Abstract
The division of labour between reproductive queens and mostly sterile workers is among the defining characteristics of social insects. Queen-produced chemical signals advertising her presence and fertility status, i.e. queen pheromones, are normally used to assert the queen's reproductive dominance in the colony. Most queen pheromones identified to date are chemicals that stop the daughter workers from reproducing. Nevertheless, it has long been suggested that queen pheromones could also regulate reproduction in different ways. In some multiple-queen ants with obligately sterile workers, for example—such as fire ants and pharaoh ants—queen pheromones are thought to regulate reproduction by inhibiting the rearing of new sexuals. Here, we identify the first such queen pheromone in the pharaoh ant Monomorium pharaonis and demonstrate its mode of action via bioassays with the pure biosynthesized compound. In particular, we show that the monocyclic diterpene neocembrene, which in different Monomorium species is produced solely by fertile, egg-laying queens, strongly inhibits the rearing of new sexuals (queens and males) and also exerts a weakly attractive ‘queen retinue’ effect on the workers. This is the first time that a queen pheromone with such a dual function has been identified in a social insect species with obligately sterile workers.
Keywords: social insects, evolution of sociality, queen pheromones, pharaoh ants, neocembrene
1. Introduction
Social insects display a remarkable division of labour between reproductive queens and mostly sterile workers. Over the last few years, research has shown that queen pheromones play a key role in regulating this reproductive division of labour [1,2]. The available evidence suggests that such pheromones are usually honest indices of the queen's fertility, and that queen pheromones inhibit the workers from reproducing to the extent that this serves the workers’ inclusive fitness interests [1–3]. Although most queen pheromones identified to date directly stop the daughter workers from reproducing, it has been suggested that queen pheromones could also regulate reproduction in insect societies in other ways. In some multiple-queen ants with obligately sterile workers, such as argentine ants, fire ants and pharaoh ants [4–6], queen pheromones are thought to regulate reproduction by inhibiting the rearing of excess queens and males [7–9]. Several factors can select for the rearing of an excess of sexuals, including queen–queen competition over the production of sexual offspring [10–13], female larvae benefiting from biasing their development towards the queen pathway (caste fate conflict) [9,14] and ‘bet-hedging’-type benefits whereby spare sexuals could be produced as an insurance strategy [10]. If many queens are present in the colony, queen pheromones are thought to stimulate workers to cull excess sexual brood, thereby decreasing unnecessary competition among breeding queens and optimizing the ratio of reproductive and non-reproductive individuals in the colony [7–9]. However, if the colony has only a few queens left and the dose of emitted queen pheromones is low, workers will allow queens and males to be reared to adulthood and allow newly mated queens to be readopted into the colony.
The mode of action of queen pheromones mediating the rearing of new sexuals has been best documented in the fire ant Solenopsis invicta [7,8,15]. In that species, queen pheromones were shown to regulate the rearing of new queens and males in two different ways: by inducing workers to selectively kill sexual brood [15] and by making workers provide larvae with insufficient food to be able to develop as queens [7]. Similar worker cannibalism of sexual brood induced by the presence of fertile queens has been recorded in several other ant species (e.g. [16–20]). Nevertheless, the nature of the underlying queen pheromones that induce brood cannibalism have as yet not been unequivocally identified in any of these species.
In the pharaoh ant Monomorium pharaonis, the presence of fertile queens inhibits the production of new adult sexuals [19,20]. Furthermore, egg-laying queens of several species of Monomorium, all with multiple-queen nests, including M. pharaonis, M. minimum, M. chinense, M. floricola, M. destructor and M. hiten, have been shown to specifically produce the monocyclic diterpene neocembrene. This compound consequently has been suggested to act as a queen pheromone in M. pharaonis and other Monomorium species [21–23]. Nevertheless, direct evidence of the effects of neocembrene in modulating the production of sexuals is still lacking. Here, we used the transgenic yeast strain EPY300 to produce neocembrene [24], and tested the hypothesis that neocembrene is the queen pheromone regulating the production of adult sexuals in the pharaoh ant M. pharaonis. In addition, we tested whether neocembrene also played a role as a worker attracting ‘queen retinue’ pheromone, as previously hypothesized [21].
2. Material and methods
(a). Neocembrene biosynthesis and quantification
To obtain neocembrene, (1E,5E,9E,12R)-1,5,9-trimethyl-12-(1-methylethenyl)-1,5,9-cyclotetradecatriene, in sufficient quantities, we used the metabolically engineered Saccharomyces cerevisiae yeast strain EPY300 [24]. The neocembrene present in two batches of 2 l of yeast culture each was extracted using a liquid–liquid phase extraction and was further purified using preparative liquid chromatography, providing us with 1.825 mg of neocembrene per batch with a purity measured by GC of approximately 98% (for details see electronic supplementary material).
In M. pharaonis queens, neocembrene is produced in the abdominal Dufour's gland [21]. We quantified the amount of neocembrene present in a single pharaoh ant queen by analysing two samples, each containing the dissected Dufour's glands of four M. pharaonis egg-laying queens in 100 µl hexane, using GC/MS (Thermo Fisher Scientific Trace 1300 GC-ISQ MS). The integrated area of the neocembrene peak in the samples then allowed us to determine that a single queen in our population contained 377 ng of neocembrene (sample 1 = 153 ng queen−1 and sample 2 = 601 ng queen−1), which we refer to as one queen equivalent (QE).
(b). Queen pheromone bioassays
To test the bioactivity of neocembrene, we prepared mini colonies of M. pharaonis consisting of ca 500 workers and a varying amount of eggs. The exact numbers of both eggs and adult workers were later assessed through images taken from the mini colonies at the beginning of the experiment. We performed the experiments in two different trials, corresponding to our two biosynthesized batches of neocembrene, in a matched experimental design, whereby each stock colony was divided across a solvent-only control and the different treatments. In trial 1, we used five stock colonies, each divided across a control and the 0.1, 1, 2, 5 and 10 QE treatment doses. In trial 2, we again used five colonies each split across a control and a set of higher doses of 10, 20, 50 and 100 QEs. For one control mini-colony and one replicate of the 10 QE dose in trial 2, a water tube leak caused many workers to drown, and these replicate colonies were consequently discarded. The mini colonies were fed ad libitum with sugar water, mealworms and water and were kept in a climate-controlled room at 27°C. The solutions were applied onto glass microscope slides at the beginning of the experiment and reapplied after two weeks following a protocol developed in Van Oystaeyen et al. [2]. After the experimental period of four weeks, the number of adult gynes and males in each mini colony was assessed and the data were analysed using R 3.5.3 [25]. We used a binomial generalized linear model in which the proportion of the larvae that developed into sexuals was coded as the dependent variable, the different treatment doses and trial were included as fixed factors and the initial number of workers as a covariate. Colony ID and an observation-level random effect variable to cope with overdispersion were coded as random factors. We also fitted a binomial GLM with the proportion of adult sexuals as the response variable and the log10 of the neocembrene dose coded as a continuous covariate interacting with the sex of adult individuals plus trial as a fixed factor, and including colony ID and an observation-level variable as random factors in order to analyse the overall effect of the neocembrene dose on the emergence of adult sexuals.
(c). Queen retinue behaviour bioassay
To determine whether neocembrene elicited queen retinue behaviour, we used 3 × 1.5 mm round black glass beads as dummy queens. We tested five replicates, each consisting of a control and beads dosed with 0.1, 1, 2 or 5 QEs of neocembrene. The solutions, as well as the hexane control, were pipetted onto the glass beads and allowed to evaporate prior to adding the beads to 5 × 5 cm nest-boxes containing 30 workers each. We then recorded the resulting behaviours for 10 min and analysed the number of ants interacting with each bead at 30 s intervals for the first 5 min and at every minute for the last 5 min. A Poisson generalized linear mixed model was then fitted to compare the cumulative total number of interactions after 10 min of observation in the function of treatment group with the different doses coded as a continuous covariate and with colony identity, trial and an observation-level random effect variable coded as random factors.
3. Results
(a). Effect of neocembrene on the rearing of adult sexuals
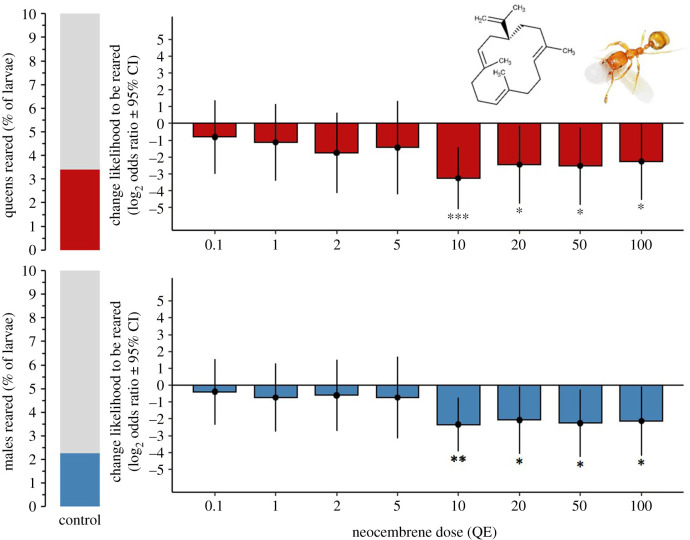
Under the queenless control conditions ca 5.7% of the larvae present at the beginning of the experiment developed into sexuals, of which around 3.4% became queens and 2.3% males (figure 1). Neocembrene significantly inhibited the production of new adult sexuals at the higher tested doses—which mimicked the presence of several queens in the nest—while the lower doses were not different from the controls. In particular, the production of adult sexuals was significantly inhibited in the treatments with doses of at least 10 QEs (log2 ratio = −3.25 p < 0.001 for queens only, and log2 ratio = −2.34 p = 0.004 for males only), and the effect plateaued with even higher treatment doses (figure 1), while no significant inhibitory effect on the production of adult sexuals was observed for the doses between 0.1 and 5 QEs (for all categories log2 odds ratio <−1.76, p > 0.05). The number of adult workers present in the mini colonies was standardized across the treatments and did not significantly affect the emergence of adult sexuals (average 665 ± 292, binominal GLMM, Wald Z score = 0.534, p = 0.593 for queens and Wald Z score = 0.439, p = 0.660 for males). If neocembrene dose was coded as a continuous covariate, there was also a strong highly significant overall inhibitory effect on the proportion of adult queens and males produced (binomial GLMM, Wald Z score = −2.742, p = 0.006). We did not observe an interaction between the neocembrene dose and the sex of emerging adults, indicating that there was no differential killing of sexual larvae based on sex (binomial GLMM, Wald Z score = 0.683, p = 0.494).
Figure 1.
The queen pheromone neocembrene inhibits the rearing of new sexuals in the pharaoh ant M. pharaonis. The figure shows the likelihood (log2 odds ratio) for adult sexuals (queens or males) to be reared when administering different doses of neocembrene (in queen equivalents, QE) compared to the control condition, with 95% confidence intervals based on a binomial GLMM. A value of zero represents no difference between treatment and control groups, a value of 1 = a twofold increase in the proportion of surviving offspring, whereas a value of −1 = a twofold decrease. The proportions of sexuals (queens and males) reared in the control groups are shown in the bar charts on the left. N = 9 colonies for the controls and 10 QE and N = 5 colonies for the remaining treatments. Significance levels: *p < 0.05, **p < 0.01, ***p < 0.001.
(b). Effect of neocembrene as a queen retinue pheromone
In addition to regulating the emergence of adult sexuals in the colony, neocembrene also had a weakly attractive ‘queen retinue’ effect on the workers (figure 2). At the highest tested dose of 5 QE, for example, we observed 67% more workers interacting with the glass bead when compared to the solvent control after the 10 min experimental period (N = 4 colonies per group and 20 colonies in total, model predictions 0 QE = 21.4 ± 2.1 s.e., 0.1 QE = 21.6 ± 2.0 s.e., 1 QE = 23.7 ± 1.8 s.e., 2 QE = 26.3 ± 1.9 s.e., 5 QE = 35.9 ± 4.9 s.e., Poisson GLMM, Wald Z score = 2.818, p = 0.004).
Figure 2.
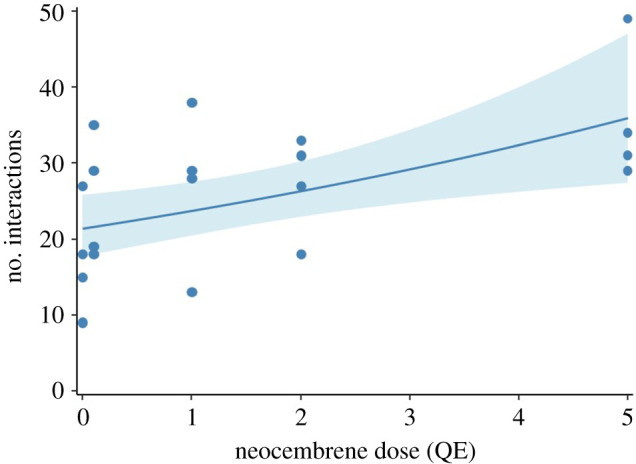
The queen pheromone neocembrene doubles up as an attractive ‘queen retinue’ pheromone in pharaoh ant workers. The line represents the fit of the cumulative total number of workers that interacted with the glass bead over a period of 10 min with 95% confidence intervals based on a Poisson GLMM. Circles represent the raw data from the four colonies tested with the different doses of neocembrene.
4. Discussion
Chemical signals produced by queens have been shown to mediate a myriad of behavioural or physiological responses in both workers and males of social insects [26,27]. A remarkable feature of queen signals is the regulation of worker reproduction, whereby workers will usually refrain from reproducing when detecting these signals in the colony [1,2]. In species where workers are permanently sterile, however, workers are considered to be in an evolutionary dead-end in terms of direct reproduction but might still maximize their inclusive fitness [28] by regulating the ratio of reproductive and non-reproductive individuals in the colony, maximizing long-term colony productivity [8,17]. This regulation can be achieved both via differential feeding of brood and sexual brood cannibalism, as described in several ant species, including M. pharaonis [7,9,15,20]. Here, we provided the first direct evidence that workers of the pharaoh ant make use of both the presence and quantity of the queen-specific compound neocembrene to regulate the number of adult sexuals produced. Interestingly, the strongest inhibitory effect of neocembrene was observed at a dose of 10 QEs, where the proportion of larvae that were reared into sexuals was ca sixfold smaller than in the controls, and with the effect stabilizing with further increases in dose (figure 1). This implies a threshold response, where sexual brood cannibalism would cease if the number of egg-laying queens fell below a certain critical number. The highly dose-dependent response may also explain why some previous studies erroneously concluded that there was no evidence for any pheromonally mediated regulation of sexual production in M. pharaonis [20,29]. Interestingly, we observed that neocembrene inhibited the rearing of new queens and males in similar amounts. A possible explanation lies in the fact that M. pharaonis mates locally inside the nest [30] and that males and females are hence required to be produced at the same time, when colonies have only few mother queens left.
We also observed that neocembrene exerted a weakly attractive effect on the workers (figure 2). Previously, only in the fire ant S. invicta, a queen retinue pheromone has been tentatively identified as a poison-gland derived α-pyrone [31], even though subsequent work showed that the fire ant queen retinue pheromone is in fact likely a blend derived from multiple glandular sources [32]. Whether additional queen retinue pheromones could also be produced by other exocrine glands in the pharaoh ant is also possible and would deserve further study. The fact that neocembrene has a dual function, regulating both the rearing of new sexuals and inducing a queen retinue response, could be a mechanism by which queen pheromones spread more effectively throughout the colony. Another interesting and not mutually exclusive possibility is that queen pheromones are spread through the colony by being deposited onto the eggs [19,33]. Previous studies have shown it to be common for social insect queen pheromones to have multiple functions. For example, in the well-studied honeybee Apis mellifera, the queen mandibular pheromone blend induces worker sterility, promotes queen retinue behaviour and acts as a sex attractant pheromone [34–38]. Likewise, in both honeybees and the common wasp Vespula vulgaris queen pheromones that stop workers from reproducing are also used as egg-marking pheromones [39–41]. Convergent evolution has also caused neocembrene to be used both as a sex pheromone and a trail-following pheromone in several termite species [42].
In conclusion, our study is the first to successfully and unambiguously identify a pheromone that both mediates the rearing of sexuals and induces a worker attracting queen retinue response in ants. The fact that neocembrene is a highly conserved pheromone specific for egg-laying queens across several species of Monomorium [22] suggests that these behavioural effects may also be conserved within the genus [31,32].
Supplementary Material
Acknowledgements
We would like to thank James Kirby for proving the yeast strain to D.S.-D. and Pieter Verlooy (INNOmater) for carrying out the phase separation that comprised the first part of the neocembrene purification.
Ethics
According to the country's law, research with insects is exempt from ethics committee approval.
Data accessibility
The original datasets as well as the R script used to analyse the data are publicly available on https://doi.org/10.5061/dryad.w0vt4b8nb and https://datadryad.org/stash/share/fy60ikVX1hgVDs64WMZ4-kQAWtplYZQJmnhvuTFLi9w [43].
Authors' contributions
R.C.O. and T.W. conceived the original idea and analysed the data. R.C.O. and J.W. conducted the bioassays. D.S.-D. provided the yeast strain. B.H.-M. and K.V. cultured the yeast and J.G.M. performed the preparative liquid chromatography. All authors revised and approved the final version of the manuscript. All authors agree to be held accountable for the work performed therein.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Research Foundation Flanders (FWO project GNM-C9018-G.0A51.15 to T.W., research grant 1502119N and postdoctoral grant 12R9619N to R.C.O.) and by KU Leuven (C3 grant C32/16/020).
References
- 1.Oi CA, van Zweden JS, Oliveira RC, Van Oystaeyen A, Nascimento FS, Wenseleers T. 2015. The origin and evolution of social insect queen pheromones: novel hypotheses and outstanding problems. Bioessays 37, 808–821. ( 10.1002/bies.201400180) [DOI] [PubMed] [Google Scholar]
- 2.Van Oystaeyen A, et al. 2014. Conserved class of queen pheromones stops social insect workers from reproducing. Science 343, 287–290. ( 10.1126/science.1244899) [DOI] [PubMed] [Google Scholar]
- 3.Holman L. 2018. Queen pheromones and reproductive division of labor: a meta-analysis. Behav. Ecol. 29, 1199–1209. [Google Scholar]
- 4.Passera L, Keller L, Suzzoni JP. 1988. Control of brood male production in the Argentine ant Iridomyrmex humilis (Mayr). Insectes Soc. 35, 19–33. ( 10.1007/BF02224135) [DOI] [Google Scholar]
- 5.Ross KG, Fletcher DJ. 1985. Comparative study of genetic and social structure in two forms of the fire ant Solenopsis invicta (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 17, 349–356. ( 10.1007/BF00293212) [DOI] [Google Scholar]
- 6.Schmidt AM, Linksvayer TA, Boomsma JJ, Pedersen JS. 2011. Queen–worker caste ratio depends on colony size in the pharaoh ant (Monomorium pharaonis). Insectes Soc. 58, 139–144. ( 10.1007/s00040-010-0126-x) [DOI] [Google Scholar]
- 7.Vargo EL. 1998. Primer pheromones in ants. In Pheromone communication in social insects: ants, wasps, bees, and termites (eds R Vander Meer, M Breed, M Winston, K Espelie), pp. 293–313. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Vargo EL, Fletcher DJ. 1986. Evidence of pheromonal queen control over the production of male and female sexuals in the fire ant, Solenopsis invicta. J. Comp. Physiol. A 159, 741–749. ( 10.1007/BF00603727) [DOI] [Google Scholar]
- 9.Bourke AF, Ratnieks FL. 1999. Kin conflict over caste determination in social Hymenoptera. Behav. Ecol. Sociobiol. 46, 287–297. ( 10.1007/s002650050622) [DOI] [Google Scholar]
- 10.Bourke AFG, Franks NR. 1995. Social evolution in ants. Princeton, NJ: Princeton University Press. [Google Scholar]
- 11.de Menten L, Fournier D, Brent C, Passera L, Vargo EL, Aron S. 2005. Dual mechanism of queen influence over sex ratio in the ant Pheidole pallidula. Behav. Ecol. Sociobiol. 58, 527–533. ( 10.1007/s00265-005-0964-0) [DOI] [Google Scholar]
- 12.Ratnieks FLW. 2006. The evolution of cooperation and altruism: the basic conditions are simple and well known. J. Evol. Biol. 19, 1413–1414. ( 10.1111/j.1420-9101.2006.01172.x) [DOI] [PubMed] [Google Scholar]
- 13.Bargum K, Helanterä H, Sundström L. 2007. Genetic population structure, queen supersedure and social polymorphism in a social Hymenoptera. J. Evol. Biol. 20, 1351–1360. ( 10.1111/j.1420-9101.2007.01345.x) [DOI] [PubMed] [Google Scholar]
- 14.Wenseleers T, Ratnieks FLW, Billen J. 2003. Caste fate conflict in swarm-founding social Hymenoptera: an inclusive fitness analysis. J. Evol. Biol. 16, 647–658. ( 10.1046/j.1420-9101.2003.00574.x) [DOI] [PubMed] [Google Scholar]
- 15.Klobuchar EA, Deslippe RJ. 2002. A queen pheromone induces workers to kill sexual larvae in colonies of the red imported fire ant (Solenopsis invicta). Naturwissenschaften 89, 302–304. ( 10.1007/s00114-002-0331-1) [DOI] [PubMed] [Google Scholar]
- 16.Passera L. 1969. Biologie de la reproduction chez Plagiolepis pygmaea Latr. et ses deux parasites sociaux, Plagiolepis grassei Le Mas. Pas. et Plagiolepis xène St. (Hymenoptera formicidae). Toulouse.
- 17.Vargo EL, Passera L. 1991. Pheromonal and behavioral queen control over the production of gynes in the Argentine ant Iridomyrmex humilis (Mayr). Behav. Ecol. Sociobiol. 28, 161–169. ( 10.1007/BF00172167) [DOI] [Google Scholar]
- 18.Brian M. 1973. Caste control through worker attack in the ant Myrmica. Insectes Soc. 20, 87–102. ( 10.1007/BF02223340) [DOI] [Google Scholar]
- 19.Edwards J. 1987. Caste regulation in the pharaoh's ant Monomorium pharaonis: the influence of queens on the production of new sexual forms. Physiol. Entomol. 12, 31–39. ( 10.1111/j.1365-3032.1987.tb00721.x) [DOI] [Google Scholar]
- 20.Edwards JP. 1991. Caste regulation in the pharaoh's ant Monomorium pharaonis: recognition and cannibalism of sexual brood by workers. Physiol. Entomol. 16, 263–271. ( 10.1111/j.1365-3032.1991.tb00565.x) [DOI] [Google Scholar]
- 21.Edwards J, Chambers J. 1984. Identification and source of a queen-specific chemical in the Pharaoh's ant, Monomorium pharaonis (L.). J. Chem. Ecol. 10, 1731–1747. ( 10.1007/BF00987358) [DOI] [PubMed] [Google Scholar]
- 22.Zhao R, Lu L, Shi Q, Chen J, He Y. 2018. Volatile terpenes and terpenoids from workers and queens of Monomorium chinense (Hymenoptera: Formicidae). Molecules 23, 2838 ( 10.3390/molecules23112838) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen J, Cantrell CL, Oi D, Grodowitz MJ. 2016. Update on the defensive chemicals of the little black ant, Monomorium minimum (Hymenoptera: Formicidae). Toxicon 122, 127–132. ( 10.1016/j.toxicon.2016.09.009) [DOI] [PubMed] [Google Scholar]
- 24.Kirby J, et al. 2010. Cloning of casbene and neocembrene synthases from Euphorbiaceae plants and expression in Saccharomyces cerevisiae. Phytochemistry 71, 1466–1473. ( 10.1016/j.phytochem.2010.06.001) [DOI] [PubMed] [Google Scholar]
- 25.R Development Core Team. 2019. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- 26.Smith AA, Liebig J. 2017. The evolution of cuticular fertility signals in eusocial insects. Curr. Opin. Insect Sci. 22, 79–84. ( 10.1016/j.cois.2017.05.017) [DOI] [PubMed] [Google Scholar]
- 27.Oliveira RC, Oi CA, do Nascimento MM, Vollet-Neto A, Alves DA, Campos MC, Nascimento F, Wenseleers T. 2015. The origin and evolution of queen and fertility signals in Corbiculate bees. BMC Evol. Biol. 15, 254 ( 10.1186/s12862-015-0509-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boomsma JJ, Huszár DB, Pedersen JS. 2014. The evolution of multiqueen breeding in eusocial lineages with permanent physically differentiated castes. Anim. Behav. 92, 241–252. ( 10.1016/j.anbehav.2014.03.005) [DOI] [Google Scholar]
- 29.Boonen S, Billen J. 2017. Caste regulation in the ant Monomorium pharaonis (L.) with emphasis on the role of queens. Insectes Soc. 64, 113–121. ( 10.1007/s00040-016-0521-z) [DOI] [Google Scholar]
- 30.Petersen M, Buschinger A. 1971. Das Begattungsverhalten der Pharaoameise, Monomorium pharaonis (L.) 1. Z. Angew. Entomol. 68, 168–175. ( 10.1111/j.1439-0418.1971.tb03140.x) [DOI] [Google Scholar]
- 31.Rocca J, Tumlinson J, Glancey B, Lofgren C. 1983. The queen recognition pheromone of Solenopsis invicta, preparation of (E-6-(1-pentenyl)-2H-pyran-2-one. Tetrahedron Lett. 24, 1889–1892. ( 10.1016/S0040-4039(00)81798-0) [DOI] [Google Scholar]
- 32.Vargo E, Hulsey C. 2000. Multiple glandular origins of queen pheromones in the fire ant Solenopsis invicta. J. Insect. Physiol. 46, 1151–1159. ( 10.1016/S0022-1910(99)00226-7) [DOI] [PubMed] [Google Scholar]
- 33.Holman L, Leroy C, Jørgensen C, Nielsen J, d'Ettorre P. 2013. Are queen ants inhibited by their own pheromone? Regulation of productivity via negative feedback. Behav. Ecol. 24, 380–385. ( 10.1093/beheco/ars174) [DOI] [Google Scholar]
- 34.Nagaraja N, Brockmann A. 2009. Drones of the dwarf honey bee Apis florea are attracted to (2E)-9-oxodecenoic acid and (2E)-10-hydroxydecenoic acid. J. Chem. Ecol. 35, 653–655. ( 10.1007/s10886-009-9648-y) [DOI] [PubMed] [Google Scholar]
- 35.Law JH, Regnier FE. 1971. Pheromones. Annu. Rev. Biochem. 40, 533–548. ( 10.1146/annurev.bi.40.070171.002533) [DOI] [PubMed] [Google Scholar]
- 36.Slessor KN, Kaminski L-A, King G, Borden JH, Winston ML. 1988. Semiochemical basis of the retinue response to queen honey bees. Nature 332, 354 ( 10.1038/332354a0) [DOI] [Google Scholar]
- 37.Slessor KN, Winston ML, Le Conte Y. 2005. Pheromone communication in the honeybee (Apis mellifera L.). J. Chem. Ecol. 31, 2731–2745. ( 10.1007/s10886-005-7623-9) [DOI] [PubMed] [Google Scholar]
- 38.Kaminski LA, Slessor KN, Winston ML, Hay NW, Borden JH. 1990. Honeybee response to queen mandibular pheromone in laboratory bioassays. J. Chem. Ecol. 16, 841–850. ( 10.1007/BF01016494) [DOI] [PubMed] [Google Scholar]
- 39.Oi CA, Van Oystaeyen A, Oliveira RC, Millar J, Verstrepen K, van Zweden J, Wenseleers T. 2015. Dual effect of wasp queen pheromone in regulating insect sociality. Curr. Biol. 25, 1638–1640. ( 10.1016/j.cub.2015.04.040) [DOI] [PubMed] [Google Scholar]
- 40.Princen SA, Oliveira RC, Ernst UR, Millar JG, van Zweden JS, Wenseleers T. 2019. Honeybees possess a structurally diverse and functionally redundant set of queen pheromones. Proc. R. Soc. B 286, 20190517 ( 10.1098/rspb.2019.0517) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martin SJ, Chaline N, Oldroyd BP, Jones GR, Ratnieks FLW. 2004. Egg marking pheromones of anarchistic worker honeybees (Apis mellifera). Behav. Ecol. 15, 839–844. ( 10.1093/beheco/arh089) [DOI] [Google Scholar]
- 42.Sillam-Dussès D. 2010. Trail pheromones and sex pheromones in termites. New York, NY: Nova Science Publishers. [Google Scholar]
- 43.Oliveira RC, Warson J, Sillam-Dussès D, Herrera-Malaver B, Verstrepen K, Millar JG, Wenseleers T. 2020. Data from: Identification of a queen pheromone mediating the rearing of adult sexuals in the pharaoh ant Monomorium pharaonis Dryad Digital Repository ( 10.5061/dryad.w0vt4b8nb) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Oliveira RC, Warson J, Sillam-Dussès D, Herrera-Malaver B, Verstrepen K, Millar JG, Wenseleers T. 2020. Data from: Identification of a queen pheromone mediating the rearing of adult sexuals in the pharaoh ant Monomorium pharaonis Dryad Digital Repository ( 10.5061/dryad.w0vt4b8nb) [DOI] [PMC free article] [PubMed]
Supplementary Materials
Data Availability Statement
The original datasets as well as the R script used to analyse the data are publicly available on https://doi.org/10.5061/dryad.w0vt4b8nb and https://datadryad.org/stash/share/fy60ikVX1hgVDs64WMZ4-kQAWtplYZQJmnhvuTFLi9w [43].