Abstract
Shikonin has been reported to exhibit a wide variety of medical functions. However, the strong non-selective cytotoxicity of shikonin can restrict its clinical application. The aim of the present study was to investigate the effects of shikonin at non-cytotoxic doses on the pro-inflammation functions of monocytes and macrophages. The present results suggested that the non-cytotoxic doses of shikonin effectively inhibited lipopolysaccharide (LPS)-induced reactive oxygen species production, NF-κB activation and TNF-α expression in RAW 264.7 mouse macrophages via AMP-activated protein kinase (AMPK) signaling pathway. In addition, the non-cytotoxic doses of shikonin downregulated LPS-induced TNF-α expression via AMPK signaling activation in primary murine bone marrow-derived macrophages, and also in monocytes cultured ex vivo from patients with chronic obstructive pulmonary disease (COPD). The present in vivo results indicated that the low-toxic dose of shikonin suppressed LPS-induced endotoxin shock and TNF-α expression in mice. Collectively, the present results may provide clinical and translational relevance for treating COPD and other TNF-α-related inflammatory disorders.
Keywords: shikonin, AMP-activated protein kinase, tumor necrosis factor-α, reactive oxygen species, chronic obstructive pulmonary disease
Introduction
Chronic obstructive pulmonary disease (COPD) occurs in >380 million individuals globally, especially amongst adults aged >30 years (1). The global prevalence of COPD is expected to increase continuously during the next few decades due to an ageing population, uncontrolled smoking prevalence in developing countries and additional environmental exposures such as air pollution and biomass fuel exposure (2). It is estimated that in 2030, more than 4.5 million deaths annually will be attributable to COPD and related conditions worldwide (2), and by 2060 there may be over 5.4 million deaths annually from COPD and related conditions globally (3). COPD is a progressive lung disease characterized by persistent airway inflammation, accompanied by irreversible airflow obstruction, including chronic bronchitis and emphysema (4,5). Despite the rising the incidence of COPD, there are currently no specific drugs to treat and protect against COPD (5-7). Therefore, the identification of novel agents for targeting this disease is urgently needed.
Elevated levels of pathogen-associated molecular patterns (PAMPs) are often associated with progressive inflammation in COPD (8,9). Lipopolysaccharide (LPS) is one of the most common PAMPs, which is produced from the cell walls of Gram-negative bacteria (4). LPS has been recognized as an essential contributor to lung inflammation and injury in COPD (10,11). Moreover, LPS can activate inflammatory cells, such as monocytes and macrophages, to produce tumor necrosis factor-α (TNF-α) and other related inflammatory cytokines (8,9). The levels of TNF-α are significantly increased in the sputum, bronchoalveolar lavage fluid, plasma and lung tissue of patients with COPD, which may serve as a therapeutic target for chronic lung inflammation (4). Strategies to reduce the secretion of TNF-α may effectively alleviate the inflammation in patients with COPD (11-13).
AMP-activated protein kinase (AMPK) plays a vital role in maintaining cellular energy homeostasis (14). Studies have suggested that AMPK also exerts potent anti-inflammatory effects (15-18). A plethora of studies have demonstrated that AMPK inhibits the inflammatory response by indirect inhibition of NF-κB activation (19-22). In addition, a study suggested that AMPK exerted anti-inflammatory effects in immune cells by switching metabolic activity from a glycolysis driven process to a mitochondrial oxidative metabolic process, such as fatty acid oxidation (FAO) (23,24). Furthermore, an additional study indicated that transformation of pro-inflammatory M1 macrophages to anti-inflammatory M2 macrophages is dependent on AMPK and FAO (25). This evidence indicates that AMPK has a functional role in attenuating the inflammatory response (15,24,26). The well-established AMPK activators 5-Aminoimidazole-4-carboxamide ribonucleotide (AICAR) and A769662 have been reported to suppress LPS-induced cytokine production and NF-κB activation (27,28). An additional classic AMPK activator and widely used antidiabetic agent, metformin, has been indicated to decrease the expression levels of pro-inflammation and adhesion molecules by activating the AMPK signaling pathway (29). Furthermore, it has been reported that perifosine and cordycepin activate the AMPK signaling pathway, and subsequently inhibit LPS-induced TNF-α expression (27,30). Therefore, AMPK activation may serve as an effective and novel therapeutic strategy for inhibiting LPS-induced inflammatory responses (31).
Zicao is a commonly used herbal medicine in China (32). Zicao is believed to possess detoxification properties and has been used for the treatment of macular eruptions, measles, sore-throat, carbuncles and burns (33). Shikonin, a naphthoquinone compound, is the primary active constituent of Zicao, which can be produced from the dried root of Lithospermum erythrorhizon (34). Shikonin has been reported to exert multiple pharmacological effects, including both anti-cancer and anti-inflammatory properties (35,36). However, the strong non-selective cytotoxicity of shikonin restricts its clinical application (36). Thus, it is essential to investigate the pharmacological effects of shikonin at low cytotoxic doses. The aims of the present study were to examine the effects of shikonin at non-cytotoxic doses on the pro-inflammation functions of monocytes and macrophages, as well as identifying the underlying molecular mechanisms.
Materials and methods
Reagents
Shikonin was obtained from Yuanye Biotechnology Co., Ltd. (cat. no. B21682). LPS (cat. no. L6143), compound C (an AMPK inhibitor; cat. no. 171261) and D-galactosamine (cat. no. G0500-5G) were obtained from Sigma-Aldrich (Merck KGaA). MTT was purchased from Beyotime Institute of Biotechnology (cat. no. C0009). Cell culture reagents, including: DMEM (Dulbecco's modified Eagle's medium; cat. no. 11965084), FBS (fetal bovine serum; cat. no. 16140071), glutamine (cat. no. 25030081) and penicillin-streptomycin (cat. no. 15070063) were supplied by Gibco (Thermo Fisher Scientific, Inc.).
Culture of RAW 264.7 cells
The mouse macrophage cell line RAW 264.7 was obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (cat. no. TCM13). The cells were cultured in DMEM with 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin, and 2 mM glutamine at 37˚C in a 5% CO2 humidified incubator as described previously (30).
Preparation of bone marrow-derived macrophages (BMDMs)
All animal experiments were approved by the Animal Ethics Committee of The Fourth Military Medical University (Xi'an, Shaanxi, China). The present study used 2-month-old male C57BL/6J mice (supplied by Beijing Vital River Laboratory, Beijing, China; n=10; 20-25 g). Mice were maintained under specific pathogen-free conditions. All mice were provided free access to food and water and were maintained at a temperature of 23±2˚C, humidity of 40-80% and on a 12 h light/dark cycle. Mice were euthanized with sodium pentobarbital (250 mg/kg; cat. no. P3761; Sigma-Aldrich; Merck KGaA), and their femurs and tibias were isolated and rinsed in 75% (vol/vol) ethanol. Bone marrow cells were flushed out with PBS and passed through 40 µM filters, then centrifuged at 450 x g for 5 min at room temperature. Cell pellets were suspended in ammonium-chloride-potassium (ACK) hypotonic buffer (cat. no. C3702; Beyotime Institute of Biotechnology) at a volume ratio of cell pellet to ACK hypotonic buffer of 1:5 at room temperature for 1 min, then washed with serum-free DMEM. After centrifugation at 450 x g for 10 min at room temperature, the isolated bone marrow cells were resuspended and cultured in DMEM containing 10% FBS and 30% L929-conditioned media at 37˚C in a 5% CO2 incubator to allow for differentiation. The L929-conditioned media was obtained from L929 fibroblasts (cat. no. GNM28; Cell Bank of Type Culture Collection of the Chinese Academy of Sciences) that were grown in DMEM (cat. no. 11965084; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no. 16140071; Gibco; Thermo Fisher Scientific, Inc.) at 37˚C in a 5% CO2 incubator for 3 days following confluency. The medium was filtered through a 0.22-µm filter (cat. no. FF362-100pcs; Beyotime Institute of Biotechnology) and kept at 4˚C for the subsequent applications, as previously described (37). After culturing for 7 days, the adherent macrophages were trypsinized and sub-cultured in DMEM supplemented with 10% FBS and 15% L929-conditioned media, 2 mM glutamine, 100 U/ml penicillin and 100 µg/ml streptomycin at 37˚C in a 5% CO2 incubator for subsequent experiments. The animal experimental procedures were carried out strictly in accordance with the guidelines approved by The Animal Ethics Committee of The Fourth Military Medical University.
Ex vivo culture of human peripheral blood mononuclear cells (PBMCs)
After obtaining their written informed consent, PBMCs were collected from patients with COPD. A total of 10 COPD patients were recruited at the Xijing Hospital of Fourth Military Medical University between October 2017 and March 2018. The patients were male, and aged 52-65 years old (mean age, 61.1; median age, 62.5). Cells were collected using lymphocyte separation medium (cat. no. 10771; Sigma-Aldrich; Merck KGaA) as previously described (4,28). The collected PBMCs were cultured in DMEM containing 10% FBS and other essential nutrients (38). The study protocol involving clinical specimens was approved by The Institutional Review Board of Xijing Hospital Affiliated to The Fourth Military Medical University.
Assessment of cytotoxicity
The survival rates of RAW 264.7 cells, BMDMs and PBMCs were assessed by MTT assay. Cells were cultured in 96-well plates at a density of 1x104 cells/well and incubated with 5% CO2 at 37˚C for 24 h, Subsequently, the cells were treated with gradually increasing doses of shikonin (0, 0.5, 1.0, 1.5, and 2.0 µM) at 37˚C for a further 24 h. After shikonin treatment, 20 µl/well of MTT (5 mg/ml) was added into each well, and then incubated at 37˚C for 4 h. Then, the culture supernatant was removed from each well, and DMSO (150 µl/well) was added to dissolve the formazan crystals (30). The optical density (OD) values were determined at 570 nm using a microplate reader (ELX 800; BioTek Instruments, Inc.).
The percentage of cell death was determined by trypan blue staining (4,39). After shikonin treatment, the cells were collected, centrifuged at 1,000 x g for 1 min at room temperature and resuspended in 0.4% trypan blue (cat. no. 15250061; Gibco; Thermo Fisher Scientific, Inc.), stained for 3 min at room temperature and then manually counted using a hemocytometer. The numbers of stained cells were presented as a ratio of the total (stained and unstained) cells.
Detection of apoptosis
The level of apoptosis in RAW 264.7 cells was evaluated using a TUNEL assay kit (cat. no. C1088; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. Briefly, RAW 264.7 cells were cultured in 24-well plates at a density of 1.5x105 cells/well and incubated with 5% CO2 at 37˚C for 24 h, subsequently, the cells were treated with gradually increasing doses of shikonin (0, 0.5, 1.0, 1.5 and 2.0 µM) at 37˚C for a further 24 h. After treatment, the cells were fixed in 4% paraformaldehyde for 20 min at room temperature. Subsequently, the cells were washed with PBS three times and permeabilized with 0.3% Triton X-100 for 5 min at room temperature before the cells were incubated with TUNEL working solution for 60 min in a humidified atmosphere at 37˚C in the dark. The cells were then counterstained with 5 µg/ml DAPI (cat. no. D8417; Sigma-Aldrich; Merck KGaA) for 5 min at room temperature and mounted using antifade mounting medium (cat. no. P0126; Beyotime Institute of Biotechnology). The cells were observed using a fluorescence microscope (magnification, x100), in which five fields were randomly selected, and the TUNEL and DAPI stained nuclei in the cells were counted manually (40). The percentage of positive cells was calculated using the following equation: TUNEL-positive cells (%)=(number of TUNEL positive cells/total number of cells) x 100%, as previously described (41). The apoptosis of RAW 264.7 cells was also assessed using a Dead Cell Annexin-V-FITC Propidium iodide (PI) apoptosis detection kit (cat. no. V13242; Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. The rate of apoptotic cells was evaluated using a standard EPICS Elite flow cytometer (Beckman Coulter, Inc.) as previously described (42), and data were analyzed using CXP Analysis Software version 1.0 (Beckman Coulter, Inc.).
ELISA assay
Cells (RAW 264.7 cells, BMDMs and PBMCs) were cultured in 6-well plates at a density of 1x106 cells/well and incubated with 5% CO2 at 37˚C for 24 h. Subsequently, the cells were treated with LPS alone (100 ng/ml) or 10-250 ng/ml in combination with different doses of shikonin (0.5 and 1.0 µM) at 37˚C for 24 h, with or without 1 h pretreatment with 10 µM compound C. After treatment, the extracellular levels of TNF-α in DMEM were detected using commercial ELISA kits (RAW 264.7 cells, cat. no. MTA00B, R&D Systems, Inc.; BMDMs, cat. no. PT512; Beyotime Institute of Biotechnology; PBMCs, cat. no. PT518; Beyotime Institute of Biotechnology) following each manufacturer's protocol. The general procedure was as follows: The culture medium was collected by centrifuging at 500 x g for 10 min at room temperature. The culture supernatant was added into anti-TNF-α antibody-coated wells (in the corresponding kit) and incubated for 2 h at room temperature. Then, the corresponding biotinylated antibody was added into each well and incubated for a further 1 h at room temperature. Horseradish peroxidase (HRP)-streptavidin was added and incubated in the dark for 20 min at room temperature, then TMB substrate was added into each well and incubated in the dark for another 20 min at room temperature. Stop solution was added and gently mixed in the dark for 2 min at room temperature. The absorbance values at 450 nm were detected using a microplate reader (ELX 800; BioTek Instruments, Inc.), and the concentration of TNF-α was calculated by referring to the standard curve.
Reverse transcription-quantitative PCR (RT-qPCR)
The cells (RAW 264.7 cells, BMDMs and PBMCs) were cultured in 6-well plates at a density of 1x106 cells/well and incubated with 5% CO2 at 37˚C for 24 h. Subsequently, the cells were treated with LPS (100 ng/ml alone or 10-250 ng/ml), or in combination with different doses of shikonin (0.5 and 1.0 µM) at 37˚C for 24 h, with or without 1 h pretreatment of 10 µM compound C. After treatment, RNA isolation and RT-qPCR assay were carried out according to previously published methods (4,43). Briefly, total RNA was extracted from the cells using TRIzol® reagent (cat. no. 15596018; Invitrogen; Thermo Fisher Scientific, Inc.). Then, the extracted RNA (2 µg; 10 µl) was reverse transcribed using AMV reverse transcriptase (cat. no. M5101; Promega Corporation) in a 25 µl final reaction volume containing AMV Reverse Transcriptase (3 µl), AMV Reverse Transcriptase 5X Reaction Buffer (5 µl; cat. no. M5101; Promega Corporation), 10 mM dNTP (2.5 µl; cat. no. U1330; Promega Corporation), RNasin® Ribonuclease Inhibitor (1 µl; cat. no. N2511; Promega Corporation), 500 µg/ml oligo(dT)15 primer (2 µl; cat. no. C1101; Promega Corporation) and nuclease-free water (1.5 µl; cat. no. P1193; Promega Corporation), which were incubated at 42˚C for 60 min. qPCR reactions were carried out on an ABI Prism 7500 RT PCR instrument (Applied Biosystems; Thermo Fisher Scientific, Inc.) in triplicate using SYBR Premix Ex Taq (cat. no. RR420A; Takara Bio Inc.). The thermocycling conditions were as follows: 95˚C for 5 min, followed by 40 cycles of 95˚C for 15 sec and 60˚C for 1 min. The relative changes in TNF-α mRNA expression levels were calculated using the 2-ΔΔCq method after normalization to GAPDH (44). The primer sequences for TNF-α and GAPDH were as follows: Mouse TNF-α (forward, 5'-CATCTTCTCAAAATTCGAGTGAC-3' and reverse, 5'-TGGGAGTAGACAAGGTACAACCC-3'), Mouse GAPDH (forward, 5'-GGCCTTCCGTGTTCCTAC-3' and reverse, 5'-TGTCATCATATCTGGCAGGTT-3'), Human TNF-α (forward, 5'-CGAGTGACAAGCCTGTAGCC-3' and reverse, 5'-TTGAAGAGGACCTGGGAGTAG-3'), Human GAPDH (forward, 5'-AACGGATTTGGTCGTATTG-3' and reverse, 5'-GGAAGATGGTGATGGGATT-3'). All the primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd.
Western blotting analysis
Total protein was extracted from the cells with RIPA buffer (cat. no. P0013; Beyotime Institute of Biotechnology), followed by quantification using a bicinchoninic acid protein assay kit (cat. no. P0012; Beyotime Institute of Biotechnology). Equal amounts of protein (30 µg protein per lane) from each sample were separated by 10% SDS-PAGE and transferred to nitrocellulose filter membrane at 100 mV for 75 min. After blocking with 5% non-fat skim milk [diluted with Tris-buffered saline containing 0.1% Tween-20 (TBST)] for 1 h at room temperature, the membrane was incubated overnight with primary antibody [diluted with 2% bovine serum albumin (cat. no. ST023; Beyotime Institute of Biotechnology) in TBST] at 4˚C (45). The following primary antibodies were used: Anti-acetyl-CoA carboxylase (ACC; 1:1,000; cat. no. AF1867), anti-phosphorylated (p)-ACC (1:1,000; cat. no. AA110), anti-AMPK (1:2,000; cat. no. AF1627), anti-p-AMPK (1:1,000; cat. no. AA393), anti-p-IκB kinase α/β (1:1,000; IKKα/β; cat. no. AI139), anti-IKKα/β (1:1,000; cat. no. AF2221), and anti-β-actin (1:1,000; cat. no. AF0003), all of the above primary antibodies were purchased from Beyotime Institute of Biotechnology. On the next day, blots were washed and incubated with anti-rabbit (1:5,000; cat. no. SA00001-2; ProteinTech Group) or anti-mouse (1:5,000; cat. no. SA00001-1, ProteinTech Group) horseradish-peroxidase-conjugated secondary antibody for 1 h at room temperature. The protein bands were visualized with an enhanced chemiluminescence western blot detection kit (Pierce; Thermo Fisher Scientific, Inc.), and the protein expression levels were semi quantified by densitometry using ImageJ version 1.46r (National Institutes of Health) (45).
Reactive oxygen species (ROS) determination
The levels of ROS in RAW 264.7 cells, BMDMs and PBMCs were assessed using a ROS detection kit (cat. no. S0033; Beyotime Institute of Biotechnology) as described previously (43). Cells were harvested by trypsinization and then resuspended into serum-free DMEM. The cell suspension was incubated with 10 µM Dichloro-dihydro-fluorescein diacetate solution at 37˚C for 20 min in the dark, and mixed by repeatedly inverting the tube for 5 min. After being washed three times with serum-free culture medium, the cell samples were analyzed by a flow cytometry (FACScan; Becton, Dickinson and Company). Fluorescence intensity values of the treatment group were normalized as fold changes relative to the control group.
Thiobarbituric acid reactive substances (TBARS) assay
TBARS assay was used for the assessment of lipid peroxidation (28,46). RAW 264.7 cells were treated with 100 ng/ml of LPS, or in combination with different doses of shikonin (0.5 and 1.0 µM) for 2 h, with or without 1 h pretreatment of 10 µM compound C. After treatment, the cells were harvested by trypsinization, and the cell extracts were prepared by sonication (at 200 W four times, for 5 sec each time, with a 2 sec interval between pulses) at 4˚C in ice-cold RIPA buffer (cat. no. P0013; Beyotime Institute of Biotechnology). To remove debris, the lysed cells were centrifuged at 10,000 x g, at 4˚C for 20 min. After centrifugation, the malondialdehyde (MDA) levels in the supernatant were measured following the manufacturer's protocol of the TBARS assay kit (cat. no. S0131; Beyotime Institute of Biotechnology) (47). A protein assay kit (cat. no. P0012; Beyotime Institute of Biotechnology) was used to quantify the total protein concentration, and the MDA levels were then normalized to mg protein. The values of MDA in treatment groups were presented as fold changes relative to the control group.
Measurement of NF-κB p65 subunit DNA binding activity
The DNA binding activity of NF-κB p65 subunit in RAW 264.7 cells, BMDMs and PBMCs was assessed as described previously (4,31). The cells were treated with 100 ng/ml of LPS, or in combination with different doses of shikonin (0.5 and 1.0 µM) for 2vh, with or without 1 h pretreatment of 10 µM compound C. After treatment, 1 µg of nuclear extracts per treatment was analyzed by the TransAM ELISA kit (cat. no. 40098; Active Motif, Inc.) according to the manufacturer's instructions. To determine the relative changes in NF-κB p65 subunit DNA binding activities, the OD values of the treatment group were compared with the untreated group.
LPS-induced endotoxin shock
The animal experiments were approved by the Animal Ethics Committee of The Fourth Military Medical University (Xi'an, Shaanxi, China). BALB/c mice (supplied by Beijing Vital River Laboratory; male; 4-6 weeks old; weighing 18-20 g; n=30) had ad libitum access to food and water, and were maintained under a 12 h light/ dark cycle at 24±1˚C with 40-80% relative humidity. The experiment was divided into three groups (n=10 per group): Control group, D-galactosamine/LPS group, and D-galactosamine/LPS + Shikonin group. The mice were intraperitoneally injected with 30 mg/kg body weight (BW) LPS, and 300 mg/kg BW D-galactosamine or 2.5 mg/kg BW shikonin as described previously (4,28). Blood samples of ~50-150 µl were collected from the tail vein of mice 8 h after LPS induction. The blood samples were incubated at 4˚C for 2 h to allow clotting and then centrifuged at 8,000 x g to obtain serum (~15-50 µl/per mouse), as previously described (48). The levels of TNF-α in serum (diluted with the dilution buffer of the corresponding kit) were detected using ELISA (mouse TNF-α ELISA kit; cat. no. PT512; Beyotime Institute of Biotechnology) following the manufacturer's protocol (38). The survival of mice was recorded within 72 h after LPS injection, and the animal suffering was minimized based on the humane endpoints. Clinical signs of the humane endpoints included a significant decrease in locomotion, severe diarrhea, piloerection and a 20% reduction in body weight (4). Animals were weighed and monitored every hour after the initial administration of LPS/D-galactosamine. Once the animals reached the endpoints, they were euthanized with sodium pentobarbital (50 mg/kg BW, i.p; cat. no. P3761; Sigma-Aldrich; Merck KGaA), followed by cervical dislocation. The procedures of animal experimentation were carried out strictly in accordance with the guidelines approved by The Animal Ethics Committee of The Fourth Military Medical University.
Statistical analysis
Data were obtained from ≥3 independent experiments. Statistical analyses were carried out using SPSS ver. 16.0 software (SPSS, Inc.). Data are presented as the mean ± SD. Statistical differences between groups were compared using Student's t-test or one-way ANOVA with Bonferroni's post hoc test. Intergroup survival rates were analyzed using Kaplan-Meier curves with log-rank test. P<0.05 was considered to indicate a statistically significant difference.
Results
Cytotoxic effects of shikonin on RAW 264.7 mouse macrophages
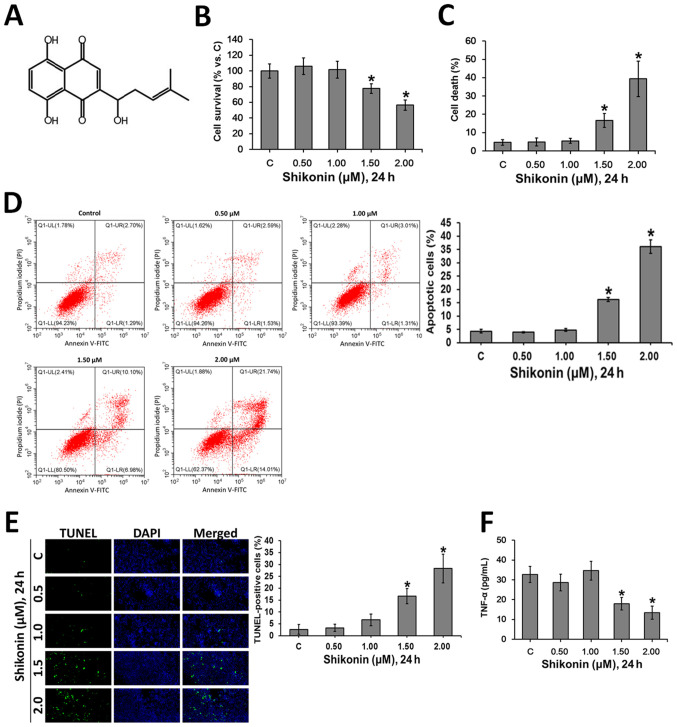
Shikonin, a naphthoquinone compound, is the primary active constituent of Zicao (32). Shikonin (Fig. 1A) has been reported to have a wide variety of medical functions, but its non-selectivity and strong cytotoxicity limits its clinical use (36). The present study investigated the cytotoxic profile of shikonin in RAW 264.7 macrophages. The cultured macrophages were treated with gradually increasing doses of shikonin for 24 h. Then, MTT (Fig. 1B) and trypan blue exclusion (Fig. 1C) assays were performed to assess the viability of cells. It was found that shikonin (1.5-2.0 µM) was cytotoxic to RAW 264.7 macrophages, however, there was no obvious cytotoxicity with 0.5 and 1.0 µM shikonin. The flow cytometry analysis (Fig. 1D) and TUNEL staining (Fig. 1E) results suggested that shikonin induced apoptosis at the concentrations of 1.5 and 2.0 µM, but did not have these effects at 0.5 and 1.0 µM. In addition, at non-cytotoxic concentrations (0.5 and 1.0 µM), shikonin did not affect the levels of TNF-α in RAW 264.7 macrophages (Fig. 1F). However, at 1.5 and 2.0 µM, shikonin significantly inhibited the production of TNF-α (Fig. 1F), which may attribute to shikonin-induced apoptosis (Fig. 1D and E).
Figure 1.
Cytotoxic profile of shikonin in RAW 264.7 mouse macrophages. (A) Chemical structure of shikonin. RAW 264.7 cells were treated with the indicated doses of shikonin for 24 h, the survival rates of cells were evaluated by (B) MTT assay and the death rates of cells were assessed using a (C) trypan blue exclusion assay. Cell apoptosis was assessed by (D) flow cytometry analysis and (E) TUNEL staining. Magnification, x100. (F) Contents of TNF-α in culture medium were examined by ELISA assay. *P<0.05 vs. control group. C, control; TNF-α, tumor necrosis factor α.
Non-cytotoxic doses of shikonin suppress LPS-induced TNF-α expression in RAW 264.7 mouse macrophages
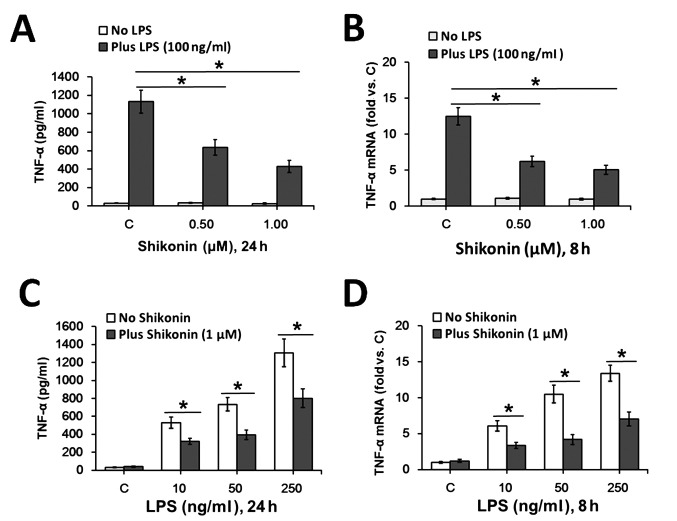
Therefore, based on the present results the non-cytotoxic doses (0.5 and 1.0 µM) of shikonin were chosen for subsequent experimentation. In line with previous studies (4,28), 100 ng/ml of LPS treatment significantly increased TNF-α expression in RAW 264.7 macrophages at both the protein (Fig. 2A) and mRNA (Fig. 2B) levels. However, co-treatment with 0.5 and 1.0 µM of shikonin significantly attenuated LPS-induced TNF-α expression levels (Fig. 2A and B). Furthermore, 1.0 µM shikonin-mediated inhibition of TNF-α expression was identified in RAW 264.7 macrophages in response to different concentrations of LPS (Fig. 2C and D). Collectively, the present results suggested that non-cytotoxic doses of shikonin inhibit LPS-induced TNF-α expression in RAW 264.7 macrophages.
Figure 2.
Non-cytotoxic doses of shikonin inhibit LPS-induced TNF-α expression in RAW 264.7 mouse macrophages. RAW 264.7 cells were treated with 10-250 ng/ml of LPS and/or the indicated doses of shikonin. (A) Contents of TNF-α in culture medium were examined using ELISA assay after 24 h of LPS treatment and shikonin. (B) After 8 h of LPS and shikonin treatment, the relative mRNA expression levels of TNF-α were detected using RT-qPCR. (C) Contents of TNF-α in culture medium were examined using ELISA assay after 24 h of varying LPS treatment and shikonin. (D) Relative mRNA expression levels of TNF-α were assessed using RT-qPCR. *P<0.05 vs. LPS alone group. Reverse transcription-quantitative PCR, RT-qPCR; C, control; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α.
Shikonin suppresses LPS-induced TNF-α expression via AMPK activation
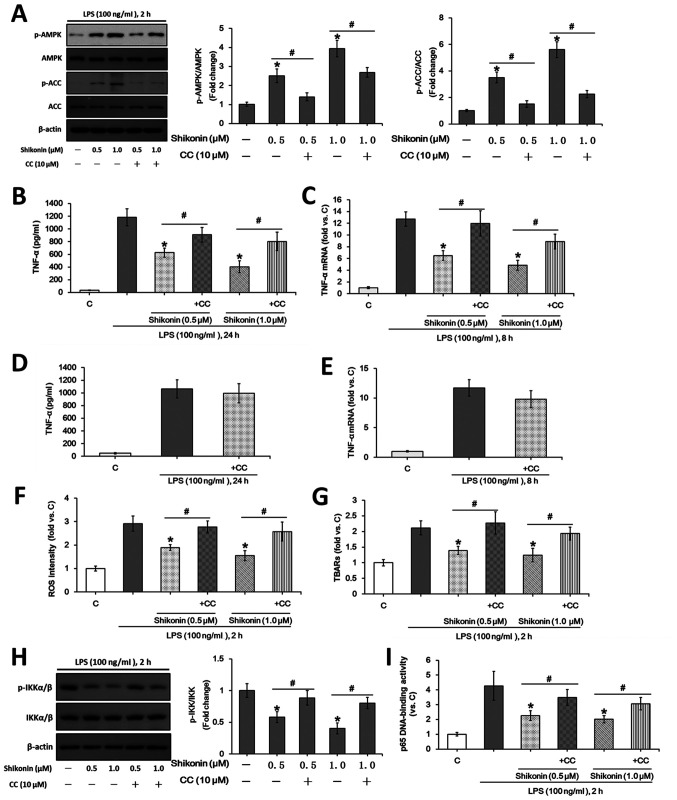
AMPK activation is involved in the suppression of inflammatory responses (31). Previous studies showed that the activation of AMPK inhibits LPS-induced TNF-α expression in mouse macrophages (28,30). However, it remains unknown whether the AMPK signaling pathway is activated in shikonin-treated RAW 264.7 macrophages. The present results indicated that the phosphorylation levels of ACC and AMPKα were significantly increased after treatment with non-cytotoxic doses of shikonin (0.5 and 1.0 µM; Fig. 3A). However, pretreatment with compound C, an AMPK inhibitor, not only reversed the AMPK activation induced by shikonin (Fig. 3A), but also attenuated the inhibitory effects of shikonin on LPS-induced TNF-α expression at both protein and mRNA levels (Fig. 3B and C). Consistent with previous results (49), in the present study compound C alone showed no obvious effect on TNF-α expression under LPS stimulation (Fig. 3D and E). Therefore, the present results suggested that the AMPK signaling pathway may play a key role in the inhibition of LPS-induced TNF-α expression after treatment with shikonin at non-cytotoxic doses.
Figure 3.
Non-cytotoxic doses of shikonin suppress LPS-induced TNF-α expression via activating AMPK pathway. RAW 264.7 cells were treated with 100 ng/ml of LPS, or in combination with the indicated doses of shikonin with and without 1 h pretreatment of 10 µM compound C. (A) Protein expression levels of ACC, p-ACC, AMPKα and p-AMPKα were determined by western blotting and then semi-quantified by densitometric analysis. (B) Extracellular contents of TNF-α were examined using ELISA assay. (C) Relative mRNA levels of TNF-α were examined using RT-qPCR assay. RAW 264.7 cells were treated with 100 ng/ml of LPS with or without 1 h pretreatment of 10 µM compound C for (D) 24 h or (E) 8 h. Extracellular concentration of TNF-α was examined using ELISA assay. Relative mRNA levels of TNF-α were examined using RT-qPCR assay. RAW 264.7 cells were treated with 100 ng/ml of LPS for 2 h, or in combination with the indicated doses of shikonin with or without 1 h pretreatment with 10 µM compound C. (F) Relative ROS intensity and (G) TBARs content were detected by flow cytometry and TBARs production assay, respectively. Activation of NF-κB was evaluated by detecting (H) p-IKKα/β and IKKα/β levels, (I) and p65 DNA-binding activity. *P<0.05 vs. LPS alone group. #P<0.05 vs. corresponding shikonin combined with LPS group. C, control; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; p-, phosphorylated; ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; IKKα/β, IκB kinase α/β; CC, Compound C; ROS, reactive oxygen species; TBARs, thiobarbituric acid reactive substances.
Previous studies have reported that LPS-induced ROS is required for subsequent TNF-α expression and NF-κB activation (28,30). AMPK activation suppresses ROS production under different stress conditions (14,43). The present results indicated that shikonin treatment at non-cytotoxic doses (0.5 and 1.0 µM) significantly attenuated LPS-induced ROS generation (Fig. 3F), lipid peroxidation (TBARs production; Fig. 3G) and NF-κB activation (Fig. 3H and I) in RAW 264.7 macrophages. Moreover, pretreatment with compound C reversed the effects of shikonin (Fig. 3F-I). However, the non-cytotoxic doses of shikonin exerted no significant effects on both NF-κB activation and ROS generation in the absence of LPS stimulation (data not shown). Therefore, shikonin, via AMPK signaling activation, inhibited LPS-induced ROS generation and NF-κB activation, which suppressed TNF-α expression.
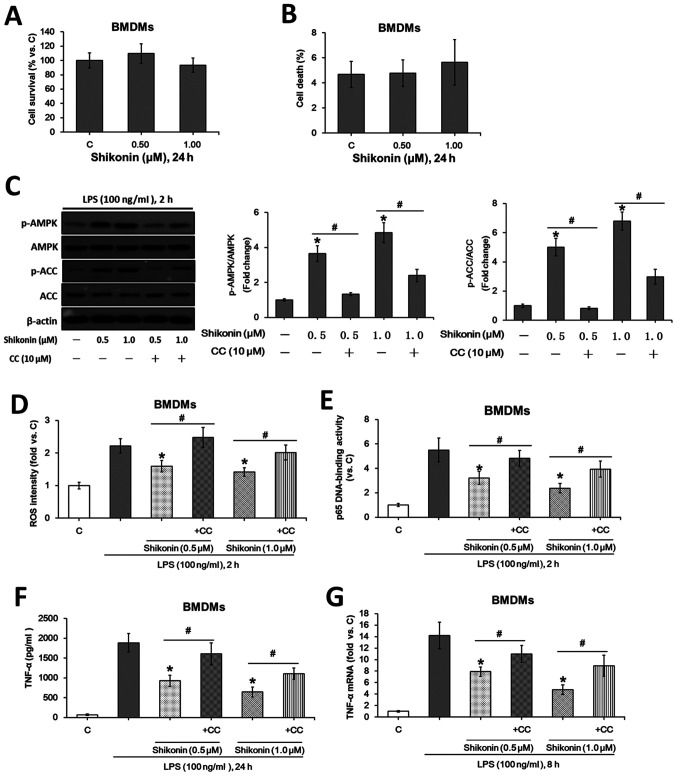
Non-cytotoxic doses shikonin inhibit LPS-induced TNF-α expression in murine BMDMs via AMPK activation
The potential role of shikonin in LPS-induced TNF-α production was investigated using the primary murine BMDMs. Similar to the results from RAW 264.7 macrophages, the indicated doses of shikonin (0.5 and 1.0 µM) were also non-cytotoxic to BMDMs (Fig. 4A and B). Moreover, the non-cytotoxic concentrations of shikonin activated AMPK signaling (Fig. 4C), suppressed LPS-induced ROS generation (Fig. 4D) and NF-κB activation (Fig. 4E), whilst downregulating TNF-α expression at both the protein (Fig. 4F) and mRNA (Fig. 4G) levels. However, pretreatment with compound C significantly attenuated such effects of shikonin (Fig. 4C-G). Collectively, the present results suggested that the non-cytotoxic doses of shikonin suppressed LPS-induced TNF-α expression in murine BMDMs via AMPK activation.
Figure 4.
Non-cytotoxic doses of shikonin suppress LPS-induced TNF-α expression in primary murine BMDMs via AMPK activation. After treating with the indicated doses of shikonin for 24 h, the survival and death rates of primary murine BMDMs were evaluated by (A) MTT and (B) trypan blue exclusion assays, respectively. (C) Expression levels of ACC, p-ACC, AMPKα and p-AMPKα were detected by western blotting. Relative ROS intensity and NF-κB activation were examined by (D) flow cytometry and (E) p65 DNA-binding activity assay, respectively. Extracellular contents and relative mRNA levels of TNF-α were examined by (F) ELISA and (G) reverse transcription-quantitative PCR, respectively. *P<0.05 vs. LPS alone group. #P<0.05 vs. corresponding shikonin combined with LPS group. C, control; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; p-, phosphorylated; ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; IKKα/β, IκB kinase α/β; CC, Compound C; ROS, reactive oxygen species; BMDMs, bone marrow-derived macrophages.
Non-cytotoxic doses of shikonin inhibit LPS-induced TNF-α expression in PBMCs from patients with COPD via AMPK activation
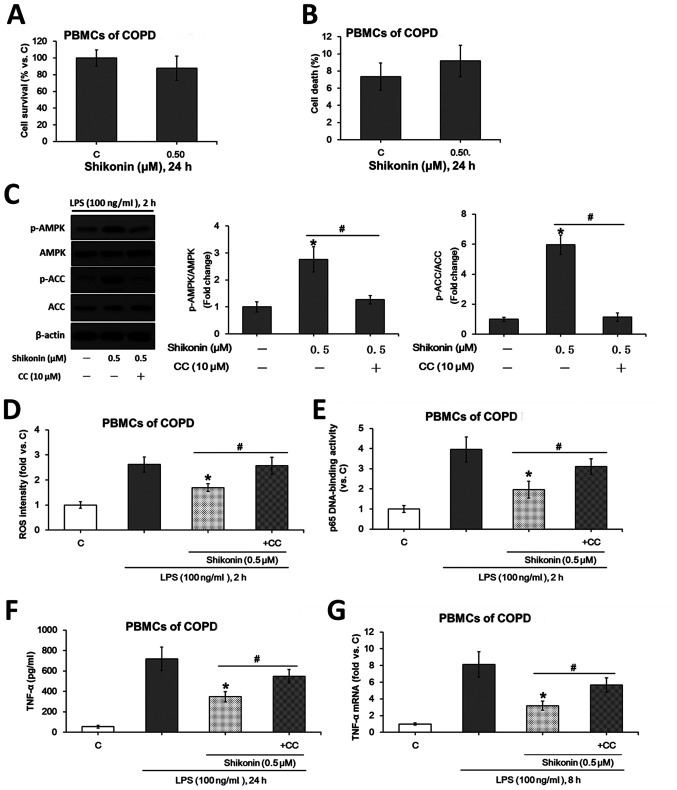
The potential role of shikonin in LPS-induced TNF-α production was assessed using ex vivo cultured primary human PBMCs collected from patients with COPD. Based on the MTT (Fig. 5A) and trypan blue exclusion (Fig. 5B) assay results, 0.5 µM was selected as the non-cytotoxic dose for shikonin. Consistent with the data of murine macrophages, the non-cytotxoic concentration of shikonin (0.5 µM) activated the AMPK signaling pathway (Fig. 5C), suppressed LPS-induced ROS generation (Fig. 5D) and NF-κB activation (Fig. 5E) in PBMCs. Moreover, 0.5 µM shikonin downregulated TNF-α expression at both protein (Fig. 5F) and mRNA (Fig. 5G) levels in the monocytes derived from patients with COPD.
Figure 5.
Non-cytotoxic dose of shikonin suppresses LPS-induced TNF-α expression via AMPK activation in the ex vivo cultured PBMCs from patients with COPD. After treating with the indicated doses of shikonin for 24 h, the survival and death rates of the ex vivo cultured PBMCs from patients with COPD were evaluated by (A) MTT and (B) trypan blue exclusion assays, respectively. (C) Protein expression levels of ACC, p-ACC, AMPKα and p-AMPKα were detected by western blotting. Relative ROS intensity and NF-κB activation were examined by (D) flow cytometry and (E) p65 DNA-binding activity assay, respectively. Extracellular contents and relative mRNA levels of TNF-α were examined using (F) ELISA and (G) reverse transcription-quantitative PCR, respectively. *P<0.05 vs. LPS alone group. #P<0.05 vs. corresponding shikonin combined with LPS group. C, control; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α; p-, phosphorylated; ACC, acetyl-CoA carboxylase; AMPK, AMP-activated protein kinase; IKKα/β, IκB kinase α/β; CC, Compound C; ROS, reactive oxygen species; COPD, Chronic obstructive pulmonary disease; PBMCs, peripheral blood mononuclear cells.
Low-toxic dose of shikonin suppresses LPS-induced endotoxin shock and TNF-α expression in mice
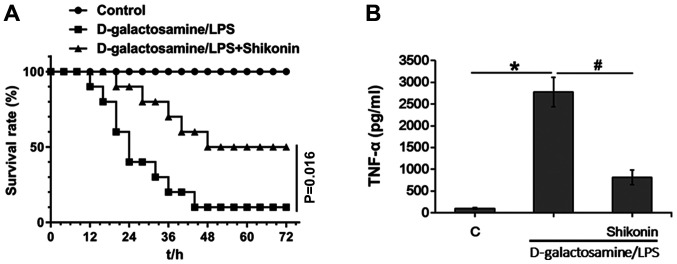
The present study investigated the protective role of shikonin in LPS-induced inflammation in vivo in mice; D-galactosamine and LPS were intraperitoneally injected into the mice. D-galactosamine is a hepatotoxic transcriptional inhibitor that strengthens the toxicity of TNF-α (50). Consistent with a previous study (28), LPS and D-galactosamine co-treatment triggered septic shock and led to mortality of mice (Fig. 6A). In addition, co-administration of shikonin (2.5 mg/kg BW) significantly protected mice against endotoxin shock (Fig. 6A). The low-toxic dose of shikonin (2.5 mg/kg BW) for in vivo application was determined based on our previous work (data not published) and results from previous study (51), wherein no obvious toxicities were observed when administered to tested mice alone. Furthermore, the present ELISA results of TNF-α in serum samples indicated that shikonin (2.5 mg/kg BW) significantly suppressed LPS/D-galactosamine-induced TNF-α expression in vivo (Fig. 6B). Collectively, the present results suggested that shikonin suppresses LPS-induced TNF-α production and protects mice against endotoxin shock.
Figure 6.
Low-toxic dose of shikonin suppresses LPS-induced endotoxin shock and TNF-α production in mice. (A) Within 72 h of initial LPS and D-galactosamine administration, the survival of mice was recorded every 4 h. (B) After 8 h of LPS injection, serum samples were collected from tail vein and the levels of TNF-α were tested by ELISA. *P<0.05 vs. control group; #P<0.05 vs. D-galactosamine and LPS group. C, control; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor α.
Discussion
This study showed that the non-cytotoxic doses of shikonin remarkably suppressed LPS-induced TNF-α expression in mouse macrophages (RAW 264.7 and primary cells). In addition, the expression of TNF-α was downregulated by shikonin in LPS-stimulated PBMCs isolated from patients with COPD. Furthermore, the underlying molecular mechanisms of shikonin-mediated anti-TNF-α activities may be regulated via activation of AMPK signaling pathway.
As a key sensor of intracellular energy status, AMPK is able to regulate vital metabolic pathways in cells (14). It was been shown that AMPK plays an important role in modulating inflammatory responses (24). A series of AMPK activators such as AICAR, A769662, GSK621 and cordycepin have been reported to suppress LPS-induced TNF-α production and NF-κB activation via AMPK activation (31). As an extract of traditional Chinese medicine, shikonin affects several important transcription factors and signaling pathways, some of which are closely related to the production of inflammatory cytokines (32,36,52). However, the present results suggested that shikonin at non-cytotoxic doses suppressed LPS-induced TNF-α production in macrophages, mainly via the activation of AMPK signaling pathway. AMPK can be activated by the upstream AMPK kinases liver kinase B1 or Ca2+/calmodulin-dependent protein kinase kinase β in cells (53). Several anti-inflammatory compounds, such as metformin, AICAR and hydrogen sulfide can activate AMPK via these two upstream kinases (54-56). A previous study speculated that the mechanism of shikonin activating AMPK is similar to that of metformin (35). However, the detailed underlying mechanisms require further investigation.
Numerous studies have suggested a vital function of AMPK for preventing oxidative stress (14,43). AMPK activation can scavenge ROS production by maintaining NADPH levels during energy stresses (14,43). AMPK activation also inhibits H2O2-induced oxidative stress by regulating the NADPH signaling pathway (57). In addition, previous studies have reported that AMPK attenuates LPS-induced ROS generation and subsequently inhibits NF-κB activation (28,30,31). Consistent with these studies, the present results indicated that shikonin-activated AMPK could scavenge LPS-induced ROS generation. Previous studies have also shown that shikonin can increase the intracellular ROS level via various mechanisms, leading to oxidative stress and cytotoxicity (34,58,59). However, the present results are inconsistent with these existing studies. These different between results may be due to the various doses used; the high doses of shikonin promote intracellular ROS production, while the non-cytotoxicity doses of shikonin scavenge ROS via activating AMPK signaling. Additionally, the present results may have valuable implications for investigating the antioxidant effects of shikonin at non-cytotoxicity doses. Moreover, AMPK and ROS production may be involve in mitochondrial function. Gasparrini et al (60) found that strawberry extract (a mixture containing 0.58 mg vitamin C per g fresh weight, 2.52 mg of polyphenol gallic acid equivalent per g fresh weight, 0.66 mg flavonoid catechin equivalent per g fresh weight and a variety of other trace ingredients) can efficiently counteract LPS-induced oxidative stress, reduce the amount of ROS and nitrite production, stimulate endogenous antioxidant enzyme activities, enhance protection against lipid, protein and DNA damage, and improve mitochondria functionality by activating the AMPK pathway. Moreover, Jung et al (61) reported that liquiritigenin, an AMPK activator, can protect hepatocytes against oxidative hepatic injury and mitochondrial dysfunction induced by nutrition deprivation. Kajiwara et al (62) demonstrated that the commonly used AMPK activator, metformin, can suppress the growth of L. pneumophila in macrophages by inducing mitochondrial ROS, but not phagosomal NADPH oxidase-derived ROS, in a time- and concentration-dependent manner. However, whether shikonin affects mitochondrial function via activating AMPK pathway requires further study.
COPD is a major health threat worldwide, affecting over 10% of the adult population and contributing to 3.2 million deaths annually (63). In patients with COPD, the content of TNF-α is significantly elevated in the bronchoalveolar lavage fluids, sputum, plasma and lung tissues, which can serve as a main cause for lung damages (11-13,28). Strategies to reduce the secretion of TNF-α can effectively prevent the inflammatory damage in patients with COPD (11-13). Shikonin has been reported to exert multiple pharmacological effects, including anti-inflammation properties (35,36). However, to the best of our knowledge, there no previous studies have investigated the potential use of shikonin in treating patients with COPD. The present results suggested that shikonin inhibited LPS-induced TNF-α production via activation of the AMPK pathway, suggesting that this herbal extract may facilitate the treatment of COPD. Several other AMPK activators have been demonstrated to exert potential therapeutic effects on COPD (28,31). However, whether shikonin has advantages over these compounds for the treatment of COPD requires more investigation.
In conclusion, shikonin has been reported to have a variety of medical functions, but the strong non-selective cytotoxicity limits its use in clinic (36). The present results suggested that the non-cytotoxic doses of shikonin inhibit LPS-induced TNF-α expression in macrophages cell line, primary murine BMDMs and ex vivo cultured PBMCs from patients with COPD. Moreover, the low-toxic dose of shikonin suppressed LPS-induced endotoxin shock and TNF-α production in mice. The present results may facilitate the clinical application of shikonin as an anti-inflammatory agent in treating COPD and other TNF-α-related inflammatory disorders.
Acknowledgements
Not applicable.
Funding
This work was supported by grants from The National Natural Science Foundation of China (grant nos. 8187102538 and 81172222), The Natural Science Foundation of Shaanxi Province (grant no. 2019JQ889), The Foundation of The State Key Laboratory of Cancer Biology of China (grant nos. CBSKL201710 and CBSKL2017Z09), and The Foundation of Xi'an Medical University (grant no. 2018DOC02).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
TP, XR and ZL conceived and designed the experiments. TP and FZ performed the study. XW and XG assisted in performing the experiments. FZ analyzed the data and created the figures. TP and FZ wrote the manuscript. XR and ZL revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by The Institutional Review Board of Xi Jing Hospital of Fourth Military Medical University. The experiments involving human subjects were performed after obtaining informed consent, and in accordance with the relevant guidelines and regulations. All animal studies were approved by The Animal Ethics Committee of Fourth Military Medical University.
Patient consent for publication
Written informed consent was obtained from all patients prior to publication.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lu Y, Chang R, Yao J, Xu X, Teng Y, Cheng N. Effectiveness of long-term using statins in COPD-a network meta-analysis. Respir Res. 2019;20(17) doi: 10.1186/s12931-019-0984-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Park HY, Kang D, Lee H, Shin SH, Kang M, Kong S, Rhee CK, Cho J, Yoo KH. Impact of chronic obstructive pulmonary disease on mortality: A large national cohort study. Respirology. 2020;25:726–734. doi: 10.1111/resp.13678. [DOI] [PubMed] [Google Scholar]
- 3.Lopez AD, Shibuya K, Rao C, Mathers CD, Hansell AL, Held LS, Schmid V, Buist S. Chronic obstructive pulmonary disease: Current burden and future projections. Eur Respir J. 2006;27:397–412. doi: 10.1183/09031936.06.00025805. [DOI] [PubMed] [Google Scholar]
- 4.Li P, Wu Y, Li M, Qiu X, Bai X, Zhao X. AS-703026 inhibits LPS-induced TNFα production through MEK/ERK dependent and independent mechanisms. PLoS One. 2015;10(e0137107) doi: 10.1371/journal.pone.0137107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barnes PJ. New anti-inflammatory targets for chronic obstructive pulmonary disease. Nat Rev Drug Discov. 2013;12:543–559. doi: 10.1038/nrd4025. [DOI] [PubMed] [Google Scholar]
- 6.Brusasco V, Martinez F. Chronic obstructive pulmonary disease. Compr Physiol. 2014;4:1–31. doi: 10.1002/cphy.c110037. [DOI] [PubMed] [Google Scholar]
- 7.Roversi S, Roversi P, Spadafora G, Rossi R, Fabbri LM. Coronary artery disease concomitant with chronic obstructive pulmonary disease. Eur J Clin Invest. 2014;44:93–102. doi: 10.1111/eci.12181. [DOI] [PubMed] [Google Scholar]
- 8.Cosio MG, Saetta M, Agusti A. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;360:2445–2454. doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 9.Lamela J, Vega F. Immunologic aspects of chronic obstructive pulmonary disease. N Engl J Med. 2009;361(1024) doi: 10.1056/NEJMra0804752. [DOI] [PubMed] [Google Scholar]
- 10.Singh D, Smyth L, Borrill Z, Sweeney L, Tal-Singer R. A randomized, placebo-controlled study of the effects of the p38 MAPK inhibitor SB-681323 on blood biomarkers of inflammation in COPD patients. J Clin Pharmacol. 2010;50:94–100. doi: 10.1177/0091270009347873. [DOI] [PubMed] [Google Scholar]
- 11.Ouagued M, Martin-Chouly CA, Brinchault G, Leportier-Comoy C, Depincé A, Bertrand C, Lagente V, Belleguic C, Pruniaux MP. The novel phosphodiesterase 4 inhibitor, CI-1044, inhibits LPS-induced TNF-alpha production in whole blood from COPD patients. Pulm Pharmacol Ther. 2005;18:49–54. doi: 10.1016/j.pupt.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 12.Rabinovich RA, Figueras M, Ardite E, Carbó N, Troosters T, Filella X, Barberà JA, Fernandez-Checa JC, Argilés JM, Roca J. Increased tumour necrosis factor-alpha plasma levels during moderate-intensity exercise in COPD patients. Eur Respir J. 2003;21:789–794. doi: 10.1183/09031936.03.00042702. [DOI] [PubMed] [Google Scholar]
- 13.Profita M, Chiappara G, Mirabella F, Di Giorgi R, Chimenti L, Costanzo G, Riccobono L, Bellia V, Bousquet J, Vignola AM. Effect of cilomilast (Ariflo) on TNF-alpha, IL-8, and GM-CSF release by airway cells of patients with COPD. Thorax. 2003;58:573–579. doi: 10.1136/thorax.58.7.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeon S-M, Chandel NS, Hay N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature. 2012;485:661–665. doi: 10.1038/nature11066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jansen T, Kvandová M, Daiber A, Stamm P, Frenis K, Schulz E, Münzel T, Kröller-Schön S. The AMP-activated protein kinase plays a role in antioxidant defense and regulation of vascular inflammation. Antioxidants (Basel) 2020;9(525) doi: 10.3390/antiox9060525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saisho Y. Metformin and inflammation: Its potential beyond glucose-lowering effect. Endocr Metab Immune Disord Drug Targets. 2015;15:196–205. doi: 10.2174/1871530315666150316124019. [DOI] [PubMed] [Google Scholar]
- 17.Salt IP, Palmer TM. Exploiting the anti-inflammatory effects of AMP-activated protein kinase activation. Expert Opin Investig Drugs. 2012;21:1155–1167. doi: 10.1517/13543784.2012.696609. [DOI] [PubMed] [Google Scholar]
- 18.Dandapani M, Hardie DG. AMPK: Opposing the metabolic changes in both tumour cells and inflammatory cells? Biochem Soc Trans. 2013;41:687–693. doi: 10.1042/BST20120351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bai A, Ma AG, Yong M, Weiss CR, Ma Y, Guan Q, Bernstein CN, Peng Z. AMPK agonist downregulates innate and adaptive immune responses in TNBS-induced murine acute and relapsing colitis. Biochem Pharmacol. 2010;80:1708–1717. doi: 10.1016/j.bcp.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Kahn BB, Shi H, Xue BZ. Macrophage alpha1 AMP-activated protein kinase (alpha1AMPK) antagonizes fatty acid-induced inflammation through SIRT1. J Biol Chem. 2010;285:19051–19059. doi: 10.1074/jbc.M110.123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, Viollet B, Yan D, Zou MH. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: Role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salminen A, Hyttinen JM, Kaarniranta K. AMP-activated protein kinase inhibits NF-κB signaling and inflammation: Impact on healthspan and lifespan. J Mol Med (Berl) 2011;89:667–676. doi: 10.1007/s00109-011-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Neill LA, Hardie DG. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature. 2013;493:346–355. doi: 10.1038/nature11862. [DOI] [PubMed] [Google Scholar]
- 24.Jeon SM. Regulation and function of AMPK in physiology and diseases. Exp Mol Med. 2016;48(e245) doi: 10.1038/emm.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galic S, Fullerton MD, Schertzer JD, Sikkema S, Marcinko K, Walkley CR, Izon D, Honeyman J, Chen ZP, van Denderen BJ, et al. Hematopoietic AMPK β1 reduces mouse adipose tissue macrophage inflammation and insulin resistance in obesity. J Clin Invest. 2011;121:4903–4915. doi: 10.1172/JCI58577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kauppinen A, Suuronen T, Ojala J, Kaarniranta K, Salminen A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell Signal. 2013;25:1939–1948. doi: 10.1016/j.cellsig.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 27.Shen J, Liang L, Wang C. Perifosine inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via regulation multiple signaling pathways: New implication for Kawasaki disease (KD) treatment. Biochem Biophys Res Commun. 2013;437:250–255. doi: 10.1016/j.bbrc.2013.06.055. [DOI] [PubMed] [Google Scholar]
- 28.Wu YH, Li Q, Li P, Liu B. GSK621 activates AMPK signaling to inhibit LPS-induced TNFα production. Biochem Biophys Res Commun. 2016;480:289–295. doi: 10.1016/j.bbrc.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Ducommun S, Ford RJ, Bultot L, Deak M, Bertrand L, Kemp BE, Steinberg GR, Sakamoto K. Enhanced activation of cellular AMPK by dual-small molecule treatment: AICAR and A769662. Am J Physiol Endocrinol Metab. 2014;306:E688–E696. doi: 10.1152/ajpendo.00672.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang JL, Xu Y, Shen J. Cordycepin inhibits lipopolysaccharide (LPS)-induced tumor necrosis factor (TNF)-α production via activating amp-activated protein kinase (AMPK) signaling. Int J Mol Sci. 2014;15:12119–12134. doi: 10.3390/ijms150712119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Li X, Wu Y, Li M, Wang X. A novel AMPK activator hernandezine inhibits LPS-induced TNFα production. Oncotarget. 2017;8:67218–67226. doi: 10.18632/oncotarget.18365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo C, He J, Song X, Tan L, Wang M, Jiang P, Li Y, Cao Z, Peng C. Pharmacological properties and derivatives of shikonin-A review in recent years. Pharmacol Res. 2019;149(104463) doi: 10.1016/j.phrs.2019.104463. [DOI] [PubMed] [Google Scholar]
- 33.Chen X, Yang L, Oppenheim JJ, Howard MZ. Cellular pharmacology studies of shikonin derivatives. Phytother Res. 2002;16:199–209. doi: 10.1002/ptr.1100. [DOI] [PubMed] [Google Scholar]
- 34.Zhou G, Yang Z, Wang X, Tao R, Zhou Y. TRAIL enhances shikonin induced apoptosis through ROS/JNK signaling in cholangiocarcinoma cells. Cell Physiol Biochem. 2017;42:1073–1086. doi: 10.1159/000478758. [DOI] [PubMed] [Google Scholar]
- 35.Velliquette RA, Rajgopal A, Rebhun J, Glynn K. Lithospermum erythrorhizon Root and its naphthoquinones repress SREBP1c and activate PGC1α through AMPKα. Obesity (Silver Spring) 2018;26:126–134. doi: 10.1002/oby.22061. [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Yin R, Zhou W, Xu D, Li S. Shikonin and its derivatives: A patent review. Expert Opin Ther Pat. 2012;22:977–997. doi: 10.1517/13543776.2012.709237. [DOI] [PubMed] [Google Scholar]
- 37.Zhang C, Zhang Y, Zhang C, Liu Y, Liu Y, Xu G. Pioglitazone increases VEGFR3 expression and promotes activation of M2 macrophages via the peroxisome proliferator-activated receptor γ. Mol Med Rep. 2019;19:2740–2748. doi: 10.3892/mmr.2019.9945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi-Lin D, Yuan X, Zhan S, Luo-Jia T, Chao-Yang T. Trametinib, a novel MEK kinase inhibitor, suppresses lipopolysaccharide-induced tumor necrosis factor (TNF)-α production and endotoxin shock. Biochem Biophys Res Commun. 2015;458:667–673. doi: 10.1016/j.bbrc.2015.01.160. [DOI] [PubMed] [Google Scholar]
- 39.Wright CJ, Agboke F, Muthu M, Michaelis KA, Mundy MA, La P, Yang G, Dennery PA. Nuclear factor-κB (NF-κB) inhibitory protein IκBβ determines apoptotic cell death following exposure to oxidative stress. J Biol Chem. 2012;287:6230–6239. doi: 10.1074/jbc.M111.318246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Si H, Zhang Y, Song Y, Li L. Overexpression of adrenomedullin protects mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the Akt/GSK3β and Bcl-2 signaling pathways. Int J Mol Med. 2018;41:3342–3352. doi: 10.3892/ijmm.2018.3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu X, Jiang Y, Shan PF, Shen J, Liang QH, Cui RR, Liu Y, Liu GY, Wu SS, Lu Q, et al. Vaspin attenuates the apoptosis of human osteoblasts through ERK signaling pathway. Amino Acids. 2013;44:961–968. doi: 10.1007/s00726-012-1425-5. [DOI] [PubMed] [Google Scholar]
- 42.Hu YB, Wu X, Qin XF, Wang L, Pan PH. Role of endoplasmic reticulum stress in silica-induced apoptosis in RAW264.7 cells. Biomed Environ Sci. 2017;30:591–600. doi: 10.3967/bes2017.078. [DOI] [PubMed] [Google Scholar]
- 43.Pan T, Zhang M, Zhang F, Yan G, Ru Y, Wang Q, Zhang Y, Wei X, Xu X, Shen L, et al. NDRG2 overexpression suppresses hepatoma cells survival during metabolic stress through disturbing the activation of fatty acid oxidation. Biochem Biophys Res Commun. 2017;483:860–866. doi: 10.1016/j.bbrc.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 45.Pan T, Zhang F, Li F, Gao X, Li Z, Li X, Ren X. Shikonin blocks human lung adenocarcinoma cell migration and invasion in the inflammatory microenvironment via the IL-6/STAT3 signaling pathway. Oncol Rep. 2020;44:1049–1063. doi: 10.3892/or.2020.7683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cortizo AM, Bruzzone L, Molinuevo S, Etcheverry SB. A possible role of oxidative stress in the vanadium-induced cytotoxicity in the MC3T3E1 osteoblast and UMR106 osteosarcoma cell lines. Toxicology. 2000;147:89–99. doi: 10.1016/s0300-483x(00)00181-5. [DOI] [PubMed] [Google Scholar]
- 47.Qian J, Jiang F, Wang B, Yu Y, Zhang X, Yin Z, Liu C. Ophiopogonin D prevents H2O2-induced injury in primary human umbilical vein endothelial cells. J Ethnopharmacol. 2010;128:438–445. doi: 10.1016/j.jep.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 48.Watcharanurak K, Zang L, Nishikawa M, Yoshinaga K, Yamamoto Y, Takahashi Y, Ando M, Saito K, Watanabe Y, Takakura Y. Effects of upregulated indoleamine 2, 3-dioxygenase 1 by interferon γ gene transfer on interferon γ-mediated antitumor activity. Gene Ther. 2014;21:794–801. doi: 10.1038/gt.2014.54. [DOI] [PubMed] [Google Scholar]
- 49.Ji G, Zhang Y, Yang Q, Cheng S, Hao J, Zhao X, Jiang Z. Genistein suppresses LPS-induced inflammatory response through inhibiting NF-κB following AMP kinase activation in RAW 264.7 macrophages. PLoS One. 2012;7(e53101) doi: 10.1371/journal.pone.0053101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dumitru CD, Ceci JD, Tsatsanis C, Kontoyiannis D, Stamatakis K, Lin JH, Patriotis C, Jenkins NA, Copeland NG, Kollias G, Tsichlis PN. TNF-alpha induction by LPS is regulated posttranscriptionally via a Tpl2/ERK-dependent pathway. Cell. 2000;103:1071–1083. doi: 10.1016/s0092-8674(00)00210-5. [DOI] [PubMed] [Google Scholar]
- 51.Thakur R, Trivedi R, Rastogi N, Singh M, Mishra DP. Inhibition of STAT3, FAK and Src mediated signaling reduces cancer stem cell load, tumorigenic potential and metastasis in breast cancer. Sci Rep. 2015;5(10194) doi: 10.1038/srep10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andújar I, Rios JL, Giner RM, Recio MC. Pharmacological properties of shikonin-a review of literature since 2002. Planta Med. 2013;79:1685–1697. doi: 10.1055/s-0033-1350934. [DOI] [PubMed] [Google Scholar]
- 53.Neumann D. Is TAK1 a direct upstream kinase of AMPK? Int J Mol Sci. 2018;19(2412) doi: 10.3390/ijms19082412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, Zhang QQ, Gao L, Shi JQ, Zhang YD, Tan L. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146–3157. doi: 10.1111/bph.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kurumbail RG, Calabrese MF. Structure and regulation of AMPK. Exp Suppl. 2016;107:3–22. doi: 10.1007/978-3-319-43589-3_1. [DOI] [PubMed] [Google Scholar]
- 56.Zhou X, Cao Y, Ao G, Hu L, Liu H, Wu J, Wang X, Jin M, Zheng S, Zhen X, et al. CaMKKβ-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid Redox Signal. 2014;21:1741–1758. doi: 10.1089/ars.2013.5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.She C, Zhu LQ, Zhen YF, Wang XD, Dong QR. Activation of AMPK protects against hydrogen peroxide-induced osteoblast apoptosis through autophagy induction and NADPH maintenance: New implications for osteonecrosis treatment? Cell Signal. 2014;26:1–8. doi: 10.1016/j.cellsig.2013.08.046. [DOI] [PubMed] [Google Scholar]
- 58.Lu B, Gong X, Wang ZQ, Ding Y, Wang C, Luo TF, Piao MH, Meng FK, Chi GF, Luo YN, Ge PF. Shikonin induces glioma cell necroptosis in vitro by ROS overproduction and promoting RIP1/RIP3 necrosome formation. Acta Pharmacol Sin. 2017;38:1543–1553. doi: 10.1038/aps.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang X, Cui JH, Meng QQ, Li SS, Zhou W, Xiao S. Advance in anti-tumor mechanisms of shikonin, alkannin and their derivatives. Mini Rev Med Chem. 2018;18:164–172. doi: 10.2174/1389557517666170228114809. [DOI] [PubMed] [Google Scholar]
- 60.Gasparrini M, Forbes-Hernandez TY, Giampieri F, Afrin S, Alvarez-Suarez JM, Mazzoni L, Mezzetti B, Quiles JL, Battino M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem Toxicol. 2017;102:1–10. doi: 10.1016/j.fct.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 61.Jung EH, Lee JH, Kim SC, Kim YW. AMPK activation by liquiritigenin inhibited oxidative hepatic injury and mitochondrial dysfunction induced by nutrition deprivation as mediated with induction of farnesoid X receptor. Eur J Nutr. 2017;56:635–647. doi: 10.1007/s00394-015-1107-7. [DOI] [PubMed] [Google Scholar]
- 62.Kajiwara C, Kusaka Y, Kimura S, Yamaguchi T, Nanjo Y, Ishii Y, Udono H, Standiford TJ, Tateda K. Metformin mediates protection against legionella pneumonia through activation of AMPK and mitochondrial reactive oxygen species. J Immunol. 2018;200:623–631. doi: 10.4049/jimmunol.1700474. [DOI] [PubMed] [Google Scholar]
- 63.Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: A systematic analysis for the global burden of disease study 2015. Lancet Respir Med. 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. GBD 2015 Chronic Respiratory Disease Collaborators. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.