Key Points
Multiple- and single-dose subcutaneous rozanolixizumab infusions were effective and well tolerated in patients with primary ITP.
Single rozanolixizumab infusion increased platelet count and decreased IgG in patients with primary ITP by day 8 without serious infection.
Abstract
Primary immune thrombocytopenia (ITP) is a predominantly immunoglobulin G (IgG)-autoantibody-mediated disease characterized by isolated thrombocytopenia. Rozanolixizumab, a subcutaneously infused humanized monoclonal anti-neonatal Fc receptor (FcRn) antibody, reduced serum IgG in healthy volunteers. In this phase 2, multicenter, open-label study, patients with persistent/chronic primary ITP received 1 to 5 once-weekly subcutaneous infusions of rozanolixizumab (cumulative doses, 15-21 mg/kg). Primary objectives were safety and tolerability, and secondary objectives were clinical efficacy (change in platelet count) and pharmacodynamic effect (change in IgG). In all, 51 (77.3%) of 66 patients reported 1 or more adverse events (AEs), all mild-to-moderate, most commonly headaches (26 [39.4%] of 66), of which 15 were treatment related. Four patients had serious AEs, but none were treatment related. No AEs resulted in discontinuation of the study drug. No serious infections occurred. Platelet counts of ≥50 × 109/L were achieved at least once at any time after multiple infusions (5 × 4, 3 × 7, or 2 × 10 mg/kg: 35.7%, 35.7%, and 45.5% of patients, respectively) or single infusions (15 or 20 mg/kg: 66.7% and 54.5% patients, respectively). Minimum mean IgG levels and maximum mean platelet counts both occurred by day 8 in the higher (15 and 20 mg/kg) single-dose cohorts and maximum platelet count occurred by day 11 onward in the multiple-dose cohorts. No clinically meaningful changes occurred in IgA, IgM, IgE, or albumin levels. In patients with persistent/chronic primary ITP, rozanolixizumab demonstrated a favorable safety profile and rapid, substantial platelet increases concordant with substantial IgG reductions, especially with single doses. By day 8, in the 15 and 20 mg/kg single-dose cohorts, >50% patients achieved clinically relevant platelet responses (≥50 × 109/L), coinciding with the lowest mean IgG levels. These data support phase 3 development of rozanolixizumab in persistent/chronic primary ITP. This trial was registered at www.clinicaltrials.gov as #NCT02718716.
Visual Abstract
Introduction
In 1964, Brambell et al1 hypothesized that immunoglobulin G (IgG) was recycled. Almost 2 decades later, the receptor responsible for IgG recycling was identified in neonatal rodent gut and was cloned and named the neonatal Fc receptor (FcRn).2,3 The roles of FcRn in prolonging the half-lives of IgG and albumin and in the transfer of passive humoral immunity from mother to fetus4,5 are well characterized (supplemental Figure 1). Greater understanding of FcRn biology has resulted in developments aimed at either increasing the half-life of therapeutic monoclonal antibodies and other fusion proteins or reducing the half-life of pathogenic IgG autoantibodies in autoimmune diseases.5 The latter approach offers a new treatment option for autoimmune diseases by selectively reducing total serum IgG, including IgG autoantibodies.
Rozanolixizumab, a subcutaneously infused humanized monoclonal antibody, specifically targets the IgG-binding region of FcRn. By blocking binding of IgG to FcRn, rozanolixizumab reduces IgG recycling, accelerates its lysosomal degradation, and lowers IgG levels.6,7 Rozanolixizumab has no effect on IgA, IgM, or IgE levels (albumin levels remain within normal range7) and does not affect IgG synthesis. In a recent phase 2 study in patients with myasthenia gravis (MG; NCT03052751), rozanolixizumab lowered pathogenic IgG autoantibody concentrations and demonstrated clinically meaningful improvements in MG outcomes,8 leading to an ongoing phase 3 trial (NCT03971422).
Primary immune thrombocytopenia (ITP)9 is an autoimmune, autoantibody-mediated disorder that typically manifests with bleeding and/or bruising with isolated thrombocytopenia.10-12 The primary drivers of ITP pathophysiology are pathogenic IgG autoantibodies,13 which target platelet surface glycoprotein (GP) complexes (eg, GPIIb/GPIIIa, GPIb/GPIX, and GPIa/GPIIa).14,15 These antibodies are thought to also target megakaryocytes, resulting in both accelerated platelet clearance and decreased platelet production.16-18 In addition to bleeding risks, another clinical feature of ITP is a paradoxically increased rate of thrombosis.19 These clinical features are combined with the long-term nature and high burden of both ITP and its treatments with substantially impaired quality of life for affected patients.20,21
Here we report final phase 2 data describing safety, tolerability, efficacy, and pharmacodynamic effects of single and multiple dosing regimens of rozanolixizumab in patients with persistent/chronic primary ITP. Comparability of different regimens was fostered by similar total doses of rozanolixizumab in each study cohort (lower-dose cohorts received more once-weekly infusions).
Methods
Study design
This 38-center, open-label, single- and multiple-dose subcutaneous infusion study (TP0001; NCT02718716) evaluated safety, tolerability, efficacy, and pharmacodynamic effect of rozanolixizumab in patients with persistent/chronic primary ITP. An initial screening period (up to 4 weeks) was followed by a dosing period (days 1-29), and an 8-week observation period, which began after patients in each cohort received their last dose (Figure 1). The study was conducted in accordance with independent ethics committee and institutional review board approvals, International Council for Harmonization Guidance for Good Clinical Practice, and the Declaration of Helsinki. Oversight of safety data was provided by a Data Monitoring Committee (DMC). Analysis of outcome data was performed by the authors. All authors had access to primary clinical trial data.
Figure 1.

TP0001 study design. Star denotes occurrence of Data Monitoring Committee meeting: safety data review of at least 6 patients in each dose cohort occurred before opening subsequent dose cohorts. In total, 127 patients were screened, 61 patients did not qualify according to the screening results (1 because of an AE, 48 because of ineligibility, 3 because consent was withdrawn, and 9 for other reasons). Overall, 66 patients were enrolled of whom 65 (98.5%) completed the study; 1 patient discontinued because of lack of efficacy. SC, subcutaneous.
Patients
Eligible patients were age 18 years or older, had primary ITP for at least 3 months, and had central laboratory platelet count determinations <30 × 109/L for screening. A local laboratory platelet count <35 × 109/L (baseline; supplemental Table 1) was required on day 1 of treatment. Patients must have previously responded to at least 1 ITP therapy; patients provided written informed consent before participation. Exclusion criteria included Karnofsky performance status <60% and IgG level ≤6 g/L. Supplemental Methods lists complete inclusion and exclusion criteria.
Treatment
Patients were sequentially allocated to 1 of 5 dose cohorts to receive either multiple once-weekly subcutaneous infusions of rozanolixizumab (5 × 4 mg/kg [cumulative dose, 20 mg/kg], 3 × 7 mg/kg [cumulative dose, 21 mg/kg], 2 × 10 mg/kg [cumulative dose, 20 mg/kg]) or a single subcutaneous infusion of rozanolixizumab (15 mg/kg or 20 mg/kg) (Figure 1). Infusion volumes were 10 to 30 mL, depending on dose, and were administered over 30 to 96 minutes. The DMC reviewed safety data for each cohort, and when the DMC was satisfied regarding safety, it recommended opening the next dose cohort (Figure 1).
Rescue or prohibited medication
Concomitant treatments prohibited during the study included thrombopoeitin-receptor agonists (TPO-RA), intravenous immunoglobulin (IVIg), rituximab, plasma exchange, immunoadsorption, and dexamethasone. Rescue medication was defined as platelet transfusion or treatment with commercially available IVIg, plasma exchange, dexamethasone, or rituximab. Patients who received those medications were excluded from the efficacy analyses from that date onward. If patients were taking rescue or prohibited medications that might potentially affect platelet counts, only the platelet data up to the start date of rescue medication were used for analyses.
Study objectives
The primary objective was to evaluate safety and tolerability of 5 dosing regimens of subcutaneous rozanolixizumab infusion in patients with persistent/chronic primary ITP. Secondary objectives assessed clinical efficacy (change in platelet count, ie, increase) and pharmacodynamic effect (change in IgG levels, ie, decrease) of rozanolixizumab. Change in bleeding score and emergence of anti-drug antibodies (ADAs) were exploratory objectives.
Study variables
The primary safety variable was occurrence of treatment-emergent adverse events (AEs), defined as AEs starting after the first rozanolixizumab infusion up to and including 8 weeks after the final infusion (referred to as AEs hereafter). Efficacy variables included number of patients achieving 1 platelet count of ≥50 × 109/L (referred to as a clinically relevant response), time to achievement of this count, and duration of platelet counts ≥50 × 109/L. All study platelet counts were from measurements made by a central laboratory, except for the baseline measurement at the time of randomization and first infusion, which used local laboratory platelet counts (central laboratory counts were also determined, resulting in 2 simultaneous platelet counts). Pharmacodynamic variables included change and maximum decrease from baseline for serum IgG levels. Other variables included change from baseline in IgA, IgM, IgE, and albumin levels, and serial measurements of C-reactive protein (CRP) and ADA.
Statistical methods
Full details of individual analysis sets are available in the supplemental Data. Statistical analyses were performed by dose cohort. Efficacy, pharmacodynamics, and other variables were summarized using descriptive statistics. SAS version 9.2 was used for data analysis. ITP bleeding score was assessed using the ITP bleeding assessment tool (ITP-BAT version 1.0).22
Results
Study population
In all, 66 adults with persistent/chronic primary ITP were enrolled: 15 in each of the 4- and 7-mg/kg cohorts (cumulative dose, 20 mg/kg and 21 mg/kg, respectively) and 12 in each of the 10-, 15-, and 20-mg/kg cohorts (cumulative dose, 20 mg/kg, 15 mg/kg, and 20 mg/kg, respectively). Sixty-five (98.5%) of 66 patients completed the study; 1 discontinued treatment on day 8 after 1 infusion of 4 mg/kg because of an acute clinical need to raise platelets. All patients were included in the safety set and full analysis set, whereas 1 patient each in the 4- and 10-mg/kg cohorts were excluded from the per protocol set (n = 64) and pharmacodynamic per protocol set (n = 64) because of prohibited medication and/or procedural noncompliance.
At baseline, patients’ median age was 54.0 years (range, 20-86 years); 63.6% were female. Patients had a median duration of ITP of 5.8 years (range, 0.3-36.2 years) and had received a median of 4.0 previous treatments (range, 1-16) (Table 1). Previous treatments included systemic corticosteroids (65.2%), TPO-RAs (overall, 39.4%; eltrombopag, 30.3%; romiplostim, 27.3%), immunoglobulins (37.9%), splenectomy (28.8%), and rituximab (15.2%) (Table 1; supplemental Table 2). Concomitant medications included systemic corticosteroids (43.9%), paracetamol (36.4%), etamsilate (22.7%), tranexamic acid (18.2%), omeprazole (15.2%), ferrous sulfate (12.1%), azathioprine (10.6%), and indapamide (10.6%) (supplemental Table 2). On the basis of central laboratory measurements (average of 3-day turnaround time from sample collection to result reporting), patients had a median baseline platelet count of 15.5 × 109/L (range, 4-53 × 109/L). Two patients had central laboratory platelet counts ≥35 × 109/L and local laboratory baseline platelet counts of <35 × 109/L with a variance of >15 × 109/L; on the basis of local platelet count (per inclusion criteria), these 2 patients were eligible.
Table 1.
Baseline patient demographics and disease characteristics (safety set)
| Characteristic | Rozanolixizumab | |||||
|---|---|---|---|---|---|---|
| Single-dose cohorts | Multiple-dose cohorts | All patients | ||||
| 20 mg/kg (n = 12) | 15 mg/kg (n = 12) | 2 × 10 mg/kg (n = 12) | 3 × 7 mg/kg (n = 15) | 5 × 4 mg/kg (n = 15) | (N = 66) | |
| Median age (range), y | 60.5 (25-78) | 49.5 (23-69) | 46.0 (23-69) | 54.0 (20-73) | 66.0 (21-86) | 54.0 (20-86) |
| Sex, n (%) | ||||||
| Female | 9 (75.0) | 7 (58.3) | 7 (58.3) | 11 (73.3) | 8 (53.3) | 42 (63.6) |
| Male | 3 (25.0) | 5 (41.7) | 5 (41.7) | 4 (26.7) | 7 (46.7) | 24 (36.4) |
| Ethnicity, n (%) * | ||||||
| Asian | 0 | 0 | 0 | 0 | 2 (13.3) | 2 (3.0) |
| White | 12 (100) | 12 (100) | 12 (100) | 15 (100) | 13 (86.7) | 64 (97.0) |
| Platelet count, × 10 9 /L † | ||||||
| Median (range) | 19.0 (4-37) | 22.5 (6-38) | 14.0 (6-53) | 11.0 (5-24) | 15.0 (5-36) | 15.5 (4-53) |
| Mean (SD) | 18.0 (10.8) | 21.2 (9.3) | 18.4 (13.8) | 13.4 (5.4) | 18.4 (10.7) | 17.7 (10.2) |
| Median duration of disease (range), y | 4.9 (0.4-10.7) | 5.8 (0.5-24.1) | 8.1 (0.4-30.8) | 5.2 (0.3-36.2) | 7.1 (1.7-28.6) | 5.8 (0.3-36.2) |
| No. of previous lines of ITP therapy (%) | 10 (83.3) | 8 (66.7) | 11 (91.7) | 12 (80.0) | 13 (86.7) | 54 (81.8) |
| Median (range) | 2.0 (1-8) | 3.0 (1-8) | 2.0 (1-6) | 6.5 (1-15) | 6.0 (1-16) | 4.0 (1-16) |
| Treatment or drug, n (%) ‡ | ||||||
| Immunoglobulins | 2 (16.7) | 5 (41.7) | 3 (25.0) | 7 (46.7) | 8 (53.3) | 25 (37.9) |
| Azathioprine | 3 (25.0) | 4 (33.3) | 3 (25.0) | 6 (40.0) | 8 (53.3) | 24 (36.4) |
| Eltrombopag | 1 (8.3) | 1 (8.3) | 5 (41.7) | 7 (46.7) | 6 (40.0) | 20 (30.3) |
| Splenectomy | 3 (25.0) | 2 (16.7) | 2 (16.7) | 7 (46.7) | 5 (33.3) | 19 (28.8) |
| Romiplostim | 2 (16.7) | 1 (8.3) | 2 (16.7) | 6 (40.0) | 7 (46.7) | 18 (27.3) |
| Cyclosporin | 1 (8.3) | 3 (25.0) | 1 (8.3) | 4 (26.7) | 5 (33.3) | 14 (21.2) |
| Dexamethasone | 2 (16.7) | 1 (8.3) | 2 (16.7) | 4 (26.7) | 3 (20.0) | 12 (18.2) |
| Danazol | 1 (8.3) | 2 (16.7) | 1 (8.3) | 2 (13.3) | 5 (33.3) | 11 (16.7) |
| Rituximab | 1 (8.3) | 1 (8.3) | 0 | 6 (40.0) | 2 (13.3) | 10 (15.2) |
| Fostamatinib | 0 | 0 | 1 (8.3) | 1 (6.7) | 6 (40.0) | 8 (12.1) |
| Prednisolone | 3 (25.0) | 1 (8.3) | 1 (8.3) | 0 | 3 (20.0) | 8 (12.1) |
SD, standard deviation.
The study was open to patients of all ethnic groups.
Based upon central laboratory measurements.
Data are shown based upon a data cutoff of >12% for the All patients column.
Safety and tolerability
Fifty-one (77.3%) of 66 patients reported ≥1 AE, most commonly headache (26 [39.4%]), diarrhea (8 [12.1%]), and vomiting (6 [9.1%]), all of which were mild-to-moderate in intensity (Table 2). Nineteen (28.8%) of 66 patients reported treatment-related AEs, including headache (15 [22.7%]), vomiting (5 [7.6%]), and diarrhea (4 [6.1%]) (Table 2; supplemental Table 3). Four (6.1%) serious AEs were reported: genital hemorrhage (4 mg/kg; severe), 2 cases of severe thrombocytopenia (1 each in the 10- and 15-mg/kg cohorts), and platelet count decrease (15 mg/kg; moderate). All serious AEs occurred in the context of the underlying disease (primary ITP), and none were considered treatment related. No AEs led to study drug discontinuation, and no deaths occurred during or after treatment with rozanolixizumab.
Table 2.
AE profile for the safety set
| AEs | Rozanolixizumab | |||||
|---|---|---|---|---|---|---|
| Single-dose cohorts | Multiple-dose cohorts | All patients | ||||
| 20 mg/kg (n = 12) | 15 mg/kg (n = 12) | 2 × 10 mg/kg (n = 12) | 3 × 7 mg/kg (n = 15) | 5 × 4 mg/kg (n = 15) | (N = 66) | |
| Any AE | 12 (100) | 11 (91.7) | 7 (58.3) | 9 (60.0) | 12 (80.0) | 51 (77.3) |
| Serious AEs | 0 | 2 (16.7) | 1 (8.3) | 0 | 1 (6.7) | 4 (6.1) |
| AEs related to rozanolixizumab | 9 (75.0) | 7 (58.3) | 1 (8.3) | 1 (6.7) | 1 (6.7) | 19 (28.8) |
| Severe AEs | 0 | 1 (8.3) | 1 (8.3) | 0 | 1 (6.7) | 3 (4.5) |
| Discontinuations as a result of AEs | 0 | 0 | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 | 0 | 0 |
| Most common AEs * | ||||||
| Headache | 9 (75.0) | 5 (41.7) | 3 (25.0) | 6 (40.0) | 3 (20.0) | 26 (39.4) |
| Diarrhea | 2 (16.7) | 2 (16.7) | 1 (8.3) | 2 (13.3) | 1 (6.7) | 8 (12.1) |
| Vomiting | 4 (33.3) | 2 (16.7) | 0 | 0 | 0 | 6 (9.1) |
| Pyrexia | 3 (25.0) | 1 (8.3) | 1 (8.3) | 0 | 0 | 5 (7.6) |
| Upper respiratory tract infection | 0 | 1 (8.3) | 1 (8.3) | 1 (6.7) | 1 (6.7) | 4 (6.1) |
| Most common AEs related to rozanolixizumab * † | ||||||
| Headache | 8 (66.7) | 5 (41.7) | 1 (8.3) | 1 (6.7) | 0 | 15 (22.7) |
| Vomiting | 3 (25.0) | 2 (16.7) | 0 | 0 | 0 | 5 (7.6) |
| Diarrhea | 2 (16.7) | 2 (16.7) | 0 | 0 | 0 | 4 (6.1) |
All data are n (%). All AEs were treatment-emergent occurring during the study after ≥1 rozanolixizumab infusions. All AEs were coded by using the Medical Dictionary for Regulatory Activities (MedDRA; version 21.1) and categorized by severity according to Common Terminology Criteria for Adverse Events (CTCAE; version 4.03) grading or, in the absence of CTCAE, according to intensity (mild, moderate, severe).
Based on a cutoff of 5% in the All patients column.
AEs that were considered related to treatment by the investigator.
Clinical chemistry and other laboratory safety findings.
Albumin levels remained within the normal range for all patients (maximum mean decrease of 4.5% observed on day 8 [20 mg/kg], which returned to baseline levels). Transient elevations in CRP >10 mg/L were observed in 10 (15.2%) of 66 patients (supplemental Table 4); there were no patterns suggestive of an association with either rozanolixizumab infusion or infection. The highest post-treatment CRP values were noted in 2 patients in the 20-mg/kg cohort with values of 126 and 27 mg/L; however, both patients had elevated baseline CRP values (43 and 40 mg/L, respectively). The patient with a CRP value of 126 mg/L reported concurrent transient knee and ankle swelling that lasted several days. Several patients with elevated CRP values at baseline had decreases in CRP values after dosing. No other clinically meaningful changes in laboratory safety findings, including liver function tests (aside from total protein decline as a result of the lower IgG levels) or hematology laboratory parameters, were observed.
Infection-related AEs and injection/infusion site reactions.
The most common infection-related AE was upper respiratory tract infection, which occurred in 4 patients (6.1%). One patient in each of the 4- and 7-mg/kg cohorts had influenza (per the investigator), and 1 each in the 4- and 10-mg/kg cohorts had an unspecified clinically diagnosed viral infection. All infections were mild-to-moderate in intensity, resolved uneventfully without further treatment, and were not considered by the investigator to be related to rozanolixizumab. One patient in the 4-mg/kg cohort had a mild injection-site reaction, and 1 patient in the 20-mg/kg cohort had mild infusion-site edema, both considered related to rozanolixizumab.
Clinical efficacy
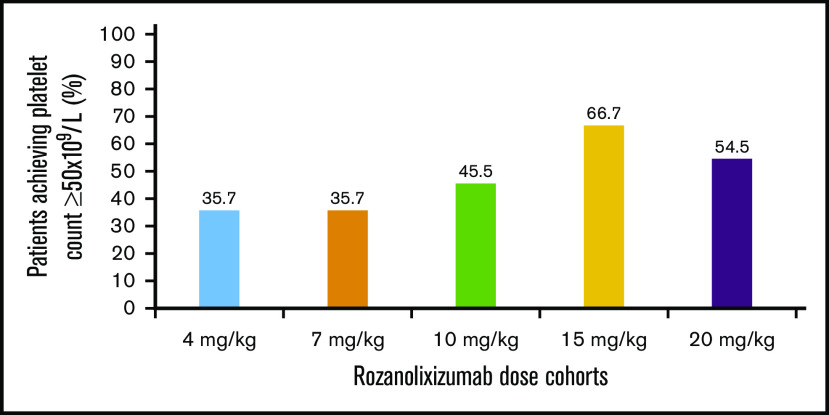
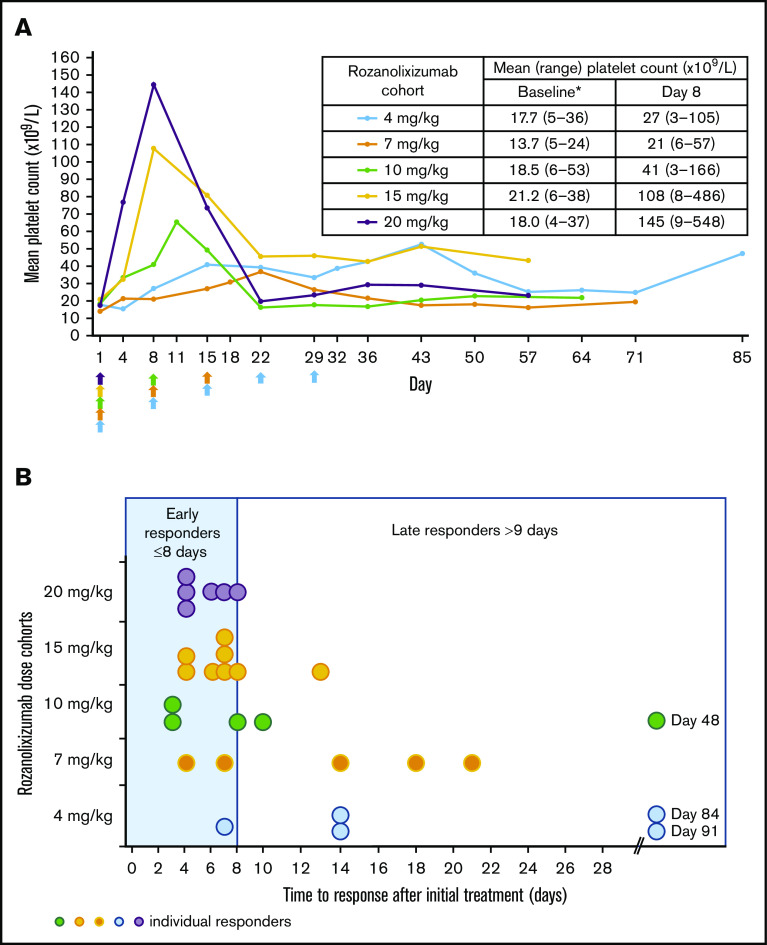
Advantages in the platelet effect for the single-dose cohorts (15 and 20 mg/kg) compared with the multiple-dose cohorts (4, 7, and 10 mg/kg) were observed in 3 ways. First, more than 50% of patients achieved at least 1 platelet count ≥50 × 109/L in the single-dose cohorts (20 mg/kg: 6 [54.5%] of 11; missing data, n = 1; 15 mg/kg: 8 [66.7%] of 12) compared with 36% to 46% of patients in the multiple-dose cohorts (10 mg/kg: 5 [45.5%] of 11; 7 mg/kg: 5 [35.7%] of 14; missing data, n = 1; and 4 mg/kg: 5 [35.7%] of 14) (Figure 2). Second, by day 8, after 1 subcutaneous infusion of rozanolixizumab, platelet counts had increased to higher mean levels in single higher-dose cohorts than in multiple-dose cohorts (Figure 3A). Third, increases in platelet counts to ≥50 × 109/L occurred more rapidly in single-dose cohorts. By day 8, dose-dependent increases in the percentage of patients achieving a platelet count ≥50 × 109/L were observed: 6 (54.5%) of 11 and 7 (58.3%) of 12 patients in the higher single-dose cohorts (20 and 15 mg/kg, respectively) compared with 3 (27.3%) of 11, 2 (14.3%) of 14, and 1 (7.1%) of 14 patients in the 10-, 7-, and 4-mg/kg multiple lower-dose cohorts, respectively. The median times to response (platelet count ≥50 × 109/L) in the single-dose cohorts were on days 5 (20 mg/kg) and 7 (15 mg/kg), whereas for the multiple-dose cohorts, the responses were on days 8 (10 mg/kg) and 14 (7 and 4 mg/kg) (Figure 3B).
Figure 2.
Percentage of patients who had a platelet count ≥50 × 109/L over all visits (per protocol set).
Figure 3.
Rozanolixizumab clinical efficacy. (A) Mean platelet count over time after rozanolixizumab subcutaneous infusion (per protocol set). Arrows indicate time of rozanolixizumab subcutaneous infusion. *Baseline platelet counts were derived from central laboratory data. (B) Time to first clinically relevant response (platelet count ≥50 × 109/L) in the patients classified as responders (per protocol set).
The one area in which the single doses were not clearly more effective than the multiple doses of rozanolixizumab was duration of response. In patients achieving a platelet count ≥50 × 109/L, duration of response was similar across all cohorts except the 4-mg/kg cohort (which received 5 infusions), in which a longer duration of response among the responders was observed. In the 20-, 15-, 10-, and 7-mg/kg cohorts, median duration of response was 10 days (range, 7-24), 12 days (range, 6-20), 12 days (range, 6-19), and 14 days (range, 3-42), respectively, compared with 35 days (range, 0-77) in the 4-mg/kg cohort.
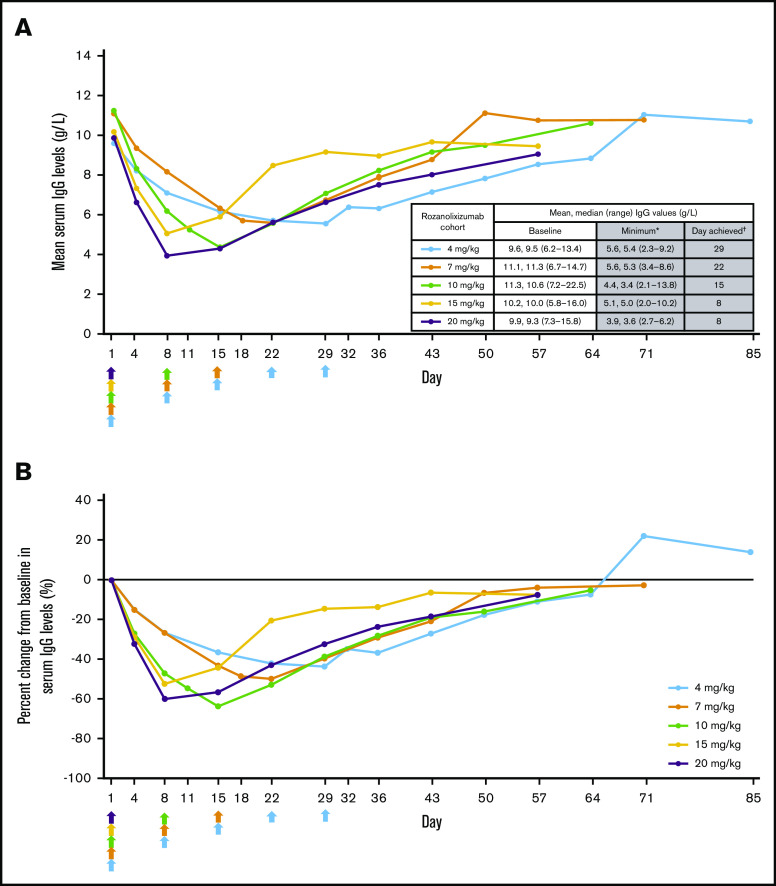
Reduction of total serum IgG levels
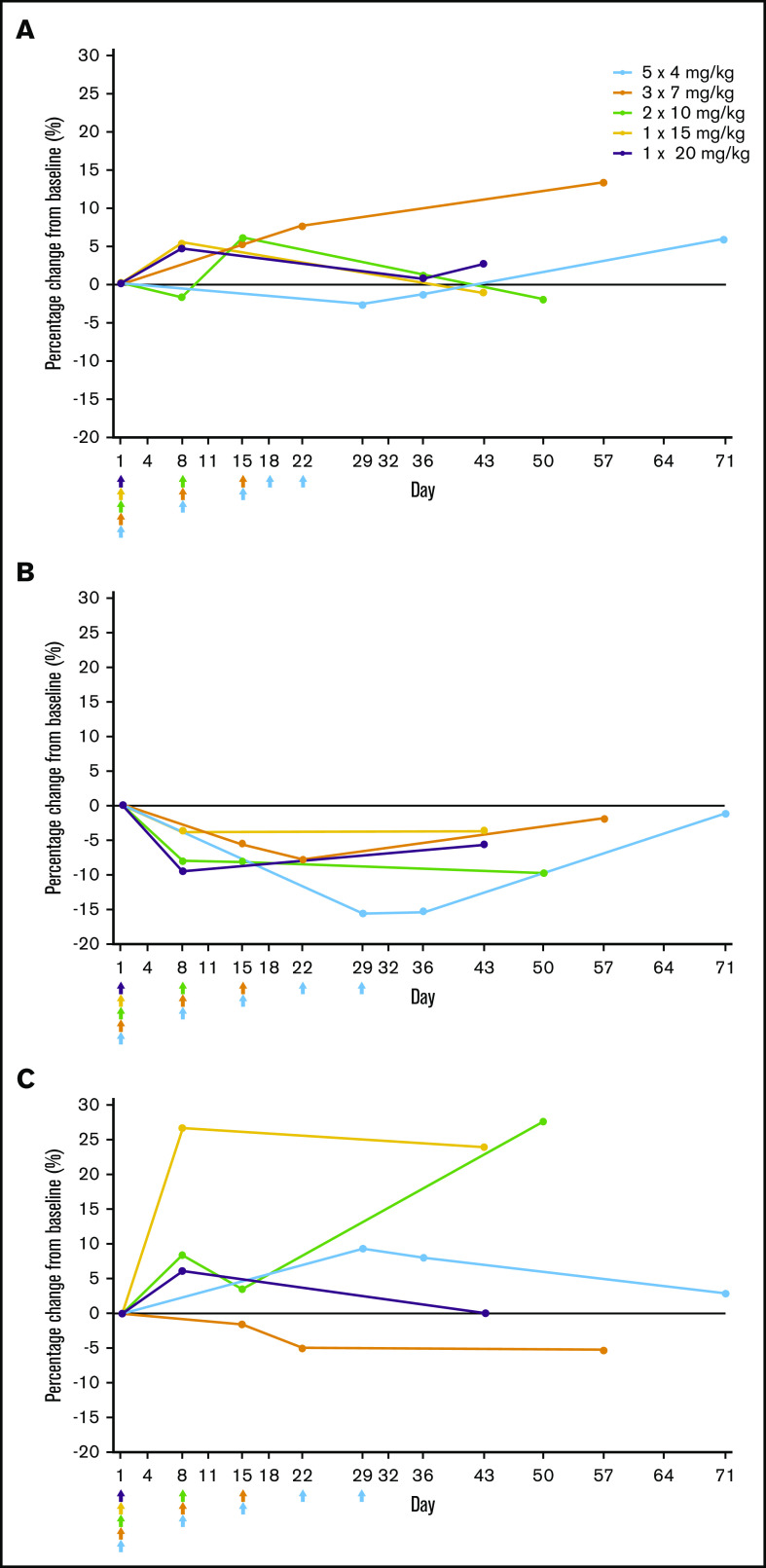
Rozanolixizumab infusion resulted in decreases in serum IgG levels that followed an approximately dose-dependent pattern after the first infusion (day 8). Minimum mean IgG was achieved by day 8 in the higher single-dose cohorts, and by days 15, 22, and 29 in the multiple-dose cohorts (2 × 10, 3 × 7, and 5 × 4 mg/kg, respectively) (Figure 4A). Percent reductions from baseline in IgG levels were 60.0% and 52.3% in the 20- and 15-mg/kg single-dose cohorts, respectively, compared with 63.8%, 49.9%, and 43.6% in the 10-, 7-, and 4-mg/kg multiple-dose cohorts, respectively (Figure 4B). For all doses, reductions in IgG subclasses mirrored those for total serum IgG. No substantial changes were observed for IgA, IgM, or IgE (Figure 5).
Figure 4.
Serum IgG levels after treatment with rozanolixizumab. (A) Mean serum IgG levels (pharmacodynamic per protocol set); arrows indicate time of rozanolixizumab subcutaneous infusion. *Minimum post-baseline mean. †Day when minimum post-baseline mean was achieved. (B) Percent change from baseline in serum IgG levels (pharmacodynamic per protocol set). Arrows indicate time of rozanolixizumab subcutaneous infusion.
Figure 5.
Percentage change from baseline in immunoglobulin levels after rozanolixizumab infusion (full analysis set). (A) IgA. (B) IgM. (C) IgE.
Exploratory end points: change in bleeding score and ADAs
The majority of patients did not experience a bleeding event at baseline. In total, 3 of 4 patients in the 20-mg/kg cohort and 3 of 3 patients in the 15-mg/kg cohort who were bleeding at baseline were no longer bleeding at day 8, whereas proportions were lower in the multiple-dose cohorts (2 of 4 patients [10 mg/kg], 3 of 6 patients [7 mg/kg], and 1 of 5 patients [4 mg/kg]).
Pre-dose measurable levels of ADA were observed in 2 of 66 patients, both in the 10-mg/kg multiple-dose cohort. Post-dose ADAs were observed at least once during the study in 31 of 66 patients (7 of 15 patients [4 mg/kg], 7 of 15 patients [7 mg/kg], 8 of 12 patients [10 mg/kg], 5 of 12 patients [15 mg/kg], and 4 of 12 patients [20 mg/kg]). ADA positivity was identified at different time points in the study. ADA did not seem to have any impact on pharmacodynamic (eg, change in IgG levels) or clinical (eg, change in platelet count) response to rozanolixizumab.
Discussion
Rozanolixizumab is the first subcutaneously infused, humanized anti-FcRn monoclonal antibody developed to treat diseases caused by pathogenic IgG autoantibodies,6 ITP, MG, and chronic inflammatory demyelinating polyradiculoneuropathy in particular. This article describes a phase 2 study of rozanolixizumab in patients with persistent/chronic primary ITP. Results show an association, at higher single doses, between immediate pronounced IgG decrease and rapid, substantial platelet count increase.
Baseline characteristics of patients in this study indicate an ITP population that is very difficult-to-treat based upon long duration of disease (median >5 years) and high numbers of previous therapies (median, 4): 41% of patients had received IVIg and 39% had received TPO-RAs. In this phase 2 study, limited prospective data on response to previous treatment were collected. The only requirement was to verify that patients had responded to at least 1 previous ITP-specific therapy as a “yes or no” response. Thus, data are lacking regarding quantification of clinical response, which treatment(s) patients had responded to, when treatments were administered, and whether response to other treatment(s) was also achieved. Furthermore, patients who had received previous TPO-RA or IVIg treatment were not balanced across dose cohorts, which made it difficult to draw conclusions from the previous response data, given that the different dose cohorts responded very differently to rozanolixizumab. With all of these caveats, very preliminarily, of the 39% of patients who had previously received TPO-RAs, at least 50% had experienced treatment failure with either romiplostim or eltrombopag or both; most of these refractory patients did not seem to respond to rozanolixizumab. Furthermore, all patients who responded to rozanolixizumab and had previously received IVIg had been assessed as responding to IVIg. This observation is consistent with the common mechanism of action of both therapies. It would have been interesting to carefully and specifically evaluate the relationship and response to previous ITP treatments, including not only TPO-RA and IVIg, but also steroids, splenectomy, and rituximab. However, even the preliminary analyses above were beyond the scope of this phase 2 trial. The response to previous therapies will be assessed in future phase 3 studies with rozanolixizumab.
Rozanolixizumab was administered in 1 of 5 sequential low- to high-dose cohorts using 3 multiple-dose regimens (resulting in cumulative doses of 20 or 21 mg/kg) and 2 single-dose regimens of 15 and 20 mg/kg. This study demonstrated that rozanolixizumab was well tolerated with an acceptable safety profile. As seen in previous studies,7,8 subcutaneous rozanolixizumab administration was generally associated with mild-to-moderate AEs, most commonly headaches, diarrhea, back pain, and vomiting. The cause of the headaches is unknown, none were severe in this trial, all were managed with standard medication (if required) of short duration, and all resolved without clinical sequelae. Headaches have also been reported in clinical studies of other FcRn inhibitors such as efgartigimod23,24 and orilanolimab.25,26 The change in rozanolixizumab administration route from IV7 to subcutaneous infusion reduced both the frequency and severity of headaches. The safety and tolerability of rozanolixizumab was further demonstrated by the absence of any AEs leading to treatment withdrawal or study discontinuation and the small number of patients (n = 2) experiencing infusion site reactions.
The dose dependency of responses observed by day 8 across the 5 dose cohorts speaks to the efficacy of rozanolixizumab. The largest maximum mean platelet count increases were observed in rozanolixizumab 15- and 20-mg/kg single-dose cohorts by day 8; more than 50% of patients in each of these cohorts achieved a clinically relevant response (platelet count ≥50 × 109/L) that lasted 10 to 12 days compared with 7% to 27% of patients in the multiple-dose cohorts. Finally, median time to response occurred earlier in single-dose cohorts (5-7 days) compared with multiple-dose cohorts (8-14 days). Considering these data overall, the single-dose cohorts demonstrated platelet count responses of greater magnitude and at an earlier time point compared with the multiple-dose cohorts. Although the single-dose cohorts had a faster onset of action, they did not result in longer duration of response. The longest duration of response, in the 5 × 4-mg/kg cohort, was likely a result of multiple infusions that prolonged the clinically relevant platelet response in the responders in these cohorts, suggesting the potential of repeated use of rozanolixizumab in future trials to maintain responses.
Reported response rates (and median time to response) with commonly used ITP treatments include 54% with rituximab (3.5 weeks),27 43% with fostamatinib (15 days),28 and 81% with IVIg (1-2 days).29 For TPO-RAs,30-35 response rates of approximately 50% to 90% (depending on definition) have been reported in similarly heavily pretreated patients with ITP (times to response varied; studies reported median time to response of 5 to 8 days,30 response within or by 2 weeks,31,35 or by 7 weeks34). Although there are obvious differences in study design and patient populations that limit comparisons, results in this phase 2 study suggest that rozanolixizumab seems to offer benefits comparable to other approved ITP therapies. Efgartigimod, an FcRn antagonist under development, also demonstrated clinically relevant increases in platelet count with associated decreases in IgG levels, further supporting this mechanism of action, although with potentially greater variability in timing of response for efgartigimod.24
If future studies demonstrate that repeated infusions of rozanolixizumab can safely maintain platelet counts, this could substantially expand the utility of this treatment for patients in various stages of ITP. Considering the burden of treatments for ITP on patients, rozanolixizumab was administered in 30 to 96 minutes as a 10- to 30-mL subcutaneous infusion. The short time and the subcutaneous route of administration minimize patients’ time and discomfort when receiving parenteral treatment. If rozanolixizumab is approved, it is anticipated that a home-infusion option may become available for rozanolixizumab, further reducing the burden of treatment on patients.
Dose-dependent decreases in serum IgG were observed on day 8. The minimum mean IgG concentration was 3.9 g/L on day 8 for the higher single-dose 20-mg/kg cohort with reduction from baseline of 60.0%. Exploratory analyses of data to day 8 appear to suggest that the magnitude of serum IgG decrease was associated with the corresponding platelet increase. The observed reduction in IgG from baseline is consistent with previous studies in healthy volunteers7 and patients with MG,8 and further confirms the hypothesis of Brambell et al1 regarding the role of FcRn in IgG recycling. Similar reductions in serum IgG levels of 50% to 75% have been observed after plasma exchange.36,37 Other anti-FcRn molecules in development have also effectively lowered IgG serum concentrations23-25,38 while simultaneously increasing platelet counts.24
Many autoimmune disorders are characterized by pathogenic IgG autoantibodies, and rozanolixizumab could offer a therapeutic alternative for a number of autoantibody-mediated disorders. The mechanism of effect of rozanolixizumab seems clear: it prevents binding of IgG to FcRn, thereby inhibiting recycling of IgG and accelerating IgG destruction through the natural lysosomal degradation pathway.6,7 Although there is not believed to be any specificity of FcRn inhibition for autoantibodies, total IgG levels are reduced by around 60%. Rozanolixizumab seems to directly reduce IgG and IgG autoantibody levels effectively in a dose-dependent and manageable manner. Reduction of IgG antiplatelet autoantibody levels is expected to not only decrease platelet destruction but also to increase platelet production.
Because of the rozanolixizumab mechanism of action and the observed decrease in serum IgG levels, the incidence of infections was of particular interest in rozanolixizumab-treated patients. Infections were uncommon (all were mild-to-moderate and none were serious or resulted in hospitalization) and were typical of those expected in the general population. Although maintenance treatment with repeated rozanolixizumab infusions is likely to lead to more prolonged IgG decrease, the perceived risk of serious infection after prolonged anti-FcRn treatment can be compensated for by the fact that IgG is not completely depleted; production and quality of IgG remains unimpaired; IgA, IgE, and IgM levels are unaltered; and cellular and innate immunity likely remains intact. CRP elevations were observed after rozanolixizumab infusions, but not consistently and/or persistently and often in conjunction with elevated baseline CRP levels; elevations were not linked to AEs except in 1 case of transient knee and ankle swelling. Long-term maintenance studies will clarify whether there are infectious or inflammatory risks related to longer-term rozanolixizumab therapy.
Post-dose ADAs were observed in all dose cohorts but the clinical impact, if any, of ADAs remains unclear. Indeed, safety and efficacy of longer-term, repeated treatment with rozanolixizumab, including ADAs, will be assessed in future trials.
Limitations of this study are inherent in its phase 2 nature: open-label, single- vs multiple-dose regimens, and non-randomized design, albeit a 66-patient study for a rare disease. Although all study participants had a history of previous ITP treatments, the previous use of TPO-RAs or IVIg were predominantly in the multiple-dose treatment groups, making it difficult to assess their impact. Inclusion of single- and multiple-dose regimens maintained relative equality of cumulative dose but influenced the timing, magnitude, and duration of responses. This was useful for suggesting the optimal dose regimen for future studies and confirmed dose-dependent efficacy and pharmacodynamics of rozanolixizumab. In contrast to several other ITP studies, this study used central laboratory platelet count reporting for data analyses with local laboratory baseline platelet counts used for inclusion only. In 2 cases, central and local platelet counts differed, which explains the apparent eligibility discrepancies in Table 1.
In conclusion, the results clearly suggest that rozanolixizumab was effective at increasing platelet counts with apparent tolerability and safety and thus it has good potential as a treatment of persistent/chronic primary ITP. Rozanolixizumab treatment at doses up to 20 mg/kg was generally well tolerated with an acceptable safety profile and demonstrable efficacy. Single doses of rozanolixizumab (15 and 20 mg/kg) demonstrated the fastest onset of action (1 week) both for platelet count increase and IgG decrease. Rozanolixizumab treatment provided substantial, transient reduction of serum IgG levels with no association with infections. These safety, tolerability, efficacy, and pharmacodynamic data support the ongoing phase 3 development of rozanolixizumab as a maintenance treatment in patients with primary ITP (NCT04200456). The rapid (1 week) onset of action observed in this study offers potential for further application of rozanolixizumab in ITP in which a platelet increase within 1 week is required.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
The authors thank all patients and their families and acknowledge Louise Prince and Laura Griffin (iMed Comms, an Ashfield company, part of UDG Healthcare plc) for medical writing support, Birgit Haier for support with data analysis and interpretation, and Linda Feighery, CMPP, and Veronica Porkess, CMPP, for publication and editorial support (all from UCB Pharma).
The study was funded by UCB Pharma (the manufacturer of rozanolixizumab). Medical writing support was funded by UCB Pharma in accordance with Good Publications Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
This study was designed by a panel that included the principal investigator (T.R.) and UCB Pharma. UCB Pharma was responsible for data collection and analysis, had a role in data interpretation, and reviewed and approved the final report. All authors had full access to the data in the study.
Footnotes
Data sharing statement: Underlying data from this manuscript may be requested by qualified researchers 6 months after product approval in the United States and/or Europe, or when global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient data and redacted trial documents which may include raw data sets, analysis-ready data sets, trial protocol, blank case report form, annotated case report form, statistical analysis plan, data set specifications, and clinical trial report. Before using the data, proposals need to be approved by an independent review panel at www.clinicalstudydatarequest.com and a signed data sharing agreement will need to be executed. All documents are available in English only for a prespecified time, typically 12 months, on a password-protected portal. Send requests to the corresponding author Tadeusz Robak at robaktad@csk.umed.lodz.pl.
Authorship
Contribution: T.R., P.K., U.M., and R.S. helped conceive and design the study and acquire and analyze/interpret the data; M.K., I.J., V.M., J.T., N.C., and J.K. helped acquire and analyze/interpret the data; F.W., G.L., and S.J. helped conceive and design the study and analyze/interpret the data; J.B.B. helped analyze/interpret the data; all authors helped write the manuscript and reviewed, revised, and approved the final version; and T.R. had final responsibility for the decision to submit the manuscript.
Conflict-of-interest disclosure: T.R. has received consultancy fees and/or research funding from AbbVie, Acerta, Amgen, BeiGene, Gilead, Janssen, Morphosys AG, Roche, and Takeda and honoraria, research funding, or for other activities from AbbVie, Janssen, and UCB Pharma. I.J. has received consultancy fees and/or has supported speaker’s bureaus for AbbVie, Alexion, Amgen, Bristol-Myers Squibb, Celgene, CellTrion, Grifols, Janssen, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Servier, Shionogi, Shire, and Takeda. V.M. is an employee of the Institute of Oncology and Arensia Exploratory Medicine. N.C. has received consultancy fees, advisory board funding, and/or honoraria from Amgen, Novartis, Principia, and Rigel. G.L. has received consultancy fees from AstraZeneca, Conatus, Creablis, Medicines for Malaria Venture, Mylan, Pfizer, Sigmoid Pharma, UCB Pharma, and Ziarco. J.B.B. has received consultancy fees from Amgen, Argenx, UCB Pharma, Dova, Kezar, Momenta, Novartis, Regeneron, and Rigel and has supported speaker’s bureaus for Novartis and 3SBio. S.J. has received support for studies and consultancy and speaker’s bureau fees and has undertaken clinical trials and provided conference support for Binding Site, Biocryst, Biotest, CSL Behring, LFB, Grifols, Octapharma, Pharming, Shire/Takeda, SOBI, UCB Pharma, Weatherden, and Zarodex. P.K., U.M., F.W., R.S., and J.K. are employees of UCB Pharma and may hold stock or stock options. The remaining authors declare no competing financial interests.
Correspondence: Tadeusz Robak, Department of Hematology, Medical University of Lodz, Copernicus Memorial Hospital, Ul. Ciolkowskiego 2, 93-510 Lodz, Poland; e-mail: robaktad@csk.umed.lodz.pl.
References
- 1.Brambell FW, Hemmings WA, Morris IG. A theoretical model of gamma-globulin catabolism. Nature. 1964;203(4952):1352-1354. [DOI] [PubMed] [Google Scholar]
- 2.Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15(7):733-738. [DOI] [PubMed] [Google Scholar]
- 3.Simister NE, Mostov KE. An Fc receptor structurally related to MHC class I antigens. Nature. 1989;337(6203):184-187. [DOI] [PubMed] [Google Scholar]
- 4.Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2(7473):1087-1093. [DOI] [PubMed] [Google Scholar]
- 5.Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7(9):715-725. [DOI] [PubMed] [Google Scholar]
- 6.Smith B, Kiessling A, Lledo-Garcia R, et al. Generation and characterization of a high affinity anti-human FcRn antibody, rozanolixizumab, and the effects of different molecular formats on the reduction of plasma IgG concentration. MAbs. 2018;10(7):1111-1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiessling P, Lledo-Garcia R, Watanabe S, et al. The FcRn inhibitor rozanolixizumab reduces human serum IgG concentration: A randomized phase 1 study. Sci Transl Med. 2017;9(414):eaan1208. [DOI] [PubMed] [Google Scholar]
- 8.Bril V, Benatar M, Brock M, et al. Proof-of-concept and safety of the anti-FcRn antibody rozanolixizumab in patients with moderate-to-severe generalized myasthenia gravis (GMG): a phase 2a study [abstract]. Neurology. 2019;92(15 suppl). Abstract S43.001.
- 9.Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386-2393. [DOI] [PubMed] [Google Scholar]
- 10.Cines DB, Bussel JB, Liebman HA, Luning Prak ET. The ITP syndrome: pathogenic and clinical diversity. Blood. 2009;113(26):6511-6521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32(10):875-887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood. 2005;106(7):2244-2251. [DOI] [PubMed] [Google Scholar]
- 13.Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). J Clin Med. 2017;6(2):16. [Google Scholar]
- 14.Brighton TA, Evans S, Castaldi PA, Chesterman CN, Chong BH. Prospective evaluation of the clinical usefulness of an antigen-specific assay (MAIPA) in idiopathic thrombocytopenic purpura and other immune thrombocytopenias. Blood. 1996;88(1):194-201. [PubMed] [Google Scholar]
- 15.McMillan R. Autoantibodies and autoantigens in chronic immune thrombocytopenic purpura. Semin Hematol. 2000;37(3):239-248. [DOI] [PubMed] [Google Scholar]
- 16.Iraqi M, Perdomo J, Yan F, Choi PY, Chong BH. Immune thrombocytopenia: antiplatelet autoantibodies inhibit proplatelet formation by megakaryocytes and impair platelet production in vitro. Haematologica. 2015;100(5):623-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arnold DM, Nazi I, Toltl LJ, et al. Antibody binding to megakaryocytes in vivo in patients with immune thrombocytopenia. Eur J Haematol. 2015;95(6):532-537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swinkels M, Rijkers M, Voorberg J, Vidarsson G, Leebeek FWG, Jansen AJG. Emerging concepts in immune thrombocytopenia. Front Immunol. 2018;9:880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodeghiero F. ITP and thrombosis: an intriguing association. Blood Adv. 2017;1(24):2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sestøl HG, Trangbæk SM, Bussel JB, Frederiksen H. Health-related quality of life in adult primary immune thrombocytopenia. Expert Rev Hematol. 2018;11(12):975-985. [DOI] [PubMed] [Google Scholar]
- 21.Cooper N, Kruse A, Kruse C, et al. Results from the ITP World IMPACT Survey (I-WISh): Patients with immune thrombocytopenia (ITP) experience impaired quality of life (QoL) regarding daily activities, social interactions, emotional well-being and working lives[abstract]. Blood. 2018;132(1). Abstract 4804. [Google Scholar]
- 22.Rodeghiero F, Michel M, Gernsheimer T, et al. Standardization of bleeding assessment in immune thrombocytopenia: report from the International Working Group. Blood. 2013;121(14):2596-2606. [DOI] [PubMed] [Google Scholar]
- 23.Howard JF Jr., Bril V, Burns TM, et al. ; Efgartigimod MG Study Group . Randomized phase 2 study of FcRn antagonist efgartigimod in generalized myasthenia gravis. Neurology. 2019;92(23):e2661-e2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newland AC, Sánchez-González B, Rejtő L, et al. Phase 2 study of efgartigimod, a novel FcRn antagonist, in adult patients with primary immune thrombocytopenia. Am J Hematol. 2020;95(2):178-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Blumberg LJ, Humphries JE, Jones SD, et al. Blocking FcRn in humans reduces circulating IgG levels and inhibits IgG immune complex-mediated immune responses. Sci Adv. 2019;5(12):eaax9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werth VP, Culton D, Blumberg L, Humphries J, Blumberg R, Hall R. FcRn blockade with SYNT001 for the treatment of pemphigus. J Invest Dermatol. 2018;138(5):S92. Abstract 538. [Google Scholar]
- 27.Cooper N, Stasi R, Cunningham-Rundles S, et al. The efficacy and safety of B-cell depletion with anti-CD20 monoclonal antibody in adults with chronic immune thrombocytopenic purpura. Br J Haematol. 2004;125(2):232-239. [DOI] [PubMed] [Google Scholar]
- 28.Bussel J, Arnold DM, Grossbard E, et al. Fostamatinib for the treatment of adult persistent and chronic immune thrombocytopenia: Results of two phase 3, randomized, placebo-controlled trials. Am J Hematol. 2018;93(7):921-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbach O, Taumberger AB, Wietek S, Cervinek L, Salama A. Efficacy and safety of a new intravenous immunoglobulin (Panzyga®) in chronic immune thrombocytopenia. Transfus Med. 2019;29(1):48-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bussel JB, Kuter DJ, George JN, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355(16):1672-1681. [DOI] [PubMed] [Google Scholar]
- 31.Bussel JB, Cheng G, Saleh MN, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237-2247. [DOI] [PubMed] [Google Scholar]
- 32.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161-2171. [DOI] [PubMed] [Google Scholar]
- 33.Bussel JB, Provan D, Shamsi T, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. Lancet. 2009;373(9664):641-648. [DOI] [PubMed] [Google Scholar]
- 34.Bussel JB, Buchanan GR, Nugent DJ, et al. A randomized, double-blind study of romiplostim to determine its safety and efficacy in children with immune thrombocytopenia. Blood. 2011;118(1):28-36. [DOI] [PubMed] [Google Scholar]
- 35.Wong RSM, Saleh MN, Khelif A, et al. Safety and efficacy of long-term treatment of chronic/persistent ITP with eltrombopag: final results of the EXTEND study. Blood. 2017;130(23):2527-2536. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan AA. Therapeutic plasma exchange: a technical and operational review. J Clin Apher. 2013;28(1):3-10. [DOI] [PubMed] [Google Scholar]
- 37.Ohkubo A, Okado T, Kurashima N, et al. Removal characteristics of immunoglobulin G subclasses by conventional plasma exchange and selective plasma exchange. Ther Apher Dial. 2015;19(4):361-366. [DOI] [PubMed] [Google Scholar]
- 38.Ling LE, Hillson JL, Tiessen RG, et al. M281, an anti-FcRn antibody: Pharmacodynamics, pharmacokinetics, and safety across the full range of IgG reduction in a first-in-human study. Clin Pharmacol Ther. 2019;105(4):1031-1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.