Abstract
The application of network methodology in anatomical structures offers new insights on the connectivity pattern of skull bones, skeletal elements and their muscles. Anatomical networks helped to improve our understanding of the water-to-land transition and how the pectoral fins were transformed into limbs via their modular disintegration. Here, we apply the same methodology to tetrapods secondarily adapted to the marine environment. We find that these animals achieved their return to the sea with four types of morphological changes, which can be grouped into two different main strategies. In all marine mammals and the majority of the reptiles, the fin is formed by the persistence of superficial and interdigital connective tissues, like a ‘baby mitten', whereas the underlying connectivity pattern of the bones does not influence the formation of the forefin. On the contrary, ichthyosaurs ‘zipped up' their fingers and transformed their digits into carpal-like elements, forming a homogeneous and better-integrated forefin. These strategies led these vertebrates into three different macroevolutionary paths exploring the possible spectrum of morphological adaptations.
Keywords: anatomical networks, marine reptiles, marine mammals, marine turtles, marine crocodiles, limb-to-fin transitions
1. Background
Tetrapods are unique among major plant and metazoan clades in showing recurrent colonizations from land or freshwater to the marine realm [1]. The colonization of land happened once and nearly 400 Mya (Devonian). However, several terrestrial lineages colonized marine ecosystems in repeated occasions since the Early Triassic (250 Mya) [2]. Iconic examples include turtles, ichthyosaurs, mosasaurs, plesiosaurs, metriorhynchid crocodylomorphs during the Mesozoic, and mainly birds and mammals during the Cenozoic (penguins, whales, dolphins, sea lions, seals and sea cows). Owing to the aquatic physical environment, all of these animals, which are secondarily adapted to the marine environment (SECAD), exhibit strong modifications in their skeletons compared to the basic terrestrial tetrapod pattern. These modifications have been extensively cited as canonical examples of convergent evolution [2,3,4,5]. Although they are not closely related, all these groups share something in common: their ancestors had fingers. Previous studies have suggested that the limb-to-forefin (to better distinguish it from pectoral fins) transition in aquatic tetrapods occurred several times and followed diverse strategies [6–11]. However, did that morphological shift influence their anatomical integration? Is there a conserved modularity pattern among SECAD tetrapods? Or did land-to-water transition trigger an array of unique appendage connectivity patterns across lineages? To address these questions, we used anatomical network analysis, a novel framework that has been demonstrated as a powerful approach to analyse the organization of anatomical structures [12,13].
Recently, this approach helped in studying the connectivity patterns of the various bones of the tetrapodomorph limb, providing a new framework for understanding the water-to-land transition. This process was characterized by less integrated and more modular appendages that were accompanied by significant muscular diversification [14,15]. The appearance of digits caused a major transformation in the connectivity pattern of the tetrapodomorph appendage, from an ‘ancestral' web-like morphology to a ‘derived' tree-like network through a process called, appropriately, the ‘disintegration' of the limb [14]. Here, we expand this framework to study the limb-to-forefin transformation in SECAD tetrapods, including a broad taxonomic sampling of extant and extinct marine reptiles and mammals.
2. Material and methods
(a). Sample analysed
We doubled the tetrapod dataset [15] by constructing networks of the forefins of 19 SECAD tetrapods (table 1). Data were selected based on the most complete published forefins and/or first-hand examinations, selecting the most representative morphotypes of each group, to have a comprehensive sample of variability. See electronic supplementary material, table S1 for details.
Table 1.
Network properties of analysed taxa. C, average clustering coefficient; D, density; E, edges; H, heterogeneity; N, nodes; P, parcellation; PL, average path length.
| taxon | classification | age | N | E | D | C | PL | H | P |
|---|---|---|---|---|---|---|---|---|---|
| Hupehsuchus | Ichthyosauromorph | Early Triassic | 37 | 49 | 0.074 | 0.230 | 5.041 | 0.446 | 0.873 |
| Nanchangosaurus | Ichthyosauromorph | Early Triassic | 56 | 76 | 0.049 | 0.160 | 6.097 | 0.495 | 0.884 |
| Petrolacosaurus | Basal diapsid | Late Carboniferous | 38 | 58 | 0.083 | 0.296 | 4.558 | 0.499 | 0.842 |
| Mixosaurus | Basal ichthyosaur | Middle Triassic | 78 | 171 | 0.057 | 0.425 | 5.861 | 0.362 | 0.804 |
| Ichthyosaurus | Ichthyosaur | Early Jurassic | 91 | 226 | 0.055 | 0.476 | 6.633 | 0.264 | 0.827 |
| Caypullisaurus | Derived ichthyosaur | Late Jurassic | 103 | 245 | 0.047 | 0.433 | 6.685 | 0.280 | 0.844 |
| Portunatasaurus | Mosasauroid | Late Cretaceous | 37 | 48 | 0.072 | 0.250 | 5.047 | 0.509 | 0.874 |
| Mosasaurus | Mosasaur | Late Cretaceous | 62 | 72 | 0.038 | 0.116 | 9.685 | 0.418 | 0.864 |
| Styxosaurus | Plesiosaur | Late Cretaceous | 95 | 106 | 0.024 | 0.074 | 14.948 | 0.400 | 0.882 |
| Dermochelys | marine turtle | extant | 33 | 44 | 0.083 | 0.248 | 4.508 | 0.510 | 0.814 |
| Cricosaurus | marine crocodylomorph | Late Jurassic | 26 | 35 | 0.108 | 0.278 | 3.920 | 0.543 | 0.822 |
| Megadyptes | penguin | extant | 12 | 19 | 0.288 | 0.683 | 2.530 | 0.352 | 0.667 |
| Zalophus | sea lion | extant | 30 | 38 | 0.087 | 0.169 | 4.487 | 0.505 | 0.844 |
| Ommatophoca | seal | extant | 30 | 40 | 0.092 | 0.229 | 4.230 | 0.568 | 0.840 |
| Dugong | Sirenid | extant | 29 | 39 | 0.091 | 0.251 | 4.096 | 0.571 | 0.828 |
| Maiacetus | Cetacean protocetid | Middle Eocene | 32 | 45 | 0.091 | 0.210 | 4.375 | 0.552 | 0.840 |
| Dorudon | Cetacean basilosaurid | Late Eocene | 24 | 37 | 0.134 | 0.354 | 3.272 | 0.565 | 0.729 |
| Lagenorhynchus | Cetacean odontocetes | extant | 35 | 48 | 0.081 | 0.183 | 5.187 | 0.554 | 0.833 |
| Megaptera | Cetacean mysticetes | extant | 35 | 47 | 0.079 | 0.176 | 5.987 | 0.508 | 0.803 |
(b). Construction of networks and analyses
All anatomical connections between bony elements of the forefins were carefully defined manually, considering either bone–bone and/or bone–cartilage connections. These models were digitalized in Gephi [16] and depicted with the Force Atlas 2 layout algorithm; see electronic supplementary material for adjacency matrices. Metrics were calculated with Gephi algorithms, excluding heterogeneity and parcellation that were calculated as in [15], but using the communities detected in Gephi; we followed Calatayud et al. [17] rationale to ensure the best community detection. The main descriptors used to analyse the networks are density (the number of connections that exist compared to the maximum number possible), heterogeneity (how the connections are distributed across the network), clustering (how well integrated the various elements are with their immediate surroundings) and parcellation (the degree of anatomical modularity of the network); see [15] for further information. Individual bones are coloured according to their betweenness centrality (how many times they are included in the shortest path between any pair of nodes), as a measure of their importance in the forefin. Principal component analysis (PCA) and PERMANOVA were performed in PAST [18].
3. Results
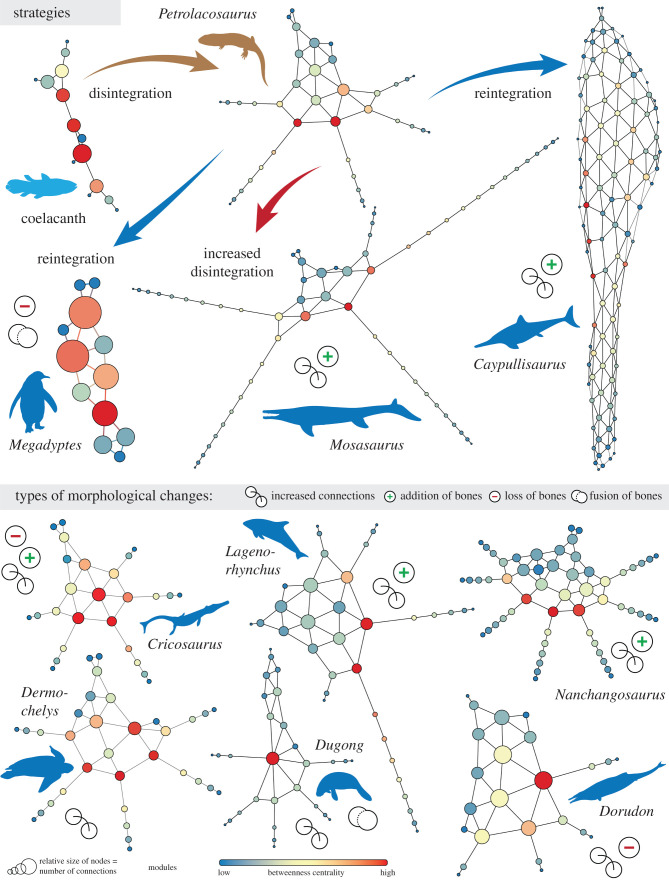
The anatomical network of the forelimb of a basic terrestrial tetrapod contains 6–7 modules. The digit modules form a tree-like appendage [14] that departs greatly from its ‘ancestral’ condition (e.g. coelacanth), which had fewer modules placed in a row, one distal to the other. Our analysis indicates that the transformation of a forelimb to a forefin in aquatic tetrapods that finally achieved pelagic lifestyle occurred, mainly, with four major morphological changes, in combinations (figure 1): increased number of connections, increased number of bones, loss of bones and fusion of bones. All SECAD taxa show, in comparison with their terrestrial tetrapod ancestor, an increased number of connections in the mesopodium, involving mainly a better integration of some of the metacarpals (usually the mid ones; figure 1). The addition of more connections and better integration are taken to the extreme in ichthyosaurs, where phalangeal elements are also connected anteroposteriorly. Several SECAD tetrapods show an increased number of bones, involving the addition of phalangeal elements (hyperphalangy) that extend the previous smaller digit modules (mosasaurs, plesiosaurs, whales, dolphins, marine crocodiles), more digits (i.e. polydactyly as in Nanchangosaurus) or both more phalanges and/or more digits forming more integrated patterns (e.g. ichthyosaurs). Few show a reduction in the number of bones, either in the mesopodium (marine crocodiles), in the digits (basilosaurids; must be corroborated with additional complete specimens), or by fusion (sirenids). These changes can be grouped into two main strategies. On one hand, mosasaurs, plesiosaurs, marine crocodiles, turtles, mammals and basal ichthyosauromorphs conserved the ancestral tree-like appendage morphology. Ichthyosaurs, on the other hand, followed a different strategy of reintegrating their digits into a fin. The case of penguins is special, because their highly modified appendages represent their ancestral condition of having wings, with mainly extreme loss and bone fusion (as a result of strong phylogenetic and functional constraints).
Figure 1.
From fin to limb and back again. Anatomical networks showing the forelimb-to-forefin transition in SECAD tetrapods stemming from a basic tetrapod limb, highlighting the main types of morphological changes. See figure 2 for silhouette credits.
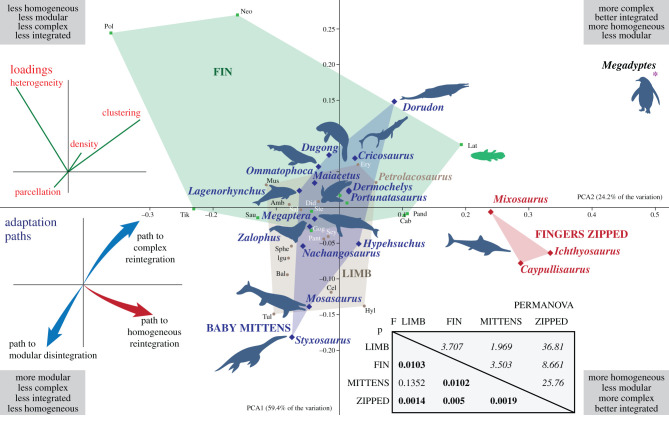
PCA (figure 2) depicts the variance across anatomical networks using four important descriptors: density, clustering, heterogeneity and parcellation. This allows SECAD tetrapods to be placed in the context of the known morphospace of tetrapodomorphs and tetrapods. The first two PCs explain 83.6% of the variation. Mysticetes, sea lions and sea turtles have forefins slightly more modular and homogeneous than terrestrial tetrapods and are placed closer to the region of the morphospace occupied mainly by terrestrial tetrapods with limbs (figure 2, brown area). This is expected for sea lions and marine turtles as they spend some time on the shore and their forefins are functional on land to support the trunk or shell, thus conserving the ancestral function of tetrapods. In the case of mysticetes, humpback whales display the longest forefin among cetaceans and increased modularity that is compensated by the loss of a digit; this allows the network to maintain its complexity and integration. Seals and sirenids are heterogeneous enough and just slightly more complex than their terrestrial tetrapod ancestor to enter in the region occupied by tetrapods with fins (figure 2, green area). Seals differentiate from sea lions in that on land they do not use their forefins as weight-bearing appendages, which might explain their displacement within the morphospace of tetrapods without tree-like appendages. Basal forms of other lineages with pelagic derived members, such as Hupehsuchus, Nanchangosaurus and Portunatasaurus, are also placed within the known LIMB morphospace, consistent with the terrestrial-like forefin topology that most of these taxa had. However, several SECAD tetrapods show some more extreme changes, following three different adaptation paths that are discussed in the following section.
Figure 2.
Principal component analysis (PCA) scatter diagram showing the position of each taxon in the morphospace defined by the first two PCAs explaining 83.727% of the variation (PC1: 59.442%, PC2: 24.285%, PC3: 15.107%, PC4: 1.1651%), including a PERMANOVA analysis of the main groups (statistically significant p < 0.05 marked with bold). Abbreviations: Amb, Ambystoma; Bal, Balanerpeton; Cab, Cabonnichthys; Cel, Celtedens; Did, Didelphis; Ery, Eryops; Gog, Gogonasus; Hyl, Hyloplesion; Igu, Iguana; Lat, Latimeria; Mus, Mus; Neo, Neoceratodus; Pand, Panderichthys; Pant, Pantylus; Pol, Polypterus; Sal, Salamandra; Sau, Sauripterus; Sey, Seymouria; Sphe, Sphenodon; Ste, Sterropterygion; Tik, Tiktaalik; Tul, Tulerpeton. Silhouettes are downloaded from phylopic.org and attributed to: Y. Wong (coelacanth), D. Bogdanov (tetrapod), S. Hartman (ichthyosaur), I. Reid (mosasaur), N. Tamura (plesiosaur), G. Monger (marine crocodile), S. Tracer (sirenian), C. Huh (dolphin, whale), M. Keesey (basilosaurid), J. R. Spotila and R. Chatterji (turtle), Jakovche (seal), S. Traver (sea lion, penguin).
PERMANOVA analysis (figure 2, inset) confirms the statistically significant difference between the fins and limbs reported previously [15]. The forefins of SECAD tetrapods that conserve the tree-like appendage (blue in figure 2) are not significantly different from the limbs of terrestrial tetrapods, whereas those that reintegrated their limbs (ichthyosaurs, red in figure 2) are significantly different from all other groups.
4. Discussion
The back-to-the-sea tetrapod transition resulted in dramatic changes in limb morphology, including the retention of the soft-tissue envelope enclosing the limb skeleton and lengthening of the distal region by the addition of bony elements, all strongly connected to early developmental stages of these animals. Resulting fin-shaped limbs can be functionally propulsive or swimming control surfaces [11]. As in the case of water-to-land transition, these morphological changes also impact the topological organization of the forefin, and anatomical networks help in recognizing these modifications in the underlying patterns of connectivity. These abstract network models could help in formulating several developmental or biomechanical hypotheses [14,15]. In our case, networks indicate that the shift from limb to forefin was also coupled with a deep modification in the anatomical integration of their forelimbs. From a tree-like network of their terrestrial ancestors, the transition to an aquatic and/or pelagic lifestyle triggered an array of connectivity patterns that could be summarized in three main adaptation paths (figure 2), as follows.
(a). Path to modular disintegration
Mosasaurs and plesiosaurs place new limits in the disintegration of the limb, a process that previously started with the conquest of the land [14]. By adding numerous new phalanges on their digits, they increase the modularity of their forefins, while reducing their density and integration.
(b). Path to complex reintegration
Marine crocodiles and, possibly, basilosaurids change their ancestral networks by losing elements and by increasing connections of the metapodials, which results in increased density (primarily) and better integration of their bones (secondarily). The extreme end to this path is the heavily reduced limb-fin of the penguin, which is also accompanied by fusion of elements.
(c). Path to homogeneous reintegration
The most impressive changes are noted in the forefins of ichthyosaurs. Although they share with other marine reptiles the addition of numerous phalangeal elements, ichthyosaurs abandoned the tree-like appendage for a new, web-like, structure. Ichthyosaurs reintegrated their digits into the mesopodium with the addition of anterior and posterior contacts and articulations. Hence, the metacarpals and the numerous phalanges of the ichthyosaurs radically adopt the connectivity pattern of carpal bones (increased clustering, betweenness centrality and degree)––this is mesopodalization [9,10,19] in network terms. These forefins are highly integrated and homogeneous, as nearly all elements are well connected to their surroundings with a similar number of connections. But at the same time, this strategy allowed ichthyosaurs to have forefins that did not lose much of their modularity.
5. Concluding remarks
The majority of the SECAD tetrapods present changes in terms of bone connectivity that include mainly the addition of a variable number of phalanges in most digits, some moderate increase in the integration of the metacarpal bones, or some minor reductions (by loss or fusion of elements). In all these cases, the limb-into-forefin transformation was actually achieved by the persistence of interdigital soft-tissue and by enclosing the limb in a broad soft-tissue envelope, which provided its form and made it functional. The underlying connectivity pattern does not influence greatly the form of the fin. This strategy is like wearing ‘baby mittens': fingers might be able to move inside the mittens but they no longer function as separate modules. With their ‘baby mittens', these tetrapods managed to explore regions outside the known morphospace of other tetrapods, attempting higher disintegration (mosasaurs and plesiosaurs) of the limb or some moderate reintegration (basilosaurs)—but without losing their digits. In the meantime, ichthyosaurs followed a different strategy and ‘zipped up' their fingers, showing a costly reintegration of their limb to a modular pattern that is analogous to fishes, with the addition of interdigital bony elements and lateral connections.
Anatomical networks help understanding that all these secondary adaptations to the marine environment are not the same, and to speculate that they are the result of different developmental mechanisms, but also physical, phylogenetic and morphological constraints. Most of these tetrapods underwent less drastic changes and are groups that still survive today mammals, turtles and crocodiles), while other, now-extinct, groups (like plesiosaurs, mosasaurs and ichthyosaurs) approached and even exceeded the limits of the potential morphological changes. Further work and detailed element- and clade-specific network analyses will allow associating this underlying bone connectivity and the functionality of these forefins.
Supplementary Material
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We would like to thank the Editorial Board, B. Esteve-Altava, J. Calatayud and E. Maxwell for valuable comments that improved this manuscript. We thank E. Crespo and N. Garcia (CESIMAR, CCT CONICET-CENPAT) for access to the collection of odontocetes' flippers.
Data accessibility
All necessary data to replicate the networks analysed herein are provided in the electronic supplementary material, in the form of adjacency matrices. Additional information on how to replicate other analyses and additional information is given in the pdf supplementary file.
Authors' contributions
E.V., M.S.F. and M.R.B. conceived, designed the study and wrote the first draft. M.S.F., L.C. and Y.H. conceived the network models of ichthyosauromorphs, ichthyosaurs, marine crocodiles, mosasaurs, plesiosaurs, M.R.B., L.A. and F.P. those of marine mammals and penguin, and J.S. that of the marine turtle. E.V. created and analysed the networks and designed the figures. All authors contributed to and approved the final version of the manuscript.
Competing interests
We declare we have no competing interests.
Funding
M.S.F. received funds from ANPCyT-PICT-2016-1039 and UNLP-N853, and M.R.B. from ANPCyT-PICT-2015-0792.
References
- 1.Vermeij GJ, Dudley RO. 2000. Why are there so few evolutionary transitions between aquatic and terrestrial ecosystems? Biol. J. Linn. Soc. 70, 541–554. ( 10.1111/j.1095-8312.2000.tb00216.x) [DOI] [Google Scholar]
- 2.Vermeij GJ, Motani R. 2018. Land to sea transitions in vertebrates: the dynamics of colonization. Paleobiology 44, 237–250. ( 10.1017/pab.2017.37) [DOI] [Google Scholar]
- 3.Pyenson ND, Kelley NP, Parham JF. 2014. Marine tetrapod macroevolution: physical and biological drivers on 250 Ma of invasions and evolution in ocean ecosystems. Palaeogeogr. Palaeoclimatol. Palaeoecol. 400, 1–8. ( 10.1016/j.palaeo.2014.02.018) [DOI] [Google Scholar]
- 4.Kelley NP, Motani R. 2015. Trophic convergence drives morphological convergence in marine tetrapods. Biol. Lett. 11, 20140709 ( 10.1098/rsbl.2014.0709) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley NP, Pyenson ND. 2015. Evolutionary innovation and ecology in marine tetrapods from the Triassic to the Anthropocene. Science 348, aaa3716 ( 10.1126/science.aaa3716) [DOI] [PubMed] [Google Scholar]
- 6.Caldwell MW. 1997. Modified perichondral ossification and the evolution of paddle-like limbs in ichthyosaurs and plesiosaurs. J. Vertebr. Paleontol. 17, 534–547. ( 10.1080/02724634.1997.10011000) [DOI] [Google Scholar]
- 7.Caldwell MW. 1997. Limb osteology and ossification patterns in Cryptoclidus (Reptilia: Plesiosauroidea) with a review of sauropterygian limbs. J. Vertebr. Paleontol. 17, 295–307. ( 10.1080/02724634.1997.10010976) [DOI] [Google Scholar]
- 8.Caldwell MW. 2002. From fins to limbs to fins: limb evolution in fossil marine reptiles. Am. J. Med. Genet. 112, 236–249. ( 10.1002/ajmg.10773) [DOI] [PubMed] [Google Scholar]
- 9.Maxwell EE. 2012. Unraveling the influences of soft tissue flipper development on skeletal variation using an extinct taxon. J. Exp. Zool. A Comp. Exp. Biol. 318, 545–554. ( 10.1002/jez.b.22459) [DOI] [PubMed] [Google Scholar]
- 10.Fedak TJ, Hall BK. 2004. Perspectives on hyperphalangy: patterns and processes. J. Anat. 204, 151–163. ( 10.1111/j.0021-8782.2004.00278.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeBlois MC, Motani R. 2019. Flipper bone distribution reveals flexible trailing edge in underwater flying marine tetrapods. J. Morphol. 280, 908–924. ( 10.1002/jmor.20992) [DOI] [PubMed] [Google Scholar]
- 12.Esteve-Altava B, Marugán-Lobón J, Botella H, Rasskin-Gutman D. 2011. Network models in anatomical systems. J. Anthropol. Sci. 89, 175–184. [DOI] [PubMed] [Google Scholar]
- 13.Rasskin-Gutman D, Esteve-Altava B. 2014. Connecting the dots: anatomical network analysis in morphological EvoDevo. Biol. Theory. 9, 178–193. ( 10.1007/s13752-014-0175-x) [DOI] [Google Scholar]
- 14.Esteve-Altava B, Molnar JL, Johnston P, Hutchinson JR, Diogo R. 2018. Anatomical network analysis of the musculoskeletal system reveals integration loss and parcellation boost during the fins-to-limbs transition. Evolution 72, 601–618. ( 10.1111/evo.13430) [DOI] [PubMed] [Google Scholar]
- 15.Esteve-Altava B, Pierce SE, Molnar JL, Johnston P, Diogo R, Hutchinson JR. 2019. Evolutionary parallelisms of pectoral and pelvic network-anatomy from fins to limbs. Sci. Adv. 5, eaau7459 ( 10.1126/sciadv.aau7459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bastian M, Heymann S, Jacomy M. 2009. Gephi: an open source software for exploring and manipulating networks. In International AAAI Conference on Weblogs and Social Media, Paris ( 10.13140/2.1.1341.1520) [DOI] [Google Scholar]
- 17.Calatayud J, Bernardo-Madrid R, Neuman M, Rojas A, Rosvall M. 2019. Exploring the solution landscape enables more reliable network community detection. Phys. Rev. E. 100, 052308 ( 10.1103/PhysRevE.100.052308) [DOI] [PubMed] [Google Scholar]
- 18.Hammer Ø, Harper DA, Ryan PD. 2001. PAST-palaeontological statistics, ver. 1.89. Palaeontol. Electronica. 4, 1–9. [Google Scholar]
- 19.Wagner GP, Chiu CH. 2001. The tetrapod limb: a hypothesis on its origin. J. Exp. Zool. 291, 226–240. ( 10.1002/jez.1100) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All necessary data to replicate the networks analysed herein are provided in the electronic supplementary material, in the form of adjacency matrices. Additional information on how to replicate other analyses and additional information is given in the pdf supplementary file.