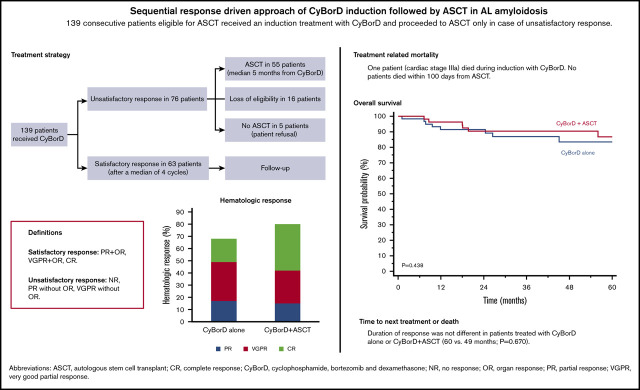
Key Points
A sequential approach offering stem cell transplant to patients not responding satisfactorily to bortezomib is feasible in AL amyloidosis.
This approach results in low (<1%) treatment-related mortality, deep hematologic response rate >60%, and estimated 5-year survival >80%.
Abstract
Autologous stem cell transplant (ASCT) is highly effective in selected patients with light chain (AL) amyloidosis. Bortezomib, preceding or following ASCT, improves responses. Satisfactory responses, including at least a partial response, very good partial response (VGPR) with organ response, or complete response, can be observed after induction therapy alone. We report 139 patients treated upfront with cyclophosphamide/bortezomib/dexamethasone (CyBorD), followed by ASCT only if response was unsatisfactory. Only 1 treatment-related death was observed. After CyBorD, hematologic response (HR) rate was 68% (VGPR or better, 51%), with 45% satisfactory responses. Transplant was performed in 55 (40%) subjects and resulted in an 80% HR rate (65% ≥ VGPR). Five-year survival was 86% and 84% in patients treated with ASCT or CyBorD alone, respectively (P = .438). Also, 6- and 12- month landmark analyses did not show differences in survival. Duration of response was not different in the 2 groups (60 vs 49 months; P = .670). Twenty-one (15%) patients with an unsatisfactory response to CyBorD could not undergo ASCT because of ineligibility or refusal; instead, they received rescue chemotherapy, with HR in 38% of cases and 51% 5-year survival. This sequential response-driven approach, offering ASCT to patients who do not attain satisfactory response to upfront CyBorD, is very safe and effective in AL amyloidosis.
Visual Abstract
Introduction
Autologous stem cell transplantation (ASCT) is very effective in light chain (AL) amyloidosis. Transplant-related mortality decreased with refinement of eligibility criteria; now, ∼70% of patients achieve a complete response (CR) or a very good partial response (VGPR).1-4 This progress was accompanied by advancements in nontransplant chemotherapy.5,6 Bortezomib is usually offered to subjects who are not eligible for ASCT.7 However, bortezomib can also be used after ASCT to improve quality of response and extend survival8,9 and before ASCT, aiming at decreasing plasma cell and light chain burden.10 This latter approach can result in deeper hematologic responses (HRs) and longer survival than ASCT alone and has been studied in recent trials.11-13 In particular, bortezomib-based induction can result in deep HRs and organ improvement, considered a satisfactory end point in AL amyloidosis, before ASCT.7,14 At our center, transplant-eligible patients are treated upfront with cyclophosphamide/bortezomib/dexamethasone (CyBorD) and they proceed to ASCT only in case of an unsatisfactory response. We report the outcome of 139 consecutive subjects treated according to this sequential response-driven approach.
Methods
Starting in 2009 we offered upfront therapy with weekly cyclophosphamide (300 mg/m2), bortezomib (1.3 mg/m2), and dexamethasone (40 mg) (CyBorD) to all patients with AL amyloidosis who were eligible for ASCT. Eligibility requirements included age <65 years, N-terminal pronatriuretic-peptide type B <5000 ng/L, glomerular filtration rate >50 mL/min, New York Heart Association class <3, Eastern Cooperative Oncology Group Performance Status ≤2, and ejection fraction >45%.15 HR and organ response (OR) were assessed after every 2 CyBorD cycles and 3 months after ASCT, according to validated criteria.16,17 Subjects attaining partial response (PR) or better after 2 CyBorD cycles continued chemotherapy, for up to 6 cycles, until the response plateaued. Patients who did not achieve satisfactory response after CyBorD proceeded to ASCT (melphalan, 200 mg/m2), if still eligible. Stem cell collection was performed only in patients who proceeded to ASCT and was done with granulocyte colony-stimulating factor alone. Satisfactory response was defined as CR, VGPR plus OR, or PR plus OR. Data were obtained from a prospectively maintained database, and 139 consecutive patients were included. Patients gave written informed consent for their clinical data to be used for research purposes. Overall survival (OS) and time to next treatment or death (TNTD) were calculated from diagnosis. Six- and 12-month landmark analyses were also performed. Deaths occurring in the first 100 days after initiation of CyBorD or ASCT were classified as treatment related. This study was approved by Fondazione Istituto di Ricovero e Cura a Carattere Scientifico Policlinico S. Matteo’s Ethical Committee.
Results
Between 2009 and 2018, 139 consecutive patients (Table 1) were included, representing 15% of all of our patients. They received a median of 4 cycles of CyBorD (range, 2-6 cycles). Twenty subjects (14%) experienced grade 3-4 adverse events (fluid retention in 7 patients, cytopenia in 5 patients, infection in 3 patients, and acute renal failure, deep venous thrombosis, angina, hypokalemia, and neuropathy in 1 patient each). One patient (cardiac stage IIIa) died suddenly within 100 days. Overall HR was 68% (95 patients), with 26 (19%) CRs and 45 (32%) VGPRs. Cardiac and renal responses were observed in 13 of 43 (30%) and in 31 of 100 (31%) evaluable cases, respectively. Overall, 63 patients (45%) achieved a satisfactory response. None of them was transplanted at relapse. Sixteen subjects (11%) did not respond satisfactorily but did not proceed to ASCT because of organ progression (supplemental Table 1). Five additional subjects (4%) with an unsatisfactory response refused to proceed to ASCT. None of these 21 patients received ASCT at relapse.
Table 1.
Characteristics of 139 patients with newly diagnosed AL amyloidosis eligible for ASCT
| Variables | Data |
|---|---|
| Age, median (IQR), y | 56 (50-61) |
| Sex, male | 77 (55) |
| Organ involvement | |
| Heart | 66 (47) |
| Kidney | 104 (75) |
| Liver | 16 (11) |
| Soft tissues | 19 (14) |
| Autonomic nervous system | 6 (4) |
| Peripheral nervous system | 9 (6) |
| More than 2 organs involved | 15 (12) |
| Cardiac stage * | |
| I | 62 (45) |
| II | 71 (51) |
| IIIa | 3 (22) |
| IIIb | 0 (0) |
| NT-proBNP, median (IQR), ng/L | 360 (133-840) |
| Renal stage † | |
| I | 70 (51) |
| II | 69 (49) |
| III | 0 (0) |
| Proteinuria, median (IQR), g/24 h | 4.80 (0.67-7.00) |
| eGFR, median (IQR), mL/min/1.72 m2 | 84 (64 to >90) |
| Bone marrow plasma cells, median (IQR), % | 10 (6-15) |
Unless otherwise noted, data are n (%).
cTnI, cardiac troponin I; eGFR, estimated glomerular filtration rate; IQR, interquartile range; NT-proBNP, N-terminal natriuretic propeptide type B.
Cardiac stage is based on troponin level and NT-proBNP: thresholds for cTnI and NT-proBNP are <0.1 ng/mL and <332 ng/L, respectively. Stage I, cTnI <0.1 ng/mL and NT-proBNP <332 ng/L; stage II, cTnI >0.1 ng/mL or NT-proBNP >332 ng/L; stage IIIa, cTnI >0.1 ng/mL and NT-proBNP >332 ng/L but <8500 ng/L; and stage IIIb, cTnI >0.1 ng/mL and NT-proBNP >8500 ng/L.
Renal stage is based on proteinuria and eGFR levels: thresholds for proteinuria are >5 g/24 h and eGFR <50 mL/min/1.73 m2. Stage I, proteinuria ≤5 g/24 h and eGFR ≥50 mL/min/1.73 m2; stage II, proteinuria >5 g/24 h or eGFR <50 mL/min/1.73 m2; and stage III, proteinuria >5 g/24 h and eGFR <50 mL/min/1.73 m2.
The remaining 55 subjects (40%) underwent ASCT after a median of 5 months from CyBorD initiation. Thirteen had achieved a VGPR after CyBorD and 6 had attained a PR, whereas the remaining were nonresponders. Grade 3-4 adverse events were infection in 10 patients (18%), renal insufficiency in 3 patients (5%), heart failure in 3 patients (5%), and cytopenia, syncope, and deep venous thrombosis in 2 (4%) cases each. No patient died within 100 days. Overall HR rate was 80%, with 21 (38%) CRs and 15 (27%) VGPRs. Cardiac or renal response was observed in 2 of 9 (22%) and 17 of 37 (46%) evaluable patients, respectively.
In the overall cohort, HR rate after CyBorD, with or without ASCT, was 76%, with 47 (34%) CRs and 40 (29%) VGPRs. Cardiac response was achieved in 15 of 43 (35%) patients, and renal response was achieved in 48 of 100 evaluable subjects (48%). Among the 21 patients with unsatisfactory response after CyBorD who did not proceed to ASCT, 12 were rescued with lenalidomide, 4 were rescued with daratumumab, and 5 were rescued with melphalan/dexamethasone. Overall, 8 patients (38%) attained HR, with 2 CRs (10%) and 3 VGPRs (14%). Cardiac response was observed in 1 of 6 patients, and renal response was achieved in 2 of 8 evaluable patients.
After a median follow-up of living patients of 48 months, 27 subjects died, and OS was 80% at 5 years. Five-year OS was 86% in patients who proceeded to ASCT and 84% in subjects who satisfactorily responded to CyBorD (Figure 1A-B; P = .438). Survival also was not different between the 2 groups in 6- and 12-month landmark analyses accounting for different treatment durations (supplemental Figure 1) or when considering only patients who achieved a VGPR or better (5-year OS 92% vs 96% with CyBorD alone or followed by ASCT, P = .425; Figure 1C-D). Likewise, TNTD was not different between patients treated with CyBorD alone and those who also received ASCT (median, 49 vs 60 months, P = .670; Figure 1E-F). This was confirmed when the analysis was limited to patients in CR (median, 54 months vs not reached, P = .692; supplemental Figure 2). In patients who did not proceed to ASCT despite failing to satisfactorily respond to CyBorD, OS was 51% at 5 years (P < .001 vs other groups; supplemental Figure 3).
Figure 1.
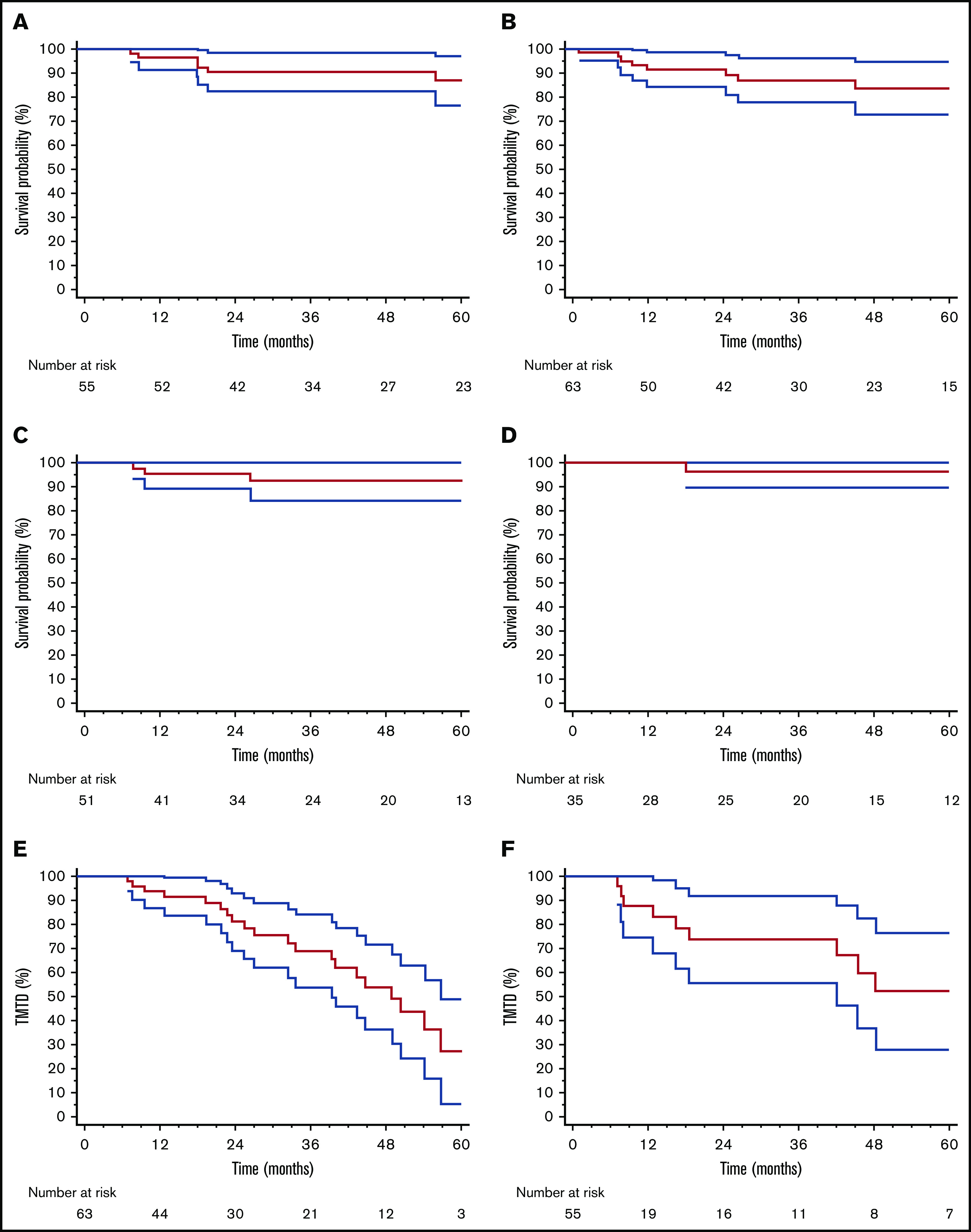
OS and TNTD in patients treated with CyBorD and ASCT and in those who received CyBorD alone. (A) OS in patients treated with CyBorD and ASCT. (B) OS in patients with a satisfactory response to CyBorD. Six-month landmark plotted OS in patients treated with CyBorD (C) or with CyBorD and ASCT (D) who achieved a VGPR or better. Six-month landmark plotted TNTD in patients who received only CyBorD (E) or CyBorD and ASCT (F).
Discussion
This sequential treatment approach was effective, resulting in VGPR/CR in 63% of patients, as well as cardiac or renal response in 35% or 48% of cases, respectively. Stem cell transplant was not required in 45% of subjects. Five-year OS (>80%) and duration of response (median, 4.5 years) were not different between patients who achieved satisfactory response after CyBorD alone or after CyBorD followed by ASCT. Importantly, VGPR/CR rate was comparable to that observed (50%) in the HOVON-104 trial with bortezomib induction and ASCT, and the duration of response was similar to that reported by the Boston University group for ASCT (median, 4.3 years).12,18
A single treatment-related death occurred during CyBorD treatment. This indicates that careful biomarker-based selection of transplant candidates and sequential therapy that limits treatment intensity based on the quality of response dramatically reduces early mortality.
Sixteen subjects (11%), who did not satisfactorily respond to CyBorD, lost their eligibility for ASCT. One cannot exclude that these subjects could have had a better outcome if they had been transplanted upfront. Further studies are warranted to identify subjects who are less likely to respond to CyBorD and should be considered for upfront ASCT. For instance, patients with t(11;14) have less frequent and less profound responses and shorter survival when treated with CyBorD, whereas they have better outcomes with melphalan.19 Unfortunately, cytogenetics data were not available in our study. Moreover, it is possible that alternative definitions of satisfactory response could better identify patients who would benefit most from ASCT.
A sequential response-driven approach offering ASCT to patients failing to achieve a satisfactory response to upfront CyBorD is very safe and effective in AL amyloidosis. More powerful regimens, including newer drugs, such as daratumumab,20-25 will likely make a sequential approach even more efficient, keeping ASCT incorporated in a rapidly changing landscape.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by Fondazione Cariplo (grants 2014-0700, 2015-0591, 2016-0489), Associazione Italiana per la Ricerca sul Cancro special program “5 per mille” (no. 9965), the Italian Ministry of Health (grants RF-2013-02355259 and RF-2016-02361756), the Italian Medicines Agency (grant AIFA-2016-02364602), and E-Rare JTC 2016 grant ReDox. This research was also funded by the Italian Ministry of Education, University, and Research to the Departments of Molecular Medicine and Biology and Biotechnologies of the University of Pavia under the initiative “Dipartimenti di Eccellenza (2018-2022)." G.P. is supported in part by the Bart Barlogie Young Investigator Award from the International Myeloma Society. P.M. is supported in part by a fellowship award from Ghislieri College. M.N. is supported in part by a grant from the Amyloidosis Foundation.
Footnotes
Presented in abstract form at the 61st annual meeting of the American Society of Hematology, Orlando, FL, 7 December 2019.
For original data, please contact Giovanni Palladini (giovanni.palladini@unipv.it).
Authorship
Contribution: G.P. designed the study; M.B and G.P. evaluated patients, collected and analyzed data, and wrote the manuscript; P.M., M.N., A.F., F.B., L.R., and G.M. evaluated patients and critically reviewed the manuscript; and all authors approved the final version of the manuscript for publication.
Conflict-of-interest disclosure: G.P. receives honoraria from Janssen Pharmaceuticals, honoraria and travel support from Prothena, and travel support from Celgene. P.M. receives speaker honoraria from Pfizer and Jansen Pharmaceuticals and travel support from Celgene. M.N. receives honoraria from Janssen Pharmaceuticals. G.M. is a consultant for Millennium Pharmaceuticals Inc., Pfizer, and Janssen Pharmaceuticals. The remaining authors declare no competing financial interests.
Correspondence: Giovanni Palladini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, Viale Golgi, 19, 27100 Pavia, Italy; e-mail: giovanni.palladini@unipv.it.
References
- 1.Gertz MA, Lacy MQ, Dispenzieri A, et al. Refinement in patient selection to reduce treatment-related mortality from autologous stem cell transplantation in amyloidosis. Bone Marrow Transplant. 2013;48(4):557-561. [DOI] [PubMed] [Google Scholar]
- 2.D’Souza A, Dispenzieri A, Wirk B, et al. Improved outcomes after autologous hematopoietic cell transplantation for light chain amyloidosis: a Center for International Blood and Marrow Transplant research study. J Clin Oncol. 2015;33(32):3741-3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sanchorawala V, Sun F, Quillen K, Sloan JM, Berk JL, Seldin DC. Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015;126(20):2345-2347. [DOI] [PubMed] [Google Scholar]
- 4.Sidiqi MH, Aljama MA, Buadi FK, et al. Stem cell transplantation for light chain amyloidosis: decreased early mortality over time. J Clin Oncol. 2018;36(13):1323-1329. [DOI] [PubMed] [Google Scholar]
- 5.Palladini G, Sachchithanantham S, Milani P, et al. A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015;126(5):612-615. [DOI] [PubMed] [Google Scholar]
- 6.Manwani R, Cohen O, Sharpley F, et al. A prospective observational study of 915 patients with systemic AL amyloidosis treated with upfront bortezomib. Blood. 2019;134(25):2271-2280. [DOI] [PubMed] [Google Scholar]
- 7.Merlini G, Dispenzieri A, Sanchorawala V, et al. Systemic immunoglobulin light chain amyloidosis. Nat Rev Dis Primers. 2018;4(1):38. [DOI] [PubMed] [Google Scholar]
- 8.Landau H, Smith M, Landry C, et al. Long-term event-free and overall survival after risk-adapted melphalan and SCT for systemic light chain amyloidosis. Leukemia. 2017;31(1):136-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Al Saleh AS, Sidiqi MH, Sidana S, et al. Impact of consolidation therapy post autologous stem cell transplant in patients with light chain amyloidosis. Am J Hematol. 2019;94(10):1066-1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwa YL, Kumar SK, Gertz MA, et al. Induction therapy pre-autologous stem cell transplantation in immunoglobulin light chain amyloidosis: a retrospective evaluation. Am J Hematol. 2016;91(10):984-988. [DOI] [PubMed] [Google Scholar]
- 11.Huang X, Wang Q, Chen W, et al. Induction therapy with bortezomib and dexamethasone followed by autologous stem cell transplantation versus autologous stem cell transplantation alone in the treatment of renal AL amyloidosis: a randomized controlled trial. BMC Med. 2014;12(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Minnema MC, Nasserinejad K, Hazenberg B, et al. Bortezomib-based induction followed by stem cell transplantation in light chain amyloidosis: results of the multicenter HOVON 104 trial. Haematologica. 2019;104(11):2274-2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanchorawala V, Brauneis D, Shelton AC, et al. Induction therapy with bortezomib followed by bortezomib-high dose melphalan and stem cell transplantation for light chain amyloidosis: results of a prospective clinical trial. Biol Blood Marrow Transplant. 2015;21(8):1445-1451. [DOI] [PubMed] [Google Scholar]
- 14.Dispenzieri A, Buadi F, Kumar SK, et al. Treatment of immunoglobulin light chain amyloidosis: Mayo stratification of myeloma and risk-adapted therapy (mSMART) consensus statement. Mayo Clin Proc. 2015;90(8):1054-1081. [DOI] [PubMed] [Google Scholar]
- 15.Palladini G, Merlini G. What is new in diagnosis and management of light chain amyloidosis? Blood. 2016;128(2):159-168. [DOI] [PubMed] [Google Scholar]
- 16.Palladini G, Dispenzieri A, Gertz MA, et al. New criteria for response to treatment in immunoglobulin light chain amyloidosis based on free light chain measurement and cardiac biomarkers: impact on survival outcomes. J Clin Oncol. 2012;30(36):4541-4549. [DOI] [PubMed] [Google Scholar]
- 17.Palladini G, Hegenbart U, Milani P, et al. A staging system for renal outcome and early markers of renal response to chemotherapy in AL amyloidosis. Blood. 2014;124(15):2325-2332. [DOI] [PubMed] [Google Scholar]
- 18.Browning S, Quillen K, Sloan JM, Doros G, Sarosiek S, Sanchorawala V. Hematologic relapse in AL amyloidosis after high-dose melphalan and stem cell transplantation. Blood. 2017;130(11):1383-1386. [DOI] [PubMed] [Google Scholar]
- 19.Bochtler T, Hegenbart U, Kunz C, et al. Translocation t(11;14) is associated with adverse outcome in patients with newly diagnosed AL amyloidosis when treated with bortezomib-based regimens. J Clin Oncol. 2015;33(12):1371-1378. [DOI] [PubMed] [Google Scholar]
- 20.Chung A, Kaufman GP, Sidana S, et al. Organ responses with daratumumab therapy in previously treated AL amyloidosis. Blood Adv. 2020;4(3):458-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kimmich CR, Terzer T, Benner A, et al. Daratumumab for systemic AL amyloidosis: prognostic factors and adverse outcome with nephrotic-range albuminuria. Blood. 2020;135(18):1517-1530. [DOI] [PubMed] [Google Scholar]
- 22.Roussel M, Merlini G, Chevret S, et al. A prospective phase 2 trial of daratumumab in patients with previously treated systemic light-chain amyloidosis. Blood. 2020;135(18):1531-1540. [DOI] [PubMed] [Google Scholar]
- 23.Sanchorawala V, Sarosiek S, Schulman A, et al. Safety, tolerability, and response rates of daratumumab in relapsed AL amyloidosis: results of a phase 2 study. Blood. 2020;135(18):1541-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palladini G, Kastritis E, Maurer MS, et al. Daratumumab plus CyBorD for patients with newly diagnosed AL amyloidosis: safety run-in results of ANDROMEDA. Blood. 2020;136(1):71-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milani P, Fazio F, Basset M, et al. High rate of profound clonal and renal responses with daratumumab treatment in heavily pre-treated patients with light chain (AL) amyloidosis and high bone marrow plasma cell infiltrate. Am J Hematol. 2020;95(8):900-905. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.