Abstract
The current pandemic COVID-19, caused by the SARS-CoV-2 virus, has been affecting thousands of people worldwide, promoting high numbers of deaths. With this, the world population is going through a process of changing habits, with social distance, improvement of hygiene techniques, to reduce the spread of the SARS-CoV-2 virus and, consequently, reduce the number of hospitalized people in serious condition, as well as the mortality rate. This scenario has been promoting a continuous search for researchers, in the most varied areas, for possible methods of prevention or cure. Specifically, in the field of pharmaceutical nanotechnology, a variety of products are being developed against SARS-CoV-2. Under these circumstances, we propose here an exposition of some of the nanotechnological products (nanoscale between 1 to 1000 nm) currently designed for the detection of the virus, for the prevention and treatment of COVID-19, in addition to equipment for personal protection. We believe that pharmaceutical nanotechnology will be a valuable tool in the disease from the development of products that guarantee our protection against the SARS-CoV-2 virus.
Keywords: Nanomedicine, Nanotechnology products, COVID-19, SARS-CoV-2
Introduction
Pharmaceutical nanotechnology is an emerging technology that can be used in a wide range of products, including medical, food, and cosmetics (FDA 2014). These products are the ones in the nanoscale range, with sizes ranging from 1 to 1000 nm. They may have different chemical or physical properties or biological effects when compared to products with a larger scale (Melo et al. 2015; Jeevanandam et al. 2018; Khan et al. 2019). Currently, there are 8879 nanotechnology products registered in the Nanotechnology Products Database, of which 2467 companies are responsible for the production, spread in 62 countries worldwide (Nanotechnology Products Database 2020).
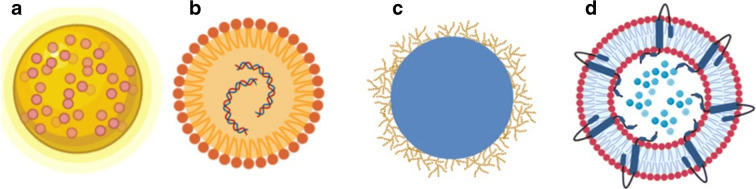
Nanotechnology allows the increase of the bioavailability of a drug, as well as reduction of the dose and improvement of the therapeutic effect, in addition to the decrease of its toxicity. In the food area, it can be used in the production of food packaging, microbiological protection, or improved delivery of a functional ingredient or nutrient. Besides, it can also be applied in the detection of pathogens (He and Hwang 2016; Singh et al. 2017; Jampilek et al. 2019). Currently, as will be detailed in this manuscript, several nanosystems are being developed and tested in the most diverse areas, such as virology, medicine, chemistry, biomedical, pharmaceutical, engineering, computational science, and technological, focusing on the development of protective and prevention equipment for SARS-CoV-2, as well as in the diagnosis and treatment of COVID-19 (Weiss et al. 2020). Figure 1 illustrates examples of the main nanosystems chosen by several companies for the development of their products.
Fig. 1.
Examples of nanosystems intended as an alternative against COVID-19. Gold nanoparticles containing proteins on the surface (a). Lipid nanoparticles for carrying genetic material (b). Chitosan nanoparticles (c). Targeted liposomes with proteins on their surface (d)
COVID-19 is an infectious disease caused by the SAR-CoV-2 virus, belonging to the coronavirus family. It was responsible for the pandemic outbreak that began in China in 2019 and has been affecting the world, infecting 21,294,845 people and causing 761,779 deaths (data obtained on August 16, 2020) (Li et al. 2020a; Mackenzie and Smith 2020; Chakraborty and Maity 2020; WHO 2020a). Currently, there is no therapeutic choice available that inhibits the proliferation of the virus, which has caused an outbreak in health systems, leading thousands of people to be hospitalized, requiring mechanical respirators, since the virus has high attractiveness to the respiratory system (Alhazzani et al. 2020; Giwa et al. 2020).
In addition to overcrowding in the health system, exposing health professionals who are on the frontline against the pandemic, the economy has felt a significant impact due to the determination of the World Health Organization, which emphasizes that social distancing is the main form of prevention against the virus, avoiding crowding as well as contact between people (Oliveira et al. 2019; Chopra et al. 2020).
Despite the challenges in finding the most suitable therapy and the best way to reduce transmission of the virus, pharmaceutical nanotechnology can be a valuable tool. Focused not only on the development of vaccines and drugs with virus targeting but also on the development of equipment that minimizes people’s exposure to viruses and allowing their safety, maintaining the daily routine of each person in the world. The information about all nanotechnological products used in this manuscript was collected from the Nanotechnology Products Database Web site. This specialized site aids the dissemination of nanotechnological products that are being developed and tested to be inserted in the market.
Nanotechnology in the detection of SARS-CoV-2
The diagnosis of SARS-CoV-2 depends on the knowledge of the viral structure as well as its genetic material. Viral replication occurs from virus adsorption to host cells to the release of new viral particles, in which SARS-CoV-2 is released by budding and adsorbed in neighboring cells (Astuti and Ysrafil 2020; Li et al. 2020b). Currently, the tests used for the detection of SARS-CoV-2 are C-reactive protein (CRP) and serology, in which CRP confirms the presence of the virus using a piece of genetic code to identify it. At the same time, the serological is based on the immunological response to the virus (Udugama et al. 2020).
The serological examination allows detecting the levels of IgM and IgG antibodies in a blood sample of patients, and it is advisable to perform this test from the seventh day after the onset of symptoms, being necessary time for the production of antibodies (Zhao et al. 2020; Li et al. 2020c). CRP is capable of detecting the presence of the virus, being performed from material collected from the throat and nose, as well as in lung secretions, detecting the genetic material of the virus in the patient’s sample. CRP can identify the presence of the virus on average until the day of symptoms (Udugama et al. 2020; Tang et al. 2020).
Focused on the development of efficient and fast approaches for the diagnosis of COVID-19, US researchers designed a rapid test based on gold nanoparticles, thereby maximizing the sensitivity and reliability of the tests. The authors attached a specific molecule to the gold nanoparticles that detect a particular protein from the genetic sequence of the SARS-CoV-2 virus. This occurs when the biosensor binds to the virus’s gene sequence, and the gold nanoparticles respond by transforming the liquid reagent from purple to blue. According to the developers, the test, based on the use of gold nanoparticles, will not allow false positive nor negative results, as the nanoparticles interact directly with the specific genetic sequence of the virus, in addition to allowing diagnosis in 10 min (Draz and Shafiee 2018; Nanotechnology Products Database 2020). Gold nanoparticles are widely used for a biosensing application (due to their biocompatibility, optical and electronic properties, besides their relatively simple production and modification). Gold nanoparticles serve as markers when antibodies or DNA strands are attached to their surface. Also, these nanoparticles can form a powerful transduction platform for the detection of unique molecules (Holzinger et al. 2014; Malekzad et al. 2017).
Gold nanoparticles have been widely used for the diagnosis of several viruses (Holzinger et al. 2014; Malekzad et al. 2017; Draz and Shafiee 2018; Hamdy et al. 2018). This use is because they allow the coupling of biological molecules forming hybrid structures Bio-AuNP, promoting a target detection of the virus, thereby increasing the sensitivity of the test, a better detection range, and reduction in detection time (Draz and Shafiee 2018). Researchers in the UK also used gold nanoparticles for the development of a rapid detection kit for IgG and IgM antibodies, achieving the results in 10 min (Nanotechnology Products Database 2020).
Seeking to develop a biosensor for the detection of SARS-CoV-2, Qiu et al. (2020) used two-dimensional gold nanoparticles functionalized with complementary DNA receptors. This dual-functional plasmid biosensor combines the plasmatic photothermal effect with localized plasmon resonance transduction, showing high sensitivity to SARS-CoV-2 with a lower detection limit up to a concentration of 0.22 pM, in addition to allowing accurate detection in a multigenic mixture.
Zhu et al. (2020) proposed a diagnostic technique for COVID-19 using nanoparticles, to which they created an isothermal amplification mediated by a multiplex reverse transcription loop coupled to a nanoparticle-based lateral flow biosensor. The results showed that the analytical technique showed a sensitivity of 100% for SARS-CoV-2, after the analysis of 33 oropharyngeal samples from patients with COVID-19. In addition, they showed 100% specificity after analyzing 96 samples from patients without COVID-19.
For the diagnosis of COVID-19, it requires samples collected in the nose or throat, which should preferably be transported in refrigerated media. However, refrigerated means of transport are not universally available. It is subject to worldwide scarcity, leading to the risk of compromised tests due to drying, contamination, or degradation of the samples, especially if transported at room temperature (Druce et al. 2012; Nanotechnology Products Database 2020). With this in mind, researchers in Singapore developed a sample collection kit containing an RNA stabilization fluid, using nanotechnology. The kit allows viral RNA to remain stable at room temperature for up to a week, facilitating its transportation (Nanotechnology Products Database 2020). Table 1 lists COVID-19 diagnostic tests developed based on nanotechnology.
Table 1.
Nanotechnological products for the diagnosis of COVID-19
| Test | Manufacturer | Country |
|---|---|---|
| COVID-19 Rapid POC CE-IVD | NanoComposix | USA |
| COVID-19 Rapid Test Cassette | SureScreen Diagnostics Ltd | UK |
| COVID-19 point-of-need diagnostic test | Mologic Ltd | UK |
| SAFER-sample Kit | Lucence Diagnostics Pte Ltd | Singapore |
Source: Nanotechnology Products Database (2020)
Nanotechnology for the prevention and treatment of COVID-19
One of the major problems to reduce SARS-CoV-2 damage is the absence, until now, of effectively and safely treated with few unwanted side effects. The best approach to introduce rapid therapy is to test drugs that are already available on the market, as these have a safety profile previously widely studied (Dorward and Gbinigie 2020; Singh et al. 2020). Nevertheless, the pathophysiological characteristics of COVID-19 should be taken into consideration, since drugs, as an example of hydroxychloroquine and chloroquine, present a predisposition to the development of cardiotoxicity in patients with COVID-19 (Monteiro et al. 2020; Driggin et al. 2020; Mehra et al. 2020).
Several drugs are being tested and listed as possible treatments for COVID-19, including chloroquine, hydroxychloroquine, ritonavir, ribavirin, umifenovir, interferon, nitazoxanide, remdesivir, and favipiravir (Sanders et al. 2020). Despite the wide variety of drugs tested, there is still no consensus about an effective and safe therapeutic alternative. According to the literature, nanotechnology provides advantages from the use of nanosystems, such as liposomes, polymeric and lipid nanoparticles, metallic nanoparticles, and micelles, for the encapsulation of drugs, promoting the improvement of pharmacological drug properties (Youssef et al. 2019; Lombardo et al. 2019; Weiss et al. 2020). Nanotechnology could provide safety and effectiveness for the treatment of COVID-19, enabling the encapsulation of drugs, their targeting at specific sites, and the reduction of drug toxicity (Lombardo et al. 2019; Weiss et al. 2020; Peng et al. 2020).
Another challenge in the treatment of COVID-19 is that some compounds after dilution, lose their effectiveness as the virus-compound complex dissociates. As a consequence, this allows the virus, when free, restarts the replication cycle. From the use of nanoparticles, there is a possibility of irreversibly inhibiting viral infectivity, as nanoparticles permanently cause damage to the virus (Weiss et al. 2020).
Researchers from Cyprus have developed chitosan nanoparticles for aerosol application, which allows the adhesion and targeting of drugs to the epithelial tissues of the lung and ensures controlled release, thereby reducing the toxicity profile of the drugs (Novochizol 2020). The authors state that chitosan nanoparticles, called Novochizol, allow the encapsulation of several drugs to carry them to the lungs to treat severe COVID-19 infections. According to the developers of this product, Novochizol aerosols can provide a therapeutic dose to a patient for a period ranging from 25 min to 3 h (Nanotechnology Products Database 2020; Novochizol 2020).
Chitosan has been widely used for the development of nanoparticles due to its biodegradability as well as its low toxicity both in vitro and in vivo models (Mohammed et al. 2017). Chitosan nanoparticles present several applications in drug administration for the treatment of cancer, gastrointestinal diseases, carrying drugs to the brain, as well as in the treatment of lung diseases and infections (Grenha et al. 2005; Pourshahab et al. 2011; Demir and Degim 2013; Islam and Ferro 2016; Mohammed et al. 2017; Wei et al. 2020). Furthermore, studies show the applicability of chitosan nanoparticles in the transport of drugs to the lung (Grenha et al. 2005; Pourshahab et al. 2011; Islam and Ferro 2016), thus being a useful alternative for the transport of drugs focused on the treatment of COVID-19.
Due to its mucoadhesive properties, chitosan nanoparticles can also be used to treat reactions caused in the intestinal tract due to the development of COVID-19 (Ways et al. 2018). According to Zuo et al. (2020), the infection of SARS-CoV-2 in gastrointestinal tissues causes changes in the fecal microbiome, even after the elimination of SARS-CoV-2, where the evaluated patients continued to present impoverished symbionts and intestinal dysbiosis. Additionally, the presence of some microorganisms, such as Coprobacillus, Clostridium ramosum, and Clostridium hathewayi, seem to be related to the severity of COVID-19. Therefore, these chitosan nanoparticles could offer benefits to patients both at the pulmonary level, as well as in the gastrointestinal reactions caused by SARS-CoV-2.
The use of nitric oxide (NO) nanoparticles can also be an alternative in the treatment of COVID-19. A study with SARS-CoV-1 observed that NO inhibits viral replication by the cytotoxic reaction from intermediary agents such as peroxynitrite (Akerstrom et al. 2005). Because SARS-CoV-2 infects endothelial cells, which are a source of NO, carrying NO from nanoparticles may be an alternative for NO replacement, as well as a response to the viral attack on endothelial cells. In addition to inhibiting viral replication, NO can prevent the onset of inflammatory processes based on hypoxia-reoxygenation/ischemia-reperfusion, control the cytokine cascade, allow the removal of cell fragments, limit lipid peroxidation and cell damage, reduce damaging vascular permeability, and maintain adequate blood flow (Adusumilli et al. 2020).
Abo-Zeid et al. (2020) carried out a molecular docking study as a proposal to reuse iron oxide nanoparticles approved by the FDA to control SARS-CoV-2 infection. Iron oxide nanoparticles have been approved for the treatment of anemias, but some studies report possible antiviral activity. According to the authors of this work, the iron oxide nanoparticles can interact with the domain connected with the S1-RBD protein receptor, being this receptor used by SARS-CoV-2 in the infection of host cells and they can be an alternative in the treatment of COVID-19.
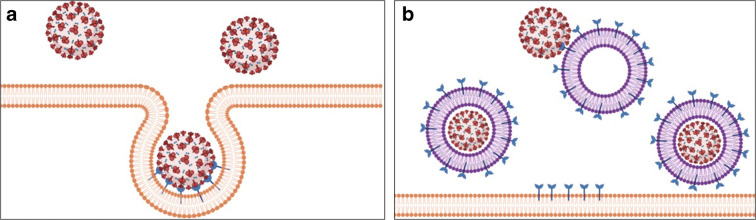
Zhang et al. (2020) developed cell nanosponges using plasma membranes derived from pulmonary type II epithelial cells or human macrophages. These nanosponges attract SARS-CoV-2, as they have on their surface binding receptors used by SARS-CoV-2 to enter the cell. After being captured by the nanosponges, SARS-CoV-2 is neutralized, preventing it from infecting other cells (Fig. 2).
Fig. 2.
Cell internalization mechanism of SARS-CoV-2 after binding with receptors (a) and mechanisms of action of nanosponges in the inactivation of SARS-CoV-2 (b)
Itani et al. (2020) discuss the implementation of theranostic nanoparticles focusing on intranasal administration for the treatment of COVID-19. Theranostic nanoparticles allow the combination of specific targeted therapy based on diagnostic tools, thus enabling the carrying of various therapeutic portions such as siRNA, peptide, and antibodies. The nanosystems allow the protection of therapeutic portions against enzyme degradation, extend their residence and release time, ensure their co-delivery with adjuvants, increase the concentration of conjugated materials in the target cells, offer receptor-binding-mediated targeting delivery, and potentiate the immune system. Several types of theranostic nanoparticles have been proposed as promising for intranasal administration, such as nanoparticles of organic, inorganic, and virus-like or automatic-mounted proteins. These nanoparticles can be an alternative both for the development of vaccines as well as in the development of drugs against the SARS-CoV-2 virus.
The development of vaccines is one of the great hopes of researchers, as it would be a powerful weapon to prevent COVID-19 and provide a reduction in the number of infections with a consequent reduction in mortality. Nanotechnology has been a promising ally for the development of vaccine prototypes. Researchers at the University of Waterloo in Canada are developing a DNA-based vaccine delivered through a nasal spray, in which the goal is to administer modified bacteriophages that stimulate an immune response in the nasal cavity and tissues of the lower respiratory tract (Erdmann and Barciszewski 2013; Nanotechnology Products Database 2020). The vaccine provides designed nanomedicine that allows its administration in a noninvasive manner, promoting immunization and decreasing COVID-19 infection. The virus-like particle (VLP) will be similar to the structure of SARS-CoV-2, being harmless, thus allowing the activation of the body’s natural immune response and providing protection to viral infections compatible with VLP, including SARS-CoV-2. Also, this nanosystem may bind specific receptors to SARS-CoV-2, thereby limiting possible transmission sites (Nanotechnology Products Database 2020).
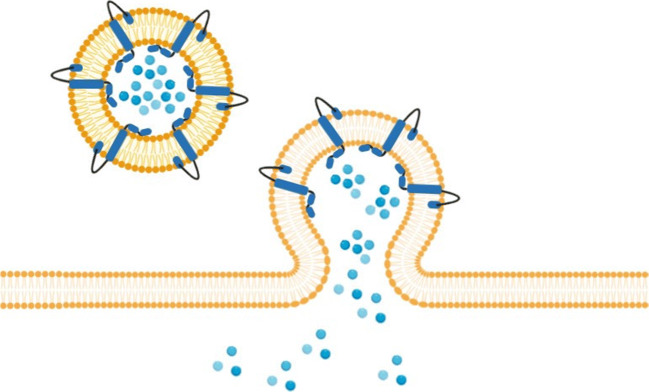
Also, seeking to develop a DNA vaccine, Entos Pharmaceuticals is developing a Fusogenix DNA vaccine to prevent COVID-19 infection. The goal is to promote the direct introduction of an antigen-encoding plasmid that stimulates the immune response by stimulating B and T cells. Entos’ Fusogenix platform is a proteolipid vehicle that uses a new fusogenix mechanism to provide its genetic load directly inside cells, allowing it to generate protection mechanisms against various structural components of SARS-CoV-2 (Nanotechnology Products Database 2020). Fusogenix is a formulation of lipid nanoparticles that supplies intact and unmodified molecules, directing in the cytosol of target cells (Fig. 3). It can be applied to a variety of therapeutic types, including gene therapy, mRNA, miRNA, RNAi, CRISPR, and small molecule medications (Kaczmarek et al. 2017; Nanotechnology Products Database 2020; Entos Pharma 2020). The use of plasmid DNA in a vaccine will allow the development of an advanced optimized payload that encodes various protein epitopes of the crucial immunogenic proteins of SARS-CoV-2. These protein epitopes will activate the natural production of antibodies in the body, as well as the protective immune response for the prevention of COVID-19 (Donnelly and Ulmer 1999; Kaczmarek et al. 2017; Entos Pharma 2020; Saylor et al. 2020).
Fig. 3.
Mechanism of action of fusogenix based on lipid nanoparticles
US researchers are developing a vaccine applying the modified smallpox virus, designed to express a virus protein that causes COVID-19. While researchers in Canada have produced a particle similar to the coronavirus virus, called VLP, they are also in search of antibodies against SARS-CoV-2 for use as a vaccine. Also focused on strengthening the immune system, GeoVax has used its experience with GV-MVA-VLPTM vaccines to design and produce vaccine candidates using genetic sequences of SARS-CoV-2, in which on this platform, the MVA, a large virus covers carrying various antigens of the vaccine, expresses proteins that group in the VLP immunogens of the person receiving the vaccine, where the production of VLPs in the vaccinated person mimics the production of viruses in a natural infection, with this stimulating the immune system (Nanotechnology Products Database 2020).
Ufovax has developed an automatic-mount protein nanoparticle vaccine platform (1c-SApNP) for a vaccine against SARS-CoV-2. The vaccine prototype presents parts of SARS-CoV-2 proteins that protrude from a scaffold of protein nanoparticles, aiming to stimulate the immune response and stimulate the development of antibodies to neutralize SARS-CoV-2. This same platform has already been used as a promising candidate for vaccines to cope with HIV, HCV, Ebola, and RSV (Nanotechnology Products Database 2020). The vaccine consists of self-assembling nanoparticles made of proteins, identical to SARS-CoV-2, which proteins are synthesized by inserting a single plasmid encoding the relevant gene into a host cell, followed by one-step expression and two subsequent purifications. The company points out that the large-scale production process of these vaccines has been validated by external industrial partners (Nanotechnology Products Database 2020).
Another proposal vaccine is being developed by Arcturus Therapeutics in the USA. The proposal is to combine a self-replicating RNA with LUNAR®, which is a non-viral release system of nanoparticles, to produce proteins within the human body. In preclinical models, the researchers found that STARRTM technology is effective at extraordinarily low doses—30 times more efficient than traditional mRNA (Nanotechnology Products Database 2020). Table 2 shows examples of vaccine proposals that were designed and are currently being tested against COVID-19.
Table 2.
Vaccines developed based on nanotechnological products to prevent COVID-19
| Vaccine | Manufacturer | Country |
|---|---|---|
| Vaccine with DNA | Mediphage Bioceuticals Inc | Canada |
| TNX-801 | Tonix Pharmaceuticals Holding Corp. | USA |
| VLP | Medicago Inc | Canada |
| GV-MVA-VLPTM | GeoVax Inc | USA |
| DNA Fusogenix | Entos Pharmaceuticals | Canada |
| Matrix-M™ | Novavax Inc | USA |
| Vaccine mRNA-1273 | Moderna Inc | USA |
| Ad5-nCoV | Cansino Biologics Inc | China |
| 1c-SApNP | Ufovax LLC | USA |
| STARR™ | Arcturus Therapeutics Ltd | USA |
Source: Nanotechnology Products Database (2020)
Nanotechnology for the development of personal protective equipment
It is known that the main route of transmission of SARS-CoV-2 is through the nasal route. With the lack of effective treatment and the high spread of the SARS-CoV-2 virus, efforts have arisen to stop the virus transmission either through social distancing as well as the reinforcement of hygiene methods. As an example is advised the use of personal safety equipment for the entire population, with the recommendation of the use of masks, restriction of contact between people and care with the hygiene of hands and shared use objects (WHO 2020b; Oliveira et al. 2020; Kissler et al. 2020).
Centered on the individual and collective care, numerous companies are investing in products from the nanotechnology field, aiming for the creation of personal protective equipment, as well as for the development of cleaning products that protect against COVID-19. Studies highlight the importance of silver nanoparticles in antimicrobial infections, presenting long-lasting activity and a safety profile (Durán et al. 2010; Marassi et al. 2018; Lee et al. 2019; Simbine et al. 2019; Dung et al. 2020). With this, silver nanoparticles have been considered the most useful metal disinfectant against bacteria, viruses, and other eukaryotic microorganisms due to the constant release of silver ions by slow oxidation (Wang et al. 2019). Silver nanoparticles can be applied in a practical way in our daily life, in different sectors, such as silver-based air/water filters, textiles, animal husbandry, biomedical and food packaging, among other applications (Deshmukh et al. 2019).
SHEPROS has developed a silver nanoparticle-based disinfectant that can be used in handwashing, which ensures that it kills 99% of germs and bacteria. Still aiming to seek product disinfection, NanoTouch has developed scarves that allow the disinfection of door handles, elevator buttons, and rear telephone, protecting them against the SARS-CoV-2 virus. The scarves are based on mineral nanocrystals that create an oxidation reaction, continuously oxidizing organic contaminants for 24/7 (King et al. 2018; Nanotechnology Products Database 2020).
Graphene has a good capacity for viral inhibition. The large graphene surface provides the largest contact area of the ligand for the adsorption of negatively charged sulfates, where they can interact with the positively charged residues of the virions and block these microorganisms (Palmieri and Papi 2020). Graphene has shown activity against African swine fever virus, orthopoxvirus strains, and herpesviruses (Ziem et al. 2016; Ziem et al. 2017). When functionalized in nanoparticles, graphene presented activities against herpes simplex viruses type 1 (Ziem et al. 2016) and respiratory syncytial virus (Deokar et al. 2017; Yang et al. 2017). The use of graphene is extensive, including the area of textile products to control the spread of the pandemic (Palmieri and Papi 2020). US researchers have developed a mask using graphene that prevents the growth of bacteria on its surface and is effective at stopping 99% of particles above 0.3 μm and 80% of particles smaller than 0.3 μm. Researchers in the UK have also developed a graphene mask to protect against coronavirus. Promethean Particles has been developing, in association with textile companies, using copper nanoparticles designed for use in personal protective equipment in fabrics (Nanotechnology Products Database 2020).
Following another path, the University of Houston has proposed coating respiratory masks with a hydrophobic material, and this material can provide the most resistant mask to SARS-CoV-2. Another mask developed was the MVX Nano Mask™, which according to the manufacturers, is a sanitizing and self-cleaning mask capable of killing 99.9% of all viruses and bacteria that come into contact, being effective against coronavirus. Another mask also developed based on nanoparticles is ReSpimask®, which uses copper oxide nanoparticles, where, according to producers, they have a filtration efficiency of 99.9% for viruses and bacteria (Nanotechnology Products Database 2020).
In addition to allowing the development of masks, nanoparticles are also being used to simulate the exposure of masks to the SARS-CoV-2 virus. Lustig et al. (2020) developed aerosols containing fluorescent virus-like nanoparticles to track transmission through materials that help greatly in the accuracy of detection. The authors noted that materials with absorbent hydrophilic layers and hydrophobic barrier layers are effective in preventing the transmission of the virus.
Besides masks and cleaning products, some purifiers are also being provided to prevent airborne infection by SARS-CoV-2. TEQOYA has developed the TeqAir 200 air ionizer purifier. Another purifier proposal is produced in Finland that allows the purification of buildings and public offices, based on AAVI technology® IonJet. American researchers have also developed a purifier that captures 99.5% of viruses and bacteria from 6 to 12 air exchanges per hour (Nanotechnology Products Database 2020). Given the above information, we summarize in Table 3 several products used to protect against SARS-CoV-2 infection.
Table 3.
Nanotechnological products for individual and collective prevention against COVID-19
| Products | Manufacturer | Country |
|---|---|---|
| Nano Silver Multipurpose Sanitiser | SHEPROS SDN. BHD. | Malaysia |
| Guardian G-Volt | LIGC Applications Ltd. | USA |
| G+Fibrics | Directa Plus PLC | UK |
| Antiviral fabrics | Promethean Particles Ltd | UK |
| Respiratory masks | Integricote Inc | USA |
| NanoSeptic | NanoTouch Materials, LLC | USA |
| Nano Mask™ | MVX Prime Ltd | UK |
| ReSpimask® | RESPILON Group s.r.o. | Czech Republic |
| Nanofiber mask | YAMASHIN-FILTER CORP. | Japan |
| NanoHack | Copper 3D Antibacterial Innovations | Chile |
| TeqAir 200 air ionizer | TEQOYA | France |
| AAVI Leaf® | AAVI Technologies Co. | Finland |
| HealthPro® Compact Air Purifier | Turn-Key Environmental Consultants | USA |
| Mack Antonoff HVAC | Mack Antonoff HVAC | USA |
| Air Decontamination Units | Genano Ltd | Finland |
Source: Nanotechnology Products Database (2020)
Conclusion
Since the discovery of nanotechnology, the development of nanoscale products has enabled a growth in several fields. Initially, occurred an evolution in the area of technology. However, recently with the implementation of nanomedicine, that aims to enhance the therapeutic effects of drugs, as well as to reduce their toxicity, it was observed a significant improvement on therapeutic care of numerous diseases, from cancer to several infections. Amid the pandemic we are experiencing, and with the extensive knowledge that scientists have developed over the years, nanotechnology seems to play a key role against COVID-19.
The appropriate knowledge of pharmaceutical nanotechnology has promoted the development of controlled release nanosystems that target drugs to the respiratory system, as well as the development of products that assist in the rapid and effective diagnosis of COVID-19, as well as in the development of protective equipment that prevents infection by SARS-CoV-2, being in this sense hope against COVID providing the resumption of the routine of people around the world.
Acknowledgments
Availability of data and material
Not applicable.
Code availability
Not applicable.
Funding
Iago Dillion Lima Cavalcanti and Mariane Cajubá de Britto Lira Nogueira are supported by the Council for Scientific and Technological Development (CNPq), CNPq #141307/2020-0 and CNPq #427243/2016-5, respectively.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Iago Dillion Lima Cavalcanti, Email: iagodillion@hotmail.com.
Mariane Cajubá de Britto Lira Nogueira, Email: mariane.lira@ufpe.br.
References
- Abo-Zeid Y, Ismail NSM, McLean GR, Hamdy NM (2020) A molecular docking study repurposes FDA approved iron oxide nanoparticles to treat and control COVID-19 infection. Eur J Pharm Sci v.153. 10.1016/j.ejps.2020.105465 [DOI] [PMC free article] [PubMed]
- Adusumilli NC, Zhang D, Friedman JM, Friedman AJ. Harnessing nitric oxide for preventing limiting and treating the severe pulmonary consequences of COVID-19. Nitric Oxide. 2020;103:4–8. doi: 10.1016/j.niox.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akerstrom S, Mousavi-Jazi M, Klingstrom J, Leijon M, Lundkvist A, Mirazimi A. Nitric oxide inhibits the replication cycle of severe acute respiratory syndrome coronavirus. J Virol. 2005;79:1966–1969. doi: 10.1128/JVI.79.3.1966-1969.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alhazzani W, Moller MH, Arabi YM, Loed M, Gong MN, Fan E, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astuti I, Ysrafil (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. 5. 10.1016/j.dsx.2020.04.020 [DOI] [PMC free article] [PubMed]
- Chakraborty I, Maity P (2020) COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci Total Environ 728. 10.1016/j.scitotenv.2020.138882 [DOI] [PMC free article] [PubMed]
- Chopra V, Toner E, Waldhorn R, Washer L. How should U.S. hospitals prepare for coronavirus disease 2019 (COVID-19)? 2020. p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demir GM, Degim IT. Preparation of chitosan nanoparticles by nano spray drying technology. 2013. [Google Scholar]
- Deokar AR, Nagvenkar AP, Kalt I, Shani L, Yeshurun Y, Gedanken A, et al. Graphene-based “Hotplate” for the capturing and destruction of the herpes simplex virus type. Bioconjug Chem. 2017;28:4. doi: 10.1021/acs.bioconjchem.7b00030. [DOI] [PubMed] [Google Scholar]
- Deshmukh SP, Patil SM, Mullani SB, Delekar SD. Silver nanoparticles as an effective disinfectant: a review. Mat Sci Eng C-Mater. 2019;97:954–965. doi: 10.1016/j.msec.2018.12.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JJ, Ulmer JB (1999) DNA vaccines for viral diseases. Braz J Med Biol Res 32(2) [DOI] [PubMed]
- Dorward J, Gbinigie K (2020) There is currently no strong evidence for the efficacy of lopinavir/ritonavir in the treatment of COVID-19. University of Oxford
- Draz MS, Shafiee H. Applications of gold nanoparticles in virus detection. Theranostics. 2018;8(7):1985–2017. doi: 10.7150/thno.23856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driggin E, Madhavan MV, Bikdeli B, Chuich T, Laracy J, Biondi-Zoccai G, Brown TS, der Nigoghossian C, Zidar DA, Haythe J, Brodie D, Beckman JA, Kirtane AJ, Stone GW, Krumholz HM, Parikh SA. Cardiovascular considerations for patients, health care workers, and health systems during the COVID-19 pandemic. J Am Coll Cardiol. 2020;75(18):2352–2371. doi: 10.1016/j.jacc.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druce J, Garcia K, Tran T, Papadakis G, Birch C. Evaluation of swabs, transport media, and specimen transport conditions for optimal detection of viruses by PCR. J Clin Microbiol. 2012;50(3):1064–1065. doi: 10.1128/JCM.06551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dung TTN, Nam VN, Nhan TT, Ngoc TTB, Minh LQ, Nga BTT, Le VP, Quang DV. Silver nanoparticles as potential antiviral agents against African swine fever virus. Mater Res Express. 2020;6:12. [Google Scholar]
- Durán N, Marcato PD, Conti RD, Alves OL, Costa FTM, Brocchi M. Potential use of silver nanoparticles on pathogenic bacteria, their toxicity and possible mechanisms of action. J Braz Chem Soc. 2010;21:6. [Google Scholar]
- Entos Pharma. The challenge: effective drug delivery. 2020. Available in: https://www.entospharma.com/fusogenix.
- Erdmann VA, Barciszewski J. DNA and RNA nanobiotechnologies in medicine: diagnosis and treatment of diseases. Berlin: Springer Science & Business Media; 2013. [Google Scholar]
- FDA . Food and Drug Administration. Guidance for industry considering whether an FDA-regulated product involves the application of nanotechnology. 2014. [Google Scholar]
- Giwa A, Desai A, Duca A. Novel 2019 coronavirus SARS-CoV-2 (COVID-19): na overview for emergency clinicians. 2020. [PubMed] [Google Scholar]
- Grenha A, Seijo B, Remuñán-López C. Microencapsulated chitosan nanoparticles for lung protein delivery. Eur J Pharm Sci. 2005;25:427–437. doi: 10.1016/j.ejps.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Hamdy ME, Carlo MD, Hussein HA, Salah TA, El-Deeb AH, Emara MM, Pezzoni G, Compagnone D. Development of gold nanoparticles biosensor for ultrasensitive diagnosis of foot and mouth disease virus. J Nanobiotechnology. 2018;16:48. doi: 10.1186/s12951-018-0374-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Hwang HM. Nanotechnology in food science: functionality, applicability, and safety assessment. J Food Drug Anal. 2016;24(4):671–681. doi: 10.1016/j.jfda.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzinger M, Goff AL, Cosnier S (2014) Nanomaterials for biosensing applications: a review. Front Chem 2. 10.3389/fchem.2014.00063 [DOI] [PMC free article] [PubMed]
- Islam N, Ferro V. Recent advances in chitosan-based nanoparticles pulmonary drug delivery. Nanoscale. 2016;8(30):14341–14358. doi: 10.1039/c6nr03256g. [DOI] [PubMed] [Google Scholar]
- Itani R, Tobaiqy M, Faraj AA. Optimizing use of theranostic nanoparticles as a life-saving strategy for treating COVID-19 patients. Theranostics. 2020;10(13):5932–5942. doi: 10.7150/thno.46691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jampilek J, Kos J, Kralova K. Potential of nanomaterial applications in dietary supplements and foods for special medical purposes. Nanomaterials (Basel) 2019;9:2. doi: 10.3390/nano9020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeevanandam J, Barhoum A, Chan YS, Dufresne A, Danquah MK. Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. Beilstein J Nanotechnol. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczmarek JC, Kowalski PS, Anderson DG (2017) Advances in the delivery of RNA therapeutics: from concept to clinical reality. Genome Med 9. 10.1186/s13073-017-0450-0 [DOI] [PMC free article] [PubMed]
- Khan I, Saeed K, Khan I. Nanoparticles: properties, applications and toxicities. Arab J Chem. 2019;12(7):908–931. [Google Scholar]
- King T, Osmond-McLeod MJ, Duffy LL. Nanotechnology in the food sector and potential applications for the poultry industry. Trends Food Sci Technol. 2018;72:62–73. [Google Scholar]
- Kissler SM, Tedijanto C, Goldstein E, Grad YH, Lipsitch M. Projecting the transmission dynamics of SARS-CoV-2 through the postpandemic period. Science. 2020;368:860–868. doi: 10.1126/science.abb5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NY, Ko WC, Hsueh PR (2019) Nanoparticles in the treatment of infections caused by muldidrug-resistant organisms. Front Pharmacol 10. 10.3389/fphar.2019.01153 [DOI] [PMC free article] [PubMed]
- Li H, Liu SM, Yu XH, Tang SL, Tang CK. Coronavirus disease 2019 (COVID-19): current status and future perspectives. Int J Antimicrob Agents. 2020;55:5. doi: 10.1016/j.ijantimicag.2020.105951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Fan Y, Lai Y, Han T, Li Z, Zhou P, Pan P, Wang W, Hu D, Liu X, Zhang Q, Wu J. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. doi: 10.1002/jmv.25685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Yi Y, Luo X, Xiong N, Liu Y, Li S, Sun R, Wang Y, Hu B, Chen W, Zhang Y, Wang J, Huang B, Lin Y, Yang J, Cai W, Wang X, Cheng J, Chen Z, Sun K, Pan W, Zhan Z, Chen L, Ye F. Development and clinical application of a rapid IgM-IgG combined antibody test for SARS-CoV-2 infection diagnosis. J Med Virol. 2020;92:1518–1524. doi: 10.1002/jmv.25727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo D, Kiselev MA, Caccamo MT. Smart nanoparticles for drug delivery application: development of versatile nanocarrier platforms in biotechnology and nanomedicine. J Nanomater. 2019;2019:1–26. [Google Scholar]
- Lustig SR, Biswakarma JJH, Rana D, Tilford SH, Hu W, Su M, Rosenblatt MS. Effectiveness of common fabrics to block aqueous aerosols of virus-like nanoparticles. ACS Nano. 2020;14(6):7651–7658. doi: 10.1021/acsnano.0c03972. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Smith DW. COVID-19: a novel zoonotic disease caused by a coronavirus from China: what we know and what we don’t. Microbiol Aust, v.17, 2020. 10.1071/MA20013 [DOI] [PMC free article] [PubMed]
- Malekzad H, Zangabad PS, Mirshekari H, Karimi M, Hamblin MR. Noble metal nanoparticles in biosensors: recent studies and applications. Nanotechnol Rev. 2017;6(3):301–329. doi: 10.1515/ntrev-2016-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marassi V, Cristo LD, Smith SGJ, Ortelli S, Blosi M, Costa AL, Reschiglian P, Volkov Y, Prina-Mello A. Silver nanoparticles as a medical device in healthcare settings: a five-step approach for candidate screening of coating agents. R Soc Open Sci, v.5, n.1, 2018. 10.1098/rsos.171113 [DOI] [PMC free article] [PubMed]
- Mehra MR, Desai SS, Ruschitzka F, Patel AN (2020) Hydroxychloroquine or chloroquine with or without a macrolide for treatment of COVID-19: a multinational registry analysis. Lancet. 10.1016/S0140-6736(20)31180-6 [DOI] [PMC free article] [PubMed] [Retracted]
- Melo A, Amadeu MS, Lancellotti M, Hollanda LM, Machado D. The role of nanomaterials in cosmetics: national and international legislative aspects. Quim. Nova, v.38, n.4, 2015. 10.5935/0100-4042.20150042
- Mohammed MA, Syeda JTM, Wasan KM, Wasan EK. An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics, v.9, n.4, 2017. 10.3390/pharmaceutics90400053 [DOI] [PMC free article] [PubMed]
- Monteiro WM, Brito-Sousa JD, Baía-da-Silva D, Melo GC, Siqueira AM, Val F et al. Driving forces for COVID-19 clinical trials using chloroquine: the need to choose the right research questions and outcomes. Rev. Soc. Bras. Med. Trop., v.53, 2020. 10.1590/0037-8682-0155-2020 [DOI] [PMC free article] [PubMed]
- Nanotechnology Products Database. COVID-19. 2020. Available in: https://product.statnano.com/.
- Novochizol. Aerosol formulation of COVID-19. 2020. Available in: https://www.novochizol.ch/.
- Oliveira AC, Lucas TC, Iquiapaza RA. What has the COVID-19 pandemic taught us about adopting preventive measures? Texto Contexto, v.29, 2019. 10.1590/1980-265x-tce-2020-0106
- Oliveira TC, Abranches MV, Lana RM. Food (in)security in Brazil in the contexto of the SARS-CoV-2 pandemic. Cad. Saúde Pública, v.36, n.4, 2020. 10.1590/0102-311x00055220 [DOI] [PubMed]
- Palmieri V, Papi M. Can graphene take part in the fight against COVID-19? Nano Today, v.33, 2020. 10.1016/j.nantod.2020.100883 [DOI] [PMC free article] [PubMed]
- Peng Y, Chen L, Ye S, Kang Y, Liu J, Zeng S, Yu L. Research and development of drug delivery systems based on drug transporter and nanoformulation. Asian J Pharm Sci. 2020;15(2):220–236. doi: 10.1016/j.ajps.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourshahab PS, Gilani K, Moazeni E, Eslahi H, Fazeli MR, Jamalifar H. Preparation and characterization of spray dried inhalable powders containing chitosan nanoparticles for pulmonary delivery of isoniazid. J Microencapsul. 2011;28(7):605–613. doi: 10.3109/02652048.2011.599437. [DOI] [PubMed] [Google Scholar]
- Qiu G, Gai Z, Tao Y, Schmitt J, Kullak-Ublick GA, Wang J. Dual-functional plasmonic photothermal biosensors for highly accurate severe acute respiratory syndrome coronavirus 2 detection. ACS Nano. 2020;14(5):5268–5277. doi: 10.1021/acsnano.0c02439. [DOI] [PubMed] [Google Scholar]
- Sanders JM, Monogue ML, Jodlowski TZ. Pharmacologic treatments for coronavirus disease 2019 (COVID-19) a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Saylor K, Gillam F, Lohneis T, Zhang C (2020) Designs of antigen structure and composition for improved protein-based vaccine efficacy. Front Immunol 11. 10.3389/fimmu.2020.00283 [DOI] [PMC free article] [PubMed]
- Simbine EO, Rodrigues LC, Lapa-Guimarães J, Kamimura ES, Corassin CH, Oliveira CAF. Application of silver nanoparticles in food packages: a review. Food Sci Technol. 2019;39:4. [Google Scholar]
- Singh T, Shukla S, Kumar P, Wahla V, Bajpai VK, Rather IA. Application of nanotechnology in food science: perception and overview. Front Microbiol., v.8, 2017. 10.3389/fmicb.2017.01501 [DOI] [PMC free article] [PubMed]
- Singh AK, Singh A, Shaikh A, Singh R, Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang A, Tong ZD, Wang HL, Dai YX, Li KF, Liu JN, Wu WJ, Yuan C, Yu ML, Li P, Yan JB. Detection of novel coronavirus by RT-PCR in stool specimen from asymptomatic child, China. Emerg Infect Dis. 2020;26(6):1337–1339. doi: 10.3201/eid2606.200301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC, Chen H, Mubareka S, Gubbay JB, Chan WCW. Diagnosing COVID-19: the disease and tools for detection. ACS Nano. 2020;14:3822–3835. doi: 10.1021/acsnano.0c02624. [DOI] [PubMed] [Google Scholar]
- Wang X, Sun W, Yang W, Gao S, Sun C, Li Q. Mesoporous silica-protected silver nanoparticle disinfectant with controlled Ag+ ion release, efficient magnetic separation, and effective antibacterial activity. Nanoscale Adv. 2019;1:840–848. doi: 10.1039/c8na00275d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways TMM, Lau WM, Khutoryanskiy VV. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers, v.10, n.3, 2018. 10.3390/polym10030267 [DOI] [PMC free article] [PubMed]
- Wei Y, Huang YH, Cheng KC, Song YL. Investigations of the influences of processing conditions on the properties of spray dried chitosan-tripolyphosphate particles loaded with theophylline. Scientific Reports, v.10, 2020. 10.1038/s41598-020-58184-3 [DOI] [PMC free article] [PubMed]
- Weiss C, Carriere M, Fusco L, Capua I, Regla-Nava JA, Pasquali M, Scott JA, Vitale F, Unal MA, Mattevi C, Bedognetti D, Merkoçi A, Tasciotti E, Yilmazer A, Gogotsi Y, Stellacci F, Delogu LG. Toward nanotechnology-enabled approaches against the COVID-19 pandemic. ACS Nano. 2020;14(6):6383–6406. doi: 10.1021/acsnano.0c03697. [DOI] [PubMed] [Google Scholar]
- WHO. World Health Organization. Coronavirus disease 2019 (COVID-19) Situation Report – 84. 2020a. Available in: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports.
- WHO. World Health Organization. Water, sanitation, hygiene, and waste management for the COVID-19 virus: interim guidance. 2020b
- Yang XX, Li CM, Li YF, Wang J, Huang CZ. Synergistic antiviral effect of curcumin functionalized graphene oxide against respiratory syncytial virus infection. Nanoscale. 2017;9:16086–16092. doi: 10.1039/c7nr06520e. [DOI] [PubMed] [Google Scholar]
- Youssef FS, El-Banna HA, Elzorba HY, Galal AM. Application of some nanoparticles in the field of veterinary medicine. Int J Vet Sci Med. 2019;7(1):78–93. doi: 10.1080/23144599.2019.1691379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Honko A, Zhou J, Gong H, Downs SN, Vaques JH, et al. Cellular nanosponges inhibit SARS-CoV-2 infectivity. Nano Lett. 2020;20(7):5570–5574. doi: 10.1021/acs.nanolett.0c02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, Su Y, Wang X, Yuan J, Li T, Li J, Qian S, Hong C, Wang F, Liu Y, Wang Z, He Q, Li Z, He B, Zhang T, Fu Y, Ge S, Liu L, Zhang J, Xia N, Zhang Z (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 10.1093/cid/ciaa344 [DOI] [PMC free article] [PubMed]
- Zhu X, Wang X, Han L, Chen T, Wang L, Li H et al. Multiplex reverse transcription loop-mediated isothermal amplification combined with nanoparticle-based lateral flow biosensor for the diagnosis of COVID-19. Biosens. Bioelectron., v.166, 2020. 10.1016/j.bios.2020.112437 [DOI] [PMC free article] [PubMed]
- Ziem B, Thien H, Achazi K, Yue C, Stern D, Silberreis K, Gholami MF, Beckert F, Gröger D, Mülhaupt R, Rabe JP, Nitsche A, Haag R. Hidhly efficient multivalent 2D nanosystems for inhibition of orthopoxvirus particles. Adv Healthc Mater. 2016;5:2922–2930. doi: 10.1002/adhm.201600812. [DOI] [PubMed] [Google Scholar]
- Ziem B, Azab W, Gholami MF, Rabe JP, Osterrieder N, Haag R. Size-dependent inhibition of herpesvirus cellular entry by polyvalent nanoarchitectures. Nanoscale. 2017;9:3774–3783. doi: 10.1039/c7nr00611j. [DOI] [PubMed] [Google Scholar]
- Zuo T, Zhang F, Lui GCY, Yeoh YK, Li AYL, Zhan H, Wan Y, Chung A, Cheung CP, Chen N, Lai CKC, Chen Z, Tso EYK, Fung KSC, Chan V, Ling L, Joynt G, Hui DSC, Chan FKL, Chan PKS, Ng SC (2020) Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 10.1053/j.gastro.2020.05.048 [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.