Abstract
Purpose of Review
Angiotensin type 2 receptor (AT2R) and receptor Mas (MasR) are part of the “protective arm” of the renin angiotensin system. Gene and pharmacological manipulation studies reveal that AT2R and MasR are involved in natriuretic, vasodilatory, and anti-inflammatory responses and in lowering blood pressure in various animal models under normal and pathological conditions such as salt-sensitive hypertension, obesity, and diabetes. The scope of this review is to discuss colocalization and heterodimerization as potential molecular mechanisms of AT2R- and MasR-mediated functions including antihypertensive activities.
Recent Findings
Accumulating evidences show that AT2R and MasR are co-localized, make a heterodimer, and are functionally interdependent in producing their physiological responses. Moreover, ang-(1–7) preferably may be an AT1R-biased agonist while acting as a MasR agonist.
Summary
The physical interactions of AT2R and MasR appear to be an important mechanism by which these receptors are involved in blood pressure regulation and antihypertensive activity. Whether heteromers of these receptors influence affinity or efficacy of endogenous or synthetic agonists remains a question to be considered.
Keywords: Angiotensin II type 2 receptor, Mas receptor, Angiotensin II type 1 receptor, Dimerization, Functional interdependence, Blood pressure
Introduction
Renin angiotensin system (RAS) is an important hormone system known to regulate volume homeostasis and BP. RAS is comprised of various enzymes, bioactive peptides, and receptors, which produce diverse and opposing cellular and physiological responses. Angiotensin-converting enzyme (ACE) and angiotensin II (ang-II) and its type 1 receptor (AT1R), collectively termed as “deleterious arm” of RAS, are involved in the pathogenesis of hypertension including vasoconstriction and anti-diuresis/anti-natriuresis. Contrarily, ang-II type 2 receptor (AT2R), ACE2, ang-(1–7), and MasR, collectively termed as “protective arm” of the RAS, have been shown to play role in vasodilatation, promoting diuresis/natriuresis, and lowering BP, thus largely counteracting the effects mediated via the AT1R. Although the threeRAS receptors, namely AT1R, AT2R, and MasR, have been assigned to their specific cellular and physiological responses, evidences have been documented indicating that these receptors affect each other’s cellular expression, signaling, and response. For example, the absence of the AT2R enhances the AT1R-mediated cellular response and BP [1–4] and an increased expression of the AT2R attenuates the AT1R-mediated signaling [5] and BP [6, 7]. Similarly, AT1R-mediated responses decrease upon activation of the MasR [8]. As it relates to the expression, renal MasR expression is decreased in AT2R knockout mice [1] and the activation of the AT2R causes an increase in the kidney MasR expression [9]. Of the proposed mechanisms include physical interaction of AT2R [10] or MasR [11] with AT1R and/or post-receptor opposing signaling cross talk. Since the expression of AT1R, relative to the AT2R and MasR, is much higher in the heart, the kidney, the vasculature, and other tissues, reducing the plasma levels of ang-II and/or AT1R activation by ACE inhibitors and selective antagonists, respectively, has been the focus to tackle RAS hyperactivity and treat various renal and cardiovascular diseases, including hypertension. Interestingly, however, RAS story seems to be more complex than ever before, particularly in light of new findings as to how the AT2R and MasR may be joining forces together to oppose and counterbalance the deleterious effects mediated by the AT1R. Purpose of this review is to highlight recent discoveries on AT2R and MasR heterodimerization as a potential mechanism responsible for these receptors to amplify their cellular signal impacting RAS physiology related to cardiovascular function and BP regulation.
Role of the AT2R and the MasR in Blood Pressure Regulation
AT2R Activation and Signaling in Blood Pressure Control
AT2R is an atypical G-protein (guanine nucleotide-binding protein)-coupled receptor (GPCR) with only 30% homology with AT1R. Both the receptors are activated by ang-II with similar affinity [12]. Other studies have suggested ang-III as the preferred peptide agonist for AT2R [13]. It is unusual that activation of AT2R is linked to inhibitory (Gαi/o) [14] as well as stimulatory (Gαs) protein and even G-protein-independent pathways [15, 16]. It is the SH2 domain which predominately mediates AT2R signaling via nitric oxide (NO)-cyclic guanosine monophosphate (cGMP) pathway [16], a pathway known to cause vasodilatation and natriuresis. Additionally, the AT2R is linked to activation of tyrosine phosphatases. Recently, the AT2R has been crystallized which provides a glimpse as to the chemical nature of the receptor [17]. One notable is the feature that AT2R tends to stay in an active state without exposing with an agonist, a concept held based on early pharmacological and biochemical studies [18]. Specifically, mere increase in the AT2R expression reduces the AT1R function in terms of reducing inositol hydrolysis [10]. Consistently, the AT2R is designated as an endogenous AT1R antagonist. Moreover, unlike typical GPCRs, the AT2R is resistant to agonist-induced desensitization, endocytosis, and degradation [19] due to fewer serine residues and the inability of the receptor to recruit β-arrestin [17]. The agonist treatment triggers rapid transport of cytosolic AT2R onto the plasma membrane which is likely to intensify AT2R-mediated signaling and cellular response [20]. Collectively, these chemical and cellular regulatory features of the AT2R make AT2R an attractive potential target for agonist-based therapy. With the availability of a selective orally active AT2R agonist compound 21 (C21), it became easier to study the AT2R for its biological roles in health and disease.
The AT2Rs are expressed in various organ systems including the heart, the vasculature particularly on endothelial cells, the brain, immune cells, and kidney tubular cells. Generally, the expression of AT2R is upregulated under various pathological conditions both in humans and animal models. For example, human diabetic resistance arteries [21] and failing heart [22], as well as rodent diabetic [23] and obese [24, 25] kidney, express higher levels of the AT2Rs. Information linking the AT2R to natriuresis and BP control including inflammation and oxidative stress comes from knockout as well as pharmacological studies. Mice lacking the AT2R exhibit modest higher BP [4] and higher sensitivity to ang-II elevating systolic BP [3, 4]. Also, these mice are more susceptible to deoxycorticosterone acetate (DOCA)-salt hypertension [26, 27]. Since the AT2R-null mice express higher levels of the AT1R [1], it is difficult to clearly ascribe the changes in natriuresis and BP directly due to lack of the AT2R or increased the AT1R expression/signaling. Although some studies show that the AT2R-mediated natriuretic, vasodilatory, and depressor responses can be observed only in the presence of blockade of the AT1R [28, 29] or ACE [30], other studies demonstrate that the AT2R agonists such as peptide CGP42112a and nonpeptide C21 are able to produce natriuresis [24]. In obese Zucker rats, acute infusion of CGP42112a [24] or C21 [31] produced remarkable natriuresis. It is interesting to note that the AT2R agonist does not affect natriuresis in control lean Zucker rats [24]. Similarly, other studies show enhanced natriuretic response in female spontaneously hypertensive rats (SHRs) (not in males) upon activation of AT2R by the agonist C21 [32]. The greater response in obese Zucker rats (compared to lean) or females (compared to males) may be linked to the higher expression of the AT2R in the kidney [23–25, 33]. Likewise, greater AT2R function in females may be attributed to a positive feedback loop between the AT2R expression and estrogen levels [34]. In the long term, chronic treatment with C21 of obese Zucker rats with high salt-induced hypertension [35•] or of Sprague-Dawley rats with ang-II-induced hypertension prevented increase in systolic BP to almost normal levels [36••]. In both the studies, C21 co-administration with high salt or ang-II produced greater natriuresis compared to high salt or ang-II alone [35•, 36••], preventing sodium and water retention and body fluid buildup thus preventing rise in BP. Central role of the AT2R receptors in BP control also has been reported. Cerebroventricular infusion of the AT2R agonist C21 lowers BP in normal rats [37••] and suppresses sympathetic outflow in failing heart model by improving baroreflex sensitivity [38]. Collectively, emerging studies provide strong evidences as to the role of AT2R in fluid homeostasis and BP control/regulation. However, given that the AT2R expression is very low compared with the AT1R, it is puzzling as to how the AT2R activation produces significant biological responses particularly under pathological conditions such as obesity, diabetes, salt-sensitive hypertension, inflammation, and oxidative stress. This review highlights, in the following section, that interaction with the AT1R and MasR may be a plausible explanation to the AT2R function.
MasR Activation and Signaling in Blood Pressure Control
The MasR is a proto-oncogene, which based on conserved structural motifs is classified as a GPCR and with ang-(1–7) as its natural agonist MasR belongs to the RAS. Multiple G-proteins such Gs, Gi, and G12 have been suggested to couple with the MasR [39], but vasodilatory NO and prostaglandins are the most reported signaling pathways linked to the MasR stimulation in the vasculature [40], heart [41, 42], and kidney [43]. Unlike the AT2R, the MasR in response to its agonist ang-(1–7) stimulation internalizes through clathrin- or caveolin-1/dynamin-coated endocytic pit and slowly re-sensitizes through recycling protein Rab11. However, surprisingly, the internalized MasR does not follow classical lysosomal trafficking for degradation [44]. Another study shows that chronic treatment of normotensive Wistar-Kyoto (WKY) rats with ang-(1–7) does not affect the MasR expression in the kidney, but cardiac MasR expression is decreased [45], suggesting an organ/cell-type-specific regulation of the MasR. More studies are needed to understand the cellular regulation of MasR in response to chronic agonist exposure, particularly whether MasR degradation is tissue/cell specific. Similar to the AT2R, the MasR also is considered a physiological antagonist of the AT1R [11], i.e., the activation of the MasR produces natriuretic [46] and vasodilatory responses in various vascular beds [47–49], increases blood flow [50], and lowers BP in animal models of hypertension [8, 51, 52]. A recent article provides a thorough review on ang-(1–7), AVE0991, and CGEN-856S on vasodilation and antihypertensive activity of these MasR agonists [53]. Some of the early studies indicate that ang-(1–7) infusion produces natriuretic and diuretic responses and increases glomerular filtration rate [54–56]. Ang-(1–7) attenuates ang-II-stimulated Na+-ATPase activity in isolated proximal tubules, suggesting a direct action on tubular sodium transport [57]. Recently, we have reported that ang-(1–7) produces natriuretic and diuretic responses which are blocked by the MasR antagonist A-779 [58••]. Other studies also confirmed natriuretic role of ang-(1–7) [59, 60], but there is some evidence suggesting antidiuretic actions of ang-(1–7) blocked by MasR antagonist D-ala7-ang-(1–7) [61]. Similarly, some studies suggest that ang-(1–7) despite with a vasodilatory response does not lower BP [50, 62, 63] or increases mean arterial pressure [64–67], while other studies clearly demonstrate that acute as well as chronic infusion of agonists ang-(1–7) [68, 69], AVE0991 [70–72], or CGEN-856S [73] lowers BP in control SHRs and protected against BP elevation and end-organ damage provoked by L-NG-nitroarginine methyl ester treatment in these animals [74].
Most of the initial discoveries assigned to the MasR function come from ang-(1–7) as an agonist, which through MasR knockout studies has been established as a MasR’s natural agonist [75]. However, emerging evidences demonstrate that ang-(1–7) may not be binding to and eliciting its responses via MasR alone. For example, ang-(1–7)-elicited responses are attenuated by the AT1R antagonist losartan [51, 59, 76] or the AT2R antagonist PD123319 [76, 77, 78]. Several possibilities including heterodimerization of MasR and AT2R with AT1R (discussed in the next section) have been suggested in order to explain ang-(1–7) physiological functions. However, recent studies are of particular importance showing that ang-(1–7) also is a biased ligand for the AT1R [79, 80••]. The ang-(1–7) binds to AT1R and activates only β-arrestin pathway while blocking Gi/Go-linked pathways and thereby produces cardioprotective effects mediated by AT1R [79, 80••]. While there still exist questions related to the specificity of ang-(1–7) with MasR activation and the net responses, more selective ligands for MasR, such as AVE0991 and CGEN-856S as agonists and A779 as antagonist, have been helpful to confirm and study the MasR-mediated physiological responses, as described above.
Dimerization and Functional Interdependence of the AT2R and the MasR
As the AT2R and MasR are components of the protective arm of the RAS, a communication in terms of functional interaction has been reported in many studies. For instance, the MasR agonist ang-(1–7) induced vasoprotective and atheroprotective effects in apolipoprotein E-deficient mice [77], amelioration of right ventricular modelling in diabetic rats [78•], and vasodepressor response in SHR and WKY rats [68] which are prevented by the AT2R antagonist PD123319. Conversely, in monocrotaline-induced rat model of pulmonary hypertension, the AT2R agonist C21 reduced pulmonary and ventricular fibrosis which is prevented by MasR antagonist A-779 [81]. Ang-(1–7) and C21 have been shown to improve neurological deficits in endothelin-1-induced stroke model which is cross-inhibited by their antagonists PD123319 and A-779 [82]. Such cross-inhibition indicates that these receptors may have post-receptor signaling cross talk or may exist in close proximity which allows them to interact at receptor level by heterodimerization. Recent two studies emerged at the same time showing that the AT2R and the MasR make heterodimers that may be responsible for functional interdependence of these two receptors [58••, 83••]. Using gold standard co-immunoprecipitation and dual-immunolabelling experiments, we have reported an interaction of the AT2R and the MasR in obese Zucker rat kidney and human kidney-2 (HK-2) cells [58••]. Moreover, incubation of renal cortical homogenate with cupric-phenanthroline (CuP), an oxidative cross-linker of proteins via free thiol (–SH) group of amino acids located within < 7 Å, followed by western immunoblotting of receptor complexes reveals that the AT2R and the MasR bands on the blot shifted at higher molecular weight. This suggests a heterodimerization of AT2R and MasR through free thiol groups of cysteine residues that may have located in very close proximity [58••]. This AT2R-MasR interaction is sensitive to reducing agent, β-mercaptoethanol, suggesting that it is the disulfide formation that mediates the heterodimerization. Moreover, it seems that the AT2R-MasR constitutively exists in the heterodimer form, as the western blotting in the absence of β-mercaptoethanol (not CuP either) reveals upward shift of some of the AT2R and MasR; CuP converts 100% of the AT2R-MasR into heterodimer or heteromers of the higher order. Earlier, through mutagenesis experiments, disulfide formation within the AT2R has been shown to confer stability to AT2R and introduction of free –SH groups leads to inactive population of the AT2R [84], highlighting the importance of cysteine residues in the AT2R activity. Another study utilized fluorescence resonance energy transfer (FRET) and cross-correlation spectroscopy in HEK-293 cells transfected with vectors encoding fluorophore-tagged AT2R and MasR. The tagged receptors results in FRET with 10.8% efficiency, suggesting AT2R and MasR are capable of forming heterodimers and dimerization disappears when AT2R is mutated at Cys35 residue [83••]. Furthermore, Leonhardt et al. [83••] also show that the AT2R and the MasR in astrocytes with CX3CR1 mRNA as the functional readout are interdependent. Specifically, blocking with either of the receptor antagonists (AT2R, PD123319, and MasR, A-779) or knocking out of a single of these receptors made astrocytes unresponsive for both agonists. Consistent to these findings, our in vivo renal function experiment reveals that natriuretic and diuretic responses to the AT2R agonist C21 or MasR agonist ang-(1–7) were cross-inhibited by their selective antagonists [58••]. In light of reports that AT2R [35•, 85••] and MasR [8, 86••] activations exhibit antihypertensive activity, it remains unknown whether blocking one receptor would impair the antihypertensive activity of the other receptor and to what extent.
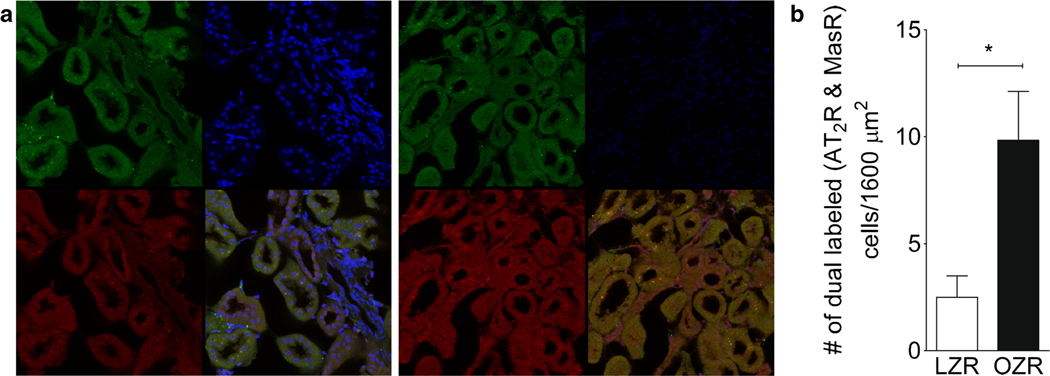
Functionally important thiol group-containing amino acids are sensitive to oxidative stress and the CuP experiment provided cues that oxidative stress may play a critical role in dimerization of the AT2R and the MasR. The treatment of HK-2 cells with glucose (25 mM), a known pro-oxidant molecule, followed by CuP reaction shows enhanced cross-linking of the AT2R and the MasR (unpublished observations). Since obese Zucker rats exhibit modest hyperglycemia, consistent to the observation in HK-2 cells exposed to higher glucose concentration, there is an increase in the AT2R-MasR dual-labelled puncta in the kidney of obese Zucker rats compared to lean Zucker rats (Fig. 1a, b). However, the increased dual labelling in obese rat kidney also could be due to the higher AT2Rs expressed in obese rat kidneys [24]. In another study, we have reported that the AT2R agonist chronic treatment of obese Zucker rats increases MasR expression [9], whether this would lead to more dimerization and enhanced the AT2R function is not known. Overall, studies to determine whether a correlation exists between the levels of the AT2R and MasR expression, heterodimer formation, and enhanced signaling and functional interdependence would have patho-physiological significance.
Fig. 1.
a The immunostaining of MasR (green, upper left) and AT2R (red, lower left) with nuclear stain DAPI (blue, upper right) and merged image showing their colocalization (yellow puncta, lower right) in kidney of lean (left) and obese (right) Zucker rat and b the number of their dual-labelled puncta
Kidney sections of obese rats and HK-2 cells also show the AT2R-MasR puncta in the cytosol and on nuclear envelop, in addition to the plasma membrane. It appears that much of the AT2R and the MasR are localized on organelles such as Golgi apparatus, mitochondria, and endoplasmic reticulum. The AT2R [24] and MasR [87], both are heavily glycosylated and it is known that only completely glycosylated receptor is delivered to plasma membrane. It is likely that considerable amount of these receptors escape glycosylation and may remain in cytosol.
It has been reported that Golgi membrane-associated AT2R-binding protein binds to specific motif within the cytoplasmic carboxy-terminal of AT2R and enables its plasma membrane transport and anti-proliferative effects [88]. Carey’s group also have reported that most of the renal AT2Rs are located within the cytosolic compartments and translocate to the plasma membrane upon agonist exposure of the proximal tubules [20]. Whether it is the dimer that translocates to the plasma membrane in response to the AT2R agonist and thus enhances the cellular signaling and response remains unknown.
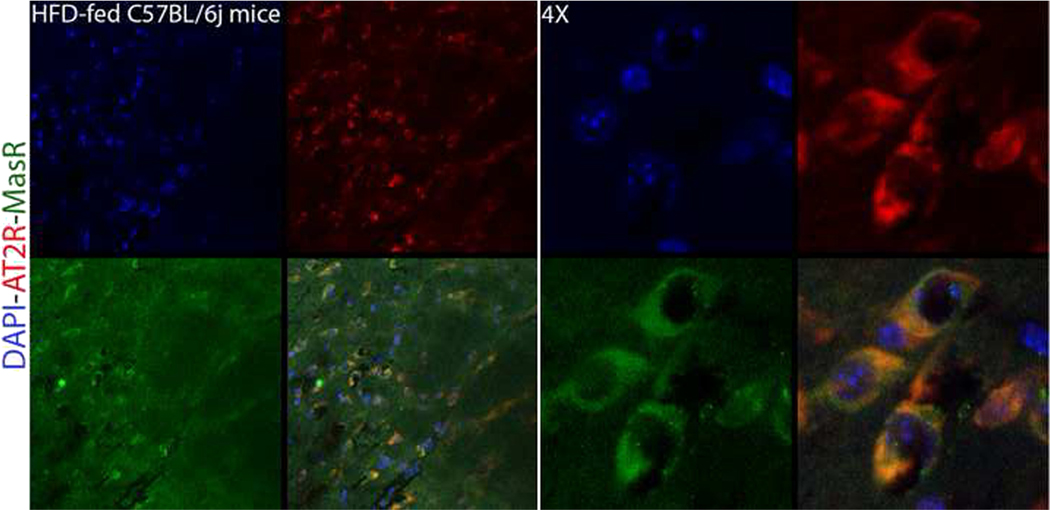
Co-localization of the AT2R-MasR is also observed in hypothalamic paraventricular (PVN) region of high-fat diet-fed male C57BL/6j mice (Fig. 2). The hypothalamus is an integrative center for numerous physiological events and hypothalamic RAS not only is involved in cardiovascular homeostasis and fluid balance [89, 90•] but also is implicated in neurohumoral [91], behavioral [92], cognitive [93], and metabolic aspects [94].
Fig. 2.
The co-localization of AT2R and MasR in paraventricular region of hypothalamus of C57BL/6j mice fed high-fat diet for 8 weeks
Interactions of the AT2R and the MasR with the AT1R
Since the AT2R and the MasR have been designated as functional antagonist of the AT1R, it is important to discuss their interaction, highlighting the functional complexity that seems to exist. The AT2R interacts with the AT1R at both on the plasma membrane and intracellularly. Several reports show ligand-independent physical interaction between the AT2R and the AT1R and their functional cross talk [10, 18, 95, 96]. The AT2R and the AT1R exists as homodimers as well as heterodimers [18, 95]. It is not clear whether the AT2R inhibits the AT1R function via homodimerization and post-receptor signaling [96]. However, the role of heterodimerization of the AT2R with the AT1R is evidently shown to inhibit the AT1R function, independent of AT2R activation or signaling [10]. Upon upregulation of the AT2R, it may heterodimerize with and inhibit the AT1R. For instance, normotensive pregnancy is linked to higher AT2R-AT1R dimers as compared to pre-eclampsia wherein the AT1R predominates [97]. Thus, it can be reasoned that the AT2R-AT1R heteromer is protective and the number of the AT2R-AT1R heteromer as compared to their respective monomer may regulate BP. However, ligand stimulation has been recently shown to enhance the AT2R-AT1R heteromerization resulting in change of conformation, co-internalization, and subsequent endosomal sorting of the AT2R with AT1R heteromer [98••] reducing the exposure of the AT1R to its ligand ang-II. Besides, the AT2R-AT1R heteromer may reduce availability of extracellular ang-II levels by inducing ang-II internalization as a complex with the AT2R-AT1R heteromer [99]. And intracrine RAS may counteract and modulate deleterious effects of extracellular paracrine RAS [100]. Activation of the intracrine AT1R is associated with increase in the expression of the AT2R which may increase the AT2R-AT1R heteromers at the cell surface and initiate positive feedback loop. These findings provide new insights into an important regulatory aspect of the AT2R in reducing the ang-II/AT1R signaling at the cell surface. Conversely, prolonged exposure of the AT1R to its ligands (ang-II, AT1R auto-antibody) has been associated with reduced AT1R internalization, sustained AT1R signaling, and hypertension [101].
The MasR has been shown to constitutively heterodimerize with the AT1R and to interfere with the ang-II/AT1R signaling [11]. The constitutively active MasR through activation of Gq/11 may sort the AT1R to the Golgi apparatus [102], plausibly reducing the ang-II-AT1R signaling. Likewise, the MasR is also internalized upon stimulation; however, it is not known whether the MasR is co-internalized with the AT1R [44]. Moreover, the pharmacologic activation of the MasR reduces the expression of the AT1R in the kidney [103, 104]. The native MasR-AT1R interactions [105] as well as opposing influence of the ang-(1–7)/MasR on the ang-II/AT1R effects have been reported [76]. The vasodepressor effects of ang-(1–7) is unmasked in the presence of the AT1R antagonist losartan, while ang-(1–7) increases perfusion pressure in the presence of the AT2R antagonist PD123319 [76] and this increase is not sensitive to the MasR antagonist A779. This suggests a shift in ang-(1–7) functional behavior when the AT2R has been blocked. Overall, it may be suggested that, in addition to monomers of the MasR, AT1R, and AT2R, a native existence of the oligomeric complex of MasR-AT1R-AT2R is a likely scenario.
Perspective
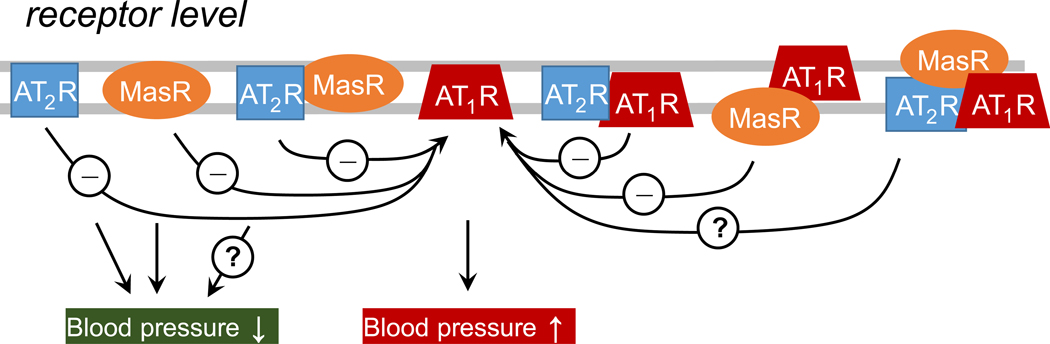
Dimerization of receptors can remarkably alter their ligand-binding properties, conformation, activation, and trafficking, which has (patho)physiological relevance [10]. In an essence, dimerization of RAS receptors still is an ill-defined, yet a central event in cross talk among these receptors and is increasingly being appreciated for the regulation of receptor function. Based on above discussion, the existence of angiotensin receptors as subset in the forms of monomer, dimer, or higher-order oligomer (Fig. 3) is a likely phenomenon, which appears to be dependent on the cellular distribution, density of the individual receptors, and pathological versus normal conditions. Unfortunately, members of GPCR undergo dimerization in uncontrolled manner. Thus, screening for disease-specific receptor complexes and of receptor complex-specific dimeric ligands should be part of the future drug discovery targeting hypertension and associated cardiovascular morbidities.
Fig. 3.
The proposed plausibilities among AT2R, MasR, and AT1R to interact in regulation of blood pressure
Acknowledgments
Funding Information This study was supported by NIH R01 grant DK61578.
Footnotes
Compliance with Ethical Standards
Conflict of Interest The authors declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Ali Q,Dhande I, Samuel P, Hussain T. Angiotensin type 2 receptor null mice express reduced levels of renal angiotensin converting enzyme-2/angiotensin (1–7)/Mas receptor and exhibit greater high-fat diet-induced kidney injury. J Renin-Angiotensin-Aldosterone Syst. 2016;17(3):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanaka M, Tsuchida S, Imai T, Fujii N, Miyazaki H, Ichiki T, et al. Vascular response to angiotensin II is exaggerated through an upregulation of AT1 receptor in AT2 knockout mice. Biochem Biophys Res Commun. 1999;258(1):194–8. [DOI] [PubMed] [Google Scholar]
- 3.Siragy HM, Inagami T, Ichiki T, Carey RM. Sustained hypersensitivity to angiotensin II and its mechanism in mice lacking the subtype-2 (AT2) angiotensin receptor. Proc Nattl Acad Sci USA. 1999;96(11):6506–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ichiki T, Labosky PA, Shiota C, Okuyama S, Imagawa Y, Fogo A, et al. Effects on blood pressure and exploratory behaviour of mice lacking angiotensin II type-2 receptor. Nature. 1995;377:748–50. [DOI] [PubMed] [Google Scholar]
- 5.Masaki H, Kurihara T, Yamaki A, Inomata N, Nozawa Y, Mori Y, et al. Cardiac-specific overexpression of angiotensin II AT2 receptor causes attenuated response to AT1 receptor-mediated pressor and chronotropic effects. J Clin Invest. 1998;101(3):527–35.9449684 [Google Scholar]
- 6.Gao L, Wang W, Wang W, Li H, Sumners C, Zucker IH. Effects of angiotensin type 2 receptor overexpression in the rostral ventrolateral medulla on blood pressure and urine excretion in normal rats. Hypertension. 2008;51(2):521–7. [DOI] [PubMed] [Google Scholar]
- 7.Blanch GT, Freiria-Oliveira AH, Speretta GF, Carrera EJ, Li H, Speth RC, et al. Increased expression of angiotensin II type 2 receptors in the solitary-vagal complex blunts renovascular hypertension. Hypertension. 2014;64(4):777–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kangussu LM, Guimaraes PS, Nadu AP, Melo MB, Santos RA, Campagnole-Santos MJ. Activation of angiotensin-(1–7)/Mas axis in the brain lowers blood pressure and attenuates cardiac remodeling in hypertensive transgenic (mRen2)27 rats. Neuropharmacology. 2015;97:58–66. [DOI] [PubMed] [Google Scholar]
- 9.Ali Q, Wu Y, Hussain T. Chronic AT2 receptor activation increases renal ACE2 activity, attenuates AT1 receptor function and blood pressure in obese Zucker rats. Kidney Int. 2013;84(5):931–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.AbdAlla S, Lother H, Abdel-tawab AM, Quitterer U. The angiotensin II AT2 receptor is an AT1 receptor antagonist. J Biol Chem. 2001;276(43):39721–6. [DOI] [PubMed] [Google Scholar]
- 11.Kostenis E, Milligan G, Christopoulos A, Sanchez-Ferrer CF, Heringer-Walther S, Sexton PM, et al. G-protein-coupled receptor Mas is a physiological antagonist of the angiotensin II type 1 receptor. Circulation. 2005;111(14):1806–13. [DOI] [PubMed] [Google Scholar]
- 12.Bosnyak S, Jones ES, Christopoulos A, Aguilar MI, Thomas WG, Widdop RE. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin Sci (Lond). 2011;121(7):297–303. [DOI] [PubMed] [Google Scholar]
- 13.Del Borgo M, Wang Y, Bosnyak S, Khan M, Walters P, Spizzo I, et al. beta-Pro7-ang-III is a novel highly selective angiotensin II type 2 receptor (AT2R) agonist, which acts as a vasodepressor agent via the AT2R in conscious spontaneously hypertensive rats. Clin Sci (Lond). 2015;129(6):505–13. [DOI] [PubMed] [Google Scholar]
- 14.da Silva Lara L, Cavalcante F, Axelband F, De Souza AM, Lopes AG, Caruso-Neves C. Involvement of the Gi/o/cGMP/PKG pathway in the AT2-mediated inhibition of outer cortex proximal tubule Na+-ATPase by ang-(1–7). Biochem J. 2006;395(1):183–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza AM, Lopes AG, Pizzino CP, Fossari RN, Miguel NC, Cardozo FP, et al. Angiotensin II and angiotensin-(1–7) inhibit the inner cortex Na+-ATPase activity through AT2 receptor. Regul Pept. 2004;120(1–3):167–75. [DOI] [PubMed] [Google Scholar]
- 16.Feng YH, Sun Y, Douglas JG. Gβγ-independent constitutive association of Gαs with SHP-1 and angiotensin II receptor AT2 is essential in AT2-mediated ITIM-independent activation of SHP-1. Proc Nattl Acad Sci USA. 2002;99(19):12049–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang H, Han GW, Batyuk A, Ishchenko A, White KL, Patel N, et al. Structural basis for selectivity and diversity in angiotensin II receptors. Nature. 2017;544(7650):327–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miura S, Karnik SS, Saku K. Constitutively active homo-oligomeric angiotensin II type 2 receptor induces cell signaling independent of receptor conformation and ligand stimulation. J Biol Chem. 2005;280(18):18237–44. [DOI] [PubMed] [Google Scholar]
- 19.Hein L, Meinel L, Pratt RE, Dzau VJ, Kobilka BK. Intracellular trafficking of angiotensin II and its AT1 and AT2 receptors—evidence for selective sorting of receptor and ligand. Mol Endocrinol. 1997;11:1266–77. [DOI] [PubMed] [Google Scholar]
- 20.Padia SH, Kemp BA, Howell NL, Gildea JJ, Keller SR, Carey RM. Intrarenal angiotensin-III infusion induces natriuresis and angiotensin type 2 receptor translocation inWistar-Kyoto but not in spontaneously hypertensive rats. Hypertension. 2009;53(2):338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savoia C, Touyz RM, Volpe M, Schiffrin EL. Angiotensin type 2 receptor in resistance arteries of type 2 diabetic hypertensive patients. Hypertension. 2007;49(2):341–6. [DOI] [PubMed] [Google Scholar]
- 22.Tsutsumi Y, Matsubara H, Ohkubo N, Mori Y, Nozawa Y, Murasawa S, et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circul Res. 1998;83(10):1035–46. [DOI] [PubMed] [Google Scholar]
- 23.Hakam AC, Siddiqui AH, Hussain T. Renal angiotensin II AT2 receptors promote natriuresis in streptozotocin-induced diabetic rats. Am J Physiol Renal Physiol. 2006;290(2):F503–8. [DOI] [PubMed] [Google Scholar]
- 24.Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/ diuresis in obese Zucker rats. Hypertension. 2005;45(2):270–5. [DOI] [PubMed] [Google Scholar]
- 25.Ali Q, Sabuhi R, Hussain T. High glucose up-regulates angiotensin II subtype 2 receptors via interferon regulatory factor-1 in proximal tubule epithelial cells. Mol Cell Biochem. 2010;344(1–2):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gross V, Obst M, Luft FC. Insights into angiotensin II receptor function through AT2 receptor knockout mice. Acta Physiol Scand. 2004;181:487–94. [DOI] [PubMed] [Google Scholar]
- 27.Gross V, Milia AF, Plehm R, Inagami T, Luft FC. Long-term blood pressure telemetry in AT2 receptor-disrupted mice. J Hypertens. 2000;18(7):955–61. [DOI] [PubMed] [Google Scholar]
- 28.Bosnyak S, Welungoda IK, Hallberg A, Alterman M, Widdop RE, Jones ES. Stimulation of angiotensin AT2 receptors by the non-peptide agonist, Compound 21, evokes vasodepressor effects in conscious spontaneously hypertensive rats. Br J Pharmacol. 2010;159(3):709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carey RM, Howell NL, Jin X-H, Siragy HM. Angiotensin type 2 receptor-mediated hypotension in angiotensin type-1 receptor-blocked rats. Hypertension. 2001;38(6):1272–7. [DOI] [PubMed] [Google Scholar]
- 30.Brouwers S, Smolders I, Massie A, Dupont AG. Angiotensin II type 2 receptor-mediated and nitric oxide-dependent renal vasodilator response to compound 21 unmasked by angiotensin-converting enzyme inhibition in spontaneously hypertensive rats in vivo. Hypertension. 2013;62(5):920–6. [DOI] [PubMed] [Google Scholar]
- 31.Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res. 2012;35(6):654–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hilliard LM, Chow CL, Mirabito KM, Steckelings UM, Unger T, Widdop RE, et al. Angiotensin type 2 receptor stimulation increases renal function in female, but not male, spontaneously hypertensive rats. Hypertension. 2014;64(2):378–83. [DOI] [PubMed] [Google Scholar]
- 33.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, et al. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62(3):587–93. [DOI] [PubMed] [Google Scholar]
- 34.Sampson AK, Hilliard LM, Moritz KM, Thomas MC, Tikellis C, Widdop RE, et al. The arterial depressor response to chronic low-dose angiotensin II infusion in female rats is estrogen dependent. Am J Physiol Regul Integr Comp Physiol. 2012;302(1):R159–65. [DOI] [PubMed] [Google Scholar]
- 35.Ali Q, Patel S, Hussain T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese Zucker rats. Am J Physiol Renal Physiol. 2015;308(12):F1379–85.•This is the first study showing the antihypertensive effects of the AT2R agonist C21 in high sodium diet-fed obese Zucker rats
- 36.Kemp BA, Howell NL, Keller SR, Gildea JJ, Padia SH, Carey RM. AT2 receptor activation prevents sodium retention and reduces blood pressure in angiotensin II-dependent hypertension. Circ Res. 2016;119(4):532–543.••This manuscript demonstrates that AT2R agonist C21, both systemically and intrarenally, is effective in producing diuretic-natriuretic response through renal proximal tubules and prevented rise in arterial pressure. This effect is additive to the actions of diuretics (thiazide and amiloride) and does not require blockade of AT1R
- 37.Gao J, Zhang H, Le KD, Chao J, Gao L. Activation of central angiotensin type 2 receptors suppresses norepinephrine excretion and blood pressure in conscious rats. Am J Hypertens. 2011;24(6): 724–30.••This study demonstrates that centrally administered AT2R agonist reduced sympathetic outflow and hypertension through pathways that involve nitric oxide
- 38.Gao J, Zucker IH, Gao L. Activation of central angiotensin type 2 receptors by compound 21 improves arterial baroreflex sensitivity in rats with heart failure. Am J Hypertens. 2014;27(10):1248–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tirupula KC, Desnoyer R, Speth RC, Karnik SS. A typical signaling and functional desensitization response of MAS receptor to peptide ligands. PLoS One. 2014;9(7):e103520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sampaio WO, Souza dos Santos RA, Faria-Silva R, da Mata Machado LT, Schiffrin EL, Touyz RM. Angiotensin-(1–7) through receptor Mas mediates endothelial nitric oxide synthase activation via Akt-dependent pathways. Hypertension. 2007;49(1):185–92. [DOI] [PubMed] [Google Scholar]
- 41.Costa MA, Lopez Verrilli MA, Gomez KA, Nakagawa P, Pena C, Arranz C, et al. Angiotensin-(1–7) upregulates cardiac nitric oxide synthase in spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2010;299(4):H1205–11. [DOI] [PubMed] [Google Scholar]
- 42.Dias-Peixoto MF, Santos RA, Gomes ER, Alves MN, Almeida PW, Greco L, et al. Molecular mechanisms involved in the angiotensin-(1–7)/Mas signaling pathway in cardiomyocytes. Hypertension. 2008;52(3):542–8. [DOI] [PubMed] [Google Scholar]
- 43.Stegbauer J, Potthoff SA, Quack I, Mergia E, Clasen T, Friedrich S, et al. Chronic treatment with angiotensin-(1–7) improves renal endothelial dysfunction in apolipoproteinE-deficient mice. Br J Pharmacol. 2011;163(5):974–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cerniello FM, Carretero OA, Longo Carbajosa NA, Cerrato BD, Santos RA, Grecco HE, et al. MAS1 receptor trafficking involves ERK1/2 activation through a beta-arrestin2-dependent pathway. Hypertension. 2017;70(5):982–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tan Z, Wu J, Ma H. Regulation of angiotensin-converting enzyme 2 and Mas receptor by ang-(1–7) in heart and kidney of spontaneously hypertensive rats. J Renin-Angiotensin-Aldosterone Syst. 2011;12(4):413–9. [DOI] [PubMed] [Google Scholar]
- 46.e Silva AC, Bello AP, Baracho NC, Khosla MC, Santos RA. Diuresis and natriuresis produced by long term administration of a selective angiotensin-(1–7) antagonist in normotensive and hypertensive rats. Regul Pept. 1998;74:177–84. [DOI] [PubMed] [Google Scholar]
- 47.Porsti I, Bara AT, Busse R, Hecker M. Release of nitric oxide by angiotensin-(1–7) from porcine coronary endothelium: implications for a novel angiotensin receptor. Br J Pharmacol. 1994;111:652–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brosnihan KB, Li P, Ferrario CM. Angiotensin-(1–7) dilates canine coronary arteries through kinins and nitric oxide. Hypertension. 1996;27(3):523–8. [DOI] [PubMed] [Google Scholar]
- 49.Silva DM, Vianna HR, Cortes SF, Campagnole-Santos MJ, Santos RA, Lemos VS. Evidence for a new angiotensin-(1–7) receptor subtype in the aorta of Sprague-Dawley rats. Peptides. 2007;28(3):702–7. [DOI] [PubMed] [Google Scholar]
- 50.Sampaio WO, Nascimento AA, Santos RA. Systemic and regional hemodynamic effects of angiotensin-(1–7) in rats. Am J Physiol Heart Circ Physiol 2003;284:H1985–H1994. [DOI] [PubMed] [Google Scholar]
- 51.Kuczeriszka M, Kompanowska-Jezierska E, Sadowski J, Prieto M, Navar LG. Modulating role of ang-(1–7) in control of blood pressure and renal function in ang-II-infused hypertensive rats. Am J Hypertens. 2018;31(4):504–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Benter IF, Diz DI, Ferrario CM. Cardiovascular actions of angiotensin(1–7). Peptides. 1993;14:679–84. [DOI] [PubMed] [Google Scholar]
- 53.Savergnini SQ, Fraga-Silva RA, Ferreira AJ, RASd S. Mas receptor agonists as novel antihypertensive agents. Curr Hypertens Rev. 2012;8:24–34. [Google Scholar]
- 54.DelliPizzi A, Hilchey SD, Bell-Quilley CP. Natriuretic effect of angiotensin(1–7). Br J Pharmacol. 1994;111:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Handa RK, Ferrario CM, Strandhoy JW. Renal actions of angiotensin-(1–7)—in vivo and in vitro studies. Am J Physiol Renal Physiol. 1996;39:F141–7. [DOI] [PubMed] [Google Scholar]
- 56.Vallon V, Heyne N, Richter K, Khosla MC, Fechter K. [7-D-ala] angiotensin 1–7 blocks renal actions of angiotensin-(1–7) in the anesthetized rat. J Cardiovasc Pharmacol. 1998;32:164–7. [DOI] [PubMed] [Google Scholar]
- 57.Caruso-Neves C, Lara LS, Rangel LB, Grossi AL, Lopes AG. Angiotensin-(1–7) modulates the ouabain-insensitive Na+-ATPase activity from basolateral membrane of the proximal tubule. Biochim Biophys Acta. 2000;1467(1):189–97. [DOI] [PubMed] [Google Scholar]
- 58.Patel SN, Ali Q, Samuel P, Steckelings UM, Hussain T. Angiotensin II Type 2 receptor and receptor Mas are colocalized and functionally interdependent in obese Zucker rat kidney. Hypertension. 2017;71(2): 831–8.••Experiments performed in this study showed ligand independent interactions of naturally occurring angiotensin II type 2 receptor and MasR in obese Zucker rat model and human kidney-2 (HK-2) cells, and their inter-dependency in formation of nitrites and in producing diuretic-natriuretic response that is relevant in obesity hypertension
- 59.O’Neill J, Healy V, Johns EJ. Intrarenal Mas and AT1 receptors play a role in mediating the excretory actions of renal interstitial angiotensin-(1–7) infusion in anaesthetized rats. Exp Physiol. 2017;102(12):1700–15. [DOI] [PubMed] [Google Scholar]
- 60.Iyer SN, Averill DB, Chappell MC, Yamada K, Allred AJ, Ferrario CM. Contribution of angiotensin-(1–7) to blood pressure regulation in salt-depleted hypertensive rats. Hypertension. 2000;36:417–22. [DOI] [PubMed] [Google Scholar]
- 61.Santos RAS, Simoes e Silva AC, Magaldi AJ, Khosla MC, Cesar KR, Passaglio KT, et al. Evidence for a physiological role of angiotensin-(1–7) in the control of hydroelectrolyte balance. Hypertension. 1996;27(4):875–84. [DOI] [PubMed] [Google Scholar]
- 62.Grobe JL, Mecca AP, Mao H, Katovich MJ. Chronic angiotensin-(1–7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am J Physiol Heart Circ Physiol. 2006;290(6):H2417–23. [DOI] [PubMed] [Google Scholar]
- 63.Collister JP, Nahey DB. Simultaneous administration of ang-(1–7) or A-779 does not affect the chronic hypertensive effects of angiotensin II in normal rats. J Renin-Angiotensin-Aldosterone Syst. 2010;11(2):99–102. [DOI] [PubMed] [Google Scholar]
- 64.Ren X, Zhang F, Zhao M, Zhao Z, Sun S, Fraidenburg DR, et al. Angiotensin-(1–7) in paraventricular nucleus contributes to the enhanced cardiac sympathetic afferent reflex and sympathetic activity in chronic heart failure rats. Cell Physiol Biochem. 2017;42(6):2523–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han Y, Sun HJ, Li P, Gao Q, Zhou YB, Zhang F, et al. Angiotensin-(1–7) in paraventricular nucleus modulates sympathetic activity and cardiac sympathetic afferent reflex in renovascular hypertensive rats. PLoS One. 2012;7(11):e48966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li P, Sun HJ, Cui BP, Zhou YB, Han Y. Angiotensin-(1–7) in the rostral ventrolateral medulla modulates enhanced cardiac sympathetic afferent reflex and sympathetic activation in renovascular hypertensive rats. Hypertension. 2013;61(4):820–7. [DOI] [PubMed] [Google Scholar]
- 67.Li P, Zhang F, Sun HJ, Zhang F, Han Y. Angiotensin-(1–7) enhances the effects of angiotensin II on the cardiac sympathetic afferent reflex and sympathetic activity in rostral ventrolateral medulla in renovascular hypertensive rats. J Am Soc Hypertens. 2015;9(11):865–77. [DOI] [PubMed] [Google Scholar]
- 68.Walters PE, Gaspari TA, Widdop RE. Angiotensin-(1–7) acts as a vasodepressor agent via angiotensin II type 2 receptors in conscious rats. Hypertension. 2005;45(5):960–6. [DOI] [PubMed] [Google Scholar]
- 69.Santiago NM, Guimaraes PS, Sirvente RA, Oliveira LA, Irigoyen MC, Santos RA, et al. Lifetime overproduction of circulating angiotensin-(1–7) attenuates deoxycorticosterone acetate-salt hypertension-induced cardiac dysfunction and remodeling. Hypertension. 2010;55(4):889–96. [DOI] [PubMed] [Google Scholar]
- 70.Pinheiro SV, Simoes e Silva AC, Sampaio WO, de Paula RD, Mendes EP, Bontempo ED, et al. Nonpeptide AVE 0991 is an angiotensin-(1–7) receptor Mas agonist in the mouse kidney. Hypertension. 2004;44(4):490–6. [DOI] [PubMed] [Google Scholar]
- 71.Lemos VS, Silva DMR, Walther T, Alenina N, Bader M, Santos RAS. The endothelium-dependent vasodilator effect of the nonpeptide ang(1–7) mimic AVE 0991 is abolished in the aorta of Mas-knockout mice. J Cardiovasc Pharmacol. 2005;46:274–9. [DOI] [PubMed] [Google Scholar]
- 72.Carvalho MB, Duarte FV, Faria-Silva R, Fauler B, da Mata Machado LT, de Paula RD, et al. Evidence for Mas-mediated bradykinin potentiation by the angiotensin-(1–7) nonpeptide mimic AVE 0991 in normotensive rats. Hypertension. 2007;50(4):762–7. [DOI] [PubMed] [Google Scholar]
- 73.Savergnini SQ, Beiman M, Lautner RQ, de Paula-Carvalho V, Allahdadi K, Pessoa DC, et al. Vascular relaxation, antihypertensive effect, and cardioprotection of a novel peptide agonist of the MAS receptor. Hypertension. 2010;56(1):112–20. [DOI] [PubMed] [Google Scholar]
- 74.Benter IF, Yousif MH, Anim JT, Cojocel C, Diz DI. Angiotensin(1–7) prevents development of severe hypertension and end-organ damage in spontaneously hypertensive rats treated with L-NAME. Am J Physiol Heart Circ Physiol. 2006;290(2):H684–91. [DOI] [PubMed] [Google Scholar]
- 75.Santos RAS, Simoes e Silva AC, Maric C, Silva DMR, Machado RP, Id B, et al. Angiotensin-(1–7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc Nattl Acad Sci USA. 2003;100(14):8258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Castro CH, Santos RA, Ferreira AJ, Bader M, Alenina N, Almeida AP. Evidence for a functional interaction of the angiotensin-(1–7) receptor Mas with AT1 and AT2 receptors in the mouse heart. Hypertension. 2005;46(4):937–42. [DOI] [PubMed] [Google Scholar]
- 77.Tesanovic S, Vinh A, Gaspari TA, Casley D, Widdop RE. Vasoprotective and atheroprotective effects of angiotensin (1–7) in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2010;30(8):1606–13. [DOI] [PubMed] [Google Scholar]
- 78.Hao PP, Yang JM, Zhang MX, Zhang K, Chen YG, Zhang C, et al. Angiotensin-(1–7) treatment mitigates right ventricular fibrosis as a distinctive feature of diabetic cardiomyopathy. Am J Physiol Heart Circ Physiol. 2015;308(9):H1007–19. [DOI] [PubMed] [Google Scholar]
- 79.Teixeira LB, Parreiras ESLT, Bruder-Nascimento T, Duarte DA, Simoes SC, Costa RM, et al. Ang-(1–7) is an endogenous beta-arrestin-biased agonist of the AT1 receptor with protective action in cardiac hypertrophy. Sci Rep. 2017;7(1):11903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Galandrin S, Denis C, Boularan C, Marie J, M’Kadmi C, Pilette C, et al. Cardioprotective angiotensin-(1–7) peptide acts as a natural-biased ligand at the angiotensin II type 1 receptor. Hypertension. 2016;68(6): 1365–1374.••This is the first report showing biased signaling of ang-(1–7) through AT1R/beta-arrestin pathway in cardioprotection.
- 81.Bruce E, Shenoy V, Rathinasabapathy A, Espejo A, Horowitz A, Oswalt A, et al. Selective activation of angiotensin AT2 receptors attenuates progression of pulmonary hypertension and inhibits cardiopulmonary fibrosis. Br J Pharmacol. 2015;172(9):2219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Villela D, Leonhardt J, Patel N, Joseph J, Kirsch S, Hallberg A, et al. Angiotensin type 2 receptor (AT2R) and receptor Mas: a complex liaison. Clin Sci (Lond). 2015;128(4):227–34. [DOI] [PubMed] [Google Scholar]
- 83.Leonhardt J, Villela DC, Teichmann A, Munter LM, Mayer MC, Mardahl M, et al. Evidence for heterodimerization and functional interaction of the angiotensin type 2 receptor and the receptor MAS. Hypertension. 2017;69(6):1128–35.••This is the first in vitro report showing heterodimerization of AT2R with MasR through Cys35 residue and their inter-dependence in astrocytes
- 84.Feng Y-H, Saad Y, Karnik SS. Reversible inactivation of AT2 angiotensin II receptor from cysteine-disulfide bond exchange. FEBS Lett. 2000;484:133–8. [DOI] [PubMed] [Google Scholar]
- 85.Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT(2) receptor activation induces natriuresis and lowers blood pressure. Circ Res. 2014;115(3):388–399.••This paper shows that in renal proximal tubule, the C21-activated AT2R translocates to apical membrane, facilitates internalization of sodium transporters, induces natriuresis, and lowers blood pressure.
- 86.Xue B, Zhang Z, Johnson RF, Guo F, Hay M, Johnson AK. Central endogenous angiotensin-(1–7) protects against aldosterone/NaCl-induced hypertension in female rats. Am J Physiol Heart Circ Physiol. 2013;305(5):H699–705.••This study shows that central stimulation of MasR with ang-(1–7) reduces the development of aldosterone-salt hypertension in female rats
- 87.Forte BL, Slosky LM, Zhang H, Arnold MR, Staatz WD, Hay M, et al. Angiotensin-(1–7)/Mas receptor as an antinociceptive agent in cancer-induced bone pain. Pain. 2016;157(12):2709–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wruck CJ, Funke-Kaiser H, PufeT, Kusserow H,Menk M,Schefe JH, et al. Regulation of transport of the angiotensin AT2 receptor by a novel membrane-associated Golgi protein. Arterioscler Thromb Vasc Biol. 2005;25(1):57–64. [DOI] [PubMed] [Google Scholar]
- 89.de Kloet AD, Pitra S, Wang L, Hiller H, Pioquinto DJ, Smith JA, et al. Angiotensin type-2 receptors influence the activity of vasopressin neurons in the paraventricular nucleus of the hypothalamus in male mice. Endocrinology. 2016;157(8):3167–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.de Kloet AD, Wang L, Ludin JA, Smith JA, Pioquinto DJ, Hiller H, et al. Reporter mouse strain provides a novel look at angiotensin type-2 receptor distribution in the central nervous system. Brain Struct Funct. 2016;221(2):891–912.•This study characterizes the distribution of the AT2R in AT2R-eGFP mouse model and reports that AT2R is localized with AT1R in some brain nuclei, particularly in paraventricular, median preoptic nucleus, nucleus of the solitary tract, and the area postrema
- 91.Coleman CG, Anrather J, Iadecola C, Pickel VM. Angiotensin II type 2 receptors have a major somatodendritic distribution in vasopressin-containing neurons in the mouse hypothalamic paraventricular nucleus. Neuroscience. 2009;163(1):129–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Diniz C, Casarotto PC, Fred SM, Biojone C, Castren E, Joca SRL. Antidepressant-like effect of losartan involves TRKB transactivation from angiotensin receptor type 2 (AGTR2) and recruitment of FYN. Neuropharmacology. 2018;135:163–71. [DOI] [PubMed] [Google Scholar]
- 93.Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018;19(3):e876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.de Kloet AD, Pati D, Wang L, Hiller H, Sumners C, Frazier CJ, et al. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus protect against diet-induced obesity. J Neurosci. 2013;33(11):4825–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miura S, Matsuo Y, Kiya Y, Karnik SS, Saku K. Molecular mechanisms of the antagonistic action between AT1 and AT2 receptors. Biochem Biophys Res Commun. 2010;391(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Horiuchi M, Hayashida W, Akishita M, Tamura K, Daviet L, Lehtonen JYA, et al. Stimulation of different subtypes of angiotensin II receptors, AT1 and AT2 receptors, regulates STAT activation by negative crosstalk. Circul Res. 1999;84:876–82. [DOI] [PubMed] [Google Scholar]
- 97.Anguiano-Robledo L, Reyes-Melchor PA, Bobadilla-Lugo RA, Perez-Alvarez VM, Lopez-Sanchez P. Renal angiotensin-II receptors expression changes in a model of preeclampsia. Hypertens Pregnancy. 2007;26(2):151–61. [DOI] [PubMed] [Google Scholar]
- 98.Inuzuka T, Fujioka Y, Tsuda M, Fujioka M, Satoh AO, Horiuchi K, et al. Attenuation of ligand-induced activation of angiotensin II type 1 receptor signaling by the type 2 receptor via protein kinase C. Sci Rep. 2016;6:21613.••Using FRET analysis, specifically, this study has confirmed that upon ang-II stimulation, the conformation of AT2R-AT1R is modulated that results in internalization of the AT2R-AT1R heteromer
- 99.Ferrao FM, Cardoso LHD, Drummond HA, Li XC, Zhuo JL, Gomes DS, et al. Luminal ang-II is internalized as a complex with AT1R/ AT2R heterodimers to target endoplasmic reticulum in LLC-PK1 cells. Am J Physiol Renal Physiol. 2017;313(2):F440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Villar-Cheda B, Costa-Besada MA, Valenzuela R, Perez-Costas E, Melendez-Ferro M, Labandeira-Garcia JL. The intracellular angiotensin system buffers deleterious effects of the extracellular paracrine system. Cell Death Dis. 2017;8(9):e3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bian J, Zhang S, Yi M, Yue M, Liu H. The mechanisms behind decreased internalization of angiotensin II type 1 receptor. Vasc Pharmacol 2018. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 102.Canals M, Jenkins L, Kellett E, Milligan G. Up-regulation of the angiotensin II type 1 receptor by the MAS proto-oncogene is due to constitutive activation of Gq/G11 by MAS. J Biol Chem. 2006;281(24):16757–67. [DOI] [PubMed] [Google Scholar]
- 103.Clark MA, Tallant EA, Diz DI. Downregulation of the AT1a receptor by pharmacologic concentrations of angiotensin-(1–7). J Cardiovasc Pharmacol. 2001;37:437–48. [DOI] [PubMed] [Google Scholar]
- 104.Clark MA, Tallant EA, Tommasi E, Bosch S, Diz DI. Angiotensin-(1–7) reduces renal angiotensin II receptors through a cyclooxygenase-dependent mechanism. J Cardiovasc Pharmacol. 2003;41:276–83. [DOI] [PubMed] [Google Scholar]
- 105.Halbach OV, Walther T, Bader M, Albrecht D.Interaction between Mas and the angiotensin AT1 receptor in the amygdala. J Neurophysiol. 2000;83:2012–21. [DOI] [PubMed] [Google Scholar]