with constitutive elevation of salicylic acid levels and expression of pathogenesis-related (PR) genes, but reduced PCD in the hypersensitive response (Yu et al., 1998; Clough et al., 2000; Moeder et al., 2011). In the absence of pathogens, Arabidopsis cngc2 and cngc4 mutants also show conditional spontaneous lesions. A very similar lesion mimic phenotype was observed for the barley null mutant of CNGC4, necrotic leaf spot1 (Rostoks et al., 2006), and the rice mutant, cell death and susceptible1, which lacks a functional OsCNGC9 gene (Wang et al., 2019). The rice cell death and susceptible1 mutant shows impaired blast fungus resistance and reduced calcium influx, oxidative burst, and PTI-related gene expression, indicating that OsCNGC9 has a significant role in PTI (Wang et al., 2019). Furthermore, the rice receptor-like cytoplasmic kinase (RLCK) OsRLCK185 physically interacts with and phosphorylates OsCNGC9 and this phosphorylation activates channel function (Tables 1 and 2; Wang et al., 2019). OsRLCK185 and OsRLCK176 interact with the chitin receptor OsCHITIN ELICITOR RECEPTOR KINASE1 (Miya et al., 2007). Therefore, CNGCs represent a common downstream target of phosphorylation during PTI. Because the closest Arabidopsis homolog of OsCNGC9 is CNGC14, which has been associated with Ca2+ entry during auxin-regulated growth (Shih et al., 2015; Zhang et al., 2017; Dindas et al., 2018; Brost et al., 2019), and not CNGC2 or 4, it will be interesting to investigate the functional diversification of CNGCs in different plant species.
In the third new study from 2019, Yu et al. (2019) used a suppressor screen to identify an Arabidopsis CNGC as a key player in the mediation of cellular homeostasis, which is regulated by BAK1 and its closest homolog, SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE4 (SERK4). Like CNGC2 and CNGC4, the two closely related group-I channels CNGC11 and CNGC12 have been implicated in immunity and PCD (Yoshioka et al., 2006; Moeder et al., 2011). Likewise, a role in immunity, wound signaling, and insect resistance has been proposed for CNGC19 and CNGC20, which comprise subclade IVa in the CNGC family (Moeder et al., 2011; Meena et al., 2019). BAK1 and SERK4 are involved in a wide variety of physiological phenomena and the bak1-4 serk4-1 double mutant develops severe postembryonic lethality due to hyper-activation of PCD (He et al., 2007; de Oliveira et al., 2016). Yu et al. (2019) conducted a nonbiased suppressor screen of cell death in RNA interference-BAK1/SERK4-silenced plants and found that a knockout mutant of CNGC20 suppressed this cell death phenotype. Furthermore, they showed that BAK1 phosphorylates the CT cytosolic domain of CNGC20 (Table 2). These sites are conserved in CNGC19, which can also be phosphorylated by BAK1. This breakthrough study revealed a novel mechanism of CNGC regulation in which phosphorylation of the C terminus regulates CNGC19 and CNGC20 protein stability. According to their model, CNGC19 and CNGC20 form a hetero-tetrameric channel that positively regulates cell death, and BAK1/SERK4 phosphorylation accelerate CNGC19/20 turnover to maintain cellular homeostasis (Fig. 1).
These three recent studies showed that phosphorylation of CNGCs plays key roles in their regulation (Table 1). One important question will be whether phosphorylation itself activates/inactivates the channel or whether a conformational change will alter the accessibility or sensitivity for cNMPs or CaM. Further investigation of other CNGCs may identify common patterns and will tell us whether each CNGC subunit undergoes unique regulation of either activity or turnover by phosphorylation.
CNGC GATING BY THE CALCIUM-SENSOR PROTEIN CAM AND ITS ROLE IN SHAPING CA2+ OSCILLATIONS
The partial overlap of cNMP-binding domains and the CaM binding site suggested that these signaling compounds competitively regulate CNGCs (Arazi et al., 2000; Hua et al., 2003b; Kaplan et al., 2007). However, a second CaM-binding domain adjacent to the cNMP-binding domain, which is formed by an Ile-Gln (IQ) motif and is conserved among CNGCs (Fischer et al., 2013), challenged this model. Moreover, additional CaM binding sites were mapped within the NT cytosolic domain and distal of the IQ-domain, and both positive and negative regulation of CNGCs by CaM was shown for CNGC12 (Fig. 2; Chin et al., 2010; DeFalco et al., 2016). The finding that the IQ motif binds CaM in a Ca2+-independent manner and is required for channel function paved the way for revisiting the gating mechanisms of CNGCs, because CaM could serve as a built-in subunit that senses local changes in Ca2+ concentration upon channel opening, and induces rapid, Ca2+-dependent feedback regulation (DeFalco et al., 2016; Fischer et al., 2017; Demidchik et al., 2018). However, additional data are required, especially about the role of cNMPs and their possible interplay with CaM, to gain a better understanding of the gating mechanisms of CNGCs.
Figure 2.
Three different modules of CaM regulation in CNGC complexes. A, When apo-CaM is bound to the IQ domain in the presence of low Ca2+-concentration, the homo-tetrameric CNGC12 channel or the CNGC8-CNGC18 hetero-complex can be gated open upon hyperpolarization to allow Ca2+ entry. B, Elevation of cytosolic Ca2+-concentration induces dissociation of Ca2+-CaM, which leads to closure of CNGC8-CNGC18. C, Alternatively, as shown for CNGC14 and CNGC2-CNGC4 hetero-complexes, binding of (Ca2+)-CaM induces conformational rearrangements resulting in channel closure. Red dots and red color indicate Ca2+ ions and high Ca2+ concentration, respectively. CaM is shown with its NT and CT lobes with two apo (white) or Ca2+ (red) loaded EF-hands. For clarity, two CT domains of CNGC subunits are shown for each complex only. The conserved helical parts of the cyclic nucleotide binding domain (CNBD) are represented by green rods, the helical IQ domain in purple. The open state (A) is symbolized by a Ca2+-occupied pore and a compact arrangement of the CNBD with the transmembrane part of the channel, in analogy to known structures (Li et al., 2017). In B and C, the pore is closed and the CNBD is separated from the membrane via the C-linker, to illustrate the closed state.
Negative Regulation by CaM Binding
In X. laevis oocytes, CaM binding to the C terminus of CNGC14 and to the CNGC2/CNGC4 complex inhibits ion channel function (Tian et al., 2019; Zeb et al., 2020). Unfortunately, it is not clear which CaM binding site is involved and whether this inhibition required an elevation of [Ca2+]cyt. Interestingly, in both of the abovementioned studies, CaM7 functioned as a negative regulator (Fig. 2) and CaM7, but not CaM2, specifically inhibited currents through CNGC14 (Zeb et al., 2020). The mechanism by which CaMs exert their isoform-specific regulation remains unclear, because all Arabidopsis CaM isoforms interacted with the C terminus of CNGC14 and CNGC6 in Y2H assays (Fischer et al., 2017).
The CaM2.1 and CaM7.1 isoforms have identical protein sequences except for one conserved K to R change, while the CaM2.2 splice variant used by Zeb et al. (2020) contains 12 additional residues. CaM is highly conserved across kingdoms, which poses questions about the expression levels and physiological relevance of the extended splice variant described by Zeb et al. (2020). If the negative regulation of CNGCs depends on Ca2+-loading of the respective CaM, this will assist in shaping the Ca2+ signature in vivo. In the case of CNGC14, this will relate to Ca2+ oscillations in root hairs and auxin-dependent growth (Shih et al., 2015; Dindas et al., 2018; Brost et al., 2019).
In vivo, Ca2+-dependent binding of CaM to the NT binding site of CNGC12 negatively regulates its channel activity (DeFalco et al., 2016). Ectopic expression of CNGC12 with a mutated NT domain, which cannot bind Ca2+-CaM, constitutively induced PCD, similar to the phenotype produced by a constitutively active channel. Therefore, CaM may regulate channel activity via binding to the NT and CT domains. As channel activity is dependent on heteromeric subunit assembly (Pan et al., 2019; Tian et al., 2019), one urgent task in Ca2+ signaling research is determining the stoichiometry of natural channel assemblies, including their associated CaM subunit(s).
Positive Regulation by CaM Binding
CNGC12 contains three CaM-binding sites: the NT-CaMBD and the CT-CaMBD (which interact with Ca2+-CaM), and the IQ-CaMBD (which associates with apo-CaM; DeFalco et al., 2016). A mutation in a CT motif that had been shown to be crucial for CaM binding (Arazi et al., 2000) resulted in a loss-of-function of CNGC12, indicating CaM binding to this site positively regulates CNGC function (Abdel-Hamid et al., 2010; Chin et al., 2010). Mutation of the core IQ sequence to DA disrupts the interaction with CaM and channel function. When CNGC11/12, a chimeric channel composed of the NT half of CNGC11 and the CT half of CNGC12, is expressed in Nicotiana benthamiana leaves, PCD is induced by constitutively activated Ca2+ flux (Yoshioka et al., 2006, Moeder et al., 2019). By contrast, expression of the CNGC11/12DA mutant does not induce PCD (DeFalco et al., 2016). If only the Ca2+-dependent interaction of CaM with the CNGC12 IQ domain was disrupted, channel function could be partially retained, suggesting that the IQ domain-calcium–free CaM (apo-CaM) complex supports channel function (Fischer et al., 2017). This conclusion was further substantiated by heterologous expression of CNGC11 and CNGC12 in X. laevis oocytes. CNGC12-mediated hyperpolarization-dependent Ca2+ currents were enhanced by ∼3-fold upon coexpression with CaM1 or apo-CaM1, which was kept in the apo state by mutating all four Ca2+-binding sites (Zhang et al., 2019). In comparison, CNGC11 was inactive as a channel in X. laevis oocytes, both in the presence and absence of CaM (Zhang et al., 2019). Only CaM1, which is identical to CaM4, was able to activate CNGC12 in X. laevis oocytes, but CaM6 was not (Zhang et al., 2019). This again points to isoform-specific CaM functions, despite the ability of CaM2 and CaM6 to bind to the C terminus as well as to the isolated IQ domain of CNGC12 in yeast (Fischer et al., 2017). The CaM1 and CaM6 protein sequences (protein models CaM1.1 and CaM6.1) differ in five positions with conserved exchanges (E/D; K/R; T/S; I/V); this led us to question how such subtle differences in protein sequence produce the observed functional differences.
Furthermore, interaction of apo-CaM to CNGCs suggest the concept that CaM may function as a built-in Ca2+ sensor of CNGCs. CaM has two lobes (C and N) with two EF-hands each connected by a flexible linker. Both lobes bind Ca2+ with different affinities, which contributes to the ability of CaM to regulate many target proteins (Villarroel et al., 2014). Apo-CaM can interact with the IQ domain of CNGCs via its C-lobe, indicating that apo-CaM attaches to CNGCs in the resting state and plays a role as a Ca2+-sensing subunit for the channel complex (Fischer et al., 2017). Indeed, apo-CaM association is required for Ca2+ sensing and for channel opening, at least in some channels such as CNGC12 (DeFalco et al., 2016; Fischer et al., 2017; Zhang et al., 2019) and CNGC8/CNGC18 heteromers (Fig. 2; Pan et al., 2019), where the channel–CaM complex may support sustained Ca2+ oscillations during pollen tube growth. As both proximal and distal regions of the core IQ motif play critical roles in CaM accommodation (Fischer et al., 2017), the observation that some CNGCs are activated by CaM (probably by binding of apo-CaM to their IQ domain), while others are not, poses new questions about the complexity of the interaction of CaM with different CNGC subunits and heteromeric CNGC complexes (Fig. 2). Therefore, more quantitative and dynamic analyses of CaM isoform-specific interactions with CNGCs are required to improve our understanding of any CaM-induced gating mechanism.
Role in Shaping Ca2+ Oscillations
Many recent publications have shed light on the role of CNGCs as central elements of plant Ca2+ oscillators. This is not unexpected, because their intrinsic CaM-binding properties make CNGCs function as Ca2+-feedback–regulated elements (Fig. 2).
In pollen tubes, CNGC18 is essential for guidance and tip growth (Frietsch et al., 2007; Gao et al., 2016), while CNGC7 and CNGC8 have partially redundant functions in controlling pollen tube growth (Tunc-Ozdemir et al., 2013). In different heterologous expression systems, CNGC18 mediates hyperpolarization-activated calcium currents, but the regulation of channel activities appears to be complex (Table 1). In HEK293T cells, addition of cAMP and cGMP activated CNGC7, CNGC8, and CNGC18 to produce inward calcium currents at hyperpolarized potentials (Gao et al., 2016). In another report, CNGC18 expressed in X. laevis oocytes could be activated by coexpression of a constitutively active form of the Ca2+-dependent protein kinase CPK32 (Zhou et al., 2014). The authors therefore suggested that Ca2+-dependent feed-forward stimulation of calcium entry occurs via CPK32 during Ca2+ oscillations in growing pollen tubes (Zhou et al., 2014). In later experiments, CNGC18 was highly active in X. laevis oocytes in the absence of plant kinases and without addition of membrane-permeable cyclic nucleotides (Pan et al., 2019), leaving us to question the impact of CNGC18 regulation by CPK32 and cyclic nucleotides in pollen tubes.
A recent study presented a novel mechanism for regulation by heteromeric channel assembly and CaM in the absence of elevated levels of cyclic nucleotides (Table 1; Pan et al., 2019). In X. laevis oocytes, CNGC18 currents were inhibited by coexpression of CNGC7 or CNGC8, and this inhibition was relieved in the presence of CaM2. By contrast, CNGC7 and CNGC8 produced nonfunctioning homomeric channels in the presence and absence of CaM2. Biochemical studies revealed that the CNGC C-termini interacted with each other and with apo-CaM2 or Ca2+-CaM2. Ca2+ loading of CaM2 lowered the affinity for the CNGC8 and CNGC18 C-termini from 50 to >800 nm, suggesting that Ca2+ induced the dissociation of CaM2 from the heteromeric channel complex, which leads to channel inactivation. In this scenario, the heteromeric CNGC18–CNGC8 complex would be active at low [Ca2+]cyt, when apo-CaM is associated, but a rise in [Ca2+]cyt would trigger CaM release and channel closure. Pan et al. (2019) thus suggest a new model in which the dissociation of Ca2+-CaM2 induces inhibition of the channel complex (Fig. 2).
This type of Ca2+ feedback regulation perfectly meets the theoretical expectations for the situation in growing pollen tubes. However, no oscillatory calcium current (or free-running membrane potential) was measured in oocytes, where the “oscillator” had been reconstituted. Despite the presence of high extracellular Ca2+ concentrations of 30 mm, current amplitudes in the presence of CaM2 with nonfunctional EF-hands (CaM21234) were only ∼20% higher than those with Ca2+-sensitive CaM2. The study by Pan et al. (2019) provides new and essential data for future modeling of the Ca2+ oscillator, if the suggested mechanism can be validated in vivo. Modeling of Ca2+ oscillations could then also integrate knowledge about feedback-control by membrane voltage, as well as on- and off-rates of CaM binding.
Loss of CNGC14 causes root hair defects, including swelling and branching, as well as bursting of the root hair tip (Zhang et al., 2017; Brost et al., 2019), indicating its role in the regulation of cell integrity during polar growth. CNGC5, CNGC6, and CNGC9 also contribute to the robustness of unidirectional cell expansion and stability of cytosolic Ca2+ oscillations (Brost et al., 2019; Tan et al., 2020). Loss of CNGC14 had the strongest effect by destabilizing the calcium oscillations and inducing growth defects. The typical Ca2+-oscillation period of ∼30 s found in wild-type root hairs was not established in the cngc14 cngc6 and cngc14 cngc9 double mutants (Brost et al., 2019). However, this period was still present in cngc6 cngc9 double mutants and cngc9 single mutants, although with much less robustness, identifying CNGC14 as the major pacemaker in vivo. Finally, under the experimental conditions used, the cngc6 cngc9 cngc14 triple mutants initiated root hair bulges, which rapidly burst after transition to the rapid growth phase. In another study, growth defects of the cngc5 cngc6 cngc9 triple mutant could be complemented by overexpression of each of the CNGC subunits, indicating similar functions of these channels (Tan et al., 2020). Similar to the results for CNGC14, heterologous expression of CNGC5-, CNGC6-, or CNGC9-induced hyperpolarization-activated Ca2+ currents in HEK293T cells, although the role of cyclic nucleotides required for channel activation differs between individual studies (Table 1; Gao et al., 2016; Tan et al., 2020). In addition to cytosolic Ca2+ oscillations, the participation of CNGCs in nuclear Ca2+ oscillations has also been reported (Charpentier et al., 2016; Leitão et al., 2019). CNGC15 homologs from Medicago truncatula and Arabidopsis are the only CNGCs so far that are localized to the nuclear envelope, where they participate in nuclear Ca2+ oscillations, which are crucial for root growth and symbiosis establishment (Charpentier et al., 2016; Leitão et al., 2019).
CNGC HETERO-TETRAMERIZATION AND LOCALIZATION TO MEMBRANE NANODOMAINS
Ca2+ signals participate in many physiological responses; therefore, one important question is how specific stimuli generate unique signals to maintain signaling specificity. The rates of Ca2+ entry and export, Ca2+ buffering and binding to target proteins, and the respective reaction volumes determine the shape of the “Ca2+ signature” (Clapham, 2007; McAinsh and Pittman, 2009). Hetero-tetramerization of plant CNGCs thus provides a versatile tool to generate unique patterns of Ca2+ signatures. Based on the examples described above, it is reasonable to hypothesize that each subunit has a unique mode of regulation by phosphorylation and CaM binding, but also a certain degree of functional redundancy.
The Arabidopsis CNGC family has 20 members subdivided into five groups (Mäser et al., 2001). Some species have fewer family members, such as maize (Zea mays; 12 CNGCs) or castor bean (Ricinus communis; 11 CNGCs), but other species have many different channel subunits, such as soybean (Glycine max; 35 CNGCs) or apple (Malus domestica; 44 CNGCs), according to the presence of a family-specific sequence motif (Saand et al., 2015). Hetero-tetramerization or subunit interactions have been observed or suggested for CNGC2-CNGC4, CNGC7/CNGC8-CNGC18, CNGC19-CNGC20, CNGC6-9, CNGC6-CNGC14, and CNGC9-CNGC14 (Chin et al., 2013; Brost et al., 2019; Pan et al., 2019; Tian et al., 2019). Pan et al. (2019) reported an intriguing inhibitory effect of CNGC7 or CNGC8 on CNGC18 heteromeric channel function, indicating that some CNGC members may inhibit or modify the activity of their respective heteromeric channel complexes (Fig. 2; Table 2). This observation indicates that the composition of hetero-tetramers has a substantial influence on channel function and regulation, thus determining their physiological function.
Another possible mechanism for creating unique Ca2+ signatures is the formation of protein complexes at the plasma membrane acting as specific sensing modules (Fig. 1). This idea is supported by the observation of interactions of CNGCs with receptor kinases and other membrane-localized proteins as discussed above (Table 2; Ladwig et al., 2015; Wang et al., 2019; Yu et al., 2019; Meng et al., 2020). Following the membrane raft hypothesis proposed by Simons and Ikonen (1997), subcompartmentalization of plasma membrane proteins in nanodomains or microdomains may produce signaling hubs that give specificity in plant signaling (Keinath et al., 2010; Demir et al., 2013; Jaillais and Ott, 2020). For example, the FLS2 coreceptor BAK1 is also a coreceptor of the major brassinosteroid (BR) receptor BRI1. Upon sensing BR, BRI1 forms an active receptor complex with BAK1, thereby initiating BR signaling (Kim and Wang, 2010). Interestingly, Wang et al. (2015) showed that BRI1 localizes to membrane nanodomains and that this partitioning of BRI1 is essential for proper BR signal transduction. Furthermore, Bücherl et al. (2017) showed that FLS2 and BRI1 localize to distinct plasma membrane nanodomains and such spatiotemporal separation of two receptor kinases could contribute to their signaling specificity in immunity and growth regulation. Thus, it is plausible to hypothesize that CNGC hetero-tetrameric channels are parts of sensing complexes, together with specific receptors and downstream decoder proteins, to create specific downstream outputs (Fig. 1).
CONCLUDING REMARKS
As discussed above, recent studies have substantially enriched the field of CNGC research (see Advances). As expected, these new data and concepts raise further questions for deepening our understanding of this channel group and its role in plant calcium signaling (see Outstanding Questions).
The interaction of CNGCs with receptor-like kinases and other membrane-localized proteins, as recently reported for Mildew Locus O (MLO) proteins (Meng et al., 2020), would allow specific CNGCs together with CaMs to be part of different nanodomains associated with their respective receptors. These membrane domains may include decoder proteins such as CPKs. The exploration of how such “channelosomes” generate stimulus-specific Ca2+ signatures that are decoded instantly by the attached decoder proteins will be an exciting future direction for CNGC research (Fig. 1). Structural modeling using solved animal CNGC/HCN structures has improved our understanding (Hua et al., 2003b; Baxter et al., 2008; Niu et al., 2020) but cannot accurately predict the structure of the important CT CaM binding domains. Therefore, resolving high-resolution structures of plant CNGCs will help our understanding of their gating and regulation mechanisms.
The first CNGC family member was identified in 1998 in a screen for CaM binding targets in a complementary DNA library from barley (Hordeum vulgare) aleurone cells, and this CNGC was therefore named H. vulgare CaM Binding Transporter 1, HvCBT1 (Schuurink et al., 1998). Around the same time, two Arabidopsis genes homologous to animal CNGCs were identified and named CNGC1 and CNGC2 (Köhler and Neuhaus, 1998). The CNGC nomenclature was adopted for future family members, following the suggestion of Mäser et al. (2001). Indeed, within the presumed C terminus of each channel, a cyclic nucleotide-binding domain represents the most conserved sequence. Despite this clear domain classification, binding affinities of cAMP or cGMP to this site have not been measured, and the exact role of these nucleotides, both for channel opening and for physiological functions, is still unclear. Furthermore, there is a fierce, ongoing debate about the production of cNMP in plants (Qi et al., 2010; Ashton, 2011). In light of recent advances in understanding CNGC assembly, function, and regulation by CaM, it is time to conduct more quantitative analyses by using (genetically encoded) reporters for cNMPs, as well as biochemical methods, single-channel recordings, and structural approaches to assess (CaM and cNMP) ligand affinities, gating behavior, and composition of membrane domains containing CNGCs. These analyses will provide us with a better understanding of the role of cNMP for CNGC regulation.
In this Update, we summarized exciting new findings on the molecular functions of plant CNGCs and discussed their significance. With this remarkable progress, we are entering a new era of research on CNGCs and calcium signaling, and we anticipate that more advances in this research field will emerge in the near future.
Footnotes
This work was supported by the Natural Science and Engineering Research Council of Canada (grant no. PGPIN–2014–04114), the Canadian Foundation for Innovation, and the Ontario Research Fund (to K.Y.).
Articles can be viewed without a subscription.
References
- Abdel-Hamid H, Chin K, Shahinas D, Moeder W, Yoshioka K(2010) Calmodulin binding to Arabidopsis cyclic nucleotide gated ion channels. Plant Signal Behav 5: 1147–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GJ, Chu SP, Harrington CL, Schumacher K, Hoffmann T, Tang YY, Grill E, Schroeder JI(2001) A defined range of guard cell calcium oscillation parameters encodes stomatal movements. Nature 411: 1053–1057 [DOI] [PubMed] [Google Scholar]
- Arazi T, Kaplan B, Sunkar R, Fromm H(2000) Cyclic-nucleotide- and Ca2+/calmodulin-regulated channels in plants: Targets for manipulating heavy-metal tolerance, and possible physiological roles. Biochem Soc Trans 28: 471–475 [PubMed] [Google Scholar]
- Ashton AR. (2011) Guanylyl cyclase activity in plants? Proc Natl Acad Sci USA 108: E96, author reply E97–E98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balagué C, Lin B, Alcon C, Flottes G, Malmström S, Köhler C, Neuhaus G, Pelletier G, Gaymard F, Roby D(2003) HLM1, an essential signaling component in the hypersensitive response, is a member of the cyclic nucleotide-gated channel ion channel family. Plant Cell 15: 365–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baxter J, Moeder W, Urquhart W, Shahinas D, Chin K, Christendat D, Kang HG, Angelova M, Kato N, Yoshioka K(2008) Identification of a functionally essential amino acid for Arabidopsis cyclic nucleotide gated ion channels using the chimeric AtCNGC11/12 gene. Plant J 56: 457–469 [DOI] [PubMed] [Google Scholar]
- Bender KW, Zielinski RE, Huber SC(2018) Revisiting paradigms of Ca2+ signaling protein kinase regulation in plants. Biochem J 475: 207–223 [DOI] [PubMed] [Google Scholar]
- Brost C, Studtrucker T, Reimann R, Denninger P, Czekalla J, Krebs M, Fabry B, Schumacher K, Grossmann G, Dietrich P(2019) Multiple cyclic nucleotide-gated channels coordinate calcium oscillations and polar growth of root hairs. Plant J 99: 910–923 [DOI] [PubMed] [Google Scholar]
- Bücherl CA, Jarsch IK, Schudoma C, Segonzac C, Mbengue M, Robatzek S, MacLean D, Ott T, Zipfel C(2017) Plant immune and growth receptors share common signalling components but localise to distinct plasma membrane nanodomains. eLife 6: e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CWM, Schorrak LM, Smith RK Jr., Bent AF, Sussman MR(2003) A cyclic nucleotide-gated ion channel, CNGC2, is crucial for plant development and adaptation to calcium stress. Plant Physiol 132: 728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CWM, Wohlbach DJ, Rodesch MJ, Sussman MR(2008) Transcriptional changes in response to growth of Arabidopsis in high external calcium. FEBS Lett 582: 967–976 [DOI] [PubMed] [Google Scholar]
- Charpentier M, Sun J, Vaz Martins T, Radhakrishnan GV, Findlay K, Soumpourou E, Thouin J, Véry AA, Sanders D, Morris RJ, et al. (2016) Nuclear-localized cyclic nucleotide-gated channels mediate symbiotic calcium oscillations. Science 352: 1102–1105 [DOI] [PubMed] [Google Scholar]
- Chiasson DM, Haage K, Sollweck K, Brachmann A, Dietrich P, Parniske M(2017) A quantitative hypermorphic CNGC allele confers ectopic calcium flux and impairs cellular development. eLife 6: e25012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, DeFalco TA, Moeder W, Yoshioka K(2013) The Arabidopsis cyclic nucleotide-gated ion channels AtCNGC2 and AtCNGC4 work in the same signaling pathway to regulate pathogen defense and floral transition. Plant Physiol 163: 611–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin K, Moeder W, Abdel-Hamid H, Shahinas D, Gupta D, Yoshioka K(2010) Importance of the αC-helix in the cyclic nucleotide binding domain for the stable channel regulation and function of cyclic nucleotide gated ion channels in Arabidopsis. J Exp Bot 61: 2383–2393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE.(2007) Calcium signaling. Cell 131: 1047–1058 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Fengler KA, Yu IC, Lippok B, Smith RK Jr., Bent AF(2000) The Arabidopsis dnd1 “defense, no death” gene encodes a mutated cyclic nucleotide-gated ion channel. Proc Natl Acad Sci USA 97: 9323–9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couto D, Zipfel C(2016) Regulation of pattern recognition receptor signalling in plants. Nat Rev Immunol 16: 537–552 [DOI] [PubMed] [Google Scholar]
- Curran A, Chang IF, Chang CL, Garg S, Miguel RM, Barron YD, Li Y, Romanowsky S, Cushman JC, Gribskov M, et al. (2011) Calcium-dependent protein kinases from Arabidopsis show substrate specificity differences in an analysis of 103 substrates. Front Plant Sci 2: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFalco TA, Marshall CB, Munro K, Kang HG, Moeder W, Ikura M, Snedden WA, Yoshioka K(2016) Multiple calmodulin-binding sites positively and negatively regulate Arabidopsis CYCLIC NUCLEOTIDE-GATED CHANNEL12. Plant Cell 28: 1738–1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demidchik V, Shabala S, Isayenkov S, Cuin TA, Pottosin I(2018) Calcium transport across plant membranes: Mechanisms and functions. New Phytol 220: 49–69 [DOI] [PubMed] [Google Scholar]
- Demir F, Horntrich C, Blachutzik JO, Scherzer S, Reinders Y, Kierszniowska S, Schulze WX, Harms GS, Hedrich R, Geiger D, et al. (2013) Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc Natl Acad Sci USA 110: 8296–8301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira MV, Xu G, Li B, de Souza Vespoli L, Meng X, Chen X, Yu X, de Souza SA, Intorne AC, de A Manhães AM, et al. (2016) Specific control of Arabidopsis BAK1/SERK4-regulated cell death by protein glycosylation. Nat Plants 2: 15218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindas J, Scherzer S, Roelfsema MRG, von Meyer K, Müller HM, Al-Rasheid KAS, Palme K, Dietrich P, Becker D, Bennett MJ, et al. (2018) AUX1-mediated root hair auxin influx governs SCFTIR1/AFB-type Ca2+ signaling. Nat Commun 9: 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felle H.(1988) Auxin causes oscillations of cytosolic free calcium and pH in Zea mays coleoptiles. Planta 174: 495–499 [DOI] [PubMed] [Google Scholar]
- Fischer C, DeFalco TA, Karia P, Snedden WA, Moeder W, Yoshioka K, Dietrich P(2017) Calmodulin as a Ca2+-sensing subunit of Arabidopsis cyclic nucleotide-gated channel complexes. Plant Cell Physiol 58: 1208–1221 [DOI] [PubMed] [Google Scholar]
- Fischer C, Kugler A, Hoth S, Dietrich P(2013) An IQ domain mediates the interaction with calmodulin in a plant cyclic nucleotide-gated channel. Plant Cell Physiol 54: 573–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frietsch S, Wang YF, Sladek C, Poulsen LR, Romanowsky SM, Schroeder JI, Harper JF(2007) A cyclic nucleotide-gated channel is essential for polarized tip growth of pollen. Proc Natl Acad Sci USA 104: 14531–14536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao QF, Gu LL, Wang HQ, Fei CF, Fang X, Hussain J, Sun SJ, Dong JY, Liu H, Wang YF(2016) Cyclic nucleotide-gated channel 18 is an essential Ca2+ channel in pollen tube tips for pollen tube guidance to ovules in Arabidopsis. Proc Natl Acad Sci USA 113: 3096–3101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KA, et al. (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Gou X, Yuan T, Lin H, Asami T, Yoshida S, Russell SD, Li J(2007) BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroid-independent cell-death pathways. Curr Biol 17: 1109–1115 [DOI] [PubMed] [Google Scholar]
- Herrmann S, Schnorr S, Ludwig A(2015) HCN channels--modulators of cardiac and neuronal excitability. Int J Mol Sci 16: 1429–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdaway-Clarke TL, Feijo JA, Hackett GR, Kunkel JG, Hepler PK(1997) Pollen tube growth and the intracellular cytosolic calcium gradient oscillate in phase while extracellular calcium influx is delayed. Plant Cell 9: 1999–2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Leng Q, Berkowitz GA(2003a) Plants do it differently. A new basis for potassium/sodium selectivity in the pore of an ion channel. Plant Physiol 132: 1353–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua BG, Mercier RW, Zielinski RE, Berkowitz G(2003b) Functional interaction of calmodulin with a plant cyclic nucleotide gated cation channel. Plant Physiol Biochem 41: 945–954 [Google Scholar]
- Isner JC, Maathuis FJ(2013) In vivo imaging of cGMP in plants. Methods Mol Biol 1016: 57–65 [DOI] [PubMed] [Google Scholar]
- Jaillais Y, Ott T(2020) The nanoscale organization of the plasma membrane and its importance in signaling: A proteolipid perspective. Plant Physiol 182: 1682–1696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegla T, Busey G, Assmann SM(2018) Evolution and structural characteristics of plant voltage-gated K+ channels. Plant Cell 30: 2898–2909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang JY, Falcone JL, Curci S, Hofer AM(2017) Interrogating cyclic AMP signaling using optical approaches. Cell Calcium 64: 47–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadota Y, Shirasu K, Zipfel C(2015) Regulation of the NADPH oxidase RBOHD during plant immunity. Plant Cell Physiol 56: 1472–1480 [DOI] [PubMed] [Google Scholar]
- Kaplan B, Sherman T, Fromm H(2007) Cyclic nucleotide-gated channels in plants. FEBS Lett 581: 2237–2246 [DOI] [PubMed] [Google Scholar]
- Kaupp UB, Seifert R(2002) Cyclic nucleotide-gated ion channels. Physiol Rev 82: 769–824 [DOI] [PubMed] [Google Scholar]
- Keinath NF, Kierszniowska S, Lorek J, Bourdais G, Kessler SA, Shimosato-Asano H, Grossniklaus U, Schulze WX, Robatzek S, Panstruga R(2010) PAMP (pathogen-associated molecular pattern)-induced changes in plasma membrane compartmentalization reveal novel components of plant immunity. J Biol Chem 285: 39140–39149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TW, Wang ZY(2010) Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu Rev Plant Biol 61: 681–704 [DOI] [PubMed] [Google Scholar]
- Kintzer AF, Stroud RM(2016) Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature 531: 258–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler B, Blatt MR(2002) Protein phosphorylation activates the guard cell Ca2+ channel and is a prerequisite for gating by abscisic acid. Plant J 32: 185–194 [DOI] [PubMed] [Google Scholar]
- Köhler C, Neuhaus G(1998) Cloning and partial characterization of two putative cyclic nucleotide-regulated ion channels from Arabidopsis thaliana, designated CNGC1 and CNGC2. Plant Physiol 116: 1604 [Google Scholar]
- Ladwig F, Dahlke RI, Stührwohldt N, Hartmann J, Harter K, Sauter M(2015) Phytosulfokine regulates growth in Arabidopsis through a response module at the plasma membrane that includes CYCLIC NUCLEOTIDE-GATED CHANNEL17, H+-ATPase, and BAK1. Plant Cell 27: 1718–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitão N, Dangeville P, Carter R, Charpentier M(2019) Nuclear calcium signatures are associated with root development. Nat Commun 10: 4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Hua BG, Fromm H, Berkowitz GA(2002) Electrophysiological analysis of cloned cyclic nucleotide-gated ion channels. Plant Physiol 128: 400–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Yao W, Berkowitz GA(1999) Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol 121: 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CH, Zhang Q, Teng B, Mustafa SJ, Huang JY, Yu HG(2008) Src tyrosine kinase alters gating of hyperpolarization-activated HCN4 pacemaker channel through Tyr531. Am J Physiol Cell Physiol 294: C355–C362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li M, Yu L, Zhou Z, Liang X, Liu Z, Cai G, Gao L, Zhang X, Wang Y, et al. (2014) The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15: 329–338 [DOI] [PubMed] [Google Scholar]
- Li M, Zhou X, Wang S, Michailidis I, Gong Y, Su D, Li H, Li X, Yang J(2017) Structure of a eukaryotic cyclic-nucleotide-gated channel. Nature 542: 60–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Zhou JM(2018) Receptor-like cytoplasmic kinases: Central players in plant receptor kinase-mediated signaling. Annu Rev Plant Biol 69: 267–299 [DOI] [PubMed] [Google Scholar]
- Liu J, Lenzoni G, Knight MR(2019) Design principles for decoding calcium signals to generate specific gene expression via transcription. Plant Physiol 182: 1743–1761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love J, Dodd AN, Webb AA(2004) Circadian and diurnal calcium oscillations encode photoperiodic information in Arabidopsis. Plant Cell 16: 956–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maierhofer T, Lind C, Hüttl S, Scherzer S, Papenfuß M, Simon J, Al-Rasheid KA, Ache P, Rennenberg H, Hedrich R, et al. (2014) A single-pore residue renders the Arabidopsis root anion channel SLAH2 highly nitrate selective. Plant Cell 26: 2554–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins TV, Evans MJ, Woolfenden HC, Morris RJ(2013) Towards the physics of calcium signalling in plants. Plants (Basel) 2: 541–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäser P, Thomine S, Schroeder JI, Ward JM, Hirschi K, Sze H, Talke IN, Amtmann A, Maathuis FJ, Sanders D, et al. (2001) Phylogenetic relationships within cation transporter families of Arabidopsis. Plant Physiol 126: 1646–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAinsh MR, Pittman JK(2009) Shaping the calcium signature. New Phytol 181: 275–294 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Webb A, Taylor JE, Hetherington AM(1995) Stimulus-induced oscillations in guard cell cytosolic free calcium. Plant Cell 7: 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meena MK, Prajapati R, Krishna D, Divakaran K, Pandey Y, Reichelt M, Mathew MK, Boland W, Mithöfer A, Vadassery J(2019) The Ca2+ channel CNGC19 regulates Arabidopsis defense against spodoptera herbivory. Plant Cell 31: 1539–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng JG, Liang L, Jia PF, Wang YC, Li HJ, Yang WC(2020) Integration of ovular signals and exocytosis of a Ca2+ channel by MLOs in pollen tube guidance. Nat Plants 6: 143–153 [DOI] [PubMed] [Google Scholar]
- Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, Narusaka Y, Kawakami N, Kaku H, Shibuya N(2007) CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA 104: 19613–19618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeder W, Phan V, Yoshioka K(2019) Ca2+ to the rescue—Ca2+channels and signaling in plant immunity. Plant Sci 279: 19–26 [DOI] [PubMed] [Google Scholar]
- Moeder W, Urquhart W, Ung H, Yoshioka K(2011) The role of cyclic nucleotide-gated ion channels in plant immunity. Mol Plant 4: 442–452 [DOI] [PubMed] [Google Scholar]
- Molokanova E, Krajewski JL, Satpaev D, Luetje CW, Kramer RH(2003) Subunit contributions to phosphorylation-dependent modulation of bovine rod cyclic nucleotide-gated channels. J Physiol 552: 345–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Messerli MA, Gilroy S(2008) Imaging of the Yellow Cameleon 3.6 indicator reveals that elevations in cytosolic Ca2+ follow oscillating increases in growth in root hairs of Arabidopsis. Plant Physiol 147: 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu WT, Han XW, Wei SS, Shang ZL, Wang J, Yang DW, Fan X, Gao F, Zheng SZ, Bai JT, et al. (2020) Arabidopsis cyclic nucleotide-gated channel 6 is negatively modulated by multiple calmodulin isoforms during heat shock. J Exp Bot 71: 90–104 [DOI] [PubMed] [Google Scholar]
- Pan Y, Chai X, Gao Q, Zhou L, Zhang S, Li L, Luan S(2019) Dynamic interactions of plant CNGC subunits and calmodulins drive oscillatory Ca2+ channel activities. Dev Cell 48: 710–725 e715 [DOI] [PubMed] [Google Scholar]
- Qi Z, Verma R, Gehring C, Yamaguchi Y, Zhao Y, Ryan CA, Berkowitz GA(2010) Ca2+ signaling by plant Arabidopsis thaliana Pep peptides depends on AtPepR1, a receptor with guanylyl cyclase activity, and cGMP-activated Ca2+ channels. Proc Natl Acad Sci USA 107: 21193–21198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranf S.(2017) Sensing of molecular patterns through cell surface immune receptors. Curr Opin Plant Biol 38: 68–77 [DOI] [PubMed] [Google Scholar]
- Ronzier E, Corratgé-Faillie C, Sanchez F, Prado K, Brière C, Leonhardt N, Thibaud JB, Xiong TC(2014) CPK13, a noncanonical Ca2+-dependent protein kinase, specifically inhibits KAT2 and KAT1 shaker K+ channels and reduces stomatal opening. Plant Physiol 166: 314–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostoks N, Schmierer D, Mudie S, Drader T, Brueggeman R, Caldwell DG, Waugh R, Kleinhofs A(2006) Barley necrotic locus nec1 encodes the cyclic nucleotide-gated ion channel 4 homologous to the Arabidopsis HLM1. Mol Genet Genomics 275: 159–168 [DOI] [PubMed] [Google Scholar]
- Saand MA, Xu YP, Munyampundu JP, Li W, Zhang XR, Cai XZ(2015) Phylogeny and evolution of plant cyclic nucleotide-gated ion channel (CNGC) gene family and functional analyses of tomato CNGCs. DNA Res 22: 471–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuurink RC, Shartzer SF, Fath A, Jones RL(1998) Characterization of a calmodulin-binding transporter from the plasma membrane of barley aleurone. Proc Natl Acad Sci USA 95: 1944–1949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold H, Trempel F, Ranf S, Scheel D, Romeis T, Lee J(2014) Ca2+ signalling in plant immune response: From pattern recognition receptors to Ca2+ decoding mechanisms. New Phytol 204: 782–790 [DOI] [PubMed] [Google Scholar]
- Shih HW, DePew CL, Miller ND, Monshausen GB(2015) The cyclic nucleotide-gated channel CNGC14 regulates root gravitropism in Arabidopsis thaliana. Curr Biol 25: 3119–3125 [DOI] [PubMed] [Google Scholar]
- Simons K, Ikonen E(1997) Functional rafts in cell membranes. Nature 387: 569–572 [DOI] [PubMed] [Google Scholar]
- Stoelzle S, Kagawa T, Wada M, Hedrich R, Dietrich P(2003) Blue light activates calcium-permeable channels in Arabidopsis mesophyll cells via the phototropin signaling pathway. Proc Natl Acad Sci USA 100: 1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talke IN, Blaudez D, Maathuis FJ, Sanders D(2003) CNGCs: Prime targets of plant cyclic nucleotide signalling? Trends Plant Sci 8: 286–293 [DOI] [PubMed] [Google Scholar]
- Tan Y-Q, Yang Y, Zhang A, Fei C-F, Gu L-L, Sun S-J, Xu W, Wang L, Liu H, Wang YF(2020) Three CNGC family members, CNGC5, CNGC6, and CNGC9, are required for constitutive growth of Arabidopsis root hairs as Ca2+-permeable channels. Plant Communications 1: 100001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W, Hou C, Ren Z, Wang C, Zhao F, Dahlbeck D, Hu S, Zhang L, Niu Q, Li L, et al. (2019) A calmodulin-gated calcium channel links pathogen patterns to plant immunity. Nature 572: 131–135 [DOI] [PubMed] [Google Scholar]
- Tunc-Ozdemir M, Rato C, Brown E, Rogers S, Mooneyham A, Frietsch S, Myers CT, Poulsen LR, Malhó R, Harper JF(2013) Cyclic nucleotide gated channels 7 and 8 are essential for male reproductive fertility. PLoS One 8: e55277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel A, Taglialatela M, Bernardo-Seisdedos G, Alaimo A, Agirre J, Alberdi A, Gomis-Perez C, Soldovieri MV, Ambrosino P, Malo C, et al. (2014) The ever changing moods of calmodulin: how structural plasticity entails transductional adaptability. J Mol Biol 426: 2717–2735 [DOI] [PubMed] [Google Scholar]
- Wang J, Liu X, Zhang A, Ren Y, Wu F, Wang G, Xu Y, Lei C, Zhu S, Pan T, et al. (2019) A cyclic nucleotide-gated channel mediates cytoplasmic calcium elevation and disease resistance in rice. Cell Res 29: 820–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li H, Lv X, Chen T, Li R, Xue Y, Jiang J, Jin B, Baluška F, Šamaj J, et al. (2015) Spatiotemporal dynamics of the BRI1 receptor and its regulation by membrane microdomains in living Arabidopsis cells. Mol Plant 8: 1334–1349 [DOI] [PubMed] [Google Scholar]
- Wang YF, Munemasa S, Nishimura N, Ren HM, Robert N, Han M, Puzõrjova I, Kollist H, Lee S, Mori I, et al. (2013) Identification of cyclic GMP-activated nonselective Ca2+-permeable cation channels and associated CNGC5 and CNGC6 genes in Arabidopsis guard cells. Plant Physiol 163: 578–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kang Y, Ma C, Miao R, Wu C, Long Y, Ge T, Wu Z, Hou X, Zhang J, Qi Z(2017) CNGC2 is a Ca2+ influx channel that prevents accumulation of apoplastic Ca2+ in the leaf. Plant Physiol 173: 1342–1354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, McAinsh-Martin R, Taylor Jane E, Hetherington AM(1996) Calcium ions as intracellular second messengers in higher plants. Adv Bot Res 22: 45–96 [Google Scholar]
- Xu Y, Yang J, Wang Y, Wang J, Yu Y, Long Y, Wang Y, Zhang H, Ren Y, Chen J, et al. (2017) OsCNGC13 promotes seed-setting rate by facilitating pollen tube growth in stylar tissues. PLoS Genet 13: e1006906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka K, Moeder W, Kang HG, Kachroo P, Masmoudi K, Berkowitz G, Klessig DF(2006) The chimeric Arabidopsis CYCLIC NUCLEOTIDE-GATED ION CHANNEL11/12 activates multiple pathogen resistance responses. Plant Cell 18: 747–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu IC, Parker J, Bent AF(1998) Gene-for-gene disease resistance without the hypersensitive response in Arabidopsis dnd1 mutant. Proc Natl Acad Sci USA 95: 7819–7824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Xu G, Li B, de Souza Vespoli L, Liu H, Moeder W, Chen S, de Oliveira MVV, Ariádina de Souza S, Shao W, et al. (2019) The receptor kinases BAK1/SERK4 regulate Ca2+ channel-mediated cellular homeostasis for cell death containment. Curr Biol 29: 3778–3790.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeb Q, Wang X, Hou C, Zhang X, Dong M, Zhang S, Zhang Q, Ren Z, Tian W, Zhu H, et al. (2020) The interaction of CaM7 and CNGC14 regulates root hair growth in Arabidopsis. J Integr Plant Biol 62: 887–896 [DOI] [PubMed] [Google Scholar]
- Zhang S, Pan Y, Tian W, Dong M, Zhu H, Luan S, Li L(2017) Arabidopsis CNGC14 mediates calcium influx required for tip growth in root hairs. Mol Plant 10: 1004–1006 [DOI] [PubMed] [Google Scholar]
- Zhang Z, Hou C, Tian W, Li L, Zhu H(2019) Electrophysiological studies revealed CaM1-mediated regulation of the Arabidopsis calcium channel CNGC12. Front Plant Sci 10: 1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Lan W, Jiang Y, Fang W, Luan S(2014) A calcium-dependent protein kinase interacts with and activates a calcium channel to regulate pollen tube growth. Mol Plant 7: 369–376 [DOI] [PubMed] [Google Scholar]
- Zipfel C, Oldroyd GE(2017) Plant signalling in symbiosis and immunity. Nature 543: 328–336 [DOI] [PubMed] [Google Scholar]