Abstract
The rapid spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is due to the high rates of transmission by individuals who are asymptomatic at the time of transmission1,2. Frequent, widespread testing of the asymptomatic population for SARS-CoV-2 is essential to suppress viral transmission and key for safely reopening society. Despite increases in testing capacity, multiple challenges remain in deploying traditional reverse transcription and quantitative PCR (RT-qPCR) tests at the scale required for population screening of asymptomatic individuals. We have developed SwabSeq, a high-throughput testing platform for SARS-CoV-2 that uses next-generation sequencing as a readout. SwabSeq employs sample-specific molecular barcodes to enable thousands of samples to be combined and simultaneously analyzed for the presence or absence of SARS-CoV-2 in a single run. Importantly, SwabSeq incorporates an in vitro RNA standard that mimics the viral amplicon, but can be distinguished by sequencing. This standard allows for end-point rather than quantitative PCR, improves quantitation, reduces requirements for automation and sample-to-sample normalization, enables purification-free detection, and gives better ability to call true negatives. We show that SwabSeq can test nasal and oral specimens for SARS-CoV-2 with or without RNA extraction while maintaining analytical sensitivity better than or comparable to that of fluorescence-based RT-qPCR tests. We have tested 1215 clinical samples: purified RNA from nasal swabs (n=380), and extraction free protocols for nasal swabs (n=298) and extraction free saliva (n=537) that combined show 96.6% agreement, high sensitivity and specificity when compared to high sensitivity nasal swab qPCR assays. SwabSeq is simple, sensitive, flexible, rapidly scalable, inexpensive enough to test widely and frequently, and, in clinical operation, provides a turn around time of 12 to 18 hours.
INTRODUCTION
In the absence of an effective vaccine or prophylactic treatment, public health strategies remain the only tools for controlling the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19. In contrast to SARS-CoV-1, for which infectivity is associated with symptoms3,4, infectivity of SARS-CoV-2 is high during the asymptomatic/presymptomatic phase5,6. As a consequence, containing transmission based solely on symptoms is impossible, which makes molecular screening for SARS-CoV-2 essential for pandemic control.
As regional lockdowns have been lifted and people have returned to work and resumed other activities, rates of infection have started to rise again7. In many parts of the United States, the rise in cases has overwhelmed the capacity of quantitative RT-PCR (qRT-PCR) tests that make up the majority of FDA-authorized tests for COVID-19. Delays in obtaining test results, which are due to capacity constraints rather than assay times8, render testing ineffective for the public health aims of preventing viral transmission and suppressing local outbreaks. Even where expanded capacity exists, the ~$100 price of tests (current Medicare reimbursement rates9) prohibits the widespread adoption by large employers and schools on a regular basis for effective viral suppression10,11. Frequent, low-cost population testing, combined with contact tracing and isolation of infected individuals, would help to halt the spread of COVID-19 and reopen society12,13. Here we describe SwabSeq, a SARS-CoV-2 testing platform that leverages next-generation sequencing to massively scale up testing capacity14,15.
SwabSeq improves on one-step reverse transcription and polymerase chain reaction (RT-PCR) approaches in several key areas. Like other sequencing approaches, SwabSeq utilizes molecular barcodes that are embedded in the RT-PCR primers to uniquely label each sample and allow for simultaneous sequencing of hundreds to thousands of samples in a single run (LampSeq16, Illumina CovidSeq17, DxSeq18). SwabSeq uses very short reads, reducing sequencing times so that results can be returned in less than 24 hours.
To deliver robust and reliable results at scale, SwabSeq adds to every sample a synthetic in vitro RNA standard that is almost the same as that of the virus, but can be distinguished easily by sequencing. SARS-CoV-2 detection is based on the ratio of the counts of true viral sequencing reads to those from the in vitro viral standard. Since every sample contains the synthetic RNA, SwabSeq controls for failure of amplification: samples with no SARS-CoV-2 detected are those in which only in vitro viral standard reads are observed, while those without viral or in vitro viral standard reads are inconclusive.
The RNA control confers a number of additional advantages on the SwabSeq assay. Since we are only interested in the ratio of real virus to in vitro standard, the PCR can be run to the endpoint, where all primers are consumed, rather than for a set number of cycles. By driving the reaction to endpoint, we overcome the presence of varying amounts of RT and PCR inhibitors and effectively force each sample to have similar amounts of final product. Using in vitro standard RNA with end-point PCR has two important consequences. First, by overcoming the heterogeneity that inevitably occurs with clinical samples, we can pool reaction products after PCR, without the need to normalize each sample individually. Second, it enables direct processing of extraction-free samples. Inhibitors of RT and PCR present in mucosal tissue or saliva should affect both the virus and the in vitro standard equally. Endpoint PCR overcomes the effect of inhibition, while keeping the ratio of reads between the two RNA species approximately constant, and so avoids the need for extraction.
Here we show that SwabSeq has extremely high sensitivity and specificity for the detection of viral RNA in purified samples. We also demonstrate a low limit of detection in extraction-free lysates from mid-nasal swabs and oral fluids. These results demonstrate the potential of SwabSeq to be used for SARS-CoV-2 testing on an unprecedented scale, offering a potential solution to the need for population-wide testing to stem the pandemic.
RESULTS
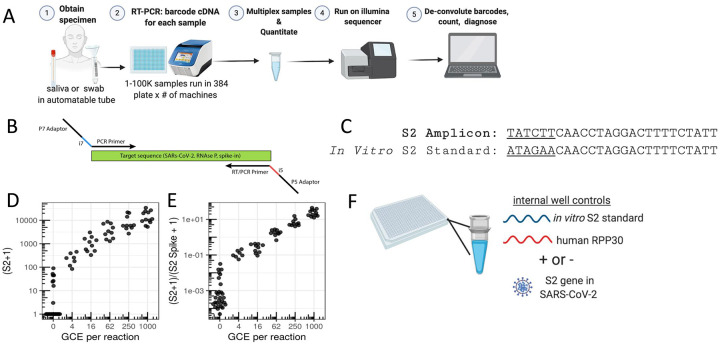
SwabSeq is a simple and scalable protocol, consisting of 5 steps (Figure 1A): (1) sample collection, (2) reverse transcription and PCR using primers that contain unique molecular indices at the i7 and i5 positions (Figure 1B, Figure S1) as well as in vitro standards, (3) a simple pooling (no normalization) and cleanup of the uniquely barcoded samples for library preparation, (4) sequencing of the pooled library, and (5) computational assignment of barcoded sequencing reads to each sample for counting and viral detection.
Figure 1. SwabSeq Diagnostic Testing Platform for COVID19.
A) The workflow for SwabSeq is a five step process that takes approximately 12 hours from start to finish. B) In each well, we perform RT-PCR on clinical samples. Each well has two sets of indexed primers that generate cDNA and amplicons for SARS-CoV-2 S2 gene and the human RPP30 gene. Each primer is synthesized with the P5 and P7 adaptors for Illumina sequencing, a unique i7 and i5 molecular barcodes, and the unique primer pair. Importantly, every well has a synthetic in vitro S2 standard that is key to allowing the method to work at scale. C) The in vitro S2 standard (abbreviated as S2-Spike) differs from the virus S2 gene by 6 base pairs that are complemented (underlined). (D) Read count at various viral concentrations (E) Ratiometric normalization allow for in-well normalization for each amplicon (F) Every well has two internal well controls for amplification, the in vitro S2 standard and the human RPP30. The RPP30 amplicon serves as a control for specimen collection. The in vitro S2 standard is critical to SwabSeq’s ability to distinguish true negatives.
Our assay consists of two primer sets that amplify two genes: the S2 gene of SARS-CoV-2 and the human Ribonuclease P/MRP Subunit P30 (RPP30). We include a synthetic in vitro RNA standard that is identical to the viral sequence targeted for amplification, except for the most upstream 6 bp (Figure 1C), which allows us to distinguish sequencing reads corresponding to the in vitro standard from those corresponding to the target sequence. The primers amplify both the viral and the synthetic sequences with equal efficiency (Figure S2). We have also added a second RNA standard for RPP30 with a similar design. The ratio of the number of native reads to the number of in vitro standard reads provides a more accurate and quantitative measure of the number of viral genomes in the sample than native read counts alone (Figure 1D,E). The in vitro standard also allows us to retain linearity over a large range of viral input despite the use of endpoint PCR (Figure S3). With this approach, the final amount of DNA in each well is largely defined by the total primer concentration rather than by the viral input—negative samples have high amounts of in vitro S2 standard (abbreviated as S2-spike) and low/zero amounts of viral reads, and positive samples have low amounts of in vitro S2 standard and high amounts of viral reads (Figure S3). In addition to viral S2, we reverse-transcribe and amplify a human housekeeping gene to control for specimen quality, as in traditional qPCR assays (Figure 1F). The i5/i7 barcodes used are designed to be at least several base-pair edits away from one another, allowing for assignment even in the face of sequencing errors.
After RT-PCR, samples are combined at equal volumes, purified, and used to generate one sequencing library. We have used both the Illumina MiSeq and the Illumina NextSeq 550 to sequence these libraries (Figure S4). We minimize instrument sequencing time by sequencing only the minimum required 26 base pairs (Methods). Each read is classified as deriving from native or in vitro standard S2, or RPP30, and assigned to a sample based on the associated index sequences (barcodes). To maximize specificity and avoid false-positive signals arising from incorrect classification or assignment, conservative edit distance thresholds are used for this matching operation (Methods and Supplemental Results). A sequencing read is discarded if it does not match one of the expected sequences. Counts for native and in vitro standard S2 and RPP30 reads are obtained for each sample and used for downstream analyses (Methods).
We have estimated that approximately 5,000 reads per well are sufficient to detect the presence or absence of viral RNA in a sample (Methods). This translates to at least 1,500 samples per run on a MiSeq or MiniSeq, 20,000 samples per run on a NextSeq 550, and up to 150,000 samples per run on a NovaSeq S2 flow cell. Computational analysis takes only minutes per run19. The MiniSeq newly released rapid output flow cell has a turn around time of 2.5 hours, which significantly improves our throughput and turn-around time. Our optimized protocols allow for a single person to process 6–384 well plates in a single hour; which is equivalent to 2304 samples an hour. This process cloned over multiple liquid handlers can rapidly scale to 10,000 samples per hour with a staff of 5 people.
We have optimized the SwabSeq protocol by identifying and eliminating multiple sources of noise (Supplemental Results) to create a streamlined and scalable protocol for SARS-CoV-2 testing.
Validation of SwabSeq as a diagnostic platform
We first validated SwabSeq on purified RNA nasopharyngeal (NP) samples that were previously tested by the UCLA Clinical Microbiology Laboratory with a standard RT-qPCR assay (ThermoFisher Taqpath COVID19 Combo Kit). To determine our analytical limit of detection, we diluted inactivated virus with pooled, remnant clinical NP swab specimens. The remnant samples were all confirmed to be negative for SARS-CoV-2. In these remnant samples, we performed a serial, 2-fold dilution of heat-inactivated SARS-CoV-2 (ATCC® VR-1986HK), from 8,000 to 125 genome copy equivalents (GCE) per mL. We detected SARS-CoV-2 in 34/34 samples down to 250 GCE per mL, and in 28/34 samples down to 125 GCE per mL (Figure 2A). These results established that SwabSeq is highly sensitive, with an analytical limit of detection (LOD) of 250 GCE per mL for purified RNA from nasal swabs. This limit of detection is lower than those of many currently FDA authorized and highly sensitive RT-qPCR assays for SARS-CoV-2.
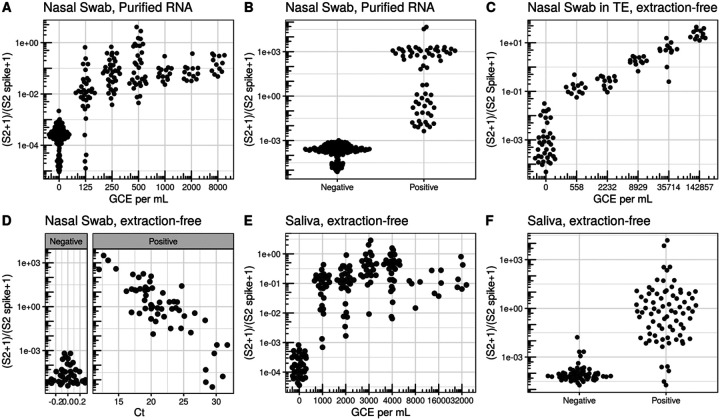
Figure 2. Validation in clinical specimens demonstrate a limit of detection equivalent to sensitive RT-qPCR reactions.
A) Limit of Detection in nasal swab samples with no SARS-CoV2 were pooled and ATCC inactivated virus was added at different concentrations. Nasal Swab sample was RNA purified and using SwabSeq showed a limit of detection of 250 genome copy equivalents (GCE) per mL. B) RNA-purified clinical nasal swab specimens obtained through the UCLA Health Clinical Microbiology Laboratory were tested based on clinical protocols using FDA authorized platforms and then also tested using SwabSeq. We show 100% agreement with samples that tested positive for SARS-CoV-2 (n=63) and negative for SARS-CoV-2 (n=159). C) We also tested RNA purified samples from extraction-free nasopharyngeal swab and showed a limit of detection of 558 GCE/mL. D) Relationship between Ct from RT-qPCR targetting the S gene (x-axis) and SwabSeq ratio for extract-free swabs into normal saline or Tris-EDTA (y-axis) for patient samples classified as testing positive or negative for SARS-CoV-2 by the UCLA Clinical Microbiology Laboratory. Samples with no virus detected were assigned a Ct of 0 for this visualization. E) Extractio- free processing of saliva specimens show a limit or detection down to 1000 GCE per mL. F) Extraction-free processing of saliva clinical specimens using swabseq (y-axis) compared to classification of SARS-CoV-2 status from RNA-purified clinical nasal swab specimens for matched samples (x-axis).
SwabSeq detects the SARS-CoV-2 genome with high clinical sensitivity and specificity. We retested 668 RNA-purified nasopharyngeal samples from the UCLA Clinical Microbiology Laboratory in which SARS-CoV-2 positive (n=94) and negative (n=574). We observed 99.2% agreement with RT-qPCR results for all samples (Figure 2B and S5D) with 100% concordance in samples that were positive for SARS-CoV-2. We sequenced these samples on either a MiSeq, MiniSeq or on a NextSeq550 (Figure S5), with 100% concordance between the different sequencing instruments.
One of the major bottlenecks in scaling up RT-qPCR diagnostic tests is the RNA purification step. RNA extraction is challenging to automate, and supply chains have not been able to keep up with the demand for necessary reagents during the course of the pandemic. Thus, we explored the ability of SwabSeq to detect SARS-CoV-2 directly from a variety of extraction-free sample types. There are several types of media that are recommended by the CDC for nasal swab collection: viral transport medium (VTM)20, Amies transport medium21, and normal saline21. A main technical challenge arises from RT or PCR inhibition by ingredients in the collection buffers. We found that dilution of specimens with water overcame the RT and PCR inhibition and allowed us to detect viral RNA in contrived and positive clinical patient samples, at limits of detections between 4000 and 6000 GCE/mL (Figure S6). We also tested nasal swabs that were collected directly into Tris-EDTA (TE) buffer, diluted 1:1 with water. This approach yielded a limit of detection of 560 GCE/mL (Figure 2C). We next performed a comparison between traditional purified RT-qPCR protocols and our extraction free-protocol for nasopharyngeal samples collected into either normal saline (n=128) or TE (n=170). We showed 96.0% overall agreement, 92.0% positive percent agreement and 97.6% negative percent agreement for all samples and a high correlation (r2=0.68) between SwabSeq signal and RT-qPCR (Figure 2D, Figures S7–S8).
We also tested extraction-free saliva protocols in which saliva is collected directly into a matrix tube using a funnel-like collection device (Figure S9). The main technical challenges in demonstrating the detection of virus in saliva samples have been preventing degradation of the inactivated SARS-CoV-2 virus that is added to saliva and ensuring accurate pipetting of this heterogeneous and viscous sample type. We found that heating the saliva samples to 95 for 30 minutes22 reduced PCR inhibition and improved detection of the S2 amplicon (Figure S10). After heating, we diluted samples at a 1:1 volume with 2× TBE with 1% Tween-2022. Using this method, we obtained a LoD of 2000 GCE/mL (Figure 2E). In a study performed in the UCLA Emergency Department, we collected paired saliva and nasopharyngeal swab samples in patients with COVID19-like symptoms. In a comparison between our extraction-free, saliva SwabSeq protocol and purified nasopharyngeal swab samples run with high-sensitivity RT-qPCR in the UCLA Clinical Microbiology lab, we showed 95.3% overall agreement, 90.2% positive percent agreement, and 96.3% negative percent agreement (Figure S11).
With a highly scalable diagnostic platform, such as SwabSeq, specimen accessioning, securing supply chains, and processing samples all present challenges. We have redesigned the pre-analytic processes to prioritize self-registration, rapid sample collection, and leverage simple automation once samples are received in the lab (Figure S9). We have developed a web app that allows us to push the registration of samples to the individual. The second innovation is sample collection into tubes that can be uncapped in a racked, 96-tube format. This allows us to pipet batches of 96-samples at a time directly into a RT-PCR mix (Figure S9).
The development of extraction free protocols prioritized “low-touch”. Our inactivation protocol requires a simple heat step of the specimen tube, minimizing exposure and pipetting of the sample. In addition, we only use only a 1 liquid-handler tip per sample thereby minimizing supply chain challenges that continue to emerge.
We have calculated that a single person can process 6 384-well plates per hour using an automated liquid handler. In our COVID-19 testing laboratory, we have scaled this to four-liquid handlers capable of processing up to 24 plates/hour, which is equivalent to a capacity of 9,216 samples per hour. A laboratory running 24 hours a day could process well over 100,000 samples per day. Furthermore, the post-PCR multiplexing and library generation is designed such that a single person can handle thousands of samples concurrently, the number of samples is dependent only on the capacity of the sequencer. For smaller scale university settings, having an automated decapper, single liquid handler and MiniSeq, and investment of less than $150,000 in equipment, would be able to diagnose up to ~3000 samples a day.
These optimizations allow for rapid scaling of the SwabSeq diagnostic platform. We tested sample collection and processing workflows in a variety of settings, including the emergency room, return-to-school testing and university-wide population screening. These and other optimizations demonstrate a path to rapid scaling in population-dense settings such as university campuses.
DISCUSSION
Swabseq has the potential to alleviate existing bottlenecks in diagnostic clinical testing. We believe that it has even greater potential to enable testing on a scale necessary for pandemic suppression via population surveillance. The technology represents a novel use of massively parallel next-generation sequencing for infectious disease surveillance and diagnostics. We have demonstrated that SwabSeq can detect SARS-CoV-2 RNA in clinical specimens from both purified RNA and extraction-free lysates, with clinical and analytical sensitivity and specificity comparable to RT-qPCR performed in a clinical diagnostic laboratory. We have optimized SwabSeq to prioritize scale and low cost, as these are the key factors missing from current COVID-19 diagnostic platforms.
Methods for surveillance testing, such as SwabSeq, should be evaluated differently than those for clinical testing. Clinical testing informs medical decision-making, and thus requires high sensitivity and specificity. For surveillance testing, the most important factors are the breadth and frequency of testing and the turn-around-time12. Sufficiently broad and frequent testing with rapid return of results, contact tracing, and quarantining of infectious individuals can effectively contain viral outbreaks, avoiding blanket stay-at-home orders. Epidemiological modeling of surveillance testing on university campuses has shown that diagnostic tests with only 70% sensitivity, performed frequently with a short turn-around time, can suppress transmission13. However, there remain major challenges for practical implementation of frequent testing, including the cost of testing and the logistics of collecting and processing thousands of samples per day.
The use of next generation sequencing in diagnostic testing has garnered concern about turn-around-time and cost. SwabSeq uses short sequencing runs that read out the molecular indexes and 26 base pairs of the target sequence in as little as 3 hours, followed by computational analysis that can be performed on a desktop computer in 5 minutes. The cost of 1,000 samples analyzed in one MiSeq run is less than $1 per sample for sequencing reagents. Running 10,000 samples on a NextSeq550, which generates 13 times more reads per flow cell, can reduce this sequencing cost approximately 10-fold. We estimate that the total consumable cost, inclusive of the collection kit and informatics infrastructure, ranges from 4 to 6 dollars per test. The total cost of operations depends on a wide range of factors, including the costs of setting up CLIA-certified laboratory, certified personnel, logistics of sample collection, and result reporting. Ongoing optimization to decrease reaction volumes and to use less expensive RT-PCR reagents can further decrease the total cost per test.
Finally, scaling up testing for SARS-CoV-2 requires high-throughput sample collection and processing workflows. Manual processes, common in most academic clinical laboratories, are not easily compatible with simple automation. The current protocols with nasopharyngeal swabs into viral transport media, Amies buffer or normal saline are collection methods that date back to the pre-molecular-genetics era, when live viral culture was used to identify cytopathic effects on cell lines. A fresh perspective on collection methods that are easily scalable would be enormously beneficial to scaling up centralized laboratory testing approaches.
Several groups, including ours, have piloted “lightweight” sample collection approaches, which push sample registration and patient information collection directly onto the individual tested via a smartphone app. Much of the labor of sample acquisition is due to a lack of interoperability between electronic health systems, with laboratory professionals manually entering information for every sample by hand. By developing a HIPAA-compliant registration process, we aim to streamline labor-intensive sample accessioning. To promote scalability, we have also started to develop sample collection protocols that use smaller-volume tubes that are compatible with simple automation, such as automated capper-decapper and 96-head liquid handlers23,24. These approaches decrease the amount of hands-on work required in the laboratory to process and perform tests leading to higher reproducibility, faster turn-around time and decreasing exposure risk to laboratory workers.
The SwabSeq diagnostic platform complements traditional clinical diagnostics tests25, as well as the growing arsenal of point-of-care rapid diagnostic platforms26 emerging for COVID-19, by increasing test capacity to meet the needs of both diagnostic and widespread surveillance testing. Looking forward, SwabSeq is easily extensible to accommodate additional pathogens and viral targets. This would be particularly useful during the winter cold and flu season, when multiple respiratory pathogens circulate in the population and cannot be easily differentiated based on symptoms alone. Surveillance testing is likely to become a part of the new normal as we aim to safely reopen the educational, business and recreational sectors of our society.
SOFTWARE AND DATA
https://github.com/joshsbloom/swabseq
https://github.com/octantbio/SwabSeq
The core technology has been made available under the Open Covid Pledge, and software and data under the MIT license (UCLA) and Apache 2.0 license (Octant Inc.).
METHODS
Sample Collection.
All patient samples used in our study were deidentified. All samples were obtained with UCLA IRB approval. Nasopharyngeal samples were collected by health care providers from individuals whom physicians suspected to have COVID19.
Creation of Contrived Specimens.
For the clinical limit of detection experiments, we pooled confirmed, COVID-19 negative remnant nasopharyngeal swab specimens collected by the UCLA Clinical Microbiology Laboratory. Pooled clinical samples were then spiked with ATCC Inactivated Virus (ATCC 1986-HK) at specified concentrations and extracted as described below. For the clinical purified RNA samples, they were collected as nasopharyngeal swabs and purified using the KingFisherFlex (Thermofisher Scientific) instrument using the MagMax bead extraction. All extractions were performed according to manufacturer’s protocols. For extraction-free samples, we first contrived samples at specified concentrations into pooled, confirmed negative clinical samples and diluted samples in TE buffer or water prior to adding to the RT-PCR master mix.
Processing of Extraction-Free Saliva Specimens
Direct saliva is collected into a Matrix tube (Thermofisher, 3741-BR) using a small funnel (TWDRer 6565). The saliva samples were collected into a matrix tube and heated to 95 for 30 minutes. Samples were then either frozen at −80 or processed by dilution with 2X TBE with 1% Tween-20, for final concentration of 1× TBE and 0.5% Tween-2022. We also tested 1x Tween with Qiagen Protease and RNA Secure (ThermoFisher), which also works but resulted in more sample-to-sample variability and required additional incubation steps.
Processing of Extraction-Free Nasal Swab Lysates
All extraction-free lysates were inactivated using a heat inactivation at 56 for 30 minutes. Samples were then diluted with water at a ratio of 1:4 and directly added to mastermix. Dilution amounts varied depending on the liquid media that was used. We found that of the CDC recommended media, normal saline performed the most robustly. Viral Transport Media and Amies Buffer showed significant PCR inhibition that was difficult to overcome, even with dilution in water. We recommend placing the swab directly into the diluted TE buffer, which has little PCR inhibition.
Barcode Primer Design
Barcode primers were chosen from a set of 1,536 unique 10bp i5 barcodes and a set of 1,536 unique 10bp i7 barcodes. These 10 bp barcodes satisfied the criteria that there is a minimum Levenshtein27 distance of 3 between any two indices (within the i5 and i7 sets) and that the barcodes contain no homopolymer repeats greater than 2 nucleotides. Additionally, barcodes were chosen to minimize homo- and hetero-dimerization using helper functions in the python API to Primer328. Additional details and code for primer design can be found at https://github.com/octantbio/SwabSeq.
Construction of S2 and RPP30 in vitro standard
| S2_FP | TAATACGACTCACTATAGGGCTGGTGCTGCAGCTTATTATGTGGGTATAGAACAACCTAGGACTTTTCTATTAA |
| S2_RP | AACGTACACTTTGTTTCTGAGAGAGG |
| RPP30_FP_1 | CTGACCTGAAGGCTGACGCCGGACTTGTGGAGACAGC |
| RPP30_FP_2 | TAATACGACTCACTATAGGGAGATTTGGACCTGCGAGCGGGTTCTGACCTGAAGGCTGA |
| RPP30_R | GGTTTTTCAATTTCCTGTTTCTTTTCCTTAAAGTCAACG |
RT-PCR was performed using primers shown above on gRNA of SARS-CoV-2 (Twist BioSciences, #1) for construction of a in vitro S2 standard DNA template. RT PCR (FP_1, R) and a second round of PCR (FP_2, R) was performed on HEK293T lysate for construction of an in vitro RPP30 standard DNA template. Products were run on a gel to identify specific products at ~150 bp. DNA was purified using Ampure beads (Axygen) using a 1.8 ratio of beads:sample volume. The mixture was vortexed and incubated for 5 minutes at room temperature. A magnet was used to bind beads for 1 minute, washed twice with 70% EtOH, beads were air-dried for 5 minutes, and then removed from the magnet and eluted in 100 μL of IDTE Buffer. The bead solution was placed back on the magnet and the eluate was removed after 1 minute. DNA was quantified by nanodrop (Denovix).
This prepared DNA template was used for standard HiScribe T7 in vitro transcription (NEB). IVT reactions prepared according to the manufacturer’s instructions using 300 ng of template DNA per 20 uL reaction with a 16 hour incubation at 37. IVT reactions were treated with DNAseI according to the manufacturer’s instructions. RNA was purified with an RNA Clean & Concentrator-25 kit (Zymo Research) according to the manufacturer’s instructions and eluted into water. RNA standard was quantified both by nanodrop and with a RNA screen tape kit for the TapeStation according to the manufacturer’s instructions (Agilent) to verify the RNA was the correct size (~133 nt).
One-Step RT-PCR
RT-PCR were performed using either the Luna® Universal One-Step RT-qPCR Kit (New England BioSciences E3005) or the TaqPath™ 1-Step RT-qPCR Master Mix (Thermofisher Scientific, A15300) with a reaction volume of 20 μL. Both kits were used according to the manufacturer’s protocol. The final concentration of primers in our mastermix was 50 nM for RPP30 F and R primers and 400 nM for S2 F and R primers. Synthetic S2 RNA was added directly to the mastermix at a copy number of 500 copies per reaction. Sample was loaded into a 20 μL reaction. All reactions were run on a 96- or 384-well format and thermocycler conditions were run according to the manufacturer’s protocol. We observe significant differences between the amplification of samples from purified RNA versus extraction-free (unpurified swab) samples (Figure S12). For purified RNA samples we performed 40 cycles of PCR. For extraction-free samples, we performed endpoint PCR for 50 cycles.
Multiplex Library Preparation
After the RT-PCR reaction, samples were pooled using a multichannel pipet or Integra Viaflow Benchtop liquid handler. 6 μL from each well were combined in a sterile reservoir and transferred into a 15 mL conical tube and vortexed. 100 uL of the pool was transferred to a 1.7 mL eppendorf tube for a double-sided SPRI cleanup29. Briefly, 50 μL of AmpureXP beads (Beckman Coulter A63880) were added to 100 μL of the pooled PCR volume and vortexed. After 5 minutes, a magnet was used to collect beads for 1 minute and supernatant transferred to a new eppendorf tube. An additional 130 μL of Ampure XP beads were added to the 150 μL of supernatant and vortexed. After an additional 5 minutes, the magnet was used to collect beads for 1 minute and the beads were washed twice with fresh 70% EtOH. DNA was eluted off the beads in 40 μL of Qiagen EB buffer. The magnet was used to collect beads for 1 minute and 33 μL of supernatant was transferred to a new tube. Purified RNA was quantified and library quality was assessed using the Agilent TapeStation. We observe some differences in non-specific peaks in our TapeStation analysis of the final library preparation, particularly when sequencing unpurified samples out to 50 cycles (Figure S13). The presence of non-specific reads affects the quantification of the library, loading concentration, and cluster density. Therefore, we suggest quantifying the final library based on the proportion of the desired peaks.
Sequencing Protocol
Libraries were sequenced on either an Illumina MiSeq (2012), NextSeq550, or MiniSeq. Prior to each MiSeq run, a bleach wash was performed using a sodium hypochlorite solution (Sigma Aldrich, 239305) according to Illumina protocols. We also perform a maintenance wash between each run. On the NextSeq550 and MiniSeq, the post-run wash was performed automatically by the instruments, and no human intervention was required. The pooled and quantitated library was diluted to a concentration of 6 nM (based on Qubit 4 Fluorometer and Illumina’s formula for conversion between ng/μl and nM) and was loaded on the sequencer at either 25 pM (MiSeq), 1.35 pM (NextSeq), or 1.6 pM (MiniSeq). PhiX Control v3 (Illumina, FC-110–3001) was spiked into the library at an estimated 30–40% of the library. PhiX provides additional sequence diversity to Read 1, which assists with template registration and improves run and base quality.
For this application, the MiSeq and MiniSeq (Rapid Run Kit) require 2 custom sequencing primer mixes, the Read1 primer mix and the i7 primer mix. Both mixes have a final concentration of 20 μM of primers (10 μM of each amplicon’s sequencing primer). The NextSeq requires an additional sequencing primer mix, the i5 primer mix, which also has a final concentration of 20 μM. The MiSeq Reagent Kit v3 (150-cycle; MS-102–3001) is loaded with 30 μL of Read1 sequencing primer mix into reservoir 12 and 30 μL of the i7 sequencing primer primer mix into reservoir 13. The Miniseq Rapid High Output Reagent Kit (100-cycle; 20044338) is loaded with 17 μl of Read1 sequencing primer mix into reservoir 24 and 26 μl of i7 sequencing primer mix into reservoir 28. The NextSeq 500/550 Mid Output Kit (150 cycles; 20024904) is loaded with 52 μl of Read1 sequencing primer mix into reservoir 20, 85 μl of i7 sequencing primer mix into reservoir 22, and 85 μl of i5 sequencing primer mix into reservoir 22. Index 1 and 2 are each 10 bp, and Read 1 is 26 bp.
Analysis
The bioinformatic analysis consists of standard conversion of BCL files into FASTQ sequencing files using Illumina’s bcl2fastq software (v2.20.0.422). Demultiplexing and read counts per sample are performed using our custom software. Here read1 is matched to one of the three expected amplicons allowing for the possibility of a single nucleotide error in the amplicon sequence. The hamming distance is the number of positions at which the corresponding sequences are different from each other and is a commonly used measure of distance between sequences. Samples are demultiplexed using the two index reads in order to identify which sample the read originated from. Observed index reads are matched to the expected index sequences allowing for the possibility of a single nucleotide error in one or both of the index sequences. The set of three reads are discarded if both index1 and index2 have hamming distances greater than 1 from the expected index sequences. The count of reads for each amplicon and each sample is calculated. In this analysis we make use of a few custom scripts written in R that rely on the ShortRead30 and stringdist31 packages for processing fastq files and calculating hamming distances between observed and expected amplicons and indices. This approach was conservative and gave us very low level control of the sequencing analysis. However, we anticipate that continued development of the kallisto and bustools SwabSeq analysis tools19 will be a more user-friendly and computationally efficient solution for SwabSeq.
Criteria for Classification of Purified Patient Samples
For our analytic pipeline, we developed QC metrics for each type of specimen. For purified RNA, we require each sample to have at least 10 reads detected for RPP30 and that the sum of S2 and in vitro S2 standard reads exceeds 2,000 reads. If these conditions are not met, the sample is rerun one time and if there is a second fail we request a resample. To determine if SARS-CoV-2 is present, we calculate if the ratio of S2 to in vitro S2 standard exceeds 0.003. (We note that we add 1 count to both S2 and in vitro S2 standard before calculating this ratio to facilitate plotting the results on a logarithmic scale.) If the ratio is greater than 0.003 we concluded that SARS-CoV-2 is detected for that sample and if it is less than or equal to 0.003 we conclude that SARS-CoV-2 is not detected (Figure S5C).
The same pair of primers will amplify both the S2 and in vitro S2 standard amplicons. Because we run an endpoint assay, the primers will be the limiting reagent to continued amplification. In developing this assay, we observed that as S2 counts increase for a sample, the in vitro S2 standard counts decrease (Figure S3). We found that at very high viral levels, in vitro S2 standard read counts decreased to less than 1000 reads. Therefore, analysis of S2 and in vitro S2 standard together allowed our QC to call SARS-CoV-2 even at extremely high viral levels.
Since the S2 and in vitro S2 standard are derived from the same primer pair, to account for the scenario where in vitro S2 standard counts are low because S2 amplicon counts are very high and the sample contains large amounts of SARS-CoV-2 RNA (Figure S3) in the QC we require that the sum of S2 and in vitro S2 standard counts together exceeds 2000. For example, if we detected greater than 2000 S2 counts and 0 in vitro S2 standard counts this would certainly be a SARS-CoV-2 positive sample and we would result: SARS-CoV-2 detected.
Criteria for Classification of Extraction Free Patient Samples
We require that the sum of S2 and S2 synthetic spike-in reads exceeds 500 reads, or the results are considered inconclusive. With inhibitory lysates we have observed that this lower total is acceptable for maintaining sensitivity and specificity while limiting the number of tests that are considered inconclusive. If the sum of S2 and S2 synthetic spike-in reads exceeds 500, we determine if SARS-CoV-2 is detected in a sample by seeing if the ratio of S2 to S2 spike exceeds 0.003. (We note that we add 1 count to both S2 and S2 spike before calculating this ratio to facilitate plotting the results on a logarithmic scale.) If the ratio is greater than 0.003 we concluded that SARS-CoV-2 is detected for that sample and if it is less than or equal to 0.003 we conclude that SARS-CoV-2 is not detected as long as 10 reads are detected for RPP30 for that sample. This serves as a control that sample collection took place properly and contains a human specimen. If fewer than 10 reads are detected for RPP30 and the ratio of S2 to S2 spike is less than or equal to 0.003 the results are considered inconclusive. We have modified this criteria such that only samples without SARS-CoV-2 signal are considered inconclusive if RPP30 reads are fewer than 10. This ensures that we do not miss SARS-CoV-2 positive samples that may have fewer RPP30 reads (Figure S7).
Downsampling analysis
Reads were downsampled from the results for the NP purified confirmatory LoD shown in Figure S5B. We observed that downsampling down to 5,000 reads per well resulted in no instances of mis-classification of SARS-CoV-2 presence or absence. At 5,000 reads per well approximately 3% of wells would no longer pass the filter that the sum of S2 and S2 spike reads exceeded 1,000 reads and would result in a sample being classified as ‘Inconclusive’. A logistic regression classifier described elsewhere19 should robustly tolerate a small fraction of outlier samples with slightly lower read depth.
Analysis of index mis-assignment
Unique dual indices and amplicon specific indices were used to study index mis-assignment. In this scheme, each sample was assigned two unique indices for the S2 or Spike amplicon and two unique indices for the RPP30 amplicon for a total of four unique indices per sample. A count matrix with all possible pairwise combinations, i.e. a “matching matrix”, was generated for each index pair (one i7 and one i5) using kallisto and bustools32. The counts on the diagonal of the matching matrix correspond to input samples and counts off of the diagonal correspond to index swapping events. The extent of index mis-assignment for the i7 and i5 index was determined by computing the row and column sums, respectively, of the off-diagonal elements of the matching matrix. The observed rate of index swapping to wells with a known zero amount of viral RNA was determined by computing the mean of the viral S counts to spike ratio for those wells.
Supplemental Results
Improving Limit of Detection Requires Minimizing Sources of Noise
One of the major challenges in running a highly sensitive molecular diagnostic assay is that even a single contaminant or source of noise can decrease the test’s analytical sensitivity. In the process of developing SwabSeq, we observed S2 reads from control samples in which no SARS-CoV-2 RNA was present (Figure 1D). We subsequently refer to these reads as “no template control” (NTC) reads. A key part of SwabSeq optimization has been understanding and minimizing the sources of NTC reads in order to improve the limit of detection (LoD) of the assay. We identified two important sources of NTC reads: molecular contamination and mis-assignment of sequencing reads.
To minimize molecular contamination, we followed protocols and procedures that are commonly used in molecular genetic diagnostic laboratories33. To limit molecular contamination, we use a dedicated hood for making dilutions of the synthetic RNA controls and master mix. At the start of each new run, we sterilize the pipettes, dilution solutions, and PCR plates with 10% bleach, followed by UV-light treatment for 15 minutes.
To prevent post-PCR products that are at high concentration from contaminating our pre-PCR processes, we physically separated pre- and post-PCR steps of our protocol into two separate rooms, where any post-PCR plates were never opened within the pre-PCR laboratory space. To further protect from post-PCR contamination, we compared RT-PCR mastermixes with or without Uracil-N-glycosylase (UNG). The presence of UNG in the TaqPath™ 1-Step RT-qPCR Master Mix (Thermofisher Scientific) showed a significant improvement reducing post-PCR contamination of S2 reads present in the negative patient samples as compared with the Luna One Step RT-PCR Mix (New England Biosciences) (Figure S14). The RT-PCR mastermix contains a mix of dTTP and dUTP such that post-PCR amplicons are uracil containing DNA. These post-PCR that are remnants of previously run SwabSeq experiments therefore can be selectively eliminated by UNG. Importantly, this addition does not interfere with downstream sequencing.
A third source of molecular contamination was carryover contamination on the sequencer template line of the Illumina MiSeq34. Without a bleach maintenance wash, we found that indices from the previous sequencing run were identified in a subsequent experiment where those indices were not included. While the number of reads for some indices were present at a number of S2 reads, the presence of carryover contamination affects the sensitivity and specificity of our assay. After an extra maintenance and bleach wash, we substantially reduced the amount of carryover reads present to less than 10 reads (Figure S15).
Another source of NTC reads is mis-assignment of amplicons. Mis-assignment of amplicons occurs when sequencing (and perhaps at a lower rate, oligo synthesis) errors result in an amplicon sequence that originates from the in vitro S2 standard but is mistakenly assigned to the S2 sequence within a given sample. Only 6 bp distinguishes S2 from in vitro S2 standard at the beginning of read 1. Sequencing errors can result in in vitro S2 standard reads being misclassified as S2 reads as error rates appear to be higher in the beginning of the read (Figure S16A). If computational error correction of the amplicon reads is too tolerant, these reads may be inadvertently counted to the wrong category. To reduce this source of S2 read misassignment, we use a more conservative thresholding on edit distance (Figure S16B). Future redesigns or extensions to additional viral amplicons should consider engineering longer regions of sequence diversity here.
An additional source of NTC reads is when S2 amplicon reads are mis-assigned to the wrong sample based on the indexing strategy. In our assay, individual samples are identified by pairs of index reads (Figure 1B). Mis-assignment of samples to the wrong index could occur if there is contamination of index primer sequences, synthesis errors in the index sequence, sequencing errors in the index sequences, or “index hopping”35.
We leveraged multiple indexing strategies in our development of SwabSeq, from fully combinatorial indexing (where each possible combination of i5 and i7 indices was used to tag samples in the assay) to unique-dual indexing (UDI) where each sample has distinct and unrelated i7 and i5 indices (Figure S14). However, the ability to scale can be limited due to the substantial upfront cost of developing that many unique primers. Fully combinatorial indexing approaches significantly expand the number of unique primer combinations. We have also explored a compromise strategy between fully combinatorial indexing and UDI where sets of indices are only shared between small subsets of samples. Such designs reduce the effect of sample mis-assignment while facilitating scaling to tens of thousands of patient samples (Figure S17). With a fully combinatorial indexing (Figure S17) we observed that NTC read depth was correlated with the total number of S2 reads summed across all samples that shared the same i7 sequence (Figure S18A). This is consistent with the effect of index hopping from samples with high S2 viral reads to samples that share the same indices. It is possible to computationally correct for this effect, for example using a linear mixed model (Figures S18B).
Finally, the challenges associated with combinatorial and semi-combinatorial indexing strategies can be mitigated by using unique dual indexing (UDI), a known strategy to reduce the number of index-hopped reads by two orders of magnitude36. We have observed consistently lower S2 viral reads for negative control samples with this strategy. It also enables quantification of index mis-assignment by counting reads for index combinations that should not occur in our assay (Figure S19 A and B). The number of index hopping events is correlated with the total number of S2 + S2 spike reads (Figure S19 C and D), indicating that hopped reads are more likely to come from wells where the expected index has strong viral signal. We quantify the overall rate of hopping as 1–2% on a MiSeq, and expect this rate may be higher on patterned flow cell instruments.
There are many sources of noise in amplicon-based sequencing, from environmental contamination in the RT-PCR and sequencing steps to misassignment of reads based on computational correction and “index-hopping” on the Illumina flow cells. Preventing and correcting these sources of error considerably improves the limit of detection of the SwabSeq assay.
Supplementary Material
Acknowledgments.
We thank Jane Semel. Without her support this work would not have been possible. We also thank the Held Foundation for all their support of this project. We thank the UCLA David Geffen School of Medicine’s Dean’s Office for their support, the Fast Grants, Inc for funding of this work. We also thank Lea Starita, Beth Martin, Jase Gehring, Sanjay Srivatsan, Jay Shendure, and the members of the Covid Testing Scaleup Slack for their input, guidance and openness in sharing their processes. This work was supported by funding from the Howard Hughes Medical Institute (to LK) and DP5OD024579 (to VA). IL is supported by T32GM008042. We thank Marlene Berro for her guidance with the FDA EUA201963. We also thank the clinical lab specialists in the UCLA Clinical Microbiology lab for their assistance in collecting and processing the remnant specimens and data. Biorender.com was used to generate figures with cartoons. We thank all the video games and video game makers that have helped keep our loved ones sane as we spent all our time on SwabSeq.
E.M.J, M.G., N.B.L., S.W.S. and S.K. are employed by and hold equity, J.S.B. consults for and holds equity, and A.R.C holds equity in Octant Inc. which initially developed SwabSeq, and has filed for patents for some of the work here, though they have been made available under the Open Covid License: https://www.notion.so/Octant-COVID-License-816b04b442674433a2a58bff2d8288df.
Footnotes
Supplementary Documents
OptimizedProtocol.docx
EquipmentList.xlsx
SampleExperimentalDesignSetup.xlsx
SampleTrackingWorkbook.xlsx
Supplementary Figures
SupplementaryFigures.pdf
Supplementary Videos
1_PrePCR.mov
2_PostPCR.mov
Data Availability
Source data for all figures are available on https://github.com/joshsbloom/swabseq. All protocols and primers are available under an Open Covid License https://www.notion.so/Octant-COVID-License-816b04b442674433a2a58bff2d8288df.
Code Availability
All code can be accessed on https://github.com/joshsbloom/swabseq
REFERENCES
- 1.Furukawa N. W., Brooks J. T. & Sobel J. Evidence Supporting Transmission of Severe Acute Respiratory Syndrome Coronavirus 2 While Presymptomatic or Asymptomatic. Emerg. Infect. Dis. 26, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lavezzo E. et al. Suppression of a SARS-CoV-2 outbreak in the Italian municipality of Vo’. Nature (2020) doi: 10.1038/s41586-020-2488-1 [DOI] [PubMed] [Google Scholar]
- 3.Peiris J. S. M., Yuen K. Y., Osterhaus A. D. M. E. & Stöhr K. The severe acute respiratory syndrome. N. Engl. J. Med. 349, 2431–2441 (2003). [DOI] [PubMed] [Google Scholar]
- 4.Cheng C., Wong W.-M. & Tsang K. W. Perception of benefits and costs during SARS outbreak: An 18-month prospective study. J. Consult. Clin. Psychol. 74, 870–879 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Gandhi M., Yokoe D. S. & Havlir D. V. Asymptomatic Transmission, the Achilles’ Heel of Current Strategies to Control Covid-19. The New England journal of medicine vol. 382 2158–2160 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kimball A. et al. Asymptomatic and Presymptomatic SARS-CoV-2 Infections in Residents of a Long-Term Care Skilled Nursing Facility - King County, Washington, March 2020. MMWR Morb. Mortal. Wkly. Rep. 69, 377–381 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernandez M. & Mervosh S. Texas Pauses Reopening as Virus Cases Soar Across the South and West. The New York Times (2020). [Google Scholar]
- 8.News Division. HHS Details Multiple COVID-19 Testing Statistics as National Test. https://www.hhs.gov/about/news/2020/07/31/hhs-details-multiple-covid-19-testing-statistics-as-national-test-volume-surges.html.
- 9.CMS Increases Medicare Payment for High-Production Coronavirus Lab Tests. https://www.cms.gov/newsroom/press-releases/cms-increases-medicare-payment-high-production-coronavirus-lab-tests-0.
- 10.Kliff S. Most Coronavirus Tests Cost About 100. Why Did One Cost 2,315? The New York Times (2020). [Google Scholar]
- 11.Pollitz K. Free Coronavirus Testing for Privately Insured Patients? Kasier Family Foundation https://www.kff.org/coronavirus-policy-watch/free-coronavirus-testing-for-privately-insured-patients/ (2020). [Google Scholar]
- 12.Larremore D. B. et al. Surveillance testing of SARS-CoV-2. Infectious Diseases (except HIV/AIDS) (2020) doi: 10.1101/2020.06.22.20136309 [DOI] [Google Scholar]
- 13.Paltiel A. D., David Paltiel A., Zheng A. & Walensky R. P. Assessment of SARS-CoV-2 Screening Strategies to Permit the Safe Reopening of College Campuses in the United States. JAMA Network Open vol. 3 e2016818 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones Eric M, Cooper Aaron R, Bloom Joshua S, Lubock Nathan B., Simpkins Scott W., Gasperini Molly, Kosuri Sriram. Octant SwabSeq Testing. https://www.notion.so/Octant-SwabSeq-Testing-9eb80e793d7e46348038aa80a5a901fd (2020).
- 15.Jones E. M. et al. A Scalable, Multiplexed Assay for Decoding GPCR-Ligand Interactions with RNA Sequencing. Cell Syst 8, 254–260.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmid-Burgk J. L. et al. LAMP-Seq: Population-Scale COVID-19 Diagnostics Using a Compressed Barcode Space. bioRxiv 2020.04.06.025635 (2020) doi: 10.1101/2020.04.06.025635 [DOI] [Google Scholar]
- 17.COVIDSeq Test (RUO and PEO Versions). https://www.illumina.com/products/by-type/clinical-research-products/covidseq.html.
- 18.rapidmicrobiology Bioinnovation’s DxSeq™ Sequences Filoviruses. https://www.rapidmicrobiology.com/news/bioinnovations-dxseq-sequences-filoviruses.
- 19.Booeshaghi A. S. et al. Fast and accurate diagnostics from highly multiplexed sequencing assays. Health Informatics (2020) doi: 10.1101/2020.05.13.20100131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Relich R. F. PREPARATION OF VIRAL TRANSPORT MEDIUM. https://www.cdc.gov/coronavirus/2019-ncov/downloads/Viral-Transport-Medium.pdf (2020).
- 21.CDC. Information for Laboratories about Coronavirus (COVID-19). Centers for Disease Control and Prevention https://www.cdc.gov/coronavirus/2019-ncov/lab/rt-pcr-panel-primer-probes.html (2020).
- 22.Ranoa D. R. E. et al. Saliva-Based Molecular Testing for SARS-CoV-2 that Bypasses RNA Extraction. bioRxiv 2020.06.18.159434 (2020) doi: 10.1101/2020.06.18.159434 [DOI] [Google Scholar]
- 23.COVID-19 Testing at Broad. https://covid-19-test-info.broadinstitute.org/.
- 24.iSWAB Rack Format - Mawi DNA Technologies. Mawi DNA Technologies https://mawidna.com/our-products/iswab-rack-format/.
- 25.Covid T. Multiplex Diagnostic Solution| Thermo Fisher Scientific-US. (19AD).
- 26.Clark T. W. et al. Diagnostic accuracy of the FebriDx host response point-of-care test in patients hospitalised with suspected COVID-19. J. Infect. (2020) doi: 10.1016/j.jinf.2020.06.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yujian L. & Bo L. A normalized Levenshtein distance metric. IEEE Trans. Pattern Anal. Mach. Intell. 29, 1091–1095 (2007). [DOI] [PubMed] [Google Scholar]
- 28.Untergasser A. et al. Primer3--new capabilities and interfaces. Nucleic Acids Res. 40, e115 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quail M. A., Swerdlow H. & Turner D. J. Improved protocols for the illumina genome analyzer sequencing system. Curr. Protoc. Hum. Genet. Chapter 18, Unit 18.2 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morgan M. et al. ShortRead: a bioconductor package for input, quality assessment and exploration of high-throughput sequence data. Bioinformatics 25, 2607–2608 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Van der Loo M. P. J. The stringdist package for approximate string matching. R J. 6, 111–122 (2014). [Google Scholar]
- 32.Melsted P. et al. Modular and efficient pre-processing of single-cell RNA-seq. bioRxiv 673285 (2019) doi: 10.1101/673285 [DOI] [Google Scholar]
- 33.Furtado L. V., The 2013 AMP Clinical Practice Committee consisted of Matthew J. Bankowski, Milena Cankovic, Jennifer Dunlap.
- 34.Nelson M. C., Morrison H. G., Benjamino J., Grim S. L. & Graf J. Analysis, optimization and verification of Illumina-generated 16S rRNA gene amplicon surveys. PLoS One 9, e94249 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van der Valk T. et al. Index hopping on the Illumina HiseqX platform and its consequences for ancient DNA studies. Molecular Ecology Resources (2019) doi: 10.1111/1755-0998.13009 [DOI] [PubMed] [Google Scholar]
- 36.MacConaill L. E. et al. Unique, dual-indexed sequencing adapters with UMIs effectively eliminate index cross-talk and significantly improve sensitivity of massively parallel sequencing. BMC Genomics 19, 30 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.