Two multifunctional class III gibberellin 2-oxidases contribute to freezing tolerance and to the regulation of seed production.
Abstract
Many developmental processes in plants are regulated by GA hormones. GA homeostasis is achieved via complex biosynthetic and catabolic pathways. GA catabolic enzymes include GA 2-oxidases that are classified into three classes. Members of class III GA 2-oxidases typically act on GA precursors containing a C20-skeleton. Here, we identified two further members of this class of GA 2-oxidases, namely AtGA2ox9 and AtGA2ox10, in the Arabidopsis (Arabidopsis thaliana) genome. Both genes encode enzymes that have functional similarities to AtGA2ox7 and AtGA2ox8, which are class III GA 2-oxidases that 2β-hydroxylate C20-GAs. Previously unknown for GA 2-oxidases, AtGA2ox9 performs 2α-hydroxylation of C19-GAs and harbors putative desaturating activity of C20-GAs. Additionally, AtGA2ox9 and AtGA2ox10 exhibit GA 20-oxidase activity. AtGA2ox9 oxidizes carbon-20 to form tricarboxylic acid C20-GAs, whereas AtGA2ox10 produces C19-GA9. AtGA2ox9 transcript levels increase after cold treatment and AtGA2ox10 is expressed mainly in the siliques of Arabidopsis plants. Atga2ox9 loss-of-function mutants are more sensitive to freezing temperatures, whereas Atga2ox10 loss-of-function mutants produce considerably more seeds per silique than wild-type plants. We conclude that in Arabidopsis, AtGA2ox9 contributes to freezing tolerance and AtGA2ox10 regulates seed production.
Bioactive GAs are signaling molecules essential for plant growth and responses to environmental conditions (Yamaguchi, 2008; Sun, 2010; Colebrook et al., 2014). GAs regulate plant developmental processes, including flowering, fertilization, and embryo growth (Pimenta Lange and Lange, 2006; Mutasa-Göttgens and Hedden, 2009; Martínez-Bello et al., 2015; Pimenta Lange et al., 2020). A common reaction to abiotic stresses (e.g. cold, salt, osmotic stress, or touch) is a reduction in GA levels and, consequently, a decrease in plant growth. There is increasing evidence that GA inactivation is an integral part of the plant hormonal response pathway, and in particular that catabolic GA 2-oxidases are the main players in this process (Pimenta Lange and Lange, 2006, 2015; Achard et al., 2008; Colebrook et al., 2014). GA 2-oxidases belong to soluble 2-oxoglutarate-dependent dioxygenases that are encoded by multigene families (Hedden and Phillips, 2015; Lange and Pimenta Lange, 2020).
Lee and Zeevaart (2005) proposed three structural classes of GA 2-oxidases: Class I and class II enzymes utilize C19-GAs, whereas class III enzymes accept C20-GAs as substrates. However, exceptions to this rule were published recently: class I GA 2-oxidases from cucumber (Cucumis sativus) and bread wheat (Triticum aestivum) are also capable of oxidizing C20-GAs, whereas a bread wheat class III GA 2-oxidase also converts C19-GAs (Pimenta Lange et al., 2013; Pearce et al., 2015). In Arabidopsis (Arabidopsis thaliana), nine genes code for GA 2-oxidases, four of which belong to the class III enzymes, including the two characterized herein (Fig. 1A; Supplemental Fig. S1).
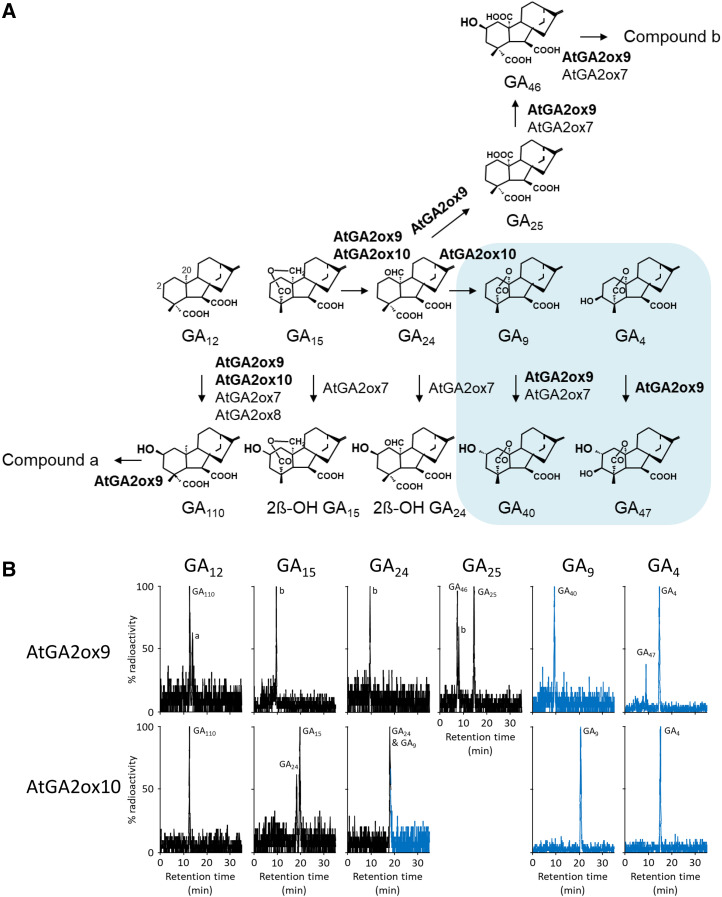
Figure 1.
Enzymatic properties of class III GA 2-oxidases in Arabidopsis. A, Scheme of reactions catalyzed by the two identified multifunctional enzymes AtGA2ox9 (At5g58660) and AtGA2ox10 (At3g47190), in bold print. C19-GAs are highlighted by the blue background. All others are C20-GAs. B, HPLC radiochromatograms of products from incubation of recombinant AtGA2ox9 and AtGA2ox10 with the C20-GAs 14C-GA12, 14C-GA15, 14C-GA24, and 14C-GA25 and the C19-GAs 14C-GA9 and 14C-GA4 as substrates. Blue traces represent C19-GAs. Products were identified by GC-MS (Supplemental Table S1). Compounds a (putative didehydro-GA110) and b (putative didehydro-GA46) are referred to as “a” and “b,” respectively.
In this study, we report two further GA 2-oxidases from Arabidopsis, designated AtGA2ox9 and AtGA2ox10, that are structurally related to class III enzymes and that, consequently, both hydroxylate C20-GA12 to GA110 in a process called 2β-hydroxylation (Fig. 1A). In addition, AtGA2ox9 catalyzes the conversion of other C20-GA precursors (including GA15, GA24, and GA25) further to putative monounsaturated GA46, which includes oxidations at carbon-2β and carbon-20. Moreover, this enzyme oxidizes the C19-GAs GA9 and GA4 to GA40 and GA47, respectively (2α-hydroxylated products; Fig. 1A). As a side reaction, AtGA2ox10 also catalyzes oxidations at carbon-20 by two sequential conversions of C20-GA15 to C19-GA9 via GA24. We demonstrate that AtGA2ox9 is involved in acquiring freezing tolerance and that AtGA2ox10 contributes to plant growth and seed production in Arabidopsis.
RESULTS
Cloning of AtGA2ox9 and AtGA2ox10 from Arabidopsis
In a phylogenetic analysis, Porco et al. (2016) identified two putative 2-oxoglutarate dependent dioxygenases, At5g58660 and At3g47190, in the Arabidopsis genome that are related to the previously identified class III GA 2-oxidases AtGA2ox7 and AtGA2ox8 (Schomburg et al., 2003). To investigate their enzymatic activities, we first cloned respective complementary DNA (cDNA) molecules in the pET101/D-TOPO expression vector. Total RNA, derived from 4-week-old Arabidopsis shoots and siliques of 35-d-old plants, was used to clone At5g58660 (designated AtGA2ox9) and At3g47190 (designated AtGA2ox10). Positive clones were sequenced on both strands and the DNA sequences obtained for both genes are identical to the respective sequences from the Arabidopsis genome (Supplemental Figs. S1 and S2). The two previously uncharacterized GA 2-oxidases AtGA2ox9 and AtGA2ox10 consist of amino acid sequences related to the previously described class III GA 2-oxidases (Supplemental Figs. S1 and S2; Schomburg et al., 2003). Several putative class III-related proteins also exist in other plant species, including cucumber and rice (Oryza sativa; Supplemental Fig. S1), but their enzymatic properties remain to be investigated.
AtGA2ox9 and AtGA2ox10 GA 2-Oxidases Are Multifunctional
Both of the AtGA2ox9 and AtGA2ox10 recombinant enzymes 2β-hydroxylated the C20-GA precursor GA12 and produced GA110 (Fig. 1; Supplemental Table S1). Moreover, AtGA2ox9 was shown to harbor activities previously unknown for GA 2-oxidases; it hydroxylates the C19-GAs GA9 and GA4 at the 2α-position to form GA40 and GA47, respectively. Both products were identified conclusively by their Kovats retention indices and mass spectra, which differ from the respective 2β-hydroxylated products (Gaskin and MacMillan, 1992; Pimenta Lange et al., 2020; Supplemental Table S1). Additionally, AtGA2ox9 and AtGA2ox10 were found to oxidize GA15 at the C-20 position to GA24 (Fig. 1; Supplemental Tables S1 and S2). Both enzymes also oxidized GA24, the next step of the GA biosynthetic pathway. AtGA2ox10 formed the C19-GA GA9 and AtGA2ox9 produced the C20-tricarboxylic acid GA25, both of which are typical products of anabolic and catabolic GA 20-oxidase activities, respectively (Lange and Pimenta Lange, 2020). Compound c, an unstable intermediate product from GA15 of AtGA2ox9, appeared to be converted nonenzymatically during derivatization to GA25 (Supplemental Table S2). AtGA2ox9 further oxidized GA25 at the C-2β position to GA46 (Fig. 1; Supplemental Tables S1 and S2) and also likely harbored desaturase activity, as its reaction products from GA110 and GA46 represented monounsaturated GAs, based on their mass spectra (compounds a and b, respectively; Fig. 1; Supplemental Tables S1 and S2).
The previously identified class III GA 2-oxidases AtGA2ox7 and AtGA2ox8 were shown to 2β-hydroxylate GA12 (Schomburg et al., 2003). It was reported that AtGA2ox7 also accepts other C20-GA precursors, including GA15 and GA24, but the resulting products were not identified (Magome et al., 2008). By reinvestigating their enzymatic properties, we found that AtGA2ox7 and AtGA2ox8 hydroxylate GA12 to GA110, confirming the results of Schomburg et al. (2003; Fig. 1A; Supplemental Fig. S3; Supplemental Table S1). Additionally, here we show that AtGA2ox7 converts C20-GAs GA15, GA24, and GA25 to 2β-hydroxylated GA15, 2β-hydroxylated GA24, and GA46, respectively. Moreover, AtGA2ox7 also produces small amounts of putative monounsaturated GA46 (compound b) from GA25 and 2α-hydroxylates C19-GA9 to GA40, similar to AtGA2ox9 (Fig. 1; Supplemental Fig. S3; Supplemental Table S1).
AtGA2ox9 and AtGA2ox10 GA 2-Oxidases Are Catabolic Enzymes
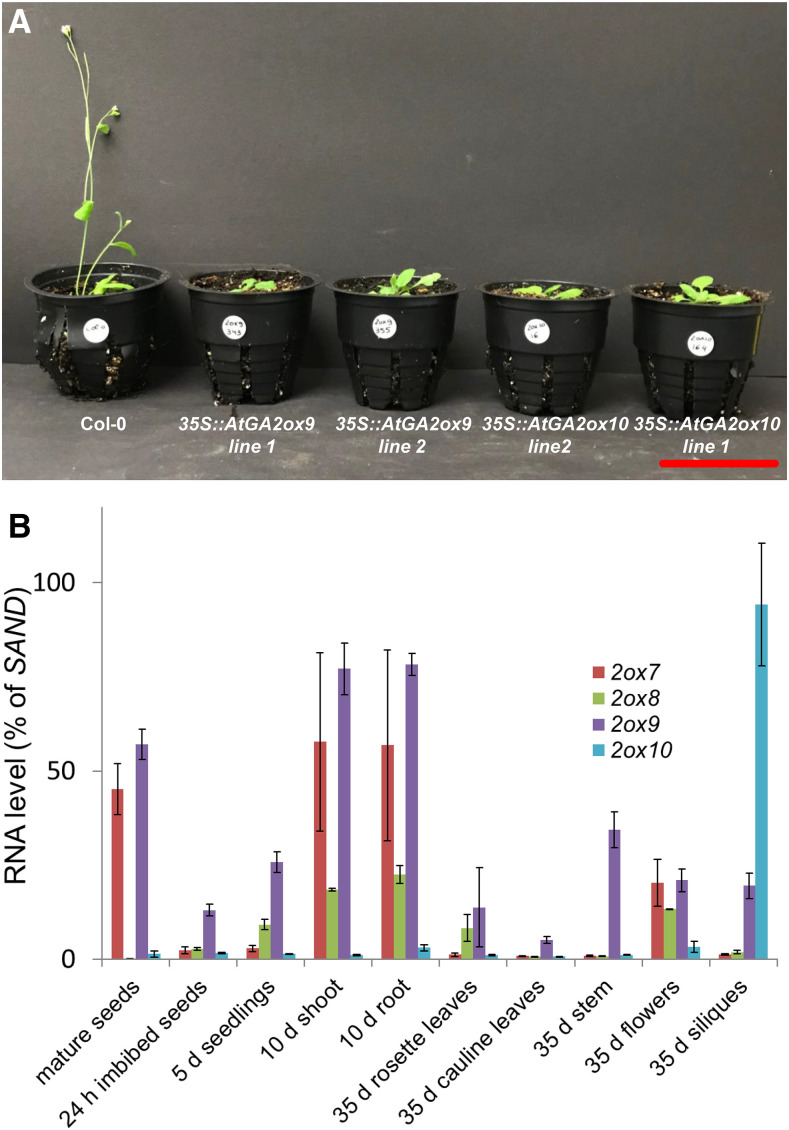
Previous studies identified class III GA2ox7 and GA2ox8 as catabolic enzymes (Schomburg et al., 2003). Similarly, overexpression of AtGA2ox9 and, less severely, of AtGA2ox10 in Arabidopsis results in a phenotype (dwarfism, late flowering, small dark green rosette leaves) that is typical for GA-deficient plants (Fig. 2A; Supplemental Fig. S4), indicating a GA-catabolic function for both enzymes in planta. Consistently, endogenous GA110 levels in the AtGA2ox9 overexpressors are extremely elevated, whereas levels of almost all other GAs in the pathway are reduced, including bioactive GA4 (Supplemental Table S3). Moreover, endogenous GA25 levels are also elevated, indicating that AtGA2ox9 functions as a GA 20-oxidase in planta and confirming the activity found for the respective recombinant enzyme (see previous section).
Figure 2.
Catabolic function and sites of expression of class III GA 2-oxidases. A, Phenotype of transgenic plants overexpressing catabolic AtGA2ox9 and AtGA2ox10. Shown from left to right are representative 32-d-old plants transferred to the soil after 18 d in Murashige and Skoog medium: Col-0 and two overexpressor lines each for AtGA2ox9OE and AtGA2ox10OE. Scale bar = 5 cm. B, Catabolic class III GA2ox9 and GA2ox10 are expressed during seedling and seed development, respectively. Transcript levels of GA2ox7, GA2ox8, GA2ox9, and GA2ox10 genes were determined in different tissues by RT-qPCR (relative to SAND) during Arabidopsis development. Plotted data are means of three biological replicates ± se.
AtGA2ox9 and AtGA2ox10 Are Expressed during Seedling and Seed Development, Respectively
To evaluate the role of the identified class III GA 2-oxidases in plant development, we analyzed their expression levels at different developmental stages of Arabidopsis by reverse transcription quantitative PCR (RT-qPCR; Fig. 2B). Expression analysis revealed that AtGA2ox9 is expressed throughout the Arabidopsis life cycle, with the highest transcript levels observed in mature seeds and in shoots and roots of 10-d-old seedlings. AtGA2ox10 was expressed mostly in siliques (Fig. 2B) and in developing seeds according to the Arabidopsis transcriptome analysis proposed by Klepikova et al. (2016). These results suggest that AtGA2ox9 and AtGA2ox10 play a role in seedling and seed development, respectively. Moreover, transcript profiles of AtGA2ox7, AtGA2ox8, and AtGA2ox9 were similar in 10-d-old seedlings and flowers of 35-d-old plants, and AtGA2ox7 expression levels were similar to those of AtGA2ox9 in mature seeds, suggesting functional redundancies.
AtGA2ox10 Regulates Fertility
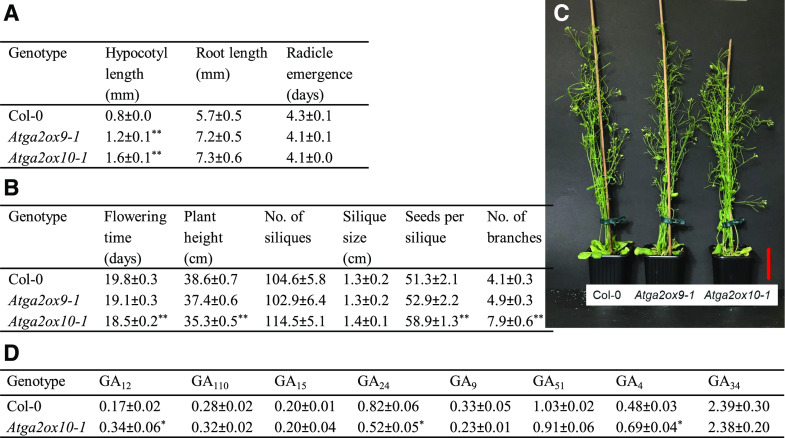
Homozygous lines were obtained for two independent alleles of loss-of-function mutants of AtGA2ox9 (Atga2ox9-1 and Atga2ox9-2) and AtGA2ox10 (Atga2ox10-1 and Atga2ox10-2) by genotype PCR screening (Supplemental Table S4). To validate the roles of AtGA2ox9 and AtGA2ox10 in plant development, we analyzed the phenotypes of Atga2ox9-1 and Atga2ox10-1 loss-of-function mutants in comparison to wild-type Arabidopsis ecotype Columbia (Col-0) plants (Fig. 3, A–C). In 7-d-old seedlings, hypocotyls of both Atga2ox9-1 and Atga2ox10-1 mutants were significantly longer compared to those of wild-type plants (Fig. 3A). However, no differences were observed in root length and radicle emergence between 7-d-old mutant and wild-type plants. The similar development of 5-week-old Atga2ox9-1 mutant and wild-type plants suggests functional redundancy among GA 2-oxidase family members in adult plants, as predicted by the expression studies (Figs. 2B and 3, B and C). However, Atga2ox10-1 knock-out mutants flowered slightly earlier and developed more side branches on the main bolt compared to wild-type plants (Fig. 3B). As suggested by transcript analysis, AtGA2ox10 plays an important role in reproductive development. Loss-of-function mutants of the two independent alleles of AtGA2ox10, namely Atga2ox10-1 and Atga2ox10-2, displayed an increased number of seeds per silique compared to wild-type plants (Fig. 3B; Supplemental Table S5). To verify the GA status, we analyzed endogenous GA levels in siliques of Atga2ox10-1 mutant and wild-type plants (Fig. 3D). Levels of GA12, the precursor of C19-GAs, significantly increased in the mutant plants, as expected, indicating a lack of AtGA2ox10 activity. Consequently, the Atga2ox10-1 mutants had slightly elevated bioactive C19-GA4 levels compared to wild-type plants, which we propose is responsible for the mutant phenotype observed, including the elevated seed production (Fig. 3, A–C).
Figure 3.
Analysis of AtGA2ox9 and AtGA2ox10 loss-of-function mutant plants. Phenotypic analysis of Atga2ox9-1 and Atga2ox10-1 mutants in comparison to wild-type Col-0 A, Plate-grown 7-d-old seedlings. B, Soil-grown 35-d-old plants. Plotted data are means of ≥15 plants, ≥90 siliques, and seeds from 20 siliques per genotype ± se. C, Representative 35-d-old plants (from left to right) Col-0, Atga2ox9-1, and Atga2ox10-1. D, Endogenous GA levels (means ± se) in siliques of 35-d-old Col-0 and Atga2ox10-1 plants. Shown are means of three biological replicates (ng·g−1 fresh weight). Statistical differences relative to Col-0 were determined by Student’s t test; *P < 0.05, **P < 0.01. Scale bar = 5 cm.
AtGA2ox9 Contributes to Freezing Tolerance
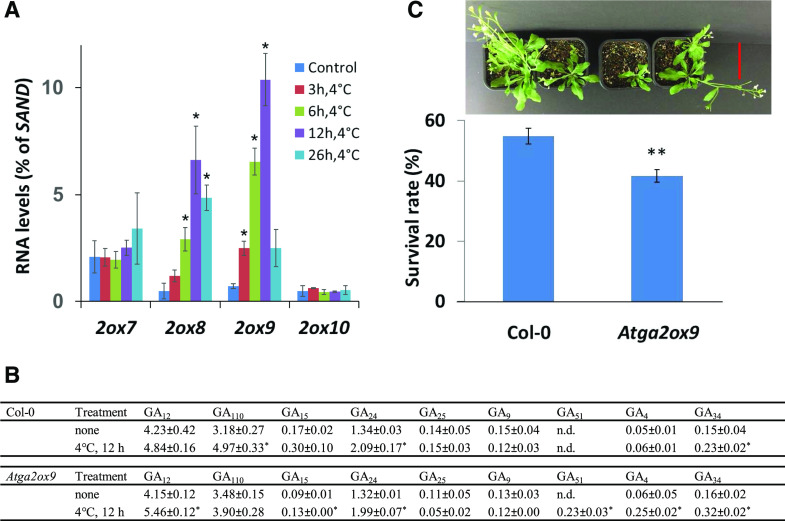
C-repeat/dehydration-responsive element binding protein (DREB) binding factors (CBFs) are transcription factors known to be involved in cold adaptation of plants (Shi et al., 2018). Transcript levels of the C-repeat/DREB binding factors CBF1, CBF2, and CBF3, as expected, increased after the onset of the cold treatment, followed by class I/II 2-oxidases AtGA2ox1 and AtGA2ox6, and the highest transcript levels of the class III GA 2-oxidases AtGA2ox8 and, particularly, AtGA2ox9 were observed much later, after 12 h of cold treatment (Fig. 4A; Supplemental Table S6). For the other two members of the GA 2-oxidase family (AtGA2ox7 and AtGA2ox10), transcript levels did not change or were much less affected by the cold treatment.
Figure 4.
AtGA2ox9 contributes to freezing tolerance in Arabidopsis. A, Cold regulation of the Arabidopsis class III GA 2-oxidase genes. Transcript levels of GA2ox7, GA2ox8, GA2ox9, and GA2ox10 genes were determined by RT-qPCR (relative to SAND) in Arabidopsis 2-week-old plants subjected to cold treatment (4°C) for the times indicated or kept at 22°C (control). Data plotted are means ± se of three biological replicates. B, Endogenous GA levels (means ± se) in 14-d-old Col-0 and Atga2ox9-1 seedlings without or with cold treatment (12 h at 4°C). Shown are means of three biological replicates (ng·g−1 fresh weight). In some samples, endogenous GA was not detected (n.d.), but the internal standard was recovered. C, Fourteen-day-old Col-0 and Atga2ox9-1 plants were exposed to −5°C for 10 h, as described in “Materials and Methods,” and survival was scored after 14 d of recovery. Top, Representative Col-0 (left two pots) and Atga2ox9-1 (right two pots) plants after freezing treatment. Each pot contained five plants. Bottom, Quantification of the results, with data plotted as means ± se of four biological replicates (n = 36 for each replicate). Statistical differences relative to Col-0 were determined by Student’s t test; *P < 0.05, **P < 0.01. Scale bar = 5 cm.
We further investigated the impact of cold treatment on the GA status of wild-type and Atga2ox9-1 knock-down plants (Fig. 4B). At normal temperature, endogenous GA levels of Col-0 and Atga2ox9-1 mutant plants were similar. Consequently, wild-type and Atga2ox9-1 mutant plants developed similarly. In wild-type plants, after chilling, catabolic GA34 and GA110 levels increased, indicating the action of class I/II and class III GA 2-oxidases, respectively, and bioactive GA4-levels remained low. However, in cold-treated Atga2ox9-1 mutant plants, only catabolic GA34 and GA51 increased, indicating class I/II GA 2-oxidase activity as proposed previously by Achard et al. (2008), and GA110 levels did not change. Consequently, precursor GA12, GA15, GA24, and bioactive GA4 levels accumulated in the Atga2ox9-1 mutant compared to wild-type plants (Fig. 4B). We further tested the role of AtGA2ox9 for freezing tolerance using two independent alleles of knock-down mutants, Atga2ox9-1 and Atga2ox9-2, and wild-type plants. The survival rate was reduced in both mutants compared to that in wild-type plants after freezing (Fig. 4C; Supplemental Table S7). We conclude that in Arabidopsis, AtGA2ox9 contributes to acquired cold stress tolerance.
DISCUSSION
Here we identify and characterize two GA 2-oxidases, AtGA2ox9 and AtGA2ox10. Both are related to the previously described class III GA 2-oxidases; however, they form a new cluster of phylogenetic relationships (Supplemental Fig. S1; Schomburg et al., 2003). Their amino acid sequences contain conserved motifs typical for 2-oxoglutarate-dependent dioxygenases, and residues involved in iron- and 2-oxoglutarate-binding (Supplemental Fig. S2; Porco et al., 2016). Previously, it was proposed that a unique peptide sequence consisting of 29 amino acids might be responsible for the specific catalytic properties of class III GA 2-oxidases (Schomburg et al., 2003). However, both AtGA2ox9 and AtGA2ox10 contain 10 extra amino acids within this peptide region, raising the question of whether their enzymatic properties are similar to those of the known class III GA 2-oxidases (Supplemental Fig. S2). Indeed, AtGA2ox9 and AtGA2ox10 recombinant enzymes were found to 2β-hydroxylate the C20-GA precursor GA12 to GA110, which is a typical reaction for this class of GA 2-oxidases (Supplemental Table S1; Schomburg et al., 2003; Lange and Pimenta Lange, 2020). In addition, AtGA2ox9 and AtGA2ox10 harbor enzymatic properties previously not associated with GA 2-oxidases. AtGA2ox9 hydroxylates the C19-GAs GA9 and GA4 at the 2α-position to form GA40 and GA47, respectively. These 2α-hydroxylated GAs were found previously in the fungus Fusarium fujikuroi, but are not commonly identified in higher plants (MacMillan, 2001). Moreover, the new AtGA2ox9 and AtGA2ox10 oxidize GA15 at the C-20 position. Such an enzymatic activity was first discovered to occur in cell extracts from spinach (Spinacia oleracea) leaves (Gilmour et al., 1986) and barley (Hordeum vulgare) seedlings (Großelindemann et al., 1992). Recently, we identified GA 20-oxidases from Arabidopsis and cucurbits (Cucurbitaceae) that catalyze this reaction as well (Lange and Pimenta Lange, 2020). The two identified Arabidopsis GA 2-oxidases also oxidize GA24, the next step in the GA biosynthetic pathway, to form either the C19-GA GA9 (AtGA2ox10) or the C20 tricarboxylic acid GA25 (AtGA2ox9). The formation of GA9 is common for anabolic GA 20-oxidases, but production of the tricarboxylic acid, as in GA25, is unusual and has been previously found for the catabolic GA 20-oxidases from pumpkin (Cucurbita maxima; CmGA20ox1 and CmGA20ox2; Lange et al., 1994; Lange, 1997) and, recently, from Arabidopsis (AtGA20ox5; Lange and Pimenta Lange, 2020). Such GA tricarboxylic acids are also endogenous to Arabidopsis and other plant species (Talon et al., 1990; MacMillan, 2001) and class III GA 2-oxidases like AtGA2ox9 might contribute to their biosynthesis. Forming GA tricarboxylic acids might be another strategy, in addition to the classical catabolic pathways, that plants develop to inactivate GAs (Magome and Kamiya, 2016), given that overexpression of the pumpkin CmGA20ox1 leads to severe dwarfism in several plant species, including Arabidopsis, lettuce (Lactuca sativa), and Solanum dulcamara (Curtis et al., 2000; Niki et al., 2001; Radi et al., 2006). Other products of AtGA2ox9 enzyme activity include putative dehydrogenated GAs (compounds a and b; Supplemental Table S1), similar activities of which have been suggested for pumpkin CmGA20ox1, since transgenic Solanum plants overexpressing this enzyme produce putative dehydrogenated GAs (Curtis et al., 2000).
Magome et al. (2008) also reported that the class III GA 2-oxidase AtGA2ox7 also converts C20-GA precursors, including GA15 and GA24. By reinvestigating the AtGA2ox7 catalytic properties, we identified the products of GA15 and GA24 incubations as 2β-hydroxy-GA15 and 2β-hydroxy-GA24, respectively. Moreover, both AtGA2ox7 and AtGA2ox9 also 2β-hydroxylate C20-GA25 to GA46, a catalytic property previously identified for a bifunctional pumpkin GA 3-oxidase, CmGA3ox1 (Lange et al., 1997). Similar to AtGA2ox9, AtGA2ox7 was shown to have 2α-hydroxylase and putative desaturase activities. These previously unassociated activities of class III GA 2-oxidases might have misguided the interpretation of results previously obtained for salt- or touch-stressed plants, in which AtGA2ox7 is strongly expressed but without a corresponding increase of endogenous 2β-hydroxylated GAs (Magome et al., 2008; Pimenta Lange and Lange, 2015). These activities might also add to the catabolic properties of multifunctional class III GA 2-oxidases. For example, tobacco (Nicotiana tabacum) plants overexpressing AtGA2ox7 display a more severe dwarf phenotype compared to those overexpressing AtGA2ox8 (an enzyme without GA 2α-hydroxylase function; Schomburg et al., 2003).
Taken together, Arabidopsis class III GA 2-oxidases have heterogeneous enzymatic properties. AtGA2ox8 is the only monofunctional member of the class III GA 2-oxidases in Arabidopsis (Fig. 1A). The other three members show broader substrate specificities: AtGA2ox7 and AtGA2ox9 also perform 2α-hydroxylation of C19-GAs, whereas AtGA2ox9 and AtGA2ox10 oxidize the C-4 to C-10 δ-lactone of GA15, leading to GA24, and produce tricarboxylic C20-GAs (GA25, GA46, and compound b) and C19-GA9, respectively.
Class III GA 2-oxidases mediate the integration of endogenous and exogenous signals into the plant life cycle program. Although the complexity of the physiological and biochemical responses after stress treatment often makes it difficult to analyze the role of GAs based on phenotypic criteria (Lantzouni et al., 2020), in this study, our phenotypical analyses support the biochemical data. We found that AtGA2ox9 (together with AtGA2ox7) is highly expressed in 10-d-old shoots and in mature seeds. AtGA2ox10 is highly expressed in siliques (Fig. 2B) and more specifically in developing seeds, as has been proposed by Klepikova et al. (2016), suggesting that these class III GA 2-oxidases play a role in GA homeostasis in these organs. Indeed, the hypocotyls of 7-d-old Atga2ox9-1 and of Atga2ox10-1 loss-of-function mutants are longer, indicating a role of class-III GA 2-oxidases in early seedling growth, similar to class I/II GA 2-oxidases (Rieu et al., 2008). Interestingly, the height of 5-week-old plants was seen to be reduced in the Atga2ox10-1 mutant, with a significant increase in the number of branches on the main bolt (Fig. 3B).
For efficient pollination, coordinated growth of pistil and stamen filaments is necessary, and this is regulated by GAs (Griffiths et al., 2006; Pimenta Lange et al., 2012; Pimenta Lange and Lange, 2016). Knock-out mutants of two C20-GA 2-oxidases in rice resulted in a low seed-set rate, indicating a potential role of class III GA 2-oxidases in fertility and seed development (Chen et al., 2019). Both elevated GA levels and GA deficiency in plants results in a loss of fertility (Jacobsen and Olszewski, 1993; Radi et al., 2006; Rieu et al., 2008). These findings indicate that to improve fertility it is necessary to achieve optimal GA levels. We found that fertility increased significantly in the Atga2ox10-1 mutant, probably due to a moderate modulation of the GA hormone pool, as we observed previously for Arabidopsis plants overexpressing pumpkin CmGA7ox or CmGA3ox1 (Radi et al., 2006).
Cold acclimatization is an important adaption of plants to low temperatures and is regulated by CBFs (Shi et al., 2018). Achard et al. (2008) demonstrated that DELLAs contribute to CBF1-induced cold acclimatization and freezing tolerance. Recent studies demonstrated that the cold-induced expression of At5g58660 (AtGA2ox9) gene is part of the CBF regulon (Zhao et al., 2016). Moreover, Lantzouni et al. (2020) showed that AtGA2ox8 expression increases after cold treatment, although gene responses were followed for only 4 h after the onset of the cold-stress treatment. Here we found that the class I/II GA 2-oxidases (AtGA2ox1 and AtGA2ox6) respond earlier to cold stress (3 to 6 h after the treatment) compared to class III GA 2-oxidases (AtGA2ox8 and AtGA2ox9; 12 h after treatment), suggesting different regulation and function of class I/II and class III GA 2-oxidases upon cold stress.
The endogenous GA levels we observed in response to freezing stress support such interplay of class I/II and class III GA 2-oxidases. In wild-type plants, GA products of class I/II GA 2-oxidases (GA34) first increased, followed by those of class III (GA110). As a result, bioactive GA4 levels remained low. In the Atga2ox9-1 mutant plants, early catabolism by class I/II GA 2-oxidases occurred and the products (GA51, GA34) accumulated after chilling stress. However, the missing late activity of catabolic AtGA2ox9 led to an accumulation of GA precursors and of bioactive GA4 levels in the mutant plant. This elevated biosynthesis of GA4 is likely due to cold stress induction of GA 20-oxidase and GA 3-oxidase expression, as has been previously observed by Achard et al. (2008). Given that wild-type plants are more freezing tolerant than the Atga2ox9 mutants, we conclude that AtGA2ox9 activity maintains low bioactive GA4 levels, which is essential for survival under such unfavorable growth conditions.
We conclude that the identified class III GA 2-oxidases AtGA2ox9 and AtGA2ox10 are catabolic enzymes that play pivotal roles in plant stress responses and developmental processes of high economic and ecologic relevance. Class III GA 2-oxidases with similar functions to AtGA2ox9 and AtGA2ox10 may exist in other plant species, but enzymatic characterization of these putative family members remains to be performed.
Fine-tuning of bioactive GAs is particularly important for plant fertility, and AtGA2ox10 substantially regulates this process. AtGA2ox9 is cold regulated and contributes to acquiring freezing tolerance, a task previously attributed to some members of class I/II GA 2-oxidases. It is now tempting to study the role of class I/II and class III GA 2-oxidases in plant development and precise adaption to stresses in a spatiotemporal manner.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) was used as the wild type. The following mutant lines were obtained from the European Arabidopsis Stock Centre (NASC) and are all in the Col-0 background: At5g58660 (Atga2ox9-1 [Salk_030540] and Atga2ox9-2, [Salk_030539]); At3g47190 (Atga2ox10-1 [Salk_053952C] and Atga2ox10-2 [Salk_071088]). Homozygous lines were obtained by genotype PCR screening (described below). For soil-grown plants, seeds were sown on soil:vermiculite (2:1 [v/v], 156 g per pot, 92% relative field capacity [FC]) in 24-pot trays, with 12 pots containing Col-0 and 12 pots the respective mutant line. To minimize positional effects within the growth chambers, pots were placed in a checkerboard-like pattern on the tray, and trays were rotated 180° every other day. Soil humidity was kept between 46% and 76% FC. Plants were grown in growth chambers with 70% relative humidity and a 16-h light/8-h dark cycle at 22°C/20°C, respectively (Atga2ox9-1 and Atga2ox10-1 at a photon fluence rate of 200 µmol m−2 s−1 [Osram Cool-White Fluorescent lamps] and Atga2ox9-2 and Atga2ox10-2 at a photon fluence rate of 300 µmol m−2 s−1 [Osram Powerstar HQI-T 400W/D daylight lamps]). For measurement of radicle emergence, hypocotyls length, and root length, seeds were sown on half-strength Murashige and Skoog agar square petri dishes, stratified at 4°C for 3 d, and then placed vertically in a controlled environment chamber (16-h light (photon fluence 200 µmol m−2 s−1)/8-h dark cycle at 22°C/20°C). Radicle emergence of at least 50 seedlings per genotype was monitored with the help of binoculars. Seven days after sowing the seeds, hypocotyl and root length were measured from at least 15 seedlings per genotype. For phenotypic analysis of adult plants, the flowering time was scored when the first flower bud was visible at the apex. The number of siliques and plant height (main inflorescence) were scored in 5-week-old plants using at least 15 plants for the measurements. For each genotype, silique length of at least 90 siliques was measured and the number of seeds counted in at least 20 siliques per genotype.
Plant Cold/Freezing Assays
Prior to stress treatment, pots containing 3–5 2-week-old plants per pot were adjusted to 61% FC. For cold treatment, plants were incubated for up to 26 h at 4°C, maintaining the photoperiod described above. Plant freezing assays were performed as described by Eremina et al. (2016) with modifications. For freezing assays, plants were incubated in the dark in a controlled temperature chamber (FitoClima 600) for 30 min at 4°C. The temperature was then decreased to 0°C at a rate of 4°C h−1, and further to –5°C at a rate of 2°C h−1. This final temperature was maintained for 10 h (Atga2ox9-1) or 6 h (Atga2ox9-2) before the temperature was increased again to 4°C at a rate of 2.25°C h−1. The plants were kept for one day at 4°C in a 16-h light (photon fluence 200 μmol m−2 s−1)/8-h dark photoperiod before they were returned to the standard growth conditions described in the previous section. Survival rates were determined 2 weeks (Atga2ox9-1) or 1 week (Atga2ox9-2) after treatment, and only those plants able to develop new leaves were counted as survivors.
Genotype PCR
Genomic DNA of the Arabidopsis mutant lines was isolated by the cetrimonium bromide method using 20 mg of plant material. Genotype PCR reactions (20 μL) were performed using 0.5 μg of DNA as a template together with gene- and T-DNA-specific primers. Primers used are listed in Supplemental Table S8.
Cloning of GA 2-Oxidases from Arabidopsis
In a phylogenetic analysis, Porco et al. (2016) identified two putative 2-oxoglutarate-dependent dioxygenases, At5g58660 and At3g47190, in the Arabidopsis genome that are closely related to the previously identified class III GA 2-oxidases AtGA2ox7 and AtGA2ox8. To investigate their enzymatic activities, we first cloned the respective cDNA molecules of putative or known GA 2-oxidases into the pET101/D-TOPO expression vector. Total RNA (100 ng) from different Arabidopsis tissues (Supplemental Table S9) was isolated as described by Pimenta Lange and Lange (2015) and was used in 10 μL reverse transcription reactions to produce cDNA molecules using the PCRBIO cDNA synthesis kit (BIOSYSTEMS) according to the manufacturer’s indications. The cDNA molecules were used as templates in 10 μL PCR reactions containing 2× Phusion High-Fidelity PCR Master Mix (Thermo Fisher Scientific) and sequence-specific primers designed according to the coding sequence of the Arabidopsis putative or known GA 2-oxidases. PCR conditions and length of the expected PCR products are listed in Supplemental Table S9. After reamplification, PCR products were purified by agarose gel electrophoresis (GeneJet TM Gel extraction kit; Thermo Fisher Scientific) and cloned into the pET101/D-TOPO expression kit (Invitrogen) following the manufacturer’s instructions. Positive clones were identified by PCR and respective plasmid DNAs were sequenced on both strands.
Heterologous Expression of Recombinant GA 2-Oxidases
Plasmid DNA of the cloned AtGA2ox7, AtGA2ox8, At5g58660 (AtGA2ox9), and At3g47190 (AtGA2ox10) was used to transform BL21 Star Escherichia coli (Invitrogen) according to the manufacturer's instructions. Production of recombinant GA 2-oxidases and protein induction were as previously described by Lange et al. (2005).
Enzyme Assays and Analysis of Incubation Products
14C-labeled GAs were prepared as described by Lange et al. (2005). 17-14C-labeled GA12 was a gift from Professor Lew Mander (Canberra, Australia). Preparations of E. coli cell lysates (70 μL) were incubated with 2-oxoglutarate and ascorbate (final concentration 100 mm each). FeSO4 (0.5 mm), catalase (1 mg/mL), and the 14C-labeled substrate [2 μL in methanol; 0.33 nmol for (1,7,12,18-14C4)-labeled GAs and 1 nmol for (17-14C)-labeled GAs] were added in a total volume of 100 μL and incubated for 4 h at 30°C. Variations in incubation conditions are specified for particular experiments. For the synthesis of 17,17-d2-labeled GA110 (giving a yield of 94% recovery), incubation mixtures contained recombinant AtGA2ox7 (36 μL E. coli cell lysate prepared as described by Lange et al. (2005), 17,17-d2-labeled GA12 (2 μg; also from Professor Lew Mander), 2-oxoglutarate (4 mm), ascorbate (4 mm), FeSO4 (0.5 mm), and catalase (1 mg/mL) in a total volume of 50 µL were incubated for 16 h at 30°C. Analyses of incubation products by HPLC and gas chromatography-mass spectrometry (GC-MS) were done as previously described by Lange et al. (2005).
Overexpression of CsGA2ox9 and CsGA2ox10 in Arabidopsis
To express AtGA2ox9 and AtGA2ox10 in Arabidopsis, cDNA molecules were PCR amplified using Phusion High-Fidelity PCR Master Mix as described in “Cloning of GA 2-Oxidases from Arabidopsis”, with primers containing restriction sites (Supplemental Table S8) and cloned at the BamH1, EcoRI sites of a modified pBE2113 vector containing a strong promoter cassette and a translational enhancer (E12-35-Ω). Agrobacterium tumefaciens-mediated transformation of Arabidopsis and selection of transgenic lines was done as previously described by Pimenta Lange et al. (2020). After scoring at T3, two homozygous lines for each gene were taken to generate T4 homozygous plants. The lines were phenotypically similar (Fig. 2), and line 1 of each construct was utilized in the further studies (Supplemental Fig. S4).
Gene Expression Analysis
Total RNA extraction has been described by Pimenta Lange and Lange (2015). First-strand cDNA synthesis was done with 500 ng of DNaseI-treated total RNA using the SuperScript IV VILO MasterMix in a 5-μL reaction volume according to the manufacturer’s protocol (Thermo Fisher Scientific). The cDNA was diluted 10 times with water and used for 10 μL RT-qPCR reactions as previously described by Pimenta Lange and Lange (2015), but with the addition of 5 μL of qPCRBIO SyGreen No-Rox mix (PCR Biosystems) in a two-step cycling program according to the manufacturer’s protocol. The At2g28390 (SAND) gene was used as the internal reference gene. The real-time PCR Miner program was used to calculate the relative expression of each gene (http://www.miner.ewindup.info/) as the average of two technical qPCR replicates. Technical replicates with a difference from the mean of cycle threshold (Ct) ≥ 0.5 were excluded. All experiments were done with at least three biological replicates. The samples were tested for the absence of genomic DNA by qPCR using RNA as template. Primers used for qPCR are listed in Supplemental Table S8.
Analysis of Endogenous GAs
For quantitative analysis of endogenous GAs, 0.5 g (fresh weight) of frozen, pulverized plant tissues were spiked with 17,17-d2-GA standards (between 0.4 and 6 ng, according to the expected amounts of endogenous GAs; from Professor Lew Mander), and 80% (v/v) methanol-water (1.5 mL) was added. The samples were stored at –20°C for 16 h and centrifuged at 4,000g for 5 min. Extraction and solvent partitioning were performed as described by Lange et al. (2005). Samples were dissolved in methanol (2 mL) and after loading onto an ion-exchange column (BondElut DEA, Varian), the column was washed with methanol (6 mL) and the GAs were eluted with methanol containing 1% (v/v) acetic acid (6 mL). The samples were dried and redissolved in methanol (100 μL) and water (2 mL) containing 1% (v/v) acetic acid. After loading the samples onto a C18-cartridge (Waters), the cartridge was washed with water (2× 10 mL) containing 1% (v/v) acetic acid. GAs were eluted from the cartridge with 80% (v/v) methanol-water containing 1% (v/v) acetic acid. The eluates were then dried, derivatized, and analyzed by GC-MS (Lange et al., 2005). The ions monitored for quantication of endogenous GAs were 300 and 302 (GA12), 298 and 300 (GA110), 239 and 241 (GA15), 314 and 316 (GA24), 312 and 314 (GA25), 298 and 300 (GA9), 268 and 270 (GA51), 284 and 286 (GA4), and 506 and 508 (GA34).
Statistical Analysis
Statistical analysis was performed using Student’s t test with significance levels of P < 0.01 for phenotypic characterization experiments and P < 0.05 for GA levels and RT-qPCR data.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers LT827066 (AtGA2ox9) and LT827067 (AtGA2ox10).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Phylogenetic relationships of the GA 2-oxidase family from Arabidopsis (At), cucumber (Cs), and rice (Os).
Supplemental Figure S2. Protein sequence alignment between the predicted AtGA2ox9 and AtGA2ox10 and the known Arabidopsis class III GA 2-oxidases.
Supplemental Figure S3. Catalytic properties of recombinant class III GA 2-oxidases AtGA2ox7 and AtGA2ox8.
Supplemental Figure S4. Phenotypic analysis of soil-grown 14-d-old AtGA2ox9 and AtGA2ox10 overexpressors.
Supplemental Table S1. Mass spectra and Kovats retention indices of products of the methyl ester trimethylsilylether derivatives from incubations of selected GAs with cell lysates from E coli expressing recombinant GA 2-oxidases AtGA2ox7, AtGA2ox8, AtGA2ox9, and AtGA2ox10 from Arabidopsis.
Supplemental Table S2. Metabolism of 14C-labeled GA substrates incubated for 4 h with different volumes of cell lysates prepared from E. coli expressing recombinant AtGA2ox9.
Supplemental Table S3. Endogenous GA levels in 23-d-old soil-grown Col-0 and 35S::AtGA2ox9, line 1 plants.
Supplemental Table S4. Characterization of knock-down and knock-out lines for Atga2ox9 and Atga2ox10, respectively.
Supplemental Table S5. Number of siliques per plant and seeds per silique of the Atga2ox10-2 knock-down mutant line in comparison to wild-type Col-0.
Supplemental Table S6. Transcript levels of Arabidopsis CBF1, CBF2, CBF3, and selected class I/II GA 2-oxidase encoding genes.
Supplemental Table S7. Survival rate after freezing of the Atga2ox9-2 knock-down mutant compared to Col-0.
Supplemental Table S8. List of primers used for overexpression in Arabidopsis, showing genotype and RT-qPCR conditions.
Supplemental Table S9. Plant tissues and PCR conditions used for cloning the GA 2-oxidases.
Acknowledgments
We thank Nils Kappe and Manuela Szperlinski for help with the characterization of GA2ox7 and maintenance of transgenic plants, respectively, Anja Liebrandt for technical assistance, Professor Lew Mander for the generous gift of 14C-labelled GA12, and Professor Peter Hedden for help with interpretation of the MS spectra. This work is dedicated to Professor Jan E. Graebe, a pioneer in GA biosynthesis, on the occasion of his 90th birthday.
Footnotes
This work was supported in part by the Volkswagen Foundation (grant no. Az. 95 475 to M.J.P.L.).
Articles can be viewed without a subscription.
References
- Achard P, Gong F, Cheminant S, Alioua M, Hedden P, Genschik P(2008) The cold-inducible CBF1 factor-dependent signaling pathway modulates the accumulation of the growth-repressing DELLA proteins via its effect on gibberellin metabolism. Plant Cell 20: 2117–2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Tian X, Xue L, Zhang X, Yang S, Traw MB, Huang J(2019) CRISPR-based assessment of gene specialization in the gibberellin metabolic pathway in rice. Plant Physiol 180: 2091–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colebrook EH, Thomas SG, Phillips AL, Hedden P(2014) The role of gibberellin signalling in plant responses to abiotic stress. J Exp Biol 217: 67–75 [DOI] [PubMed] [Google Scholar]
- Curtis IS, Ward DA, Thomas SG, Phillips AL, Davey MR, Power JB, Lowe KC, Croker SJ, Lewis MJ, Magness SL, et al. (2000) Induction of dwarfism in transgenic Solanum dulcamara by over-expression of a gibberellin 20-oxidase cDNA from pumpkin. Plant J 23: 329–338 [DOI] [PubMed] [Google Scholar]
- Eremina M, Unterholzner SJ, Rathnayake AI, Castellanos M, Khan M, Kugler KG, May ST, Mayer KFX, Rozhon W, Poppenberger B(2016) Brassinosteroids participate in the control of basal and acquired freezing tolerance of plants. Proc Natl Acad Sci USA 113: E5982–E5991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskin P, MacMillan J(1992) GC-MS of Gibberellins and Related Compounds. Cantock’s Enterprise, Bristol, UK [Google Scholar]
- Gilmour SJ, Zeevaart JAD, Schwenen L, Graebe JE(1986) Gibberellin metabolism in cell-free extracts from spinach leaves in relation to photoperiod. Plant Physiol 82: 190–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths J, Murase K, Rieu I, Zentella R, Zhang Z-L, Powers SJ, Gong F, Phillips AL, Hedden P, Sun TP, et al. (2006) Genetic characterization and functional analysis of the GID1 gibberellin receptors in Arabidopsis. Plant Cell 18: 3399–3414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Großelindemann E, Lewis MJ, Hedden P, Graebe JE(1992) Gibberellin biosynthesis from gibberellin A12-aldehyde in a cell-free system from germinating barley (Hordeum vulgare L., cv. Himalaya) embryos. Planta 188: 252–257 [DOI] [PubMed] [Google Scholar]
- Hedden P, Phillips A (2015) Gibberellin metabolism. In Hausinger RP, Schofield CJ, eds, 2-Oxoglutarate-Dependent Oxygenases, RSC Metallobiology Series No. 3. Royal Society of Chemistry, Cambridge, pp 367–384 [Google Scholar]
- Jacobsen SE, Olszewski NE(1993) Mutations at the SPINDLY locus of Arabidopsis alter gibberellin signal transduction. Plant Cell 5: 887–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepikova AV, Kasianov AS, Gerasimov ES, Logacheva MD, Penin AA(2016) A high resolution map of the Arabidopsis thaliana developmental transcriptome based on RNA-seq profiling. Plant J 88: 1058–1070 [DOI] [PubMed] [Google Scholar]
- Lange T.(1997) Cloning gibberellin dioxygenase genes from pumpkin endosperm by heterologous expression of enzyme activities in Escherichia coli. Proc Natl Acad Sci USA 94: 6553–6558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Hedden P, Graebe JE(1994) Expression cloning of a gibberellin 20-oxidase, a multifunctional enzyme involved in gibberellin biosynthesis. Proc Natl Acad Sci USA 91: 8552–8556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Kappler J, Fischer A, Frisse A, Padeffke T, Schmidtke S, Pimenta Lange MJ(2005) Gibberellin biosynthesis in developing pumpkin seedlings. Plant Physiol 139: 213–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange T, Pimenta Lange MJ(2020) The multifunctional dioxygenases of gibberellin synthesis. Plant Cell Physiol doi:10.1093/pcp/pcaa051. [DOI] [PubMed] [Google Scholar]
- Lange T, Robatzek S, Frisse A(1997) Cloning and expression of a gibberellin 2 β,3 β-hydroxylase cDNA from pumpkin endosperm. Plant Cell 9: 1459–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lantzouni O, Alkofer A, Falter-Braun P, Schwechheimer C(2020) GROWTH-REGULATING FACTORS interact with DELLAs and regulate growth in cold stress. Plant Cell 32: 1018–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DJ, Zeevaart JAD(2005) Molecular cloning of GA 2-oxidase3 from spinach and its ectopic expression in Nicotiana sylvestris. Plant Physiol 138: 243–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan J.(2001) Occurence of gibberellins in vascular plants, fungi, and bacteria. J Plant Growth Regul 20: 387–442 [DOI] [PubMed] [Google Scholar]
- Magome H, Kamiya Y (2016) Inactivation processes. In Hedden P, Thomas SG, eds, The Gibberellins, Annual Plant Reviews, Vol 49 Wiley, Hoboken, NJ, pp 73–94 [Google Scholar]
- Magome H, Yamaguchi S, Hanada A, Kamiya Y, Oda K(2008) The DDF1 transcriptional activator upregulates expression of a gibberellin-deactivating gene, GA2ox7, under high-salinity stress in Arabidopsis. Plant J 56: 613–626 [DOI] [PubMed] [Google Scholar]
- Martínez-Bello L, Moritz T, López-Díaz I(2015) Silencing C19-GA 2-oxidases induces parthenocarpic development and inhibits lateral branching in tomato plants. J Exp Bot 66: 5897–5910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutasa-Göttgens E, Hedden P(2009) Gibberellin as a factor in floral regulatory networks. J Exp Bot 60: 1979–1989 [DOI] [PubMed] [Google Scholar]
- Niki T, Nishijima T, Nakayama M, Hisamatsu T, Oyama-Okubo N, Yamazaki H, Hedden P, Lange T, Mander LN, Koshioka M(2001) Production of dwarf lettuce by overexpressing a pumpkin gibberellin 20-oxidase gene. Plant Physiol 126: 965–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S, Huttly AK, Prosser IM, Li YD, Vaughan SP, Gallova B, Patil A, Coghill JA, Dubcovsky J, Hedden P, Phillips AL(2015) Heterologous expression and transcript analysis of gibberellin biosynthetic genes of grasses reveals novel functionality in the GA3ox family. BMC Plant Biol 15: 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Knop N, Lange T(2012) Stamen-derived bioactive gibberellin is essential for male flower development of Cucurbita maxima L. J Exp Bot 63: 2681–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Lange T(2006) Gibberellin biosynthesis and the regulation of plant development. Plant Biol (Stuttg) 8: 281–290 [DOI] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Lange T(2015) Touch-induced changes in Arabidopsis morphology dependent on gibberellin breakdown. Nat Plants 1: 14025. [DOI] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Lange T(2016) Ovary-derived precursor gibberellin A9 is essential for female flower development in cucumber. Development 143: 4425–4429 [DOI] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Liebrandt A, Arnold L, Chmielewska SM, Felsberger A, Freier E, Heuer M, Zur D, Lange T(2013) Functional characterization of gibberellin oxidases from cucumber, Cucumis sativus L. Phytochemistry 90: 62–69 [DOI] [PubMed] [Google Scholar]
- Pimenta Lange MJ, Szperlinski M, Kalix L, Lange T(2020) Cucumber gibberellin 1-oxidase/desaturase initiates novel gibberellin catabolic pathways. J Biol Chem 295: 8442–8448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porco S, Pěnčík A, Rashed A, Voß U, Casanova-Sáez R, Bishopp A, Golebiowska A, Bhosale R, Swarup R, Swarup K, et al. (2016) Dioxygenase-encoding AtDAO1 gene controls IAA oxidation and homeostasis in Arabidopsis. Proc Natl Acad Sci USA 113: 11016–11021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radi A, Lange T, Niki T, Koshioka M, Lange MJ(2006) Ectopic expression of pumpkin gibberellin oxidases alters gibberellin biosynthesis and development of transgenic Arabidopsis plants. Plant Physiol 140: 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieu I, Eriksson S, Powers SJ, Gong F, Griffiths J, Woolley L, Benlloch R, Nilsson O, Thomas SG, Hedden P, et al. (2008) Genetic analysis reveals that C19-GA 2-oxidation is a major gibberellin inactivation pathway in Arabidopsis. Plant Cell 20: 2420–2436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg FM, Bizzell CM, Lee DJ, Zeevaart JAD, Amasino RM(2003) Overexpression of a novel class of gibberellin 2-oxidases decreases gibberellin levels and creates dwarf plants. Plant Cell 15: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Ding Y, Yang S(2018) Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci 23: 623–637 [DOI] [PubMed] [Google Scholar]
- Sun TP.(2010) Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol 154: 567–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talon M, Koornneef M, Zeevaart JA(1990) Endogenous gibberellins in Arabidopsis thaliana and possible steps blocked in the biosynthetic pathways of the semidwarf ga4 and ga5 mutants. Proc Natl Acad Sci USA 87: 7983–7987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi S.(2008) Gibberellin metabolism and its regulation. Annu Rev Plant Biol 59: 225–251 [DOI] [PubMed] [Google Scholar]
- Zhao C, Zhang Z, Xie S, Si T, Li Y, Zhu JK(2016) Mutational evidence for the critical role of CBF transcription factors in cold acclimation in Arabidopsis. Plant Physiol 171: 2744–2759 [DOI] [PMC free article] [PubMed] [Google Scholar]