Abstract
Whereas ornithischian dinosaurs are well known from Jurassic and Cretaceous deposits, deciphering the origin and early evolution of the group remains one of the hardest challenges for palaeontologists. So far, there are no unequivocal records of ornithischians from Triassic beds. Here, we present an alternative evolutionary hypothesis that suggests consideration of traditional ‘silesaurids' as a group of low-diversity clades representing a stem group leading to core ornithischians (i.e. unambiguous ornithischians, such as Heterodontosaurus tucki). This is particularly interesting because it fills most of the ghost lineages that emerge from the Triassic. Following the present hypothesis, the lineage that encompasses the Jurassic ornithischians evolved from ‘silesaurids' during the Middle to early Late Triassic, while typical ‘silesaurids' shared the land ecosystems with their relatives until the Late Triassic, when the group completely vanished. Therefore, Ornithischia changes from an obscure to a well-documented clade in the Triassic and is represented by records from Gondwana and Laurasia. Furthermore, according to the present hypothesis, Ornithischia was the first group of dinosaurs to adopt an omnivorous/herbivorous diet. However, this behaviour was achieved as a secondary step instead of an ancestral condition for ornithischians, as the earliest member of the clade is a faunivorous taxon. This pattern was subsequently followed by sauropodomorph dinosaurs. Indeed, the present scenario favours the independent acquisition of an herbivorous diet for ornithischians and sauropodomorphs during the Triassic, whereas the previous hypotheses suggested the independent acquisition for sauropodomorphs, ornithischians, and ‘silesaurids'.
Keywords: Dinosauria, Dinosauromorpha, evolution, feeding behaviours, Mesozoic era, phylogenetics
1. Introduction
Discoveries across the world are shedding light on the ancestral anatomy of dinosaurs and related groups [1–7]. By contrast, recently unearthed skeletons revealed peculiar combinations of traits that required the establishment of new phylogenetic interpretations. In response, the traditional phylogenetic relationships of dinosaurs have been challenged [4,8]. Therefore, not all studies agree with the classical dichotomy Saurischia/Ornithischia and also with the inner composition of these clades [7–9]. For instance, silesaurids, which are usually considered as the sister-group to Dinosauria [1], are considered as ornithischians by some authors [4,10]. Indeed, whereas ornithischian dinosaurs are well known from Jurassic and Cretaceous deposits, the origin and early evolution of the group remains one of the hardest challenges for palaeontologists. So far, there are no unequivocal records of ornithischians from Triassic deposits [11].
These phylogenetic disputes and scarce record of ornithischians hamper the establishment of a reliable framework. However, information on the origin and early evolution of dinosaurs has improved substantially over the last few years. Fieldwork initiated by several researchers has yielded a large number of new species and fossil material from previously described species, including nearly complete early dinosaurs [4,7,12] as well as several dinosaur relatives [4,6,13,14]. Among these discoveries, a lot of new information was produced regarding silesaurids [6,14,15], a group with the potential to explain the obscure origin of Ornithischia (see below) [10]. However, these new data were not combined into a single dataset. In addition, several characters with putative phylogenetic significance have not been incorporated in major phylogenetic datasets. In the present study, we combine these new data and investigate the phylogenetic information content as it pertains to the evolution of dinosaurs. Additionally, we place emphasis on the controversial relationships between ornithischians and silesaurids.
2. Material and methods
The new morphological dataset combines data from different sources, including those from Cabreira et al. [4] as well as additional operational taxonomic units (OTUs), characters and modifications from other studies [7,8,16–19]. The added OTUs are as follows: the two unnamed lagerpetids UFSM 11611 and PVSJ 883, Dromomeron gigas, Kwanasaurus williamparkeri, Lutungutali sitwensis, Technosaurus smalli, Soumyasaurus aenigmaticus, Ignotosaurus fragilis, Fruitadens haagarorum, Echinodon beckelessi, Tianyulong confuciusi, Gnathovorax cabreirai, Nhandumirim waldsangae, Bagualosaurus agudoensis, Macrocollum itaquii, Unaysaurus tolentinoi, Teleocrater rhadinus, Spondylosoma absconditum, Yarasuchus deccanensis and Dongusuchus efremovi. The OTUs were coded based on a first-hand examination, photographs and published literature [6,7,13,15,19–26]. Furthermore, we scored additional characters for Dromomeron romeri, Lewisuchus admixtus, Asilisaurus kongwe and Buriolestes schultzi based on previous studies [5,6,9,12]. Based on previous studies, ‘Marasuchus lilloensis' was treated as Lagosuchus talampayensis [27], and ‘Pseudolagosuchus major' was combined with Lewisuchus admixtus [5]. In addition, we followed the reinterpretations regarding the anatomy of Pisanosaurus mertii by [15]. Finally, morphological characters that support Ornithoscelida were incorporated following [8]. The final dataset included 277 morphological characters and 62 OTUs.
A phylogenetic analysis based on equally weighted parsimony was implemented in TNT v. 1.1 [28]. Characters 4, 13, 18, 25, 63, 82, 84, 87, 89, 109, 142, 166, 174, 175, 184, 186, 190, 201, 203, 205, 209, 212, 225, 235, 236, 239, 250 and 256 were treated as additive (i.e. ordered), whereas the other characters were treated as unordered (see supplementary materials for details). Euparkeria capensis was used to root the most parsimonious trees (MPTs) based on a random addition sequence+tree bisection reconnection, which included 1000 replicates of Wagner trees (with random seed = 0), tree bisection reconnection and branch swapping (holding 20 trees saved per replicate). Topologies retained in overflowed replicates were branch-swapped for MPTs using TBR. The strict consensus tree was generated using all trees recovered in the analysis and all OTUs. Decay indices (Bremer support values) and bootstrap values (1000 replicates) were also calculated with TNT v. 1.1 [28] (see electronic supplementary material, figure S2). In addition, two constrained analyses were performed adopting the same parameters of the former analysis. The first constrained analysis was conducted to access the required number of extra steps to recover a monophyletic Silesauridae apart from a traditional Ornithischia. This involved treatment of silesaurids and core ornithischians as being two distinct monophyletic groups. Pisanosaurus mertii was set as a floating taxon in the constrained searches. The second constrained analysis was conducted to access the required number of extra steps to recover a monophyletic Ornithoscelida. For this analysis, core ornithischians and core theropods were considered to be monophyletic. Pisanosaurus mertii, Eodromaeus murphi, Chindesaurus briansmalli, Tawa hallae and Daemonosaurus chauliodus were set as floating taxa. Finally, an ancestral state reconstruction of diet for the first topology was performed following the same approach by [4].
3. Results
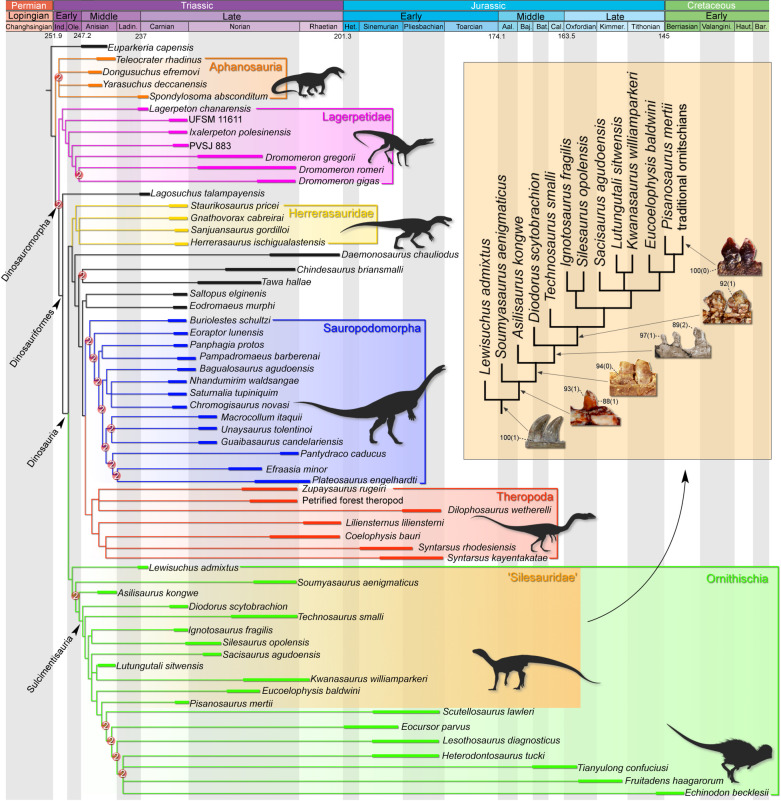
The analysis recovered 36 most parsimonious trees (MPTs) of 985 steps (consistence index = 0.320; retention index = 0.665). Lagosuchus talampayensis is sister to Dinosauria (figure 1), which is composed of a traditional Saurischia/Ornithischia arrangement (see electronic supplementary material for a list of synapomorphies and electronic supplementary material, figure S2 for support values). However, the inner affinities of Ornithischia were unusual. Unlike the traditional placement of silesaurids as sister-group to Dinosauria [1,8,9,29] or as sister-group to the core ornithischians [4,7,10], silesaurids appeared as paraphyletic within Ornithischia. Lewisuchus admixtus is the basalmost member of Ornithischia, and Pisanosaurus mertii is the sister taxon to all traditional ornithischians (e.g. Scutellosaurus lawleri; Eocursor parvus; Heterodontosaurus tucki). Sulcimentisauria [6] includes all core ornithischians and silesaurids, except by Lewisuchus admixtus, Soumyasaurus aenigmaticus and Asilisaurus kongwe. The basalmost member of the clade is Diodorus scytobrachion. The constrained analysis, assuming a monophyletic Silesauridae and a traditional Ornithischia, resulted 117 000 MPTs of 990 steps each (consistence index = 0.318; retention index = 0.663). Here, silesaurids are the sister-group to Dinosauria, which is recovered in the classical fashion of Saurischia/Ornithischia dichotomy. Pisanosaurus mertii is recovered as an ornithischian, rather than within Silesauridae. The constrained analysis, forcing a monophyletic Ornithoscelida, recovered 7632 MPTs of 1010 steps (consistence index = 0.312; retention index = 0.653). Pisanosaurus mertii nests within Silesauridae, which is recovered as the sister-group to a clade composed by Saltopus elginensis plus Dinosauria.
Figure 1.
Time-calibrated strict consensus tree depicting the phylogenetic position of traditional ‘silesaurids' with emphasis on the dental characters evolution within ‘Silesauridae'. Numbers on nodes represent Bremer support values higher than 1. Silhouettes were constructed from the composition of several sources.
4. Discussion
Silesaurids have been considered the sister-group to Dinosauria by several authors [1,8,29,30]. However, an alternative hypothesis [31] considered silesaurids as ornithischian dinosaurs. Subsequently, more comprehensive studies [4,7,10] have reinforced this hypothesis. In this scenario, silesaurids are recovered within Ornithischia as the sister-group to typical ornithischians. In addition, Pisanosaurus mertii, which is historically recognized as the basalmost ornithischian, was suggested to be a silesaurid by some authors [15,32]. However, this new hypothesis regarding the affinities of Pisanosaurus mertii has not been recovered by subsequent studies [7] that adopt the dataset of [4], which favours the scenario where silesaurids are ornithischians. Therefore, the present results provide an alternative scenario for the Triassic radiation of ornithischians dinosaurs. Here, traditional silesaurids represent a paraphyletic array of low-diversity clades that constitute stem groups leading to core ornithischians. Based on previous definitions of Silesauridae as ‘all archosaurs closer to Silesaurus opolensis than to Heterodontosaurus tucki and Marasuchus lilloensis [33]' or ‘the most inclusive clade containing Silesaurus opolensis but not Passer domesticus, Triceratops horridus and Alligator mississippiensis' [1], in our hypothesis, only Silesaurus opolensis and Ignotosaurus fragilis are strictly members of the clade. This is particularly interesting because it fills most of the ornithischian ghost lineages that emerge from the Triassic. Indeed, the fossil record of ornithischians from Triassic beds is completely scarce, with no unequivocal specimens known so far [11]. On the contrary, sauropodomorphs and theropods are well known, especially from the Norian onwards [2,4,12,25,33]. Following our hypothesis, the lineage that encompasses the Jurassic ornithischians evolved from ‘silesaurids' during the Middle to early Late Triassic, while typical ‘silesaurids' shared the land ecosystems with their relatives until the Late Triassic, when the group completely vanished.
The present alternative hypothesis explains the peculiar mosaic anatomy of Pisanosaurus mertii, which combines traits present in traditional silesaurids (e.g. possible ankylothecodont dentition, elongated popliteal fossa of the femur) and ornithischians (e.g. dorsally expanded coronoid process of the dentary). The taxon lies along a branch that connects the traditional silesaurids to core Ornithischia. The paraphyletic array indicates a gradual acquisition of traits in the branch that leads to core Ornithischia (see electronic supplementary material for inner character distribution), and therefore, Pisanosaurus mertii comprises a key-taxon in this scenario. New specimens will certainly help in our understanding of the initial evolution of the group. On the other hand, a monophyletic Silesauridae (constrained analysis) is five steps longer, representing a less parsimonious alternative. The same is true for a monophyletic Ornithoscelida, which is 25 steps longer. Nevertheless, the branch support and bootstrap values of the present topology are generally low (see electronic supplementary material, figure S2). However, it is not surprising. Low values occur in other topologies (traditional Ornithischia/Saurischia split and Ornithoscelida hypothesis) obtained by distinct datasets [29]. This condition is tentatively explained by high rates of homoplasy, as the earliest members of the major subgroups were very similar in body size and morphology [29].
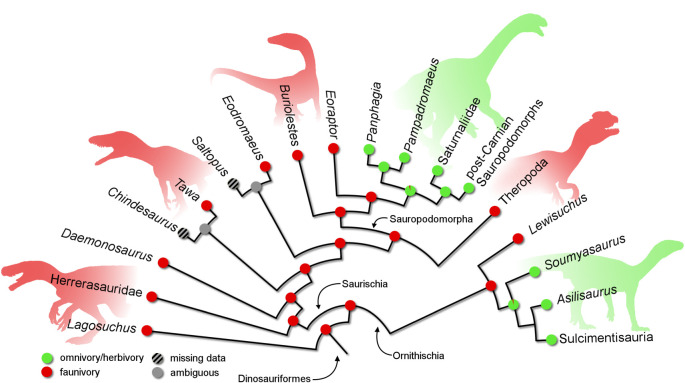
The dinosaurian affinities of Lewisuchus admixtus is another interesting result. This taxon shares with sampled dinosaurs a mediolaterally compressed basipterygoid process of the parabasisphenoid, post glenoid process of the coracoid extending caudal to glenoid, pubis length more than 70% of femoral length, a sulcus on the dorsolateral surface of the ischium, angled ‘greater trochanter' of the femur, a transverse groove on the proximal surface of the femur and a caudolateral flange on the distal portion of the tibia. In addition, the position of Lewisuchus admixtus as the basalmost member of Ornithischia sheds lights on the ancestral anatomy and diet of the clade. The clade is widely known for their highly specialized herbivorous diet [34,35]. All the previous hypotheses favour omnivory/herbivory as the feeding strategy of the earliest members of Ornithischia [1,4,8,17]. The only exception is [32], which recovered the faunivorous Daemonosaurus chauliodus as the basalmost ornithischian. Even in the studies that support the hypothesis of silesaurids being ornithischians, the omnivorous/herbivorous diet was preferred, as these studies recover Asilisaurus kongwe as the basalmost member [4,7,12]. For some authors [10], the presence of teeth with sub-triangular crowns and a constricted root and dentaries with a beak-like anterior tip suggest omnivorous/herbivorous diet for Asilisaurus kongwe. On the other hand, the recurved tooth crowns with finely serrated margins indicate a faunivorous feeding behaviour for Lewisuchus admixtus [4,5]. Therefore, the present topology implies the acquisition of an omnivorous/herbivorous diet as a secondary step instead of an ancestral condition for ornithischians (figure 2), similar to Sauropodomorpha [4,12]. The paraphyletic array reveals a gradual acquisition of dental traits related to an omnivorous/herbivorous diet across the ‘silesaurids' toward core ornithischians (figure 1). For instance, sulcimentisaurs present large serrations of middle maxillary/dentary teeth forming oblique angles with the margin of the tooth, a condition shared with omnivorous/herbivorous sauropodomorphs. However, the current scenario favours the independent acquisition of an herbivorous diet for sauropodomorphs and ornithischians during the Triassic, whereas the traditional hypotheses suggested the independent acquisition for sauropodomorphs, ornithischians and silesaurids [1,29]. The same is true for the scenario that considers Daemonosaurus chauliodus to be the earliest ornithischian, with silesaurids representing non-dinosaur dinosauriforms [32]. Therefore, according to the present hypothesis, ornithischians were the first group of dinosaurs to adopt an omnivorous/herbivorous diet, whereas during the Late Triassic, the group shared the land ecosystems with omnivorous/herbivorous sauropodomorphs. Finally, during the Jurassic, ornithischians evolved new anatomical structures that improved their feeding strategies, while sauropodomorphs became larger and typical ‘silesaurids' went extinct.
Figure 2.
Reduced strict consensus tree from the first phylogenetic analysis depicting feeding habits inference from the ancestral state reconstruction analysis. Silhouettes were constructed from the composition of several sources.
Supplementary Material
Supplementary Material
Acknowledgements
The Handling Editor of Biology Letters and anonymous reviewers provided valuable comments that greatly improved this manuscript. We thank the Willi Henning Society, for the gratuity of TNT software.
Data accessibility
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.xgxd254dm [36].
Authors' contributions
R.T.M. and M.S.G. constructed the dataset, carried out the analyses, discussed the results and wrote the paper. Both authors approved the final version of the paper and agreed to be accountable for all aspects of the work.
Competing interests
We declare we have no competing interests.
References
- 1.Nesbitt SJ, Sidor CA, Irmis RB, Angielczyk KD, Smith RM, Tsuji LA. 2010. Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature 464, 95–98. ( 10.1038/nature08718) [DOI] [PubMed] [Google Scholar]
- 2.Martínez RN, Sereno PC, Alcober OA, Colombi CE, Renne PR, Montanez IP, Currie BS. 2011. A basal dinosaur from the dawn of the dinosaur era in southwestern Pangaea. Science 331, 206–210. ( 10.1126/science.1198467) [DOI] [PubMed] [Google Scholar]
- 3.Kammerer CF, Nesbitt SJ, Shubin NH. 2011. The first silesaurid dinosauriform from the Late Triassic of Morocco. Acta Palaeontol. Pol. 57, 277–284. ( 10.4202/app.2011.0015) [DOI] [Google Scholar]
- 4.Cabreira SF, et al. 2016. A unique Late Triassic dinosauromorph assemblage reveals dinosaur ancestral anatomy and diet. Curr. Biol. 26, 3090–3095. ( 10.1016/j.cub.2016.09.040) [DOI] [PubMed] [Google Scholar]
- 5.Ezcurra MD, Nesbitt SJ, Fiorelli LE, Desojo JB. 2019. New specimen sheds light on the anatomy and taxonomy of the early Late Triassic dinosauriforms from the Chañares Formation, NW Argentina. Anat. Rec. 303, 1392–1438. ( 10.1002/ar.24243) [DOI] [PubMed] [Google Scholar]
- 6.Martz JW, Small BJ. 2019. Non-dinosaurian dinosauromorphs from the Chinle Formation (Upper Triassic) of the Eagle Basin, northern Colorado: Dromomeron romeri (Lagerpetidae) and a new taxon, Kwanasaurus williamparkeri (Silesauridae). PeerJ 7, e7551 ( 10.7717/peerj.7551) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pacheco C, Müller RT, Langer M, Pretto FA, Kerber L, da Silva SD. 2019. Gnathovorax cabreirai: a new early dinosaur and the origin and initial radiation of predatory dinosaurs. PeerJ 7, e7963 ( 10.7717/peerj.7963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baron MG, Norman DB, Barrett PM. 2017. A new hypothesis of dinosaur relationships and early dinosaur evolution. Nature 543, 501–506. ( 10.1038/nature21700) [DOI] [PubMed] [Google Scholar]
- 9.Nesbitt SJ, Langer MC, Ezcurra MD. 2019. The anatomy of Asilisaurus kongwe, a dinosauriform from the Lifua Member of the Manda Beds (∼Middle Triassic) of Africa. Anat. Rec. 303, 813–873. ( 10.1002/ar.24287) [DOI] [PubMed] [Google Scholar]
- 10.Langer MC, Ferigolo J. 2013. The Late Triassic dinosauromorph Sacisaurus agudoensis (Caturrita Formation; Rio Grande do Sul, Brazil): anatomy and affinities. Geol. Soc. Spec. Publ. 379, 353–392. ( 10.1144/SP379.16) [DOI] [Google Scholar]
- 11.Baron MG. 2019. Pisanosaurus mertii and the Triassic ornithischian crisis: could phylogeny offer a solution? Hist. Biol. 31, 967–981. ( 10.1080/08912963.2017.1410705) [DOI] [Google Scholar]
- 12.Müller RT, Langer MC, Bronzati M, Pacheco CP, Cabreira SF, Dias-Da-Silva S. 2018. Early evolution of sauropodomorphs: anatomy and phylogenetic relationships of a remarkably well preserved dinosaur from the Upper Triassic of southern Brazil. Zool. J. Linn. Soc. 184, 1187–1248. ( 10.1093/zoolinnean/zly009) [DOI] [Google Scholar]
- 13.Nesbitt SJ, et al. 2017. The earliest bird-line archosaurs and the assembly of the dinosaur body plan. Nature 544, 484–487. ( 10.1038/nature22037) [DOI] [PubMed] [Google Scholar]
- 14.Sarıgül V, Agnolin FL, Chatterjee S. 2018. Description of a multitaxic bone assemblage from the Upper Triassic Post Quarry of Texas (Dockum Group), including a new small basal dinosauriform taxon. Historia Natural 8, 5–24. [Google Scholar]
- 15.Agnolín FL, Rozadilla S. 2018. Phylogenetic reassessment of Pisanosaurus mertii Casamiquela, 1967, a basal dinosauriform from the Late Triassic of Argentina. J. Syst. Palaeontol. 16, 853–879. ( 10.1080/14772019.2017.1352623) [DOI] [Google Scholar]
- 16.Yates AM. 2007. The first complete skull of the Triassic dinosaur Melanorosaurus Haughton (Sauropodomorpha: Anchisauria). Spec. Pap. Palaeontol. 77, 9–55. [Google Scholar]
- 17.Butler RJ, Upchurch P, Norman DB. 2008. The phylogeny of ornithischian dinosaurs. J. Syst. Palaeontol. 6, 1–40. ( 10.1017/S1477201907002271) [DOI] [Google Scholar]
- 18.Nesbitt SJ. 2011. The early evolution of archosaurs: relationships and the origin of major clades. Bull. Am. Mus. Nat. Hist. 2011, 1–292. ( 10.1206/352.1) [DOI] [Google Scholar]
- 19.Martínez RN, Apaldetti C, Correa GA, Abelín D. 2016. A Norian lagerpetid dinosauromorph from the Quebrada del Barro Formation, northwestern Argentina. Ameghiniana 53, 1–13. ( 10.5710/AMGH.21.06.2015.2894) [DOI] [Google Scholar]
- 20.Peecook BR, Sidor CA, Nesbitt SJ, Smith RM, Steyer JS, Angielczyk KD. 2013. A new silesaurid from the upper Ntawere Formation of Zambia (Middle Triassic) demonstrates the rapid diversification of Silesauridae (Avemetatarsalia, Dinosauriformes). J. Vertebr. Paleontol. 33, 1127–1137. ( 10.1080/02724634.2013.755991) [DOI] [Google Scholar]
- 21.Martínez RN, Apaldetti C, Alcober OA, Colombi CE, Sereno PC, Fernandez E, Malnis PS, Correa GA, Abelin D. 2013. Vertebrate succession in the Ischigualasto Formation. J. Vertebr. Paleontol. 32, 10–30. ( 10.1080/02724634.2013.818546) [DOI] [Google Scholar]
- 22.Müller RT, Langer MC, Dias-da Silva S. 2018. Ingroup relationships of Lagerpetidae (Avemetatarsalia: Dinosauromorpha): a further phylogenetic investigation on the understanding of dinosaur relatives. Zootaxa 4392, 149–158. ( 10.11646/zootaxa.4392.1.7) [DOI] [PubMed] [Google Scholar]
- 23.Müller RT, Langer MC, Dias-da Silva S. 2018. An exceptionally preserved association of complete dinosaur skeletons reveals the oldest long-necked sauropodomorphs. Biol. Lett. 14, 20180633 ( 10.1098/rsbl.2018.0633) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pretto FA, Langer MC, Schultz CL. 2019. A new dinosaur (Saurischia: Sauropodomorpha) from the Late Triassic of Brazil provides insights on the evolution of sauropodomorph body plan. Zool. J. Linn. Soc. 185, 388–416. ( 10.1093/zoolinnean/zly028) [DOI] [Google Scholar]
- 25.Garcia MS, Müller RT, Da-Rosa ÁA, Dias-da-Silva S. 2019. The oldest known co-occurrence of dinosaurs and their closest relatives: a new lagerpetid from a Carnian (Upper Triassic) bed of Brazil with implications for dinosauromorph biostratigraphy, early diversification and biogeography. J. S. Am. Earth Sci. 91, 302–319. ( 10.1016/j.jsames.2019.02.005) [DOI] [Google Scholar]
- 26.Marsola JC, Bittencourt JS, Butler RJ, Da Rosa ÁA, Sayão JM, Langer MC. 2019. A new dinosaur with theropod affinities from the Late Triassic Santa Maria Formation, South Brazil. J. Vertebr. Paleontol. 38, e1531878 ( 10.1080/02724634.2018.1531878) [DOI] [Google Scholar]
- 27.Agnolín FL, Ezcurra MD. 2019. The validity of Lagosuchus talampayensis Romer, 1971 (Archosauria, Dinosauriformes), from the Late Triassic of Argentina. Breviora 565, 1–21. ( 10.3099/0006-9698-565.1.1) [DOI] [Google Scholar]
- 28.Goloboff PA, Farris JS, Nixon KC. 2008. TNT, a free program for phylogenetic analysis. Cladistics 24, 1–13. ( 10.1111/j.1096-0031.2007.00173.x) [DOI] [Google Scholar]
- 29.Langer MC, Ezcurra MD, Rauhut OWM, Benton MJ, Knoll F, Mcphee BW, Novas FE, Pol D, Brusatte SL. 2017. Untangling the dinosaur family tree. Nature 551, E1–E3. ( 10.1038/nature24011) [DOI] [PubMed] [Google Scholar]
- 30.Irmis RB, Nesbitt SJ, Padian K, Smith ND, Turner AH, Woody D, Downs A. 2007. A Late Triassic dinosauromorph assemblage from New Mexico and the rise of dinosaurs. Science 317, 358–361. ( 10.1126/science.1143325) [DOI] [PubMed] [Google Scholar]
- 31.Ferigolo J, Langer MC. 2007. A Late Triassic dinosauriform from south Brazil and the origin of the ornithischian predentary bone. Hist. Biol. 19, 23–33. ( 10.1080/08912960600845767) [DOI] [Google Scholar]
- 32.Baron MG, Norman DB, Barrett PM. et al. 2017. Baron et al. reply. Nature 551, E4–E5. ( 10.1038/nature24012) [DOI] [PubMed] [Google Scholar]
- 33.Langer MC, Ezcurra MD, Bittencourt JS, Novas FE. 2010. The origin and early evolution of dinosaurs. Biol. Rev. 85, 55–110. ( 10.1111/j.1469-185X.2009.00094.x) [DOI] [PubMed] [Google Scholar]
- 34.Ősi A, Prondvai E, Mallon J, Bodor ER. 2017. Diversity and convergences in the evolution of feeding adaptations in ankylosaurs (Dinosauria: Ornithischia). Hist. Biol. 29, 539–570. ( 10.1080/08912963.2016.1208194) [DOI] [Google Scholar]
- 35.Nabavizadeh A. 2015. Evolutionary trends in the jaw adductor mechanics of ornithischian dinosaurs. Anat. Rec. 299, 271–294. ( 10.1002/ar.23306) [DOI] [PubMed] [Google Scholar]
- 36.Müller RT, Garcia MS.. 2020. Data from: A paraphyletic ‘Silesauridae’ as an alternative hypothesis for the initial radiation of ornithischian dinosaurs Dryad Digital Repository. ( 10.5061/dryad.xgxd254dm) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Müller RT, Garcia MS.. 2020. Data from: A paraphyletic ‘Silesauridae’ as an alternative hypothesis for the initial radiation of ornithischian dinosaurs Dryad Digital Repository. ( 10.5061/dryad.xgxd254dm) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.xgxd254dm [36].