Plant chemicals known to mediate plant environment interactions also function as hormone-like regulators and precursors of primary metabolites.
Abstract
The plant kingdom produces hundreds of thousands of low molecular weight organic compounds. Based on the assumed functions of these compounds, the research community has classified them into three overarching groups: primary metabolites, which are directly required for plant growth; secondary (or specialized) metabolites, which mediate plant–environment interactions; and hormones, which regulate organismal processes and metabolism. For decades, this functional trichotomy of plant metabolism has shaped theory and experimentation in plant biology. However, exact biochemical boundaries between these different metabolite classes were never fully established. A new wave of genetic and chemical studies now further blurs these boundaries by demonstrating that secondary metabolites are multifunctional; they can function as potent regulators of plant growth and defense as well as primary metabolites sensu lato. Several adaptive scenarios may have favored this functional diversity for secondary metabolites, including signaling robustness and cost-effective storage and recycling. Secondary metabolite multifunctionality can provide new explanations for ontogenetic patterns of defense production and can refine our understanding of plant–herbivore interactions, in particular by accounting for the discovery that adapted herbivores misuse plant secondary metabolites for multiple purposes, some of which mirror their functions in plants. In conclusion, recent work unveils the limits of our current functional classification system for plant metabolites. Viewing secondary metabolites as integrated components of metabolic networks that are dynamically shaped by environmental selection pressures and transcend multiple trophic levels can improve our understanding of plant metabolism and plant–environment interactions.
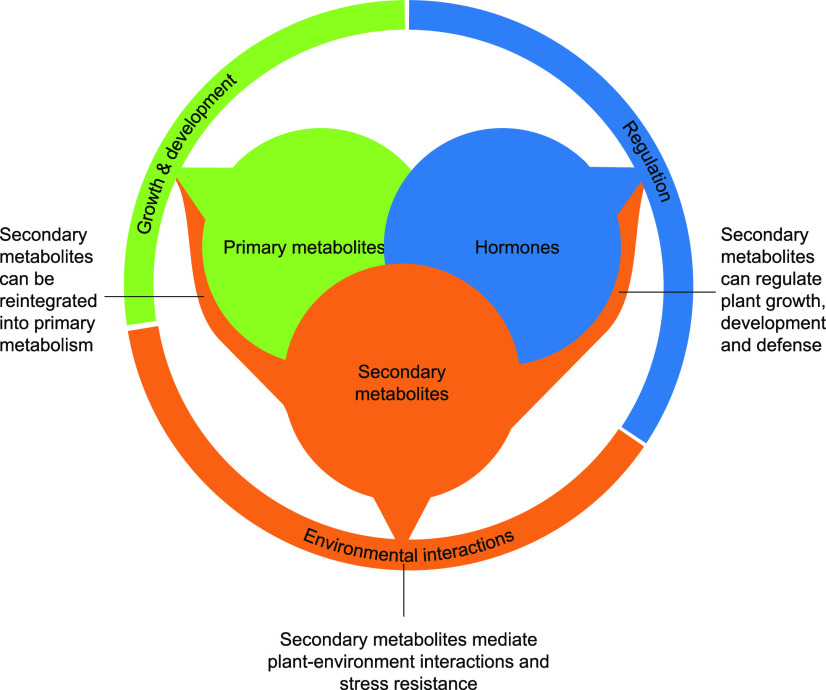
Plants can use simple, inorganic precursors to synthesize a large diversity of low Mr organic compounds. This synthetic capacity helps plants to colonize diverse and challenging environments. Low Mr organic compounds are commonly separated by perspective function into primary metabolites, secondary metabolites (also called specialized metabolites or natural products), and plant hormones (Fig. 1; Taiz et al., 2015). Primary metabolites are highly conserved and directly required for the growth and development of plants (Fernie and Pichersky, 2015). Secondary metabolites, including major groups such as phenolics, terpenes, and nitrogen-containing compounds, are often lineage specific and aid plants to interact with the biotic and abiotic environment (Hartmann, 2007). Finally, plant hormones are defined as small compounds that regulate organismal processes, including the production of the other metabolites, by interacting with receptor proteins (Davies, 2004).
Figure 1.
Low molecular weight compounds in plants are functionally classified as primary metabolites, secondary metabolites, or hormones. Present work on plant secondary metabolites demonstrates that many of them also have regulatory roles, and some are demonstrated precursors of primary metabolites. Note that primary metabolites and hormones also show functional overlap with the other metabolite classes (not discussed here). These findings blur the functional trichotomy of plant metabolism and call for a reassessment of ecological and evolutionary frameworks that are based on this model.
Despite the fact that definitions of secondary metabolites are inherently diffuse (Hartmann, 2007; Pichersky and Lewinsohn, 2011; Davies, 2013), the distinction between primary metabolites, secondary metabolites, and plant hormones has found its way into textbooks and shapes our thinking in plant biology to this day. An illustrative example is the field of plant–herbivore interactions, where major efforts have gone into disentangling how plants protect their primary metabolites (serving as nutrients for herbivores) using secondary metabolites (serving as defenses for plants), and how adapted herbivores manage to extract primary metabolites while avoiding the negative effects of secondary metabolites (Awmack and Leather, 2002; Howe and Jander, 2008; Zhou et al., 2015; Erb and Reymond, 2019). In this context, plant hormones are investigated as regulators of primary and secondary metabolism, defense, and resistance that may be manipulated by adapted herbivores (Howe and Jander, 2008; Schuman and Baldwin, 2016; Stahl et al., 2018), similar to pathogens (Kazan and Lyons, 2014). The biochemical coevolutionary arms–race theory (Ehrlich and Raven, 1964), a key concept in plant–herbivore interactions (Berenbaum and Zangerl, 2008; Jander, 2018), postulates that plant secondary metabolites evolve in response to herbivore pressure, resulting in the evolution of resistance mechanisms in herbivores. The resulting arms race is thought to drive the diversity of plant secondary metabolites and insect herbivores (Futuyma and Agrawal, 2009).
Over the last decades, the distinction between primary metabolites, secondary metabolites, and plant hormones has proven a useful approximation. However, the emergence of a more detailed understanding of plant metabolism may require us to revisit this functional partitioning (Neilson et al., 2013; Maag et al., 2015; Kliebenstein, 2018; Pichersky and Raguso, 2018; Zhou et al., 2018). In particular, an increasing number of genetic and functional studies on plant secondary metabolites are blurring the functional trichotomy by showing that plant secondary metabolites can have regulatory functions and serve as precursors for primary metabolites. In this review, we discuss this evidence, mostly focusing on examples that rely on the use of natural knockout variants, mutants, and transgenic plants altered in their capacity to produce certain secondary metabolites in combination with chemical complementation assays to demonstrate activity of the metabolites. We illustrate that for an increasing number of plant secondary metabolites, a strict functional separation from regulators and primary metabolites may not do them justice and possibly hinders our progress in understanding their roles for plant survival in hostile environments.
INTEGRATION OF PLANT SECONDARY METABOLITES INTO REGULATION AND METABOLISM
Early Evidence for Metabolic Integration of Secondary Metabolites
In 1977, David Rhoades studied the properties of creosotebush (Larrea spp.) leaf resin. He found that the resin, which contained high levels of phenylpropanoid derivatives (lignans), absorbed ultraviolet radiation, reduced evaporative water loss across cellulose membranes, and had the capacity to form complexes with proteins, thus possibly reducing the digestibility of plant materials for herbivores (Rhoades, 1977, p. 281). Rhoades (1977) thus postulated that “…any chemical system possessed by a plant must necessarily be integrated into the total metabolic scheme and multiple functions are to be expected.” In other words, Rhoades (1977) proposed that secondary metabolites are not end points, but integrated components of plant metabolism, and may, by consequence, take on any number of functions, similar to other plant metabolites. Indeed, evidence was emerging at that time that secondary metabolites may regulate growth and defense, as exogenously applied flavonoids could modulate polar auxin transport and catabolism (Stenlid, 1963; Stenlid, 1976), glucosinolate breakdown products could replace auxins in inducing hypocotyl bending (Hasegawa et al., 1986), and induced volatiles promoted resistance and defense regulation in neighboring trees (Baldwin and Schultz, 1983; Rhoades, 1983).
Secondary Metabolites as Regulators of Plant Defense
Following early preliminary evidence of secondary metabolites regulating defenses, genetic evidence followed in 2009, when it was reported that Arabidopsis (Arabidopsis thaliana) mutants defective in indole glucosinolate biosynthesis no longer mount a callose defense response following Flg22 treatment. Callose formation is rescued by adding 4-methoxy-indol-3-ylmethylglucosinolate (Clay et al., 2009). The myrosinase PEN2 is required for this phenomenon, implicating glucosinolate breakdown in callose regulation (Clay et al., 2009). Shortly thereafter, it was discovered that indole-derived benzoxazinoid secondary metabolites have a comparable callose regulatory function in cereals. Benzoxazinoid-deficient bx1 maize (Zea mays) mutants are defective in aphid- and chitosan-induced callose deposition, and callose induction is rescued by the addition of DIMBOA or DIMBOA-Glc (Ahmad et al., 2011; Meihls et al., 2013). In both cases, the capacity to regulate callose is structurally specific and depends on the modification of the indole-derived ring. In Arabidopsis, indol-3-ylmethylglucosinolate, which lacks a methylated hydroxy-group on the aromatic ring, is inactive, whereas the methylated form is active (Clay et al., 2009). In maize, DIMBOA-Glc, which lacks a methylated hydroxy-group at the nitrogen, is active, whereas the methylated form (HDMBOA-Glc) is inactive (Li et al., 2018a). Whereas the callose response to benzoxazinoids is conserved between wheat (Triticum aestivum) and maize, they do not elicit callose in Arabidopsis, and intact glucosinolates do not elicit callose in maize (Li et al., 2018a). These studies show that callose regulation by secondary metabolites is highly specific, tightly controlled, and likely evolved repeatedly. The mechanism underlying secondary metabolite–induced callose formation awaits to be elucidated. Glucosinolates and benzoxazinoids may, for instance, promote callose production by regulating hormonal pathways (Burow et al., 2015; Katz et al., 2015), through transcriptional regulation (Kim et al., 2015), or by directly initiating callose formation posttranslationally.
Interestingly, glucosinolates and benzoxazinoids also seem to regulate the accumulation of other secondary metabolites (Hemm et al., 2003; Kim et al., 2015; Li et al., 2018a). In Arabidopsis, mutants that are defective in the atypical myrosinase PEN2 release lower amounts of Trp-derived metabolites such as camalexin upon flg22 treatment (Frerigmann et al., 2016) and infection by Pseudomonas syringae (Stahl et al., 2016). Furthermore, mutants defective in the CYP83B1 enzyme required for indole glucosinolate production also show lower accumulation of the phenylpropanoid sinapoylmalate (Kim et al., 2015). The phenylpropanoid phenotype is rescued in mutants that no longer produce the substrate of CYP83B1, indole‐3‐acetaldoxime (Kim et al., 2015), suggesting that it may be the aldoxime overaccumulation rather than the lack of downstream glucosinolates that suppresses sinapoylmalate. Suppressor screens showed that the phenylpropanoid phenotype is also absent in plants that have mutated MEDa/b genes, which encode key components of a large multisubunit transcriptional complex that regulates phenylpropanoid biosynthetic genes (Kim et al., 2015; Dolan et al., 2017). A recent study demonstrates that a group of Kelch Domain F‐Box (KFB) genes that are involved in PAL inactivation (Zhang et al., 2013) are up-regulated in indole glucosinolate mutants in a MED5-dependent manner, whereas PAL-activity is suppressed (Kim et al., 2020). PAL-activity and sinapoylmalate accumulation are (partially) rescued in glucosinolate-deficient KBF mutants (Kim et al., 2020). The model emerging from these studies is that aldoximes, which accumulate in CYP83B1 mutants, increase KFB-mediated PAL degradation through MED5 transcriptional regulation as well as other, yet unknown, mechanisms (Kim et al., 2015; Kim et al., 2020). As aldoximes are produced by many different species, this form of defense regulation may also occur beyond glucosinolate-producing plants (Kim et al., 2020). Interestingly, wheat lines overexpressing a maize benzoxazinoid O-methyl transferase and thus accumulate more HDMBOA-Glc and less DIMBOA-Glc also show higher levels of the phenylpropanoid ferulic acid, despite unaltered pool sizes of amino acid precursors (Li et al., 2018a), suggesting that phenolic compounds may also be regulated by other secondary metabolite pathways.
Apart from glucosinolates and benzoxazinoids, volatile secondary metabolites such as terpenoids, green-leaf volatiles, and aromatic compounds can also regulate plant defenses (Baldwin et al., 2006; Godard et al., 2008; Erb, 2018; Bouwmeester et al., 2019). Many of these volatiles are released upon herbivore- or pathogen attack and are capable of directly inducing or priming hormonal defense signaling pathways and resistance. In maize, for instance, mutants that are defective in their capacity to produce volatile indole are unable to prime their systemic tissues to rapidly release terpenes upon herbivore attack (Erb et al., 2015). Adding indole to the headspace of maize plants restores this priming phenotype (Erb et al., 2015). Rice (Oryza sativa) plants also respond to indole through priming of early defense signaling elements such as the map kinase OsMPK3 (Ye et al., 2019). Transgenic plants that are deficient in OsMPK3 expression are no longer responsive to indole, suggesting that indole acts via the priming of early defense signaling (Ye et al., 2019). In Arabidopsis, geranylgeranyl reductase1 mutants are defective in systemic acquired resistance against P. syringae (Riedlmeier et al., 2017). Adding the pathogen-induced volatiles α- and β-pinene to the headspace of the mutant restores resistance, with the response depending on intact salicylic acid signaling and the AZELAIC ACID INDUCED (AZI1) gene (Riedlmeier et al., 2017). The precise role of other volatile secondary metabolites that can regulate defenses at physiological concentrations, including homoterpenes (Arimura et al., 2000) and green-leaf volatiles (Ameye et al., 2018), has not yet been explored using genetic approaches, but their activity has been demonstrated clearly through chemical complementation (Arimura et al., 2000; Engelberth et al., 2004; Frost et al., 2008; Meents et al., 2019). Further support for the potential regulatory role of defense volatiles comes from LOX2-silenced Nicotiana attenuata plants, which are deficient in the production of herbivory-induced, green-leaf volatiles. In contrast with the other systems where volatiles induce defense, the LOX2 mutation leads to stronger expression of defense-related genes in neighbors than wild-type plants, suggesting that volatiles can also suppress defenses (Paschold et al., 2006).
In summary, at least five classes of secondary metabolites (glucosinolates, benzoxazinoids, terpenes, aromatics, and green-leaf volatiles) are now confirmed to act as potential regulators of in planta defense. It is exciting to speculate that there are many other secondary metabolites that play similar regulatory roles. An important gap of knowledge is the mechanism by which secondary metabolites regulate defenses. As many of the secondary metabolites are chemically reactive (Farmer and Davoine, 2007; Hadacek et al., 2010), it is possible that they act indirectly by depleting detoxification enzymes, thus triggering the accumulation of known signaling molecules such as reactive oxygen species (ROS; Khokon et al., 2011). However, as discussed below, secondary metabolites may also have hormone-like properties by binding to specific receptor proteins (Katz et al., 2015). More work on the targets of secondary metabolites in planta is clearly warranted and would help to clarify the ecological and evolutionary context of their capacity to regulate defenses.
Secondary Metabolites as Regulators of Growth and Development
Plants regulate their growth dynamically and often reduce their investment into growth and development upon herbivore- or pathogen attack. This reduction in growth is thought to be largely due to the reconfiguration of a plant’s signaling network rather than a lack of resources (Kliebenstein, 2016; Machado et al., 2017; Guo et al., 2018). Strikingly, plant secondary metabolites and their breakdown products are being (re)-discovered as plant growth modulators, thus adding another layer of regulation to growth-defense patterns. Again, glucosinolates provide a mechanistic example of how secondary metabolites can modulate growth. When applied to the roots of Arabidopsis and many other plant species, the aliphatic 3-hydroxypropylglucosinolate inhibits root meristematic growth at physiological concentrations via an intact Target of Rapamycin pathway (Malinovsky et al., 2017). The exact molecular interaction partner of 3-hydroxypropylglucosinolate remains unknown. Studies on the indole glucosinolate breakdown product indole-3-carbinol have identified an unexpected target protein. Indole-3-carbinol accumulates upon wounding in Arabidopsis and rapidly reduces root growth upon exogenous application. In vitro, indole-3-carbinol interferes with the interaction between auxin and its receptor TIR1 by binding at an allosteric site (Katz et al., 2015). As the indolic glucosinolate catabolite likely binds directly to TIR1 (Katz et al., 2015), one may argue that TIR1 acts as an indolic glucosinolate receptor that mediates the regulation of growth by a plant secondary metabolite. Another link to auxin signaling was found with a structurally unrelated aliphatic glucosinolate. This was found by the initial observation that the auxin-sensitive repressors IAA5, IAA6, and IAA19 strongly regulate 4-methylsulfinylbutyl glucosinolate (4-MSOB) levels in dehydrated Arabidopsis plants (Salehin et al., 2019). Iaa5,6,19 mutants fail to close their stomata upon drought stress, a phenotype that can be reverted by adding 4-MSOB (Salehin et al., 2019). Together with the finding that glucosinolate biosynthesis and activation mutants are less tolerant to drought (Salehin et al., 2019), and that glucosinolate breakdown products can trigger stomatal closure in Arabidopsis and Vicia faba (Khokon et al., 2011; Hossain et al., 2013), these results provide evidence that aliphatic glucosinolates are involved in stomatal regulation. Interestingly, glucosinolate-mediated stomatal regulation requires a functional ROS receptor kinase (GHR1; Salehin et al., 2019). Given that the myrosinase TGG1 accumulates in guard cells and is required for stomatal regulation (Zhao et al., 2008), and that glucosinolate breakdown products can regulate stomatal closure through ROS production (Khokon et al., 2011), it is conceivable that ROS link endogenous glucosinolates to stomatal regulation (Khokon et al., 2011).
Apart from growth and stomatal opening, glucosinolates may also regulate the circadian clock and flowering time. Natural presence/absence variation in the 2-oxoglutarate-dependent dioxygenase AOP2, which converts methylsulfinylalkyl glucosinolates into alkenyl glucosinolates, is linked to variation in the expression of the major flowering gene FLC and to variation in flowering time (Kliebenstein et al., 2001; Atwell et al., 2010). Introducing a functional AOP2 into Arabidopsis Col-0 (a natural AOP2 knockout) confirmed the flowering time effect, identified a shift in the expression of circadian genes, and showed a 1-h decrease in clock periodicity (Kerwin et al., 2011). Abolishing glucosinolate production using Myb transcription factor mutants led to the same periodicity shift, suggesting that the effect may be linked to the presence of the 4-MSO glucosinolate in wild-type Col-0 (Kerwin et al., 2011). The effect of the AOP locus on flowering time depends on the genetic background (Jensen et al., 2015), supporting the hypothesis that secondary metabolites are integrated into a complex and variable regulatory network. How aliphatic glucosinolates directly regulate gene expression networks and developmental phenotypes such as flowering time remains to be tested (Burow and Halkier, 2017).
The present data suggest that glucosinolates can influence growth by multiple different mechanisms, including Target of Rapamycin regulation, auxin regulation, auxin-independent transcriptional regulation, and auxin-mediated ROS accumulation (Katz et al., 2015; Kim et al., 2015; Malinovsky et al., 2017; Salehin et al., 2019). This diversity, paired with the substantial variation in glucosinolate biosynthesis within species, creates a wealth of metabolic networks and phenotypes, which can be acted upon by natural selection. It is tempting to speculate that this diversity is a reflection of the highly diverse habitats and environments that a single species can inhabit and may provide adaptive potential beyond conserved hormonal pathways.
In addition to glucosinolates, flavonoids are implicated in regulating plant growth, development, and environmental responses. Exogenously applied flavonoids have long been known to modulate auxin transport (Stenlid, 1976). Evidence that flavonoids may also act as endogenous growth regulators came from an Arabidopsis chalcone synthase mutant, transparent testa (tt4). tt4 plants show growth alterations that are characteristic of disturbed auxin localization, including reduced root growth and gravitropism (Brown et al., 2001). The tt4 mutant also displays increased auxin transport (Murphy et al., 2000), which can be reversed by adding the flavonoid precursor naringenin (Brown et al., 2001). Further mechanistic studies suggest that flavonoids modulate auxin transport through several mechanisms, including interactions with auxin transporters and transport-regulating proteins (Peer and Murphy, 2007; Santelia et al., 2008). Arabidopsis roots grow away from light and flavonoids accumulate in their light-exposed sides (Silva-Navas et al., 2016). The tt4 mutant also shows reduced light avoidance, which was linked to reduced auxin polar transport and reduced ROS accumulation, both of which can regulate cell division and elongation (Gayomba et al., 2010; Silva-Navas et al., 2016). tt4 also displays lower accumulation of flavonols and increased ROS levels in guard cells, phenotypes associated with more rapid absisic acid–induced stomatal closure (Watkins et al., 2014). An additional link between flavonoid biosynthesis, ROS accumulation, and plant development was uncovered recently in tomato (Solanum lycopersicum; Muhlemann et al., 2018). The are mutant is defective in a flavonol 3-hydroxylase (F3H), displays reduced flavonol and increased ROS accumulation in pollen grains, and suffers from reduced pollen tube growth and integrity. The pollen tube phenotype can be rescued by the addition of antioxidants (Muhlemann et al., 2018). Flavonols are thus thought to act as antioxidants that reduce ROS accumulation and thereby regulate plant development (Hernández et al., 2009; Muhlemann et al., 2018). However, the oxidation state of a cell can directly influence signaling by altering disulfide bridge formation or other protein modifications. Thus, it is possible that flavonols also function as signals and further work is needed to differentiate between these hypotheses.
Other secondary metabolites may also regulate plant development. Diploid oat sad2 mutants that overproduce the triterpene β-amyrin produce shorter roots and significantly more root hairs than wild-type plants, phenotypes which are absent in other mutants of the pathway that do not overproduce β-amyrin (Kemen et al., 2014). However, this phenotype cannot be phenocopied by adding β-amyrin to roots, possibly because its activity requires specific spatiotemporal accumulation patterns (Kemen et al., 2014). In N. attenuata, silencing a malonyltransferase that malonylates 17-Hydroxygeranylinalool diterpene glycosides reduces floral style cell size and length (Li et al., 2018b). Knocking down diterpene glycoside production by silencing a geranylgeranyl diphosphate synthase abolishes the effect of the malonyltransferase, suggesting that specific diterpene hexose decoration patterns are responsible for the flower phenotype (Li et al., 2018b). Furthermore, a labeling experiment in poplar recently uncovered that herbivore-attacked leaves can convert benzyl cyanide, a herbivore-induced volatile, to the auxin phenylacetic acid (Günther et al., 2018), thus providing a potential link between the catabolism of volatile secondary metabolites and the regulation of plant growth and development.
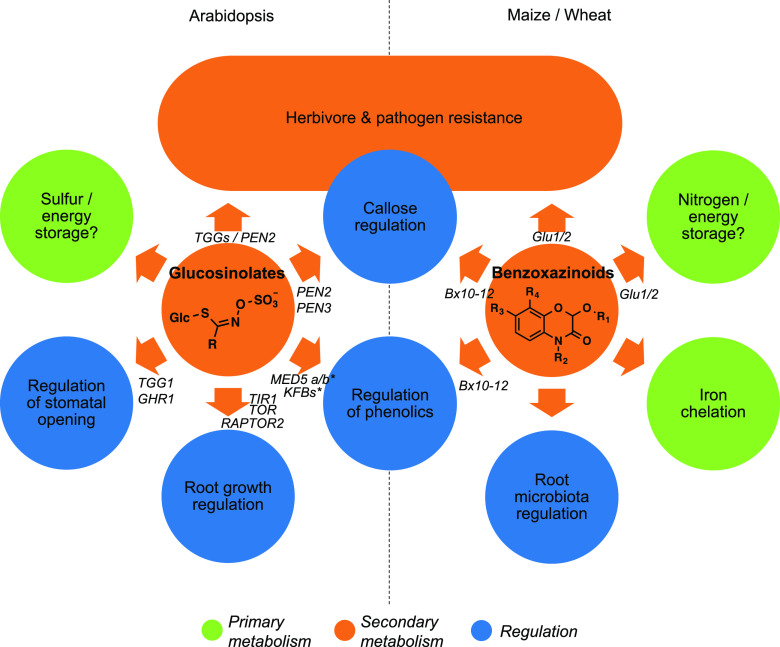
The examples above show how secondary metabolites can modulate growth and development through a variety of mechanisms, some of which are barely distinguishable from mechanisms normally assigned to plant hormones (Fig. 2). Whereas some of these secondary metabolite regulators are ancient and highly conserved (e.g. flavonoids, terpenes), others evolved more recently (e.g. glucosinolates and benzoxazinoids) and are restricted to specific plant families. Plants thus have both a conserved and a unique, variable, and flexible repertoire of regulators at their disposition to adjust growth and development, which likely contributes to their potential to colonize variable and challenging habitats.
Figure 2.
Glucosinolates and benzoxazinoids as examples of secondary metabolites that blurr the functional trichotomy of plant metabolism. Different functions of glucosinolates in Arabidopsis and benzoxazinoids in maize and wheat are depicted. Genes that are known to be involved in the different functions are indicated. Note that a direct role of benzoxazinoids and glucosinolates as plant primary metabolites (for instance, in the context of nitrogen/sulfur and/or energy storage) has not been clearly demonstrated so far. *MEDs and KFBs are likely regulated by aldoxime precursors of glucosinolates. For references, see the article.
Secondary Metabolites as Primary Metabolites
If secondary metabolites can regulate growth, development, and defense, can they also function as primary metabolites? Whereas primary metabolites are highly conserved, secondary metabolites evolve dynamically and are inherently variable in structure and production (Wink, 2008). This rapid evolution would seem to complicate their integration into the most fundamental workings of plant metabolism because it would require a rapid evolution of enzymes to connect these new structures into the more conserved metabolic pathways. However, evidence for secondary metabolites that are not strictly essential, but nevertheless contribute to primary metabolism, is emerging. In Arabidopsis, plants with mutations in the flavonoid pathway upstream of the FLAVANONE-3-HYDROXYLASE (F3H) show a reduction in the respiratory cofactor ubiquinone (coenzyme Q; Soubeyrand et al., 2018). Ubiquinone levels can be restored by adding dihydrokaempferol or kaempferol to the mutants. Labeling experiments demonstrate that the aromatic ring of kaempferol is integrated into ubiquinone, and that heme-dependent peroxidases likely use kaempferol to produce 4-hydroxybenzoate as a substrate for ubiquinone (Soubeyrand et al., 2018).
The integration of flavonoids into primary metabolism is perhaps not surpising, because they represent one of the oldest and most conserved classes of secondary metabolites (albeit with substantial interspecific variation in glycosylation patterns). Flavonoid evolution precedes the emergence of many innovations in plant primary metabolism, such as C4 photosynthesis. Whether younger, more specialized secondary metabolites can act as primary metabolites is not well understood. This lack of knowledge is closely related to a limited understanding of secondary metabolite catabolism. Where do these compounds go when they are no longer needed? One would assume that reintegrating secondary metabolites into primary metabolism is beneficial for plants (Neilson et al., 2013). Such a reintegration pathway has been proposed for cyanogenic glycosides (Selmar et al., 1988). Upon deglycosylation, HCN may be assimilated into Asn via the formation of β-cyano-Ala (Selmar et al., 1988). Indeed, two sorghum (Sorghum bicolor) nitrilases are capable of producing Asn from β-cyano-Ala (Jenrich et al., 2007). An alternative pathway not involving the release of HCN was suggested in sorghum. In this system, nitrilases are proposed to take the deglycosylated cyanogen and directly release ammonia and the corresponding acetate (Jenrich et al., 2007). Further support for the potential of cyanogenic glucosides as a primary metabolite store came from overexpression of a hydroxynitrile lyase, which is involved HCN formation in cassava (Manihot esculenta). These plants have decreased concentrations of cyanogenic glycosides and increased concentrations of total amino acids, suggesting that cyanogenic glycosides may be degraded and reintegrated into primary metabolism (Narayanan et al., 2011). The potential integration of other secondary metabolites such as glucosinolates is currently under investigation. In Arabidopsis, sulfur deficiency induces the expression of the myrosinases BGLU28 and BGLU30 (Maruyama-Nakashita et al., 2003). Under sulfur-limiting conditions, bglu28/30 double mutants accumulate higher levels of intact aliphatic glucosinolates, contain lower amounts of Cys and protein sulfur content, and grow less than wild-type plants, suggesting that glucosinolates may serve as sulfur-storage molecules (Zhang et al., 2020).
Detailed biochemical characterization and metabolic profiling of secondary-metabolite mutants, combined with complementation and labeling experiments, are required to further corroborate the potential roles of secondary metabolites in primary metabolism and to identify additional secondary metabolites that serve as primary-metabolite precursors. Degradation of many different secondary metabolites has been observed under specific environmental conditions (Negi et al., 2014; Zipor et al., 2015). Furthermore, alterations in primary metabolites are observed in various plants with altered secondary metabolism (Mayer et al., 2001; Narayanan et al., 2011; Huber et al., 2016; Machado et al., 2017; Zhang et al., 2020), and the accumulation of specific secondary metabolites has been associated with storage and growth in microevolutionary studies (Heath et al., 2014). Keeping an open mind about the capacity of plants to evolve integrated metabolic networks is warranted to gain a better comprehension of the prevalence and importance of secondary metabolites as precursors of primary metabolites.
Secondary Metabolites as Facilitators of Micronutrient Uptake
An additional example that further blurs the distinction between primary and secondary metabolism is plant micronutrient uptake. Grasses excrete low Mr compounds into the rhizosphere to chelate micronutrients such as iron and thus make them biologically available (Curie and Briat, 2003). Recent work suggests that secondary metabolites are likely important for iron uptake in both herbs and grasses. Chemical removal of excreted phenolic acids from the nutrient solution of red clover (Trifolium pretense) was found to result in iron deficiency in red clover (Jin et al., 2007). Subsequently, an Arabidopsis mutant, which is deficient in the 2-oxoglutarate-dependent dioxygenase Feruloyl-CoA 6′-Hydroxylase 1 and thus no longer able to produce coumarins, was found to suffer from iron deficiency under alkaline conditions (Schmid et al., 2014). Similarly, young maize benzoxazinoid mutants that do no longer produce and excrete benzoxazinoids were found to suffer from iron deficiency when growing in the presence of iron salts (Hu et al., 2018). Both benzoxazinoids and coumarins are able to chelate iron in vitro (Bigler et al., 1996; Mladenka et al., 2010). Because these complexes are essential for plant growth and development by providing essential micronutrients, they should, according to definition, be classified as primary metabolites, thus providing another illustration of how secondary metabolites can turn into primary metabolites under given conditions.
ADAPTIVE EXPLANATIONS FOR METABOLIC INTEGRATION OF SECONDARY METABOLITES
There is now ample evidence for secondary metabolites that are regulators and precursors of primary metabolites. But why would plants evolve an integrated metabolism in which the same metabolite class has multiple functions that incorporate growth, development, defense, and regulation? Plants have large, interconnected metabolic networks at their disposition. Natural selection acts on these metabolic networks, resulting in the evolution of network topologies that maximize fitness. Over evolutionary time, these topologies likely include dynamic transitions between secondary metabolites and hormones, for instance (Malinovsky et al., 2017; Sun et al., 2019b). Overall, the functional integration of secondary metabolites at a given point in evolution is a likely consequence of the interaction between complex environments with highly connected plant metabolic networks. Below, we discuss the potential benefits of plant secondary metabolite metabolic integration that may have favored their use as regulators and primary metabolites.
Plant Secondary Metabolites as Reliable Readouts of Defense Activation
Plants control defense activation to save metabolic energy and avoid self-damage. Defense investment is typically titrated through feedback regulation, including both positive and negative feedback loops that are built into early defense signaling (Hu et al., 2015; Li et al., 2015) and hormonal networks (Gilardoni et al., 2011; Liu et al., 2019). A limitation of these feedback loops is that they do not provide direct information about the final level of defense activation (i.e. the production of defense metabolites per se). Because herbivores and pathogens may interfere with the production of defense compounds at many levels, including in the final steps of biosynthesis (Jones et al., 2019), integrating them directly into regulatory feedback loops may allow plants to more accurately monitor and adjust defense accumulation. Using secondary metabolites as defense activation readouts may also help plants to optimize synergies between different defenses and to compensate for accidental failures of specific defense pathways. The increasing number of examples showing that plant secondary metabolites regulate defenses (see section “Secondary Metabolites as Regulators of Plant Defense”) hint at the existence of such systems.
As many secondary metabolites are compartmentalized and/or stored in inactive forms, their decompartmentalization and/or activation likely also helps plants to recognize tissue damage and other forms of environmental stress. In this case, the metabolites would be used as damage–associated molecular patterns (DAMPs). Green-leaf volatiles are an example of secondary metabolites that are also DAMPs (Tanaka et al., 2014; Quintana-Rodriguez et al., 2018). Another potential example of secondary metabolites as DAMPs is the previously discussed links between indolic glucosinolates and DIMBOA regulation of callose upon pathogen attack. Interestingly in this case, the secondary metabolite/DAMPs are linked to endogenous responses to pathogen-associated molecular patterns (e.g. FLS2; Clay et al., 2009) and stomatal closure upon drought stress (Salehin et al., 2019).
Given these considerations, secondary metabolites may be common readouts of defense activation and damage may have favored their evolution as defense regulators.
Metabolic Network Specialization as a Potential Means to Resist Manipulation
Herbivores, pathogens, and viruses can interfere with defense hormone signaling and thereby manipulate plants for their own benefit (Kazan and Lyons, 2014; Stahl et al., 2018). The high degree of conservation in defense hormone signaling may in fact favor the evolution of biotic manipulation of plant signaling (Berens et al., 2017). For example, if an attacking organism evolves the ability to alter jasmonate signaling, this may provide it a fitness benefit on a wide variety of host plants and may reduce the advantage for plants to evolve new inducible resistance mechanisms regulated by these hormones. One possibility to solve this problem would be to use less-conserved metabolites as defense regulators. If a plant had the ability to use these metabolites, it would be less likely to fall prey to host switching by hormone-manipulating enemies. The evolution of (specialized) secondary metabolites into regulatory networks may thus be promoted through the evolution of manipulation strategies in plant enemies. Clear examples supporting this hypothesis are currently lacking. As the biosynthesis of defense-regulating secondary metabolites such as glucosinolates is at least partially controlled by conserved phytohormonal pathways (Schweizer et al., 2013), plant enemies that are capable of overcoming these conserved pathways may also suppress more specific regulators. Interestingly, an opposite pattern has also been found for the tomato leaf spot fungus, which uses a hydrolase to detoxify steroidal glycoalkaloids and benefits from the defense-suppressing properties of the resulting breakdown products (Bouarab et al., 2002). This illustrates that specialized plant enemies may also misuse the regulatory properties of secondary metabolites of their host plants.
Multifunctionality as a Cost-Saving Strategy
Producing secondary metabolites has energetic and metabolic costs (Gershenzon, 1994). These costs are not always evident (Züst et al., 2011; Machado et al., 2017), and may mostly occur under specific environmental conditions such as strong competition and nutrient limitation (Cipollini et al., 2018). Plants likely manage costs of secondary metabolite production through the regulation of biosynthesis, but controlled recycling of the resulting compounds would enhance the plants ability to recoup costs in challenging environments (Neilson et al., 2013). Secondary metabolites that are induced upon environmental stress could for instance be recycled back into primary metabolism once the stress subsides. One way of testing this hypothesis is to manipulate secondary metabolite recycling by targeting enzymes involved in their degradation, such as glucosidases (Morant et al., 2008) or nitrilases (Jenrich et al., 2007). With use of this approach, a link between the degradation of cyanogenic glycosides and plant protein supply was uncovered (Narayanan et al., 2011), supporting the hypothesis that reintegration of secondary compounds into primary metabolism may be advantageous for the plant. A caveat of this approach is that it remains difficult to disentangle a direct contribution of the generated catabolites to primary metabolism from their potential regulatory roles. A more detailed understanding of secondary metabolite signaling and catabolism would help to explore the role of secondary metabolite reintegration as a cost-saving strategy.
Another way to minimize costs is to use the same secondary compound for multiple purposes (Neilson et al., 2013). As many secondary compounds are chemically reactive, they need to be managed by the plant through (potentially costly) storage, inactivation, and/or resistance mechanisms, including specialized cells, ducts, and glands (Sirikantaramas et al., 2008). By employing the same compound class for multiple purposes, plants may spread these fixed costs across more fitness components and increase their competitiveness. Metabolic costs may also be lowered by using the same biosynthetic machinery to produce different compounds for different purposes. Whereas the cost-saving aspects of multifunctionality are difficult to quantify, multifunctionality seems to be a widespread property of secondary metabolites, as discussed above, and it is difficult for this multifunctionality to evolve without benefit.
ECOLOGICAL CONSEQUENCES OF THE METABOLIC INTEGRATION OF SECONDARY METABOLITES
The separation of low Mr compounds into primary metabolites, secondary metabolites, and hormones has shaped our ecological and evolutionary thinking of plant–environment interactions. If we abolish this view in favor of a more integrated perspective (i.e. where secondary metabolites can have regulatory roles and can provide precursors for primary metabolites), we can derive new hypotheses on plant defense patterns and plant–herbivore interactions. These hypotheses are likely to improve our understanding of the ecological roles of plant secondary metabolites in the future.
Ontogenetic Patterns of Secondary Metabolite Production
Many secondary metabolites show distinct ontogenetic accumulation patterns, with concentrations varying over time and between tissues. Ecological theory explains this within-plant variation using resource constraints, allocation costs, and variation in herbivore pressure (McKey, 1974; van Dam, 2009; Meldau et al., 2012; Schuman and Baldwin, 2016; Barton and Boege, 2017). The above theories are all based on costs and benefit relationship, with the benefit typically being limited to herbivore resistance. Given the blurred trichotomy of plant secondary metabolism, the ecological balance sheet may be improved by taking into account multifunctionality (Barton and Boege, 2017). A drop in secondary metabolite levels, as is often observed a few weeks after germination or at the onset of flowering, for instance (Meldau et al., 2012; Barton and Boege, 2017), may reflect an increased need of primary metabolites and nutrients rather than a drop in herbivore pressure. Similarly, strong expression of secondary metabolites in roots may not only be the result of high tissue value and a high risk of root herbivore attack, but may simply reflect additional functions of the compounds such as micronutrient uptake and microbial conditioning (Hu et al., 2018; Stringlis et al., 2018). Our understanding of ontogenetic allocation patterns of secondary metabolites may thus improve if we take their full metabolic integration and potential multifunctionality into account and do not limit their considered benefits to herbivore resistance.
Defense Metabolites in Plant–Herbivore Interactions
The functional trichotomy used to define plant metabolites has also shaped our understanding of how these metabolites influence plant–herbivores interactions. Herbivores are assumed to forage for primary metabolites while trying to avoid the negative effects of secondary metabolites through behavioral and metabolic adaptations (Behmer, 2009; Stahl et al., 2018). If we accept that secondary metabolites can also be regulators and precursors of primary metabolites, then it becomes conceivable that they may have similar roles in herbivores. The root-feeding larvae of the western corn rootworm for instance forage for iron-benzoxazinoid complexes to acquire iron and improve their growth, thus effectively using a plant secondary metabolite as a primary metabolite (Hu et al., 2018). Several other herbivores also gain more weight in the presence of plant secondary metabolites (Meldau et al., 2009; Richards et al., 2012; Marti et al., 2013; Veyrat et al., 2016; Wetzel et al., 2016), and it is conceivable that some of these effects may be due to the capacity of the herbivores to metabolize these compounds. Recent examples also hint at the possibility that plant secondary metabolites may have hormonal functions in herbivores. In rice, knocking down CYP71A1, a gene responsible for the production of serotonin, a monoamine neurotransmitter, reduces the performance of the rice brown planthopper (Nilaparvata lugens). Adding serotonin to an artificial diet enhances its performance (Lu et al., 2018), suggesting that the herbivore may benefit from the hormonal properties of this plant metabolite. Plants may also benefit from producing secondary metabolites that act as (de)-regulators of herbivore physiology. Spinach (Spinacia oleracea), for instance, produces the molting hormone 20-hydroxyecdysone (Bakrim et al., 2008), which can interfere with caterpillar development (Kubo et al., 1983).
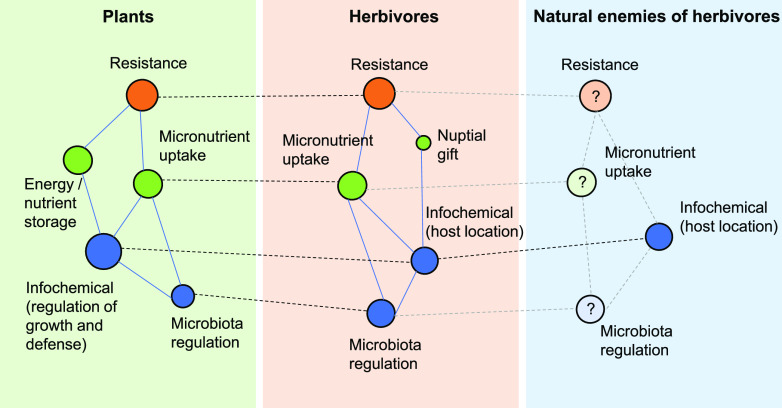
In general terms, a plant’s metabolism is shaped by a dynamic landscape of environmental selection pressure; conversely, the metabolic network of herbivores is shaped by the functional and chemical potential of plant metabolites within the herbivore’s own selection landscape. One can thus expect that, similar to what Rhoades postulated for plants (Rhoades, 1977), any chemical system taken up by a herbivore must necessarily be integrated into its total metabolic scheme, and multiple functions of plant secondary metabolites are to be expected, some of which likely mirror their multiple functions in plants (Fig. 3). Specialist herbivores are known to use secondary metabolites as infochemicals (e.g. foraging cues), and some also sequester defenses to protect themselves against herbivore natural enemies (Nishida, 2002; Opitz and Müller, 2009), in analogy to the use of these chemicals as defense regulators and resistance factors in plants (Fig. 3). Cabbage aphids (Brevicoryne brassicae) are an illustrative example in this context, as they can activate glucosinolates by producing their own myrosinases (Bridges et al., 2002; Kazana et al., 2007). This allows them to use glucosinolates as two-component defense system against predators (Kazana et al., 2007). As glucosinolate breakdown products (isothiocyanates) also increase aphid responses to alarm pheromones (Dawson et al., 1987), it was proposed that aphid-released isothiocyanates may also act as danger signals (Bridges et al., 2002). Another example where herbivores use secondary metabolites for several purposes that mirror their multiple uses by plants are again benzoxazinoids, which are used as defense metabolites and siderophores by a specialist root herbivore in maize (Box 1). Apart from mirroring plant functions, adapted herbivores can also use plant secondary metabolites for herbivore-specific functions. Cyanogenic glycosides, for instance, can be used by specialized lepidoptera as defenses and nuptial gifts (Zagrobelny et al., 2018), and glucosinolates are part of the pheromone blend of flea beetles (Phyllotreta striolata; Beran et al., 2016).
Figure 3.
Functional integration of plant secondary metabolites shapes plant–herbivore and tritrophic interactions. Schematic representation of how different functions of secondary metabolites are used by plants, herbivores, and natural enemies of herbivores is shown. Plants use secondary metabolites for multiple purposes, including resistance, regulation, and primary metabolism (see Fig. 2). Recent work suggests that this multifunctionality is mirrored in adapted herbivores, which also use secondary metabolites for multiple purposes, including similar and new functions. Little is known about how adapted natural enemies use secondary metabolites, but multifunctional integration across three trophic levels is likely (Box 2). Circles represent hypothetical individual secondary metabolites (for color code, refer to Figs. 1 and 2). Solid lines indicate metabolic connections within an organism. Dashed lines indicate similar functions of the same compounds in different organisms.
Box 1.
Case study of secondary metabolite multifunctionality. Cited articles: Glauser et al., 2011; Robert et al., 2012, 2017; Maag et al., 2016.
These examples illustrate that, as in plants, secondary metabolites can act as defenses, regulators, and precursors of primary metabolites in herbivores. Furthermore, the multifunctionality of plant secondary metabolites for plants is reflected by the multifunctional misuse of these compounds by specialized plant-feeders (Fig. 3). Whether similar phenomena can also be observed in natural enemies of herbivores, thus also shaping tritrophic interactions, is an exciting open question (Box 2).
Box 2.
Multifunctionality of plant secondary metabolites in tritrophic interactions. Cited articles: Fink and Brower, 1981; Hunter, 2003; Sarfraz et al., 2009; Sloggett and Davis, 2010; Aartsma et al., 2017; Rafter et al., 2017; Robert et al., 2017; Turlings and Erb, 2018; Sun et al., 2019a; Ugine et al., 2019; Zhang et al., 2019.
In summary, the field of plant–herbivore interactions is likely to benefit from abandoning functional preconceptions of plant secondary metabolites and to focus on a better understanding of the metabolic integration of plants and insects through untinged glasses. Comparative metabolomics of plant and herbivore tissues (Jansen et al., 2009) and parallel genome-wide screens of plants and herbivores (Nallu et al., 2018) are promising approaches to assess plant–herbivore interaction and to identify metabolite functions and effects in herbivores without prior functional assumptions.
CONCLUDING REMARKS
The functional separation of plant-derived, low Mr organic compounds into primary metabolites, secondary metabolites, and hormones has proven to be a useful approximation over the last decades. However, recent work has shown that several classes of plant secondary metabolites are highly integrated into plant metabolism and can serve as both regulators and primary metabolites. Thus, it is likely that most secondary metabolites have additional functions for plants. Taking into account these additional functions (see Outstanding Questions), we can refine key concepts in plant-environment interactions and improve our understanding of the chemical ecology of plants and their enemies.
Acknowledgments
We would like to thank Mike Blatt for the invitation to write this Inaugural Topical Review, Pierre Mateo for drawing chemical structures, and Christelle A.M. Robert, Clint Chapple, Jonathan Gershenzon, and two anonymous reviewers as well as the Twitter community for helpful comments on an earlier version of this manuscript.
Footnotes
This work was supported by the University of Bern, Swiss National Science Foundation (grant no. 155781 to M.E.), the European Research Council under the European Union’s Horizon 2020 Research and Innovation Program (grant no. ERC–2016–STG 714239 to M.E.), the National Science Foundation Division of Integrative Organismal Systems (award no. 1655810), theNational Science Foundation Division of Molecular and Cellular Biosciences (grant no. 1906486 to D.J.K.); the National Institute of Food and Agriculture (hatch project no. CA–D–PLS–7033–H to D.J.K.), and the Danish National Research Foundation (grant no. DNRF99 to D.J.K.).
Articles can be viewed without a subscription.
References
- Aartsma Y, Bianchi FJJA, van der Werf W, Poelman EH, Dicke M(2017) Herbivore-induced plant volatiles and tritrophic interactions across spatial scales. New Phytol 216: 1054–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S, Veyrat N, Gordon-Weeks R, Zhang Y, Martin J, Smart L, Glauser G, Erb M, Flors V, Frey M, et al. (2011) Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol 157: 317–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameye M, Allmann S, Verwaeren J, Smagghe G, Haesaert G, Schuurink RC, Audenaert K(2018) Green leaf volatile production by plants: A meta-analysis. New Phytol 220: 666–683 [DOI] [PubMed] [Google Scholar]
- Arimura G, Ozawa R, Shimoda T, Nishioka T, Boland W, Takabayashi J(2000) Herbivory-induced volatiles elicit defence genes in lima bean leaves. Nature 406: 512–515 [DOI] [PubMed] [Google Scholar]
- Atwell S, Huang YS, Vilhjálmsson BJ, Willems G, Horton M, Li Y, Meng D, Platt A, Tarone AM, Hu TT, et al. (2010) Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature 465: 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awmack CS, Leather SR(2002) Host plant quality and fecundity in herbivorous insects. Annu Rev Entomol 47: 817–844 [DOI] [PubMed] [Google Scholar]
- Bakrim A, Maria A, Sayah F, Lafont R, Takvorian N(2008) Ecdysteroids in spinach (Spinacia oleracea L.): Biosynthesis, transport and regulation of levels. Plant Physiol Biochem 46: 844–854 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Halitschke R, Paschold A, von Dahl CC, Preston CA(2006) Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 311: 812–815 [DOI] [PubMed] [Google Scholar]
- Baldwin IT, Schultz JC(1983) Rapid changes in tree leaf chemistry induced by damage: Evidence for communication between plants. Science 221: 277–279 [DOI] [PubMed] [Google Scholar]
- Barton KE, Boege K(2017) Future directions in the ontogeny of plant defence: Understanding the evolutionary causes and consequences. Ecol Lett 20: 403–411 [DOI] [PubMed] [Google Scholar]
- Behmer ST.(2009) Insect herbivore nutrient regulation. Annu Rev Entomol 54: 165–187 [DOI] [PubMed] [Google Scholar]
- Beran F, Jiménez-Alemán GH, Lin MY, Hsu Y-C, Mewis I, Srinivasan R, Ulrichs C, Boland W, Hansson BS, Reinecke A(2016) The aggregation pheromone of Phyllotreta striolata (Coleoptera: Chrysomelidae) revisited. J Chem Ecol 42: 748–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbaum MR, Zangerl AR(2008) Facing the future of plant-insect interaction research: Le retour à la “raison d’être”. Plant Physiol 146: 804–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens ML, Berry HM, Mine A, Argueso CT, Tsuda K(2017) Evolution of hormone signaling networks in plant defense. Annu Rev Phytopathol 55: 401–425 [DOI] [PubMed] [Google Scholar]
- Bigler L, Baumeler A, Werner C, Hesse M(1996) Detection of noncovalent complexes of hydroxamic-acid derivatives by means of electrospray mass spectrometry. Helv Chim Acta 79: 1701–1709 [Google Scholar]
- Bouarab K, Melton R, Peart J, Baulcombe D, Osbourn A(2002) A saponin-detoxifying enzyme mediates suppression of plant defences. Nature 418: 889–892 [DOI] [PubMed] [Google Scholar]
- Bouwmeester H, Schuurink RC, Bleeker PM, Schiestl F(2019) The role of volatiles in plant communication. Plant J 100: 892–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges M, Jones AME, Bones AM, Hodgson C, Cole R, Bartlet E, Wallsgrove R, Karapapa VK, Watts N, Rossiter JT(2002) Spatial organization of the glucosinolate-myrosinase system in brassica specialist aphids is similar to that of the host plant. Proc Biol Sci 269: 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK(2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126: 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burow M, Atwell S, Francisco M, Kerwin RE, Halkier BA, Kliebenstein DJ(2015) The glucosinolate biosynthetic gene AOP2 mediates feed-back regulation of jasmonic acid signaling in Arabidopsis. Mol Plant 8: 1201–1212 [DOI] [PubMed] [Google Scholar]
- Burow M, Halkier BA(2017) How does a plant orchestrate defense in time and space? Using glucosinolates in Arabidopsis as case study. Curr Opin Plant Biol 38: 142–147 [DOI] [PubMed] [Google Scholar]
- Cipollini D, Walters D, Voelckel C(2018) Costs of resistance in plants: From theory to evidence In Roberts JA, ed, Annual Plant Reviews online. Wiley, Hoboken, NJ, pp 263–307. Available at: 10.1002/9781119312994.apr0512 [DOI] [Google Scholar]
- Clay NK, Adio AM, Denoux C, Jander G, Ausubel FM(2009) Glucosinolate metabolites required for an Arabidopsis innate immune response. Science 323: 95–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Briat J-F(2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54: 183–206 [DOI] [PubMed] [Google Scholar]
- Davies J.(2013) Specialized microbial metabolites: Functions and origins. J Antibiot (Tokyo) 66: 361–364 [DOI] [PubMed] [Google Scholar]
- Davies PJ.(2004) Plant hormones Biosythesis, signal transduction, action!, 3rd ed Kluwer Academic, Dordrecht [Google Scholar]
- Dawson GW, Griffiths DC, Pickett JA, Wadhams LJ, Woodcock CM(1987) Plant-derived synergists of alarm pheromone from turnip aphid,Lipaphis (Hyadaphis) erysimi (Homoptera, Aphididae). J Chem Ecol 13: 1663–1671 [DOI] [PubMed] [Google Scholar]
- Dolan WL, Dilkes BP, Stout JM, Bonawitz ND, Chapple C(2017) Mediator complex subunits MED2, MED5, MED16, and MED23 genetically interact in the regulation of phenylpropanoid biosynthesis. Plant Cell 29: 3269–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich PR, Raven PH(1964) Butterflies and plants: A study in coevolution. Evol 18: 586 [Google Scholar]
- Engelberth J, Alborn HT, Schmelz EA, Tumlinson JH(2004) Airborne signals prime plants against insect herbivore attack. Proc Natl Acad Sci USA 101: 1781–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M.(2018) Volatiles as inducers and suppressors of plant defense and immunity-origins, specificity, perception and signaling. Curr Opin Plant Biol 44: 117–121 [DOI] [PubMed] [Google Scholar]
- Erb M, Reymond P(2019) Molecular interactions between plants and insect herbivores. Annu Rev Plant Biol 70: 527–557 [DOI] [PubMed] [Google Scholar]
- Erb M, Veyrat N, Robert CAM, Xu H, Frey M, Ton J, Turlings TCJ(2015) Indole is an essential herbivore-induced volatile priming signal in maize. Nat Commun 6: 6273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer EE, Davoine C(2007) Reactive electrophile species. Curr Opin Plant Biol 10: 380–386 [DOI] [PubMed] [Google Scholar]
- Fernie AR, Pichersky E(2015) Focus issue on metabolism: Metabolites, metabolites everywhere. Plant Physiol 169: 1421–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink LS, Brower LP(1981) Birds can overcome the cardenolide defence of monarch butterflies in Mexico. Nature 291: 67–70 [Google Scholar]
- Frerigmann H, Piślewska-Bednarek M, Sánchez-Vallet A, Molina A, Glawischnig E, Gigolashvili T, Bednarek P(2016) Regulation of pathogen-triggered tryptophan metabolism in Arabidopsis thaliana by MYB transcription factors and indole glucosinolate conversion products. Mol Plant 9: 682–695 [DOI] [PubMed] [Google Scholar]
- Frost CJ, Mescher MC, Dervinis C, Davis JM, Carlson JE, De Moraes CM(2008) Priming defense genes and metabolites in hybrid poplar by the green leaf volatile cis-3-hexenyl acetate. New Phytol 180: 722–734 [DOI] [PubMed] [Google Scholar]
- Futuyma DJ, Agrawal AA(2009) Macroevolution and the biological diversity of plants and herbivores. Proc Natl Acad Sci USA 106: 18054–18061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayomba SR, Watkins JM, Muday GK(2010) Flavonols regulate plant growth and development through regulation of auxin transport and cellular redox status In Santos-Buelga C, Escribano-Bailon MT, and Lattanzio V, eds, Recent Advances in Polyphenol Research. Wiley-Blackwell, Chichester, West Sussex, pp 143–170 [Google Scholar]
- Gershenzon J.(1994) Metabolic costs of terpenoid accumulation in higher plants. J Chem Ecol 20: 1281–1328 [DOI] [PubMed] [Google Scholar]
- Gilardoni PA, Hettenhausen C, Baldwin IT, Bonaventure G(2011) Nicotiana attenuata LECTIN RECEPTOR KINASE1 suppresses the insect-mediated inhibition of induced defense responses during Manduca sexta herbivory. Plant Cell 23: 3512–3532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glauser G, Marti G, Villard N, Doyen GA, Wolfender J-L, Turlings TCJ, Erb M(2011) Induction and detoxification of maize 1,4-benzoxazin-3-ones by insect herbivores. Plant J 68: 901–911 [DOI] [PubMed] [Google Scholar]
- Godard K-A, White R, Bohlmann J(2008) Monoterpene-induced molecular responses in Arabidopsis thaliana. Phytochemistry 69: 1838–1849 [DOI] [PubMed] [Google Scholar]
- Günther J, Irmisch S, Lackus ND, Reichelt M, Gershenzon J, Köllner TG(2018) The nitrilase PtNIT1 catabolizes herbivore-induced nitriles in Populus trichocarpa. BMC Plant Biol 18: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Q, Major IT, Howe GA(2018) Resolution of growth-defense conflict: mechanistic insights from jasmonate signaling. Curr Opin Plant Biol 44: 72–81 [DOI] [PubMed] [Google Scholar]
- Hadacek F, Bachmann G, Engelmeier D, Chobot V(2010) Hormesis and a chemical raison d’être for secondary plant metabolites. Dose Response 9: 79–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann T.(2007) From waste products to ecochemicals: Fifty years research of plant secondary metabolism. Phytochemistry 68: 2831–2846 [DOI] [PubMed] [Google Scholar]
- Hasegawa K, Noguchi H, Iwagawa T, Hase T(1986) Phototropism in hypocotyls of radish: I. Isolation and identification of growth inhibitors, cis- and trans-raphanusanins and raphanusamide, involved in phototropism of radish hypocotyls. Plant Physiol 81: 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath JJ, Kessler A, Woebbe E, Cipollini D, Stireman JO III(2014) Exploring plant defense theory in tall goldenrod, Solidago altissima. New Phytol 202: 1357–1370 [DOI] [PubMed] [Google Scholar]
- Hemm MR, Ruegger MO, Chapple C(2003) The Arabidopsis ref2 mutant is defective in the gene encoding CYP83A1 and shows both phenylpropanoid and glucosinolate phenotypes. Plant Cell 15: 179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández I, Alegre L, Van Breusegem F, Munné-Bosch S(2009) How relevant are flavonoids as antioxidants in plants? Trends Plant Sci 14: 125–132 [DOI] [PubMed] [Google Scholar]
- Hossain MS, Ye W, Hossain MA, Okuma E, Uraji M, Nakamura Y, Mori IC, Murata Y(2013) Glucosinolate degradation products, isothiocyanates, nitriles, and thiocyanates, induce stomatal closure accompanied by peroxidase-mediated reactive oxygen species production in Arabidopsis thaliana. Biosci Biotechnol Biochem 77: 977–983 [DOI] [PubMed] [Google Scholar]
- Howe GA, Jander G(2008) Plant immunity to insect herbivores. Annu Rev Plant Biol 59: 41–66 [DOI] [PubMed] [Google Scholar]
- Hu L, Mateo P, Ye M, Zhang X, Berset JD, Handrick V, Radisch D, Grabe V, Köllner TG, Gershenzon J, et al. (2018) Plant iron acquisition strategy exploited by an insect herbivore. Science 361: 694–697 [DOI] [PubMed] [Google Scholar]
- Hu L, Ye M, Li R, Zhang T, Zhou G, Wang Q, Lu J, Lou Y(2015) The rice transcription factor WRKY53 suppresses herbivore-induced defenses by acting as a negative feedback modulator of mitogen-activated protein kinase activity. Plant Physiol 169: 2907–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber M, Epping J, Schulze Gronover C, Fricke J, Aziz Z, Brillatz T, Swyers M, Köllner TG, Vogel H, Hammerbacher A, et al. (2016) A latex metabolite benefits plant fitness under root herbivore attack. PLoS Biol 14: e1002332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter MD.(2003) Effects of plant quality on the population ecology of parasitoids. Agric For Entomol 5: 1–8 [Google Scholar]
- Jander G.(2018) Revisiting plant-herbivore co-evolution in the molecular biology era In Roberts JA, ed, Annual Plant Reviews. Wiley, Hoboken, NJ, pp 361–384 [Google Scholar]
- Jansen JJ, Allwood JW, Marsden-Edwards E, van der Putten WH, Goodacre R, van Dam NM(2009) Metabolomic analysis of the interaction between plants and herbivores. Metabolomics 5: 150–161 [Google Scholar]
- Jenrich R, Trompetter I, Bak S, Olsen CE, Møller BL, Piotrowski M(2007) Evolution of heteromeric nitrilase complexes in Poaceae with new functions in nitrile metabolism. Proc Natl Acad Sci USA 104: 18848–18853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen LM, Jepsen HSK, Halkier BA, Kliebenstein DJ, Burow M(2015) Natural variation in cross-talk between glucosinolates and onset of flowering in Arabidopsis. Front Plant Sci 6: 697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin CW, You GY, He YF, Tang C, Wu P, Zheng SJ(2007) Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol 144: 278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AC, Seidl-Adams I, Engelberth J, Hunter CT, Alborn H, Tumlinson JH(2019) Herbivorous caterpillars can utilize three mechanisms to alter green leaf volatile emission. Environ Entomol 48: 419–425 [DOI] [PubMed] [Google Scholar]
- Katz E, Nisani S, Yadav BS, Woldemariam MG, Shai B, Obolski U, Ehrlich M, Shani E, Jander G, Chamovitz DA(2015) The glucosinolate breakdown product indole-3-carbinol acts as an auxin antagonist in roots of Arabidopsis thaliana. Plant J 82: 547–555 [DOI] [PubMed] [Google Scholar]
- Kazan K, Lyons R(2014) Intervention of phytohormone pathways by pathogen effectors. Plant Cell 26: 2285–2309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazana E, Pope TW, Tibbles L, Bridges M, Pickett JA, Bones AM, Powell G, Rossiter JT(2007) The cabbage aphid: A walking mustard oil bomb. Proc Biol Sci 274: 2271–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemen AC, Honkanen S, Melton RE, Findlay KC, Mugford ST, Hayashi K, Haralampidis K, Rosser SJ, Osbourn A(2014) Investigation of triterpene synthesis and regulation in oats reveals a role for β-amyrin in determining root epidermal cell patterning. Proc Natl Acad Sci USA 111: 8679–8684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerwin RE, Jimenez-Gomez JM, Fulop D, Harmer SL, Maloof JN, Kliebenstein DJ(2011) Network quantitative trait loci mapping of circadian clock outputs identifies metabolic pathway-to-clock linkages in Arabidopsis. Plant Cell 23: 471–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khokon MAR, Jahan MS, Rahman T, Hossain MA, Muroyama D, Minami I, Munemasa S, Mori IC, Nakamura Y, Murata Y(2011) Allyl isothiocyanate (AITC) induces stomatal closure in Arabidopsis. Plant Cell Environ 34: 1900–1906 [DOI] [PubMed] [Google Scholar]
- Kim JI, Dolan WL, Anderson NA, Chapple C(2015) Indole glucosinolate biosynthesis limits phenylpropanoid accumulation in Arabidopsis thaliana. Plant Cell 27: 1529–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Zhang X, Pascuzzi PE, Liu C-J, Chapple C(2020) Glucosinolate and phenylpropanoid biosynthesis are linked by proteasome-dependent degradation of PAL. New Phytol 225: 154–168 [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ.(2016) False idolatry of the mythical growth versus immunity tradeoff in molecular systems plant pathology. Physiol Mol Plant Pathol 95: 55–59 [Google Scholar]
- Kliebenstein DJ.(2018) Plant nutrient acquisition entices herbivore. Science 361: 642–643 [DOI] [PubMed] [Google Scholar]
- Kliebenstein DJ, Lambrix VM, Reichelt M, Gershenzon J, Mitchell-Olds T(2001) Gene duplication in the diversification of secondary metabolism: Tandem 2-oxoglutarate-dependent dioxygenases control glucosinolate biosynthesis in Arabidopsis. Plant Cell 13: 681–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo I, Klocke JA, Asano S(1983) Effects of ingested phytoecdysteroids on the growth and development of two Lepidopterous larvae. J Insect Physiol 29: 307–316 [Google Scholar]
- Li B, Förster C, Robert CAM, Züst T, Hu L, Machado RAR, Berset J-D, Handrick V, Knauer T, Hensel G, et al. (2018a) Convergent evolution of a metabolic switch between aphid and caterpillar resistance in cereals. Sci Adv 4: eaat6797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Schuman MC, Halitschke R, Li X, Guo H, Grabe V, Hammer A, Baldwin IT(2018b) The decoration of specialized metabolites influences stylar development. eLife 7: e38611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Zhang J, Li J, Zhou G, Wang Q, Bian W, Erb M, Lou Y(2015) Prioritizing plant defence over growth through WRKY regulation facilitates infestation by non-target herbivores. eLife 4: e04805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Du M, Deng L, Shen J, Fang M, Chen Q, Lu Y, Wang Q, Li C, Zhai Q(2019) MYC2 regulates the termination of jasmonate signaling via an autoregulatory negative feedback loop. Plant Cell 31: 106–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H-P, Luo T, Fu H-W, Wang L, Tan Y-Y, Huang J-Z, Wang Q, Ye G-Y, Gatehouse AMR, Lou Y-G, et al. (2018) Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat Plants 4: 338–344 [DOI] [PubMed] [Google Scholar]
- Maag D, Erb M, Köllner TG, Gershenzon J(2015) Defensive weapons and defense signals in plants: Some metabolites serve both roles. BioEssays 37: 167–174 [DOI] [PubMed] [Google Scholar]
- Maag D, Köhler A, Robert CAM, Frey M, Wolfender J-L, Turlings TCJ, Glauser G, Erb M(2016) Highly localized and persistent induction of Bx1-dependent herbivore resistance factors in maize. Plant J 88: 976–991 [DOI] [PubMed] [Google Scholar]
- Machado RAR, Baldwin IT, Erb M(2017) Herbivory-induced jasmonates constrain plant sugar accumulation and growth by antagonizing gibberellin signaling and not by promoting secondary metabolite production. New Phytol 215: 803–812 [DOI] [PubMed] [Google Scholar]
- Malinovsky FG, Thomsen MF, Nintemann SJ, Jagd LM, Bourgine B, Burow M, Kliebenstein DJ(2017) An evolutionarily young defense metabolite influences the root growth of plants via the ancient TOR signaling pathway. eLife 6: e2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marti G, Erb M, Boccard J, Glauser G, Doyen GR, Villard N, Robert CAM, Turlings TCJ, Rudaz S, Wolfender J-L(2013) Metabolomics reveals herbivore-induced metabolites of resistance and susceptibility in maize leaves and roots. Plant Cell Environ 36: 621–639 [DOI] [PubMed] [Google Scholar]
- Maruyama-Nakashita A, Inoue E, Watanabe-Takahashi A, Yamaya T, Takahashi H(2003) Transcriptome profiling of sulfur-responsive genes in Arabidopsis reveals global effects of sulfur nutrition on multiple metabolic pathways. Plant Physiol 132: 597–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer MJ, Narbad A, Parr AJ, Parker ML, Walton NJ, Mellon FA, Michael AJ(2001) Rerouting the plant phenylpropanoid pathway by expression of a novel bacterial enoyl-CoA hydratase/lyase enzyme function. Plant Cell 13: 1669–1682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKey D.(1974) Adaptive patterns in alkaloid physiology. Am Nat 108: 305–320 [Google Scholar]
- Meents AK, Chen S-P, Reichelt M, Lu H-H, Bartram S, Yeh K-W, Mithöfer A(2019) Volatile DMNT systemically induces jasmonate-independent direct anti-herbivore defense in leaves of sweet potato (Ipomoea batatas) plants. Sci Rep 9: 17431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meihls LN, Handrick V, Glauser G, Barbier H, Kaur H, Haribal MM, Lipka AE, Gershenzon J, Buckler ES, Erb M, et al. (2013) Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 25: 2341–2355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Erb M, Baldwin IT(2012) Defence on demand: Mechanisms behind optimal defence patterns. Ann Bot 110: 1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldau S, Wu J, Baldwin IT(2009) Silencing two herbivory-activated MAP kinases, SIPK and WIPK, does not increase Nicotiana attenuata’s susceptibility to herbivores in the glasshouse and in nature. New Phytol 181: 161–173 [DOI] [PubMed] [Google Scholar]
- Mladenka P, Macáková K, Zatloukalová L, Reháková Z, Singh BK, Prasad AK, Parmar VS, Jahodár L, Hrdina R, Saso L(2010) In vitro interactions of coumarins with iron. Biochimie 92: 1108–1114 [DOI] [PubMed] [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S(2008) Beta-glucosidases as detonators of plant chemical defense. Phytochemistry 69: 1795–1813 [DOI] [PubMed] [Google Scholar]
- Muhlemann JK, Younts TLB, Muday GK(2018) Flavonols control pollen tube growth and integrity by regulating ROS homeostasis during high-temperature stress. Proc Natl Acad Sci USA 115: E11188–E11197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Peer WA, Taiz L(2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211: 315–324 [DOI] [PubMed] [Google Scholar]
- Nallu S, Hill JA, Don K, Sahagun C, Zhang W, Meslin C, Snell-Rood E, Clark NL, Morehouse NI, Bergelson J, et al. (2018) The molecular genetic basis of herbivory between butterflies and their host plants. Nat Ecol Evol 2: 1418–1427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NN, Ihemere U, Ellery C, Sayre RT(2011) Overexpression of hydroxynitrile lyase in cassava roots elevates protein and free amino acids while reducing residual cyanogen levels. PLoS One 6: e21996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negi VS, Bingham J-P, Li QX, Borthakur D(2014) A carbon-nitrogen lyase from Leucaena leucocephala catalyzes the first step of mimosine degradation. Plant Physiol 164: 922–934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neilson EH, Goodger JQD, Woodrow IE, Møller BL(2013) Plant chemical defense: At what cost? Trends Plant Sci 18: 250–258 [DOI] [PubMed] [Google Scholar]
- Nishida R.(2002) Sequestration of defensive substances from plants by Lepidoptera. Annu Rev Entomol 47: 57–92 [DOI] [PubMed] [Google Scholar]
- Opitz SEW, Müller C(2009) Plant chemistry and insect sequestration. Chemoecol 19: 117–154 [Google Scholar]
- Paschold A, Halitschke R, Baldwin IT(2006) Using ‘mute’ plants to translate volatile signals. Plant J 45: 275–291 [DOI] [PubMed] [Google Scholar]
- Peer WA, Murphy AS(2007) Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci 12: 556–563 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Lewinsohn E(2011) Convergent evolution in plant specialized metabolism. Annu Rev Plant Biol 62: 549–566 [DOI] [PubMed] [Google Scholar]
- Pichersky E, Raguso RA(2018) Why do plants produce so many terpenoid compounds? New Phytol 220: 692–702 [DOI] [PubMed] [Google Scholar]
- Quintana-Rodriguez E, Duran-Flores D, Heil M, Camacho-Coronel X(2018) Damage-associated molecular patterns (DAMPs) as future plant vaccines that protect crops from pests. Sci Hortic (Amsterdam) 237: 207–220 [Google Scholar]
- Rafter JL, Gonda-King L, Niesen D, Seeram NP, Rigsby CM, Preisser EL(2017) Impact of consuming ‘toxic’ monarch caterpillars on adult chinese mantid mass gain and fecundity. Insects 8: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoades DF.(1977) Integrated antiherbivore, antidesiccant and ultraviolet screening properties of creosotebush resin. Biochem Syst Ecol 5: 281–290 [Google Scholar]
- Rhoades DF.(1983) Responses of alder and willow to attack by tent caterpillars and webworms: Evidence for pheromonal sensitivity of willows In Hedin PA, ed, Plant Resistance to Insects, Vol 208 American Chemical Society, Washington DC, pp 55–68 [Google Scholar]
- Richards LA, Lampert EC, Bowers MD, Dodson CD, Smilanich AM, Dyer LA(2012) Synergistic effects of iridoid glycosides on the survival, development and immune response of a specialist caterpillar, Junonia coenia (Nymphalidae). J Chem Ecol 38: 1276–1284 [DOI] [PubMed] [Google Scholar]
- Riedlmeier M, Ghirardo A, Wenig M, Knappe C, Koch K, Georgii E, Dey S, Parker JE, Schnitzler J-P, Vlot AC(2017) Monoterpenes support systemic acquired resistance within and between plants. Plant Cell 29: 1440–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert CAM, Veyrat N, Glauser G, Marti G, Doyen GR, Villard N, Gaillard MDP, Köllner TG, Giron D, Body M, et al. (2012) A specialist root herbivore exploits defensive metabolites to locate nutritious tissues. Ecol Lett 15: 55–64 [DOI] [PubMed] [Google Scholar]
- Robert CAM, Zhang X, Machado RA, Schirmer S, Lori M, Mateo P, Erb M, Gershenzon J(2017) Sequestration and activation of plant toxins protect the western corn rootworm from enemies at multiple trophic levels. eLife 6: e29307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salehin M, Li B, Tang M, Katz E, Song L, Ecker JR, Kliebenstein DJ, Estelle M(2019) Auxin-sensitive Aux/IAA proteins mediate drought tolerance in Arabidopsis by regulating glucosinolate levels. Nat Commun 10: 4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santelia D, Henrichs S, Vincenzetti V, Sauer M, Bigler L, Klein M, Bailly A, Lee Y, Friml J, Geisler M, Martinoia E(2008) Flavonoids redirect PIN-mediated polar auxin fluxes during root gravitropic responses. J Biol Chem 283: 31218–31226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarfraz M, Dosdall LM, Keddie BA(2009) Host plant nutritional quality affects the performance of the parasitoid Diadegma insulare. Biol Control 51: 34–41 [Google Scholar]
- Schmid NB, Giehl RFH, Döll S, Mock H-P, Strehmel N, Scheel D, Kong X, Hider RC, von Wirén N(2014) Feruloyl-CoA 6′-hydroxylase1-dependent coumarins mediate iron acquisition from alkaline substrates in Arabidopsis. Plant Physiol 164: 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuman MC, Baldwin IT(2016) The layers of plant responses to insect herbivores. Annu Rev Entomol 61: 373–394 [DOI] [PubMed] [Google Scholar]
- Schweizer F, Fernández-Calvo P, Zander M, Diez-Diaz M, Fonseca S, Glauser G, Lewsey MG, Ecker JR, Solano R, Reymond P(2013) Arabidopsis basic helix-loop-helix transcription factors MYC2, MYC3, and MYC4 regulate glucosinolate biosynthesis, insect performance, and feeding behavior. Plant Cell 25: 3117–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmar D, Lieberei R, Biehl B(1988) Mobilization and utilization of cyanogenic glycosides: The linustatin pathway. Plant Physiol 86: 711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva-Navas J, Moreno-Risueno MA, Manzano C, Téllez-Robledo B, Navarro-Neila S, Carrasco V, Pollmann S, Gallego FJ, Del Pozo JC(2016) Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 28: 1372–1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirikantaramas S, Yamazaki M, Saito K(2008) Mechanisms of resistance to self-produced toxic secondary metabolites in plants. Phytochem Rev 7: 467–477 [Google Scholar]
- Sloggett JJ, Davis AJ(2010) Eating chemically defended prey: Alkaloid metabolism in an invasive ladybird predator of other ladybirds (Coleoptera: Coccinellidae). J Exp Biol 213: 237–241 [DOI] [PubMed] [Google Scholar]
- Soubeyrand E, Johnson TS, Latimer S, Block A, Kim J, Colquhoun TA, Butelli E, Martin C, Wilson MA, Basset GJ(2018) The peroxidative cleavage of kaempferol contributes to the biosynthesis of the benzenoid moiety of ubiquinone in plants. Plant Cell 30: 2910–2921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl E, Bellwon P, Huber S, Schlaeppi K, Bernsdorff F, Vallat-Michel A, Mauch F, Zeier J(2016) Regulatory and functional aspects of indolic metabolism in plant systemic acquired resistance. Mol Plant 9: 662–681 [DOI] [PubMed] [Google Scholar]
- Stahl E, Hilfiker O, Reymond P(2018) Plant-arthropod interactions: Who is the winner? Plant J 93: 703–728 [DOI] [PubMed] [Google Scholar]
- Stenlid G.(1963) The effects of flavonoid compounds on oxidative phosphorylation and on the enzymatic destruction of indoleacetic acid. Physiol Plant 16: 110–120 [Google Scholar]
- Stenlid G.(1976) Effects of flavonoids on the polar transport of auxins. Physiol Plant 38: 262–266 [Google Scholar]
- Stringlis IA, Yu K, Feussner K, de Jonge R, Van Bentum S, Van Verk MC, Berendsen RL, Bakker PAHM, Feussner I, Pieterse CMJ(2018) MYB72-dependent coumarin exudation shapes root microbiome assembly to promote plant health. Proc Natl Acad Sci USA 115: E5213–E5222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun R, Jiang X, Reichelt M, Gershenzon J, Pandit SS, Giddings Vassão D(2019a) Tritrophic metabolism of plant chemical defenses and its effects on herbivore and predator performance. eLife 8: e51029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Harpazi B, Wijerathna-Yapa A, Merilo E, de Vries J, Michaeli D, Gal M, Cuming AC, Kollist H, Mosquna A(2019b) A ligand-independent origin of abscisic acid perception. Proc Natl Acad Sci USA 116: 24892–24899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taiz L, Zeiger E, Møller IM, Murphy AS(2015) Plant physiology and development. Sinauer Associates, Sunderland, MA [Google Scholar]
- Tanaka K, Choi J, Cao Y, Stacey G(2014) Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front Plant Sci 5: 446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turlings TCJ, Erb M(2018) Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annu Rev Entomol 63: 433–452 [DOI] [PubMed] [Google Scholar]
- Ugine TA, Krasnoff SB, Grebenok RJ, Behmer ST, Losey JE(2019) Prey nutrient content creates omnivores out of predators. Ecol Lett 22: 275–283 [DOI] [PubMed] [Google Scholar]
- van Dam NM.(2009) Belowground herbivory and plant defenses. Annu Rev Ecol Evol Syst 40: 373–391 [Google Scholar]
- Veyrat N, Robert CAM, Turlings TCJ, Erb M(2016) Herbivore intoxication as a potential primary function of an inducible volatile plant signal. J Ecol 104: 591–600 [Google Scholar]
- Watkins JM, Hechler PJ, Muday GK(2014) Ethylene-induced flavonol accumulation in guard cells suppresses reactive oxygen species and moderates stomatal aperture. Plant Physiol 164: 1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzel WC, Kharouba HM, Robinson M, Holyoak M, Karban R(2016) Variability in plant nutrients reduces insect herbivore performance. Nature 539: 425–427 [DOI] [PubMed] [Google Scholar]
- Wink M.(2008) Plant secondary metabolism: Diversity, function and its evolution. Nat Prod Commun 3: 1934578X0800300 [Google Scholar]
- Ye M, Glauser G, Lou Y, Erb M, Hu L(2019) Molecular dissection of early defense signaling underlying volatile-mediated defense regulation and herbivore resistance in rice. Plant Cell 31: 687–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagrobelny M, de Castro ÉCP, Møller BL, Bak S(2018) Cyanogenesis in arthropods: From chemical warfare to nuptial gifts. Insects 9: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Kawaguchi R, Morikawa-Ichinose T, Allahham A, Kim S-J, Maruyama-Nakashita A(2020) Sulfur deficiency-induced glucosinolate catabolism attributed to two β-glucosidases, BGLU28 and BGLU30, is required for plant growth maintenance under sulfur deficiency. Plant Cell Physiol 61: 803–813 [DOI] [PubMed] [Google Scholar]
- Zhang X, Gou M, Liu C-J(2013) Arabidopsis Kelch repeat F-box proteins regulate phenylpropanoid biosynthesis via controlling the turnover of phenylalanine ammonia-lyase. Plant Cell 25: 4994–5010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, van Doan C, Arce CCM, Hu L, Gruenig S, Parisod C, Hibbard BE, Hervé MR, Nielson C, Robert CAM, et al. (2019) Plant defense resistance in natural enemies of a specialist insect herbivore. Proc Natl Acad Sci USA 116: 23174–23181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Zhang W, Stanley BA, Assmann SM(2008) Functional proteomics of Arabidopsis thaliana guard cells uncovers new stomatal signaling pathways. Plant Cell 20: 3210–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Lou Y-R, Tzin V, Jander G(2015) Alteration of plant primary metabolism in response to insect herbivory. Plant Physiol 169: 1488–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S, Richter A, Jander G(2018) Beyond defense: Multiple functions of benzoxazinoids in maize metabolism. Plant Cell Physiol 59: 1528–1537 [DOI] [PubMed] [Google Scholar]
- Zipor G, Duarte P, Carqueijeiro I, Shahar L, Ovadia R, Teper-Bamnolker P, Eshel D, Levin Y, Doron-Faigenboim A, Sottomayor M, et al. (2015) In planta anthocyanin degradation by a vacuolar class III peroxidase in Brunfelsia calycina flowers. New Phytol 205: 653–665 [DOI] [PubMed] [Google Scholar]
- Züst T, Joseph B, Shimizu KK, Kliebenstein DJ, Turnbull LA(2011) Using knockout mutants to reveal the growth costs of defensive traits. Proc Biol Sci 278: 2598–2603 [DOI] [PMC free article] [PubMed] [Google Scholar]