Key Points
There is a high unmet need for new treatment options, particularly in elderly patients with relapsed/refractory FL.
Radioimmunotherapy is an underused option, and 177Lu-lilotomab satetraxetan may offer a safe and effective treatment for relapsed FL.
Abstract
For patients with indolent non-Hodgkin lymphoma who fail initial anti-CD20–based immunochemotherapy or develop relapsed or refractory disease, there remains a significant unmet clinical need for new therapeutic approaches to improve outcomes and quality of life. 177Lu-lilotomab satetraxetan is a next-generation single-dose CD37-directed radioimmunotherapy (RIT) which was investigated in a phase 1/2a study in 74 patients with relapsed/refractory indolent non-Hodgkin B-cell lymphoma, including 57 patients with follicular lymphoma (FL). To improve targeting of 177Lu-lilotomab satetraxetan to tumor tissue and decrease hematologic toxicity, its administration was preceded by the anti-CD20 monoclonal antibody rituximab and the “cold” anti-CD37 antibody lilotomab. The most common adverse events (AEs) were reversible grade 3/4 neutropenia (31.6%) and thrombocytopenia (26.3%) with neutrophil and platelet count nadirs 5 to 7 weeks after RIT. The most frequent nonhematologic AE was grade 1/2 nausea (15.8%). With a single administration, the overall response rate was 61% (65% in patients with FL), including 30% complete responses. For FL with ≥2 prior therapies (n = 37), the overall response rate was 70%, including 32% complete responses. For patients with rituximab-refractory FL ≥2 prior therapies (n = 21), the overall response rate was 67%, and the complete response rate was 24%. The overall median duration of response was 13.6 months (32.0 months for patients with a complete response). 177Lu-lilotomab satetraxetan may provide a valuable alternative treatment approach in relapsed/refractory non-Hodgkin lymphoma, particularly in patients with comorbidities unsuitable for more intensive approaches. This trial was registered at www.clinicaltrials.gov as #NCT01796171.
Visual Abstract
Introduction
Non-Hodgkin lymphoma (NHL) comprises indolent and aggressive hematologic malignancies. Follicular lymphoma (FL) is the most common indolent subtype, alongside marginal zone lymphoma, small lymphocytic lymphoma and lymphoplasmacytic lymphoma (Waldenström macroglobulinemia). FL has an annual incidence of 3.4 to 5 per 100 000 in Europe and in the United States.1 With a median age at diagnosis of 65 years, FL has a protracted course with multiple remissions and relapses. Consequently, many patients in later-stage disease will be elderly or frail, limiting feasible treatment options.
The anti-CD20 monoclonal antibody rituximab, alone or in combination with chemotherapy, has revolutionized the treatment of B-cell NHL.2,3 However, refractory disease or early relapse (within 2 years) is observed in ≤20% of patients receiving immunochemotherapy, with early relapse in FL associated with particularly poor overall survival.4 Effective treatment options other than autologous stem cell transplant for patients with relapsed and rituximab-refractory disease are needed. The anti-CD20 antibody obinutuzumab is approved for rituximab-resistant FL in combination with bendamustine,5,6 and with very promising early data in combination with lenalidomide.7 Approaches such as B-cell receptor pathway–targeting agents (including phosphatidylinositol 3-kinase [PI3K] and Bruton tyrosine kinase [BTK] inhibitors) have yielded modest response rates8,9 but remain among the few available alternatives for heavily pretreated patients.
New options for relapsed/refractory FL are urgently needed, especially for the large cohort of elderly patients with comorbidities who cannot tolerate intensive chemotherapy. In this context, radioimmunotherapy (RIT) is underutilized. CD20-directed RIT via 131I-tositumomab (Bexxar) and 90Y-ibritumomab tiuxetan (Zevalin), with predosing comprising cold antibody and rituximab, has proved effective.10,11 In patients with relapsed or refractory NHL, 90Y-ibritumomab tiuxetan was superior to rituximab (overall response rate [ORR] 80% vs 56% [P = .002] and complete response rate [CRR] 30% vs 16% [P = .04], respectively). In rituximab-refractory patients, the ORR was 74%, CRR was 15%, and time to progression was 8.7 months for responders.12
Alternative targets are necessary to overcome resistance to anti-CD20–based therapy. CD37 is a highly glycosylated transmembrane protein selectively expressed by normal B cells and the majority of B-cell lymphomas,13-15 making it an attractive therapeutic target. 177Lu-lilotomab satetraxetan (Betalutin) consists of the anti-CD37 murine monoclonal antibody lilotomab conjugated to the chelator satetraxetan (p-SCN-benzyl-DOTA) that conjugates the β-emitting isotope 177Lu. 177Lu-lilotomab satetraxetan has been extensively investigated in preclinical models,15-17 and the radionuclide 177Lu has shown efficacy in clinical trials with various tumor types.18-22
This phase 1/2a dose-escalation and expansion study (LYMRIT-37-01; NCT01796171) investigated the safety, biodistribution, and pharmacokinetics (PK) of single-dose RIT with 177Lu-lilotomab satetraxetan in patients with relapsed indolent NHL. The most appropriate dosing regimen and maximum tolerated dose were assessed, and recommended doses and regimens for expansion into phase 2 were established to further evaluate the safety and efficacy of 177Lu-lilotomab satetraxetan.
Methods
Patients
Patients ≥18 years old with histologically confirmed (World Health Organization classification) relapsed/refractory indolent non-Hodgkin B-cell lymphoma (follicular grade I-IIIA, marginal zone, small lymphocytic or lymphoplasmacytic) or mantle cell lymphoma were included. The main inclusion criteria were prestudy World Health Organization performance status of 0 to 1 and life expectancy ≥3 months; <25% tumor cells in bone marrow biopsy; measurable disease by radiological methods; platelet count ≥150 × 109/L, absolute neutrophil count ≥1.5 × 109/L; and no central nervous system lymphoma, transformed disease, or prior stem cell transplantation. Patients with human anti-mouse antibodies (HAMA+) at baseline were excluded. CD37 expression in tumor collected prior to treatment was tested by immunohistochemistry using the antibody clone CT1 (mIgG1, Leica). No biopsy specimens were collected after administration of lilotomab and 177Lu-lilotomab satetraxetan.
The lilotomab satetraxetan conjugate was manufactured by conjugating lilotomab with the chelator satetraxetan (p-SCN-bezyl-DOTA, Macrocyclics, Plano, TX). For each patient, the conjugate is mixed with noncarrier added 177Lu (ITG, Garching, Germany) to produce the final 177Lu-lilotomab satetraxetan radioimmunoconjugate, supplied ready to use to centers. Doses were calculated using patients’ bodyweight on the day of administration and corrected for physical decay of 177Lu.
Study design
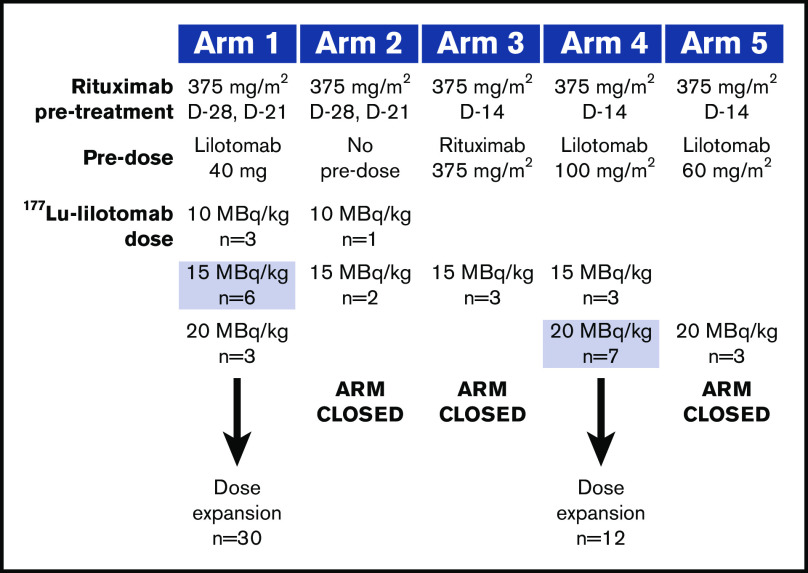
Initially, the study regimen was based on experiences from previous RIT and included pretreatment with rituximab for B-cell depletion to potentially optimize the biodistribution of 177Lu-lilotomab satetraxetan. The first (index) patient entered into the study received rituximab (Roche, Basel, Switzerland) 250 mg/m2 on days −7 and 0, with 10 MBq/kg 177Lu-lilotomab satetraxetan on day 0. Using dosimetry and safety data for the index patient, arm 1 and subsequent arms were designed to assess different pretreatment and predose regimens in a standard 3 + 3 study design (Figure 1). The dose of 177Lu-lilotomab satetraxetan was escalated if 0 out of 3 or 1 out of 6 dose-limiting toxicities (DLTs) were recorded for a cohort, and the cohort was expanded if 1 out of 3 DLTs were reported. Dose escalation was halted for ≥2 DLTs and arm closure decided by the safety review committee (SRC).
Figure 1.
Dose-escalation and expansion cohorts. Shaded doses selected for dose expansion. D, day.
Rituximab pretreatment was administered at either days −28 and −21 (arms 1 and 2) or day −14 (arms 3, 4, and 5). Predosing on day 0 with lilotomab (arms 1, 4, and 5) or rituximab (arm 3) or no predosing (arm 2) was tested to assess the impact of pre-emptively blocking the CD37 antigens (CD20 antigens in arm 3) of normal B lymphocytes on the biodistribution of 177Lu-lilotomab satetraxetan. Arm 4 was initiated to confirm whether a higher lilotomab predose would enable an increased 177Lu-lilotomab satetraxetan dose to be tolerated, and arm 5 was added to characterize the 20 MBq/kg dose and fully characterize the PK of 177Lu-lilotomab satetraxetan in addition to existing PK and dosimetry data.
Two dosing regimens from arms 1 and 4 were eventually selected for further investigation and patient enrollment in phase 2a. In the arm 1 phase 2a cohort an interim analysis was performed to review safety after 9 patients had been treated with 15 MBq/kg 177Lu-lilotomab satetraxetan.
Dosimetry
Serial whole-body or thorax/abdominal/other areas of known lesions single-photon emission computerized tomography (SPECT)/computed tomography (CT) was performed at intervals from 2 hours up to 7 days postdosing with 177Lu-lilotomab satetraxetan, with the aim of studying 3 patients at each dose level for the different pretreatment regimens. Tumor and bone marrow–absorbed doses were calculated from SPECT/CT images. A detailed description of the dosimetry methods has already been published.23-26 Volumes taken from CT images and radioactivity in tumors and lumbar vertebrae 2 to 4 derived from SPECT were used for the calculations.
Pharmacokinetics
177Lu-lilotomab satetraxetan PK was assessed by measuring the total radioactivity in blood using a γ counter. Blood samples were collected according to various schedules. For the first 2 patients, samples were collected before and 2.5, 5, 15, 30, 60, 90, and 120 minutes, 4, 8, and 20 hours, and 2, 3, 4, 7, and 28 days after administration of 177Lu-lilotomab satetraxetan). For subsequent patients, samples were collected before and 5, 60, and 120 minutes, 24 hours, and 2, 3, 4, 7, 14, 21, and 28 days after administration of 177Lu-lilotomab satetraxetan. Patients participating in the serial whole-body SPECT/CT study had additional samples collected 4 and 8 hours after 177Lu-lilotomab satetraxetan administration.
Total radioactivity in blood versus time was analyzed by PKxpert AB (Sweden) by noncompartmental analysis in Phoenix WinNonLin 64 version 8.1 build 8.1.0.3530 (Certara), using the “linear up log down” area under the curve method, and the 200-202 blood model. Maximum serum concentration and time to maximum serum concentration were taken directly from the activity-time profile.
Safety and DLTs
The SRC was responsible for dose-escalation decisions throughout the study. Adverse events (AEs) and serious AEs were collected via electronic case report forms from the signing of informed consent to 12 weeks after administration of 177Lu-lilotomab satetraxetan and then as reported to the investigator thereafter. AEs were graded according to NCI-CTCAE version 4.0.
DLTs were assessed during the first 12 weeks after administration and were initially defined as grade 4 hematologic toxicity that did not recover after 7 days, grade 3 hematologic toxicity that did not recover after 2 weeks, or grade ≥3 nonhematologic AEs at the discretion of the SRC. DLT criteria were later revised by the SRC to comprise grade 4 hematologic toxicity that did not recover to grade 3 within 7 days or bleeding due to thrombocytopenia, febrile neutropenia, failure of platelets or neutrophils to recover to grade 1 by 12 weeks after treatment, or grade ≥3 nonhematologic AEs per SRC review.
Immunogenicity assessment
Patients were monitored for the development of HAMAs after lilotomab and 177Lu-lilotomab satetraxetan administration using an in-house bridging assay, which used biotinylated and Eu-labeled lilotomab as solid-phase and tracer proteins, respectively, or the Milenia QuickLine HAMA test (Milenia Biotec). Blood samples were collected at 7 days and 1, 3, 6, and 12 months after lilotomab and 177Lu-lilotomab satetraxetan administration for patients enrolled in phase 1. Day 7 specimens were not collected for patients enrolled in phase 2a.
Efficacy
Responses were assessed periodically up to 5 years by fluorodeoxyglucose (FDG)-positron emission tomography (PET)/CT27 and contrast-enhanced CT28 (or magnetic resonance imaging for patients with allergy to CT contrast media). Baseline contrast-enhanced CT and FDG PET/CT scans were taken within 4 weeks prior to first rituximab infusion, and responses were assessed at 3 and 6 months after treatment by contrast-enhanced CT and FDG PET/CT. Repeat bone marrow biopsy was performed to confirm complete response (CR) if bone marrow biopsy was positive at baseline; progressive disease (PD) was confirmed by CT only. Follow-up CT scans were taken at 9, 12, 18, and 24 months and then every 6 months up to 5 years. Efficacy was assessed in terms of ORR (CR or partial response [PR]) at 3 months, and best ORR, progression-free survival (PFS), duration of response (DoR) and overall survival.
Ethics statement
The study protocol was approved by each hospital’s ethics committee and independent review board and/or regional ethics committees. All patients gave written, informed consent, and the study was conducted in accordance with the Declaration of Helsinki.
Results
Patients
Patients were enrolled to the study between December 2012 and December 2017 (phase 1) and October 2015 and March 2018 (phase 2a). All pretreatment biopsy specimens that were available for testing (65/74) stained positive for CD37. Patient demographics and baseline disease characteristics are shown in Table 1. The majority of patients in phase 1 and 2a had FL, and a considerable proportion (34% in phase 1 and 55% of additional patients in phase 2a) were rituximab-refractory. Patients had received a median of 2 prior therapies, including rituximab (n = 67 [91%]), alkylating agents (n = 60 [81%]), and bendamustine (n = 23 [31%]). Overall, 21 patients (28%) were refractory to their last line of therapy before study entry (n = 17 [30%] for patients with FL).
Table 1.
Patient baseline characteristics
| Phase 1 dose escalation | Phase 2a dose expansion | All FL patients | |
|---|---|---|---|
| n | 32 | 42 | 57 |
| Median age at study entry (range), y | 69.0 (38-88) | 68.0 (51-80) | 69.0 (38-80) |
| ≥65 y, n (%) | 24 (75.0) | 30 (71.4) | 41 (71.9) |
| Male, n (%) | 23 (71.9) | 18 (42.9) | 32 (56.1) |
| NHL subtype, n (%) | |||
| FL grade I | 10 (31.3) | 5 (11.9) | 15 (26.3) |
| FL grade II | 18 (56.3) | 15 (35.7) | 33 (57.9) |
| FL grade IIIa | 0 (0) | 9 (21.4) | 9 (15.8) |
| MZL | 1 (3.1) | 8 (19.0) | — |
| SLL | 0 (0) | 1 (2.4) | — |
| MCL | 3 (9.4) | 4 (9.5) | — |
| Bulky disease (>6 cm), n (%) | 10 (31.3) | 17 (40.5) | 22 (38.6) |
| BM involvement, n (%) | 7 (21.9) | 9 (21.4) | 11 (19.3) |
| Prior therapies | |||
| Median prior treatments, n (range) | 3 (1-6) | 2 (1-8) | 2 (1-7) |
| ≥2 prior regimens, n (%) | 23 (71.9) | 26 (61.9) | 38 (66.7) |
| Prior bendamustine, n (%) | 13 (40.6) | 10 (23.8) | 15 (26.3) |
| Refractory (SD or PD) to last therapy, n (%) | 9 (28.1) | 12 (28.6) | 17 (29.8) |
| Rituximab refractory, n (%) | 12 (37.5) | 19 (45.2) | 26 (45.6) |
BM, bone marrow; MCL, mantle cell lymphoma; MZL, marginal zone lymphoma; PD, progressive disease; SD, stable disease; SLL, small lymphocytic lymphoma.
Dosimetry
Some dosimetry and PK data have already been published for patients included in phase 1 of this study.23-26
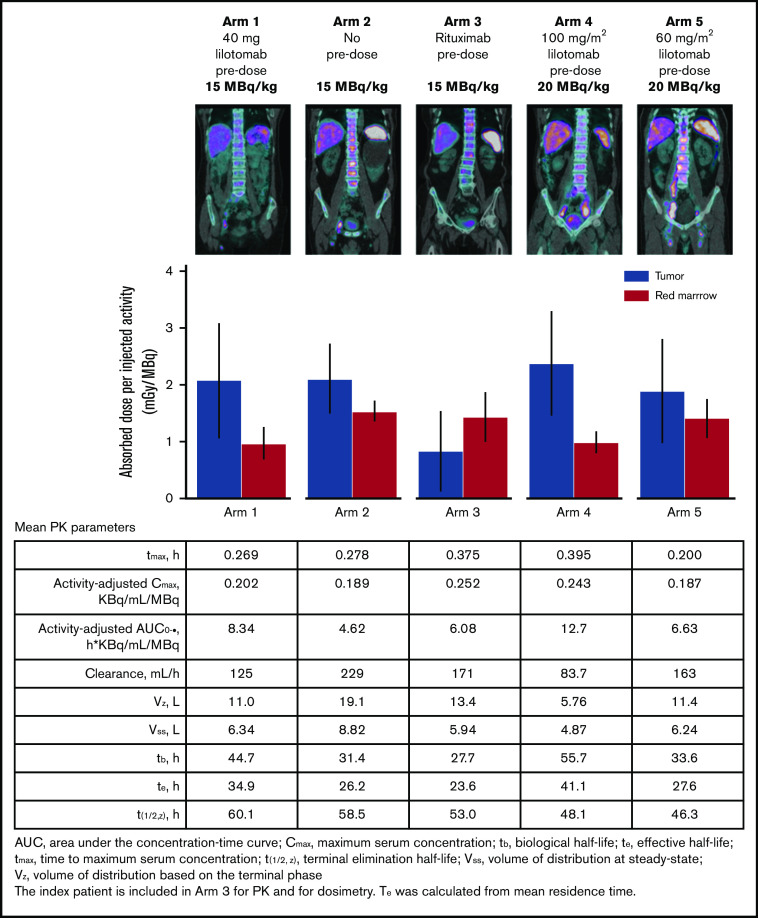
Figure 2 shows fused SPECT/CT images of activity at day 4 after 177Lu-lilotomab satetraxetan administration for 5 representative patients, the correlation between absorbed dose in red marrow and tumor, and PK profiles for 177Lu-lilotomab satetraxetan. Arms 1 and 4 showed the largest differences between tumor- and red marrow–absorbed dose. In contrast, arms 2 and 3 demonstrated the necessity of lilotomab as a predose; although arm 2 (with no predose) showed similar tumor absorption to arm 1, red marrow absorption was significantly higher, which correlated with the increased hematological toxicity reported for patients in arm 2. In arm 3 (rituximab predose), absorbed dose to red marrow was higher than that to tumor tissue. The differences in splenic uptake intensity with different lilotomab predoses vs no predose or rituximab are particularly noticeable, supporting the beneficial effect of predosing with lilotomab. These data correlate with the occurrence of DLTs observed in phase 1.
Figure 2.
SPECT, dosimetry, and PK comparison by study arm.
PK
177Lu-lilotomab satetraxetan PK was assessed as measurements of total radioactivity in blood for the index patient and patients enrolled in arm 1 (n = 9), arm 2 (n = 3), arm 3 (n = 3), arm 4 (n = 10), and arm 5 (n = 3) in the phase 1 part of the study. 177Lu-lilotomab satetraxetan blood clearance profiles of mean activity-adjusted radioactivity in blood are shown in supplemental Figure 1. Lilotomab predosing appeared to dose-dependently increase the activity-adjusted exposure (as area under the curve) of 177Lu-lilotomab satetraxetan (arm 4 > arm 1 > arm 5 > arm 3 > arm 2), with a direct impact on volume of distribution and blood clearance (Figure 2). Activity-adjusted exposure was highest (12.7 [KBq/mL]/MBq), and clearance lowest (83.7 mL/h), for arm 4. Furthermore, the biological half-life (tb) and effective half-life (te) were longest for arm 4, at 55.7 hours and 41.1 hours, respectively.
Phase 1 hematologic AEs and DLT
177Lu-lilotomab satetraxetan dose was escalated as far as 20 MBq/kg in arm 1 (with 40 mg lilotomab predosing) before being deescalated to 15 MBq/kg. DLTs during dose escalation for all study arms are shown in Table 2; arms 2 and 3 were closed due to occurrence of hematologic AEs, although only 1 patient in arm 3 experienced a DLT. For arm 4 with elevated lilotomab predosing (100 mg/m2), there was 1 DLT with 20 MBq/kg 177Lu-lilotomab satetraxetan. In conclusion, the biodistribution, tumor targeting, and hematologic toxicity profile of 177Lu-lilotomab satetraxetan was improved with lilotomab predosing compared with rituximab predosing or no predosing.
Table 2.
DLTs in phase 1 dose escalation
| Arm | Predose | 177Lu-lilotomab dose (MBq/kg) | DLT | Disease | |
|---|---|---|---|---|---|
| Index patient | Rituximab 250 mg/m2 | 10 | Thrombocytopenia | FL | |
| 1 | Lilotomab 40 mg | 10 | — | FL | |
| 10 | — | FL | |||
| 10 | — | FL | |||
| 15 | — | FL | |||
| 15 | — | FL | |||
| 15 | — | FL | |||
| 15 | Thrombocytopenia, neutropenia | FL | |||
| 15 | — | FL | |||
| 15 | Hyponatremia | FL | |||
| 20 | Neutropenia | FL | |||
| 20 | Epistaxis | MCL | |||
| 20 | Neutropenia | FL | |||
| 2 | No predose | 10 | — | FL | |
| 15 | Thrombocytopenia, neutropenia | FL | |||
| 15 | Thrombocytopenia | FL | |||
| 3 | Rituximab 375 mg/m2 | 15 | Thrombocytopenia, neutropenia | MZL | |
| 15 | — | FL | |||
| 15 | — | MCL | |||
| 4 | Lilotomab 100 mg/m2 | 15 | — | FL | |
| 15 | — | FL | |||
| 15 | — | FL | |||
| 20 | — | FL | |||
| 20 | — | FL | |||
| 20 | Hematuria with platelet count 40 × 109/L | FL | |||
| 20 | — | MCL | |||
| 20 | — | FL | |||
| 20 | — | FL | |||
| 20 | — | FL | |||
| 5 | Lilotomab 60 mg/m2 | 20 | — | FL | |
| 20 | — | FL | |||
| 20 | — | FL | |||
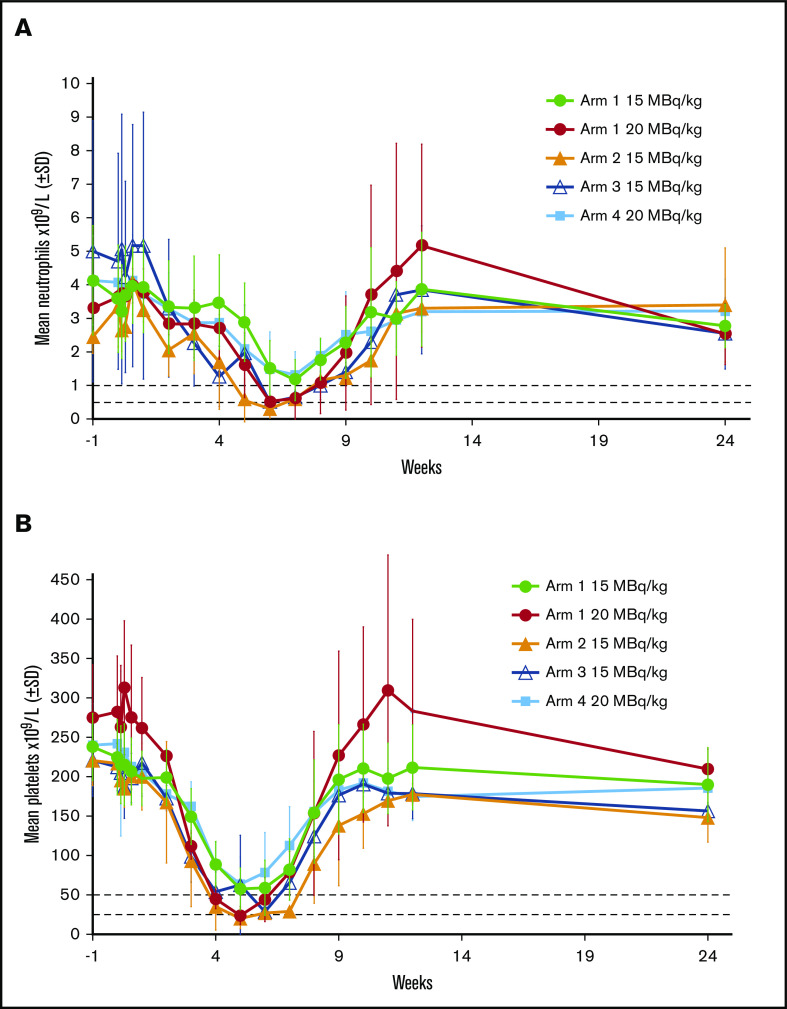
Mean neutrophil and platelet counts after dosing with 177Lu-lilotomab satetraxetan in phase 1 are shown in Figure 3. Nadirs for neutrophils (Figure 3A) and platelets (Figure 3B) with 15 MBq/kg 177Lu-lilotomab satetraxetan but no predose lilotomab (arms 2 and 3) were similar to those with 20 MBq/kg 177Lu-lilotomab satetraxetan in arm 1, and characteristic of grade 4 neutropenia and thrombocytopenia. Results for 15 MBq/kg in arm 1 and 20 MBq/kg in arm 4 were similar, for both platelets and absolute neutrophil counts. In all study arms, blood cell counts began to decrease from 2 weeks (platelets) and 4 weeks (neutrophils) after dosing, with recovery over the 4 to 5 weeks following the nadir.
Figure 3.
Effect of different phase 1 regimens on mean neutrophil and platelet counts. Horizontal dotted lines show cut-offs for grade 3 and 4 neutropenia (A) and thrombocytopenia (B).
Two regimens were selected for dose expansion in phase 2a: lilotomab 40 mg + 177Lu-lilotomab satetraxetan 15 MBq/kg (arm 1) and lilotomab 100 mg/m2 + 177Lu-lilotomab satetraxetan 20 MBq/kg (arm 4).
Phase 2a/overall AEs
Confirmatory safety data from the interim analysis of the first 15 patients to receive 15 MBq/kg 177Lu-lilotomab satetraxetan in arm 1 supported continuation of this regimen, and the general safety profile was consistent between phase 1 and phase 2a across both arms 1 and 4.
Grade 3 and 4 study drug-related treatment-emergent AEs occurring in ≥2 patients are shown in supplemental Table 1 and, as expected, primarily consisted of hematologic events. Nonhematologic events were predominantly of grade 1 or 2, with the most frequent being nausea (15.8%), upper respiratory tract infections (10.5%), and urinary tract infections (10.5%). The median duration of grade ≥3 neutropenia and thrombocytopenia was 14.0 days each. Although the overall frequencies of grade ≥3 neutropenia and thrombocytopenia were 31.6% and 26.3%, respectively, these were predominantly grade 3 events; clinically relevant grade 4 neutropenia and thrombocytopenia occurred in 11% and 8% of patients, respectively. Overall, 5 patients received platelet transfusions (2 for active bleeding and 3 as prophylaxis), and 3 patients received granulocyte colony-stimulating factor.
Fourteen patients experienced serious AEs; serious AEs in ≥2 patients comprised thrombocytopenia, atrial fibrillation, lymphoma progression, and sepsis (all n = 2), and there were no reports of febrile neutropenia. Thrombocytopenia events were considered related to study treatment. Both incidences of atrial fibrillation were of grade 2 and resolved within 24 hours with oral therapy (1 occurred 9 months after study drug administration). These events were considered possibly related to study treatment.
Two patients experienced AEs of special interest. One case of chronic myelomonocytic leukemia occurred 24 months after 177Lu-lilotomab satetraxetan administration (and 18 months after 6 courses of bendamustine-rituximab) and was fatal; this event was considered possibly related to study treatment. One case of prostate cancer diagnosed 6 months after 177Lu-lilotomab satetraxetan was not considered to be related.
Immunogenicity results
The development of a HAMA response after administration of lilotomab and 177Lu-lilotomab satetraxetan was reported for 7 out of 74 subjects overall. Five of the observed responses were detected 1 month after treatment; 3 had resolved at the 3-month visit and 1 at the 6-month visit (data not available for one patient). Two additional immune responses were detected at 12-month follow-up visits. No reported side effects could be associated with the development of HAMA.
Treatment efficacy
With a median follow-up of 24.5 months (range, 0.4-60.7 months), best ORR for all patients (N = 74) was 61% (n = 45; 22 CR [30%], 23 PR [31%], and 14 SD [19%]). For patients with FL (n = 57), ORR was 65% (n = 37; 17 CR [30%], 20 PR [35%], and 10 SD [18%]). Notably, nearly half of the clinical responses observed were CR in both the overall and FL populations. Patients with bulky disease (>6 cm; n = 27) had an ORR of 56%. For FL with ≥2 prior therapies (n = 37 evaluable), the ORR was 70% and the CRR 32%.
Of the total of 74 patients, 31 were classified as rituximab-refractory. Rituximab-refractoriness was defined as no response to single-agent rituximab (n = 6) or a rituximab-containing regimen (n = 8) or relapse/progression within 6 months (n = 3) or relapse/progression during rituximab maintenance (n = 14). For rituximab-refractory FL (n = 26), the ORR was 58%, with a CRR of 19%. For rituximab-refractory FL with ≥2 prior therapies (n = 21), the ORR was 67% and the CRR was 24%.
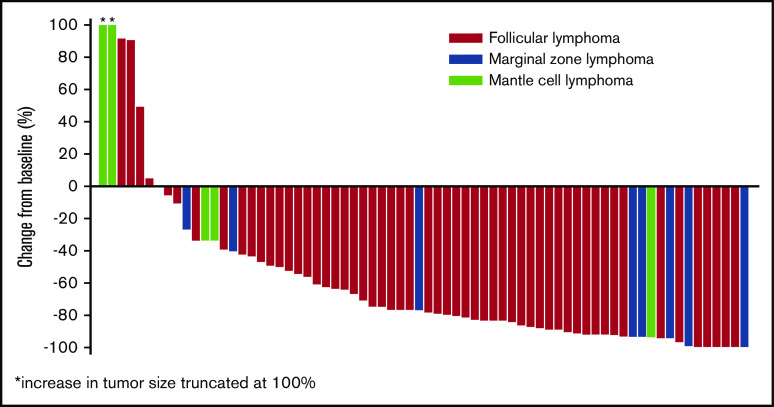
Response rates were similar in patients enrolled to phase 2a (n = 42), who received 177Lu-lilotomab satetraxetan 15 MBq/kg per arm 1 or 20 MBq/kg per arm 4; ORR was 64.3% (n = 27; 13 CR [31%], 14 PR [33%], and 7 SD [17%]). In rituximab-refractory patients (n = 19), the ORR was 53%, with a CRR of 21%. In patients with rituximab-refractory FL (n = 15), the ORR was 67% and the CRR was 27%. The majority of patients experienced a reduction in tumor size (Figure 4).
Figure 4.
Best percentage change in tumor size from baseline for evaluable patients in phases 1 and 2a.
Figure 5A shows DoR for all patients with PR or CR (n = 45) and those with CR only (n = 22). With a median follow-up time for responders of 30.0 months (range, 12.0-60.7 months), median DoR was 13.6 months (95% confidence interval [CI], 6.1, 20.5) for all responders and 32.0 months (95% CI, 14.5, 46.0) in patients achieving a CR. PFS is shown in Figure 5B. Median PFS was 8.8 months (95% CI, 6.0, 12.0) overall (n = 74) and 9.0 months (95% CI, 6.0, 15.7) in patients with FL (n = 57). Median PFS in patients without documented disease progression or death was 9.2 months overall (95% CI, 6.2, 17.7; n = 74) and 9.1 months in patients with FL (95% CI, 6.0, 17.3; n = 57).
Figure 5.
Duration of response and progression-free survival. (A) Kaplan-Meier estimate of DoR. (B) Kaplan-Meier estimate of PFS.
Discussion
This study evaluated the safety and recommended 177Lu-lilotomab satetraxetan/lilotomab regimen for further phase 2 evaluation. The single-administration regimen was administered safely with manageable toxicity. Hematologic AEs correlated well with both dosimetry and 177Lu-lilotomab satetraxetan PK data for the different study arms in phase 1 and were managed using standard therapy.
To optimize the delivery schedule for this novel RIT compound, we rigorously tested different regimens in the phase 1 part of this study and monitored PK, dose distribution, hematologic toxicity, and safety. Predosing with lilotomab was essential to mitigate hematologic toxicity; PK and dosimetry data showed reduced clearance and volume of distribution with lilotomab predosing, resulting in greater 177Lu-lilotomab satetraxetan targeting to tumor tissue. The nadir for blood cell counts occurred 5 to 7 weeks after dosing, later than would be expected with chemotherapy or immunochemotherapy. Mean neutrophil and platelet counts for patients in arms 1 (15 MBq/kg) and 4 (20 MBq/kg) remained above cutoffs indicative of grade 3 neutropenia and thrombocytopenia, while for arms 2 and 3, and the 20 MBq/kg dose in arm 1, neutrophil and platelet nadirs were near or below the levels defining grade 4 events. Correspondingly, grade 4 neutropenia and thrombocytopenia were relatively unusual in the phase 2a population, which received the arm 1 and 4 regimens.
One case of chronic myelomonocytic leukemia was observed in the study population occurring 24 months after 177Lu-lilotomab satetraxetan administration. The cumulative incidence of myelodysplastic syndrome/secondary leukemia reported for patients enrolled in Zevalin and Bexxar clinical trials is 5% to 10%.29 Since the majority of patients who received RIT had also received cytotoxic therapies, it is difficult to determine which of these treatments has primarily contributed to the occurrence of myelodysplastic syndrome and acute myeloid leukemia. However, continuous focus needs to be kept on this serious toxicity in patients treated with RIT, including 177Lu-lilotomab satetraxetan.
The ORR to 177Lu-lilotomab satetraxetan (61% in all patients and 65% in FL), particularly the high CRR (30% overall and in FL), were impressive in a cohort of heavily pretreated patients with recurrent indolent NHL, especially for those with FL histology. The importance of achieving a CR is clear given that median DoR for patients with CR (32 months) was considerably longer than the overall median DoR (13.6 months).
The ORR and CRR with 177Lu-lilotomab satetraxetan in the present study compare well with data for 90Y-ibritumomab tiuxetan RIT. In rituximab-naive patients with relapsed/refractory NHL, an ORR of 80% and CRR of 30% were reported,11 with an ORR of 74% and a CRR of 15% reported in a similar study in patients with rituximab-refractory FL.12 In these studies, although the ORR was maintained between populations, the reduction in CRR in rituximab-refractory disease was particularly noticeable, as reflected in a median DoR of 14.2 months in rituximab-naive patients and an estimated time to progression of 8.7 months in rituximab-refractory patients. Importantly, a combined analysis of 211 patients from 4 trials of 90Y-ibritumomab tiuxetan in relapsed/refractory NHL demonstrated long-term responses, with median DoR of 29 months in patients with CR or unconfirmed CR and a median time to progression of ≥12 months in 37% of patients.30 In this context, the median DoR of 13.6 months in the present study, with a mixed population of rituximab sensitivity, is noteworthy.
177Lu-lilotomab satetraxetan was granted Fast Track designation for relapsed/refractory FL by the US Food and Drug Administration in June 2018 based on efficacy and safety data from the present study31 and has since received a similar designation for relapsed/refractory marginal zone lymphoma.32 Alternative postchemotherapy options for patients with relapsed/rituximab-refractory NHL are currently limited. PI3K inhibitors have been associated with an ORR of 40% to 60%33 but with lower rates of CR (≤20%) than reported in the current study and significant nonhematologic toxicity. More recently, an ORR of 59% was shown with the pan-PI3K inhibitor copanlisib in relapsed indolent NHL and CRR of 12% to 14%.34 Similarly, overall responses to EZH2 inhibition in 76 heavily pretreated patients with FL have been promising (35%), but with low CRR (6%); in the cohort of 22 patients with an activating EZH2 mutation, ORR and CRR were 82% and 5%, respectively.35 The BTK inhibitor ibrutinib has also been investigated in relapsed/refractory FL with an ORR of 21% and CRR of 11%.36 These data highlight that deep responses with postchemotherapy agents are uncommon. Consequently, the comparatively high CRR to 177Lu-lilotomab satetraxetan and the high CRR and ORR reported for the combination of obinutuzumab and lenalidomide7 are potentially very important for this difficult-to-treat population. 177Lu-lilotomab satetraxetan therefore represents an attractive, well-tolerated alternative for a patient population that needs additional options. Furthermore, targeted RIT such as 177Lu-lilotomab satetraxetan would be an interesting partner in combinations with small-molecule inhibitors of EZH2, PI3K, BTK, and others for future development. 177Lu-lilotomab satetraxetan has been tested in combination with a panel of 384 small-molecule inhibitors, and cell-cycle kinase, topoisomerase, and histone deacetylase inhibitors emerged as potential combination partners.37 The cell-cycle kinase inhibitors JNJ-7706621, MK-1775, and PD-166285 increased the in vitro and in vivo therapeutic effect of 177Lu-lilotomab satetraxetan.37,38
RIT presents the possibility of long-term efficacy and a vital alternative option for patients with relapsed or refractory disease. 177Lu-lilotomab satetraxetan differs from prior RIT by using a different therapeutic isotope and, importantly, targeting CD37 rather than CD20. It is well placed to meet 2 current clinical needs: potential long-term efficacy in patients with recurrent disease and limited treatment options (in particular for patients refractory to CD20-directed therapy) and a highly convenient treatment with manageable and predictable toxicity. Single-dose treatment offers a meaningful improvement in quality of life for patients, especially older patients, for whom frequent hospital or clinic visits are challenging and who may have already experienced several lengthy cyclical cytotoxic regimens. The long half-life of 177Lu-lilotomab satetraxetan compared with 90Y-ibritumomab tiuxetan also simplifies logistical considerations, as this compound is prepared off site and delivered ready to use, unlike 90Y-ibritumomab, which must be coupled on site.
Two dose regimens were selected for further study: a lilotomab predose of 40 mg plus 15 MBq/kg 177Lu-lilotomab satetraxetan and a lilotomab predose of 100 mg/m2 plus 20 MBq/kg 177Lu-lilotomab satetraxetan. The global randomized phase 2b PARADIGME study is ongoing in patients with relapsed rituximab/anti-CD20 refractory FL who have received ≥2 prior therapies and will provide further data to inform the use of this novel drug.
In conclusion, 177Lu-lilotomab satetraxetan was demonstrated to be a promising, ready-to-use, single-dose RIT in heavily pretreated patients with B-cell NHL with low bone marrow infiltration and was well tolerated, with reversible uncomplicated grade 3/4 neutropenia and thrombocytopenia as the most common AEs, with limited nonhematologic toxicity. Encouraging responses, DoR, and PFS were observed in phases 1 and 2a. In particular, the preliminary efficacy observed in rituximab-refractory patients with FL warrants further investigation. RIT with 177Lu-lilotomab satetraxetan could represent an effective and convenient alternative treatment for patients with relapsed/refractory indolent NHL, a population that urgently needs effective and tolerable therapy options.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
LYMRIT-37-01 was sponsored by Nordic Nanovector ASA, Oslo, Norway.
The authors wish to thank the following Investigators for their contribution to the study: Martin Erlanson (Norrland University Hospital, Umeå, Sweden), Renate Galleberg (Haukeland University Hospital, Bergen, Norway), Roman Hájek (University Hospital Ostrava, Ostrava, Czech Republic), Ingemar Lagerlöf (University Hospital Linköping, Linköping, Sweden), and Ella Willenbacher (Medical University of Innsbruck, Innsbruck, Austria). Immunogenicity assessments were performed at Oslo University Hospital by Kari Hauge Olsen, Åge Winje Brustad, and Ann Magritt Liberg. Immunohistochemistry stainings for CD37 at Oslo University Hospital were performed by Elin Faye Borge and Inger Johanne Ryen. PK measurements were performed at Nordic Nanovector ASA by Katrine Brustad Melhus and Tatiana Reven. Assistance for study administration at Oslo University Hospital was provided by Stine Rudå Nygaard and Maren Hatteland Endresen. Medical writing assistance was provided by Martin Quinn (MQMedcomms, Nottingham, United Kingdom) and funded by Nordic Nanovector ASA, Oslo, Norway.
Footnotes
For original data, please contact jdahle@nordicnanovector.com. Individual participant data will not be shared.
Authorship
Contribution: A.K. and T.I. contributed to study conception and design, data collection and analysis, and manuscript preparation and review; H.H. contributed to study design, data collection, and manuscript review; C.S. and J.B. contributed to data collection and analysis and manuscript review; N.B., S.S., U.M., A.L., N.O., M. Beasley, W.J., U.-M.F., M.K., M. Bayne, and A.O. contributed to data collection, manuscript review; J.D. contributed to study conception and design, data collection and analysis (PK, immunogenicity, and dosimetry), and manuscript preparation and review; L.R. contributed to manuscript preparation and review; and V.P. contributed to bioanalytics data analysis and collection (PK and immunogenicity) and manuscript preparation and review.
Conflict-of-interest disclosure: A.K. declares advisory board participation for Nordic Nanovector ASA. T.I. declares advisory board participation for Nordic Nanovector ASA, Roche, and Takeda. S.S. declares lecture honoraria from Novartis and Sanofi Genzyme. W.J. declares research funding/grants from Nordic Nanovector ASA. U.-M.F. declares advisory board participation for Takeda and Roche. H.H. declares advisory board participation for Nordic Nanovector ASA, Novartis, Roche, Gilead, and Takeda and lecture honoraria from Novartis. J.D. and V.P. are employees of Nordic Nanovector ASA and owners of performance share units. J.D. also declares ownership of shares. L.R. was an employee of Nordic Nanovector ASA at the time of this study. The remaining authors declare no competing financial interests.
The current affiliation for L.R. is Viracta Therapeutics, San Diego, CA.
Correspondence: Arne Kolstad, Department of Oncology, Oslo University Hospital, Radiumhospitalet, Ullernchausseen 70, N-0379 Oslo, Norway; e-mail: arnek@ous-hf.no.
References
- 1.Matasar MJ, Luminari S, Barr PM, et al. Follicular lymphoma: recent and emerging therapies, treatment strategies, and remaining unmet needs. Oncologist. 2019;24(11):e1236-e1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16(8):2825-2833. [DOI] [PubMed] [Google Scholar]
- 3.Mohammed R, Milne A, Kayani K, Ojha U. How the discovery of rituximab impacted the treatment of B-cell non-Hodgkin’s lymphomas. J Blood Med. 2019;10:71-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casulo C, Byrtek M, Dawson KL, et al. Early relapse of follicular lymphoma after rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone defines patients at high risk for death: an analysis from the National Lymphocare Study. J Clin Oncol. 2015;33(23):2516-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sehn LH, Chua N, Mayer J, et al. Obinutuzumab plus bendamustine versus bendamustine monotherapy in patients with rituximab-refractory indolent non-Hodgkin lymphoma (GADOLIN): a randomised, controlled, open-label, multicentre, phase 3 trial. Lancet Oncol. 2016;17(8):1081-1093. [DOI] [PubMed] [Google Scholar]
- 6.Food and Drug Administration. Gazyva prescribing information revised 3/2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125486s025lbl.pdf. Accessed April 2020.
- 7.Fowler NH, Nastoupil LJ, Chin C, et al. A phase I/II study of lenalidomide plus obinutuzumab in relapsed indolent lymphoma [abstract]. Blood. 2019;134(suppl 1):348. Abstract 623. [Google Scholar]
- 8.Rodgers TD, Reagan PM. Targeting the B-cell receptor pathway: a review of current and future therapies for non-Hodgkin’s lymphoma. Expert Opin Emerg Drugs. 2018;23(2):111-122. [DOI] [PubMed] [Google Scholar]
- 9.Valla K, Flowers CR, Koff JL. Targeting the B cell receptor pathway in non-Hodgkin lymphoma. Expert Opin Investig Drugs. 2018;27(6):513-522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaminski MS, Zelenetz AD, Press OW, et al. Pivotal study of iodine I 131 tositumomab for chemotherapy-refractory low-grade or transformed low-grade B-cell non-Hodgkin’s lymphomas. J Clin Oncol. 2001;19(19):3918-3928. [DOI] [PubMed] [Google Scholar]
- 11.Witzig TE, Gordon LI, Cabanillas F, et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(10):2453-2463. [DOI] [PubMed] [Google Scholar]
- 12.Witzig TE, Flinn IW, Gordon LI, et al. Treatment with ibritumomab tiuxetan radioimmunotherapy in patients with rituximab-refractory follicular non-Hodgkin’s lymphoma. J Clin Oncol. 2002;20(15):3262-3269. [DOI] [PubMed] [Google Scholar]
- 13.Link MP, Bindl J, Meeker TC, et al. A unique antigen on mature B cells defined by a monoclonal antibody. J Immunol. 1986;137(9):3013-3018. [PubMed] [Google Scholar]
- 14.Schwartz-Albiez R, Dörken B, Hofmann W, Moldenhauer G. The B cell-associated CD37 antigen (gp40-52). Structure and subcellular expression of an extensively glycosylated glycoprotein. J Immunol. 1988;140(3):905-914. [PubMed] [Google Scholar]
- 15.Dahle J, Repetto-Llamazares AHV, Mollatt CS, et al. Evaluating antigen targeting and anti-tumor activity of a new anti-CD37 radioimmunoconjugate against non-Hodgkin’s lymphoma. Anticancer Res. 2013;33(1):85-95. [PubMed] [Google Scholar]
- 16.Repetto-Llamazares AH, Larsen RH, Patzke S, et al. Targeted cancer therapy with a novel anti-CD37 beta-particle emitting radioimmunoconjugate for treatment of non-Hodgkin lymphoma. PLoS One. 2015;10(6):e0128816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Repetto-Llamazares AHV, Malenge MM, O’Shea A, et al. Combination of 177 Lu-lilotomab with rituximab significantly improves the therapeutic outcome in preclinical models of non-Hodgkin’s lymphoma. Eur J Haematol. 2018;101(4):522-531. [DOI] [PubMed] [Google Scholar]
- 18.Forrer F, Chen J, Fani M, et al. In vitro characterization of (177)Lu-radiolabelled chimeric anti-CD20 monoclonal antibody and a preliminary dosimetry study. Eur J Nucl Med Mol Imaging. 2009;36(9):1443-1452. [DOI] [PubMed] [Google Scholar]
- 19.Sierra ML, Agazzi A, Bodei L, et al. Lymphocytic toxicity in patients after peptide-receptor radionuclide therapy (PRRT) with 177Lu-DOTATATE and 90Y-DOTATOC. Cancer Biother Radiopharm. 2009;24(6):659-665. [DOI] [PubMed] [Google Scholar]
- 20.Claringbold PG, Brayshaw PA, Price RA, Turner JH. Phase II study of radiopeptide 177Lu-octreotate and capecitabine therapy of progressive disseminated neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2011;38(2):302-311. [DOI] [PubMed] [Google Scholar]
- 21.Kunikowska J, Królicki L, Hubalewska-Dydejczyk A, Mikołajczak R, Sowa-Staszczak A, Pawlak D. Clinical results of radionuclide therapy of neuroendocrine tumours with 90Y-DOTATATE and tandem 90Y/177Lu-DOTATATE: which is a better therapy option? Eur J Nucl Med Mol Imaging. 2011;38(10):1788-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Forrer F, Oechslin-Oberholzer C, Campana B, et al. Radioimmunotherapy with 177Lu-DOTA-rituximab: final results of a phase I/II Study in 31 patients with relapsing follicular, mantle cell, and other indolent B-cell lymphomas. J Nucl Med. 2013;54(7):1045-1052. [DOI] [PubMed] [Google Scholar]
- 23.Blakkisrud J, Løndalen A, Martinsen AC, et al. Tumor-absorbed dose for non-Hodgkin lymphoma patients treated with the anti-CD37 antibody radionuclide conjugate 177Lu-lilotomab satetraxetan. J Nucl Med. 2017;58(1):48-54. [DOI] [PubMed] [Google Scholar]
- 24.Blakkisrud J, Løndalen A, Dahle J, et al. Red marrow-absorbed dose for non-Hodgkin lymphoma patients treated with 177Lu-lilotomab satetraxetan, a novel anti-CD37 antibody-radionuclide conjugate. J Nucl Med. 2017;58(1):55-61. [DOI] [PubMed] [Google Scholar]
- 25.Blakkisrud J, Holtedahl JE, Løndalen A, et al. Biodistribution and dosimetry results from a phase 1 trial of therapy with the antibody-radionuclide conjugate 177Lu-lilotomab satetraxetan. J Nucl Med. 2018;59(4):704-710. [DOI] [PubMed] [Google Scholar]
- 26.Stokke C, Blakkisrud J, Løndalen A, et al. Pre-dosing with lilotomab prior to therapy with 177Lu-lilotomab satetraxetan significantly increases the ratio of tumor to red marrow absorbed dose in non-Hodgkin lymphoma patients. Eur J Nucl Med Mol Imaging. 2018;45(7):1233-1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheson BD, Pfistner B, Juweid ME, et al. ; International Harmonization Project on Lymphoma . Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579-586. [DOI] [PubMed] [Google Scholar]
- 28.Cheson BD, Horning SJ, Coiffier B, et al. ; NCI Sponsored International Working Group . Report of an international workshop to standardize response criteria for non-Hodgkin’s lymphomas. J Clin Oncol. 1999;17(4):1244. [DOI] [PubMed] [Google Scholar]
- 29.Sachpekidis C, Jackson DB, Soldatos TG. Radioimmunotherapy in non-Hodgkin’s lymphoma: Retrospective adverse event profiling of Zevalin and Bexxar. Pharmaceuticals (Basel). 2019;12(4):E141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witzig TE, Molina A, Gordon LI, et al. Long-term responses in patients with recurring or refractory B-cell non-Hodgkin lymphoma treated with yttrium 90 ibritumomab tiuxetan. Cancer. 2007;109(9):1804-1810. [DOI] [PubMed] [Google Scholar]
- 31.Nordic Nanovector. 2018. Regulatory designations. https://www.nordicnanovector.com/node/324. Accessed March 2020.
- 32.Nordic Nanovector. 2020. Press releases. https://www.nordicnanovector.com/investors-and-media/press-releases?page=/en/pressreleases/nordic-nanovector%2527s-betalutin%2528r%2529-receives-fast-track-designation-from-us-fda-for-marginal-zone-lymphoma-1822545. Accessed July 2020.
- 33.Batlevi CL, Younes A. Revival of PI3K inhibitors in non-Hodgkin’s lymphoma. Ann Oncol. 2017;28(9):2047-2049. [DOI] [PubMed] [Google Scholar]
- 34.Krause G, Hassenrück F, Hallek M. Copanlisib for treatment of B-cell malignancies: the development of a PI3K inhibitor with considerable differences to idelalisib. Drug Des Devel Ther. 2018;12:2577-2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morschhauser F, Tilly H, Chaidos A, et al. Interim update from a phase 2 multicenter study of tazemetostat, an EZH2 inhibitor, in patients with relapsed or refractory (R/R) follicular lymphoma (FL) [oral presentation at EHA23 2018]. Abstract S100. https://library.ehaweb.org/eha/2018/stockholm/214434/gilles.salles.interim.update.from.a.phase.2.multicenter.study.of.tazemetostat.html?f=topic=1574*media=3%27. Accessed April 2020.
- 36.Gopal AK, Schuster SJ, Fowler NH, et al. Ibrutinib as treatment for patients with relapsed/refractory follicular lymphoma: Results from the open-label, multicenter, phase II DAWN study. J Clin Oncol. 2018;36(23):2405-2412. [DOI] [PubMed] [Google Scholar]
- 37.Rødland GE, Melhus K, Generalov R, et al. The dual cell cycle kinase inhibitor JNJ-7706621 reverses resistance to CD37-targeted radioimmunotherapy in activated B cell like diffuse large B cell lymphoma cell lines. Front Oncol. 2019;9:1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pichard A, Marcatili S, Karam J, et al. The therapeutic effectiveness of 177Lu-lilotomab in B-cell non-Hodgkin lymphoma involves modulation of G2/M cell cycle arrest. Leukemia. 2020;34(5):1315-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.