Abstract
Background/Aims
Endoscopic resection is the standard treatment for superficial esophageal squamous-cell neoplasia (SESCN). However, we encounter patients in whom endoscopic resection is difficult to perform. We retrospectively studied the usefulness of argon plasma coagulation (APC) in patients with SESCN.
Materials and Methods
The study comprised 45 patients with SESCN (81 lesions) who underwent APC in our hospital from March 1999 to August 2016. Clinicopathological characteristics, treatment time, the presence or absence of metastasis and recurrence, adverse events, and outcomes were studied.
Results
The median follow-up was 40 months. The median age was 70 years. The tumor diameter was 10 mm or longer in 48 lesions and less than 10 mm in 33 lesions. The median treatment time was 22 minutes. The reasons for selecting APC were as follows: technical difficulty caused by the presence of metachronous multiple lesions in the radiation field after chemoradiotherapy or close proximity to the ulcer scar remaining after endoscopic treatment in 49 lesions (60.4%), and the presence of underlying diseases in 26 lesions (32.0%). Adverse events occurred in 2 patients (4.4%) who had hypoxemia due to over-sedation. Two lesions (2.5%) recurred locally but could be controlled by additional APC. No patient had metastasis or recurrence or died of esophageal neoplasia. The 3-year overall survival rate was 87.0%, and the 3-year recurrence-free survival rate was 97.2%.
Conclusion
APC can be a useful treatment option for SESCN in patients with a limited life expectancy, poor performance status, or technical difficulty in resection of superficial neoplasms.
Keywords: Endoscopic treatment, Argon plasma coagulation, superficial esophageal squamous-cell neoplasia
INTRODUCTION
In recent years, the dissemination of upper gastrointestinal endoscopy and magnifying endoscopy with narrow-band imaging (NBI) has increased the detection rate of superficial esophageal neoplasia (1–3). In Japan, guidelines for the treatment of esophageal neoplasia recommend endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) as the treatments of choice for superficial esophageal neoplasia invading the epithelium (EP) and the lamina propria mucosae (LPM), without metastasis (1).
Argon plasma coagulation (APC) is a non-contact, high-frequency technique for thermal electrocoagulation in which high-frequency electric current is delivered to tissue by means of ionized argon gas, causing tissue coagulation and cauterization (4). In recent years, APC has been used to treat adenocarcinoma with Barrett’s esophagus and early gastric cancer, and good outcomes have been obtained (5–7). However, few studies have reported the usefulness and safety of APC in patients with superficial esophageal neoplasia that is difficult to resect endoscopically.
We previously used APC to treat 21 lesions in 17 high-risk patients with superficial esophageal neoplasia and reported that treatment could be performed safely in a relatively short period (8). Subsequently, the number of patients increased. Therefore, we retrospectively studied the safety and usefulness of APC for the treatment of superficial esophageal squamous-cell neoplasia (SESCN) in patients at high risk or who had lesions that were difficult to resect endoscopically.
MATERIALS AND METHODS
From March 1999 through till August 2016, a total of 490 patients with superficial esophageal neoplasia (594 lesions) were treated endoscopically in our hospital. ESD was performed in 315 patients (355 lesions, 59.8%), EMR was performed in 130 patients (158 lesions, 26.6%), and APC was performed in 45 patients (81 lesions, 13.6%) (Figure 1). In the present study, we evaluated the 45 patients with SESCN (81 lesions) who were treated by APC because of difficulty in endoscopic resection or high procedural risk.
Figure 1.

Endoscopic treatment of superficial esophageal squamous-cell neoplasia in our hospital.
The study variables were age, sex, performance status, and Charlson comorbidity index (9), lesion site, macroscopic type (flat and depressed type/elevated type), pathological type (squamous-cell carcinoma/high-grade intraepithelial neoplasia), tumor diameter, the preoperatively estimated depth of tumor invasion, the presence or absence of underlying diseases, the presence or absence of antithrombotic therapy, the conditions of APC (argon gas flow and high-frequency output), treatment time, number of treatment sessions performed per lesion, the duration of hospitalization, the number of lesions treated in outpatients, adverse events, local recurrence, lymph-node metastasis, distant metastasis, death as a result of esophageal neoplasia, overall-survival rates, and recurrence-free survival rates. Adverse events were evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (10).
Endoscopic resection was not performed because of the following reasons: 1) the presence of metachronous multiple lesions in the radiation field after chemotherapy or technical factors, such as the presence of lesions near the scar remaining after endoscopic treatments, including EMR, ESD, or endoscopic variceal ligation (EVL); or 2) underlying diseases, such as liver cirrhosis and chronic respiratory failure. In this study, once the EMR or ESD treatment had been confirmed to be successful, subsequent lesions occurring near the remaining scar were considered metachronous SESCN rather than recurrences. Similarly, because complete remission of the primary lesion after chemoradiotherapy had already been confirmed, subsequent lesions occurring within the radiation field were considered as metachronous SESCN.
Before APC, preoperative upper gastrointestinal endoscopy was performed in all the patients. Lesions were stained with 0.75% or 1.5% diluted iodine solution, and biopsy specimens were obtained and examined histologically to establish the diagnosis. In addition, invasion depth was evaluated using magnifying endoscopy. In the Japanese classification system, if irregularities in the vascular morphology (dilation, weaving, caliber change, and different shapes) in lesions in the region of the suspected squamous-cell carcinoma are minor, the vessels are classified as Type A, and if the irregularities are severe, they are classified as Type B. Type B irregular vessels demonstrate all four morphological factors and are subclassified as B1 if they have a loop-like formation, B2 if they have other-than-loop-like formations, and B3 if they are highly dilated and abnormal. The size of the areas with low or no vascularity surrounded by Type B vessels (AVA), denoted as AVA-S (less than 0.5 mm in diameter), AVA-M (0.5 mm to less than 3mm), and AVA-L (3 mm or larger) along with the surrounding vessel type, is predictive of invasion depth. AVA of any size surrounded by B1 vessels is predictive of EP/LPM; AVA-M surrounded by B2 vessels is predictive of MM/SM1; and AVA-L surrounded by B3 vessels is predictive of depths of SM2 or deeper. (11).
For patients in whom the depth of invasion was difficult to evaluate preoperatively, endoscopic ultrasonography (UM-DP20-25R; Olympus, Tokyo, Japan) was also performed before treatment to assess the depth of invasion. The absence of metastasis was confirmed by computed tomography (CT).
As for the criteria for discontinuing antithrombotic agents, treatment was discontinued in accordance with the Japan Gastroenterological Endoscopy Society Guidelines (12). All patients gave informed consent in accordance with the ethical guidelines of the Declaration of Helsinki. Our study protocol was approved by the ethics committee of our institution.
APC treatment
APC was performed using an Olympus videoscope, a high-frequency oscillator unit (Erbotom ICC200 from 1999 to 2004 or APC 2 from 2004 to 2016; ERBE Elektromedizin Co. Ltd, Tuebingen, Germany), an argon gas supply unit, and a flexible argon plasma coagulator with an argon gas flow of 2 L/min and a high-frequency output of 40 W (8). An FiAPC probe (ERBE Elektromedizin GmbH, Tübingen, Germany) was used. As for the APC mode, forced APC was performed in all patients. In patients undergoing APC, pethidine was used to induce analgesia, and midazolam was used to induce sedation. The treatment technique was as follows. After conventional endoscopy, the lesion was examined by magnifying endoscopy with NBI and stained with iodine (Figures 2a–d). The circumference of the lesion was marked by APC (Figure 2e). Subsequently, the entire lesion inside the marked area was ablated until it turned black (Figure 2f). If residual tumor was suspected on follow-up endoscopy one week after APC, the procedure was repeated, exercising care to avoid excessive ablation.
Figure 2. a–f.
Treatment procedures using argon plasma coagulation. a) Conventional endoscopy. b) Narrow-band imaging. c) Magnifying endoscopy. d) Iodine staining. e) Marking by APC. f) The region was completely coagulated.
Follow-up schedule
Two months after APC, upper gastrointestinal endoscopy with biopsy was performed, and scarring of the ulcer was confirmed after treatment. Subsequently, upper gastrointestinal endoscopy was performed every 6 months. In addition to white-light endoscopy, endoscopy with NBI or iodine staining was performed to evaluate the presence or absence of local recurrence. CT was performed every 6 months to evaluate lymph-node metastasis, distant metastasis, and recurrence.
Statistical Analysis
All statistical analyses were performed with the use of the Statistical Packages for the Social Sciences (SPSS), version 22 (IBM Corp.; Armonk, NY, USA). Overall survival time was defined as the time from the date of APC to the date of death or the final date of confirming survival. Survival rates were calculated with the use of Kaplan-Meier survival curves.
RESULTS
As shown in Table 1, the clinicopathological characteristics of the patients. The median follow-up period was 40 months (range, 1–158). The median age of the patients was 70 years (range, 47–88). There were 40 men and 5 women. The performance status was 0 in 26 patients, 1 in 18 patients, and 2 in 1 patient. The Charlson comorbidity index was 0 in 1 patient, 1 in 1 patient, 2 in 23 patients, 3 in 8 patients, 4 in 3 patients, 5 in 8 patients, and 6 in 1 patient. As for the lesion location, 3 lesions were located in the cervical esophagus, 12 lesions in the upper thoracic esophagus, 39 lesions in the middle thoracic esophagus, 26 lesions in the lower thoracic esophagus, and 1 lesion in the abdominal esophagus. As for the macroscopic type, 80 lesions were flat and depressed, and 1 lesion was elevated type. As for the pathological type, 56 lesions were squamous-cell carcinoma and 25 lesions were high-grade intraepithelial neoplasia. The tumor diameter was less than 10 mm in 33 lesions, 10 mm to less than 20 mm in 25 lesions, and 20 mm or greater in 23 lesions. The preoperatively estimated depth of invasion was the EP or LPM in 75 lesions and the muscularis mucosae or SM1 in 6 lesions.
Table 1.
Clinicopathological characteristics.
| 45 patients 81 lesions | ||
|---|---|---|
| Age | Median (years) (range) | 70 (47–88) |
| Sex | Male/Female | 40/5 |
| PS | 0/1/2 | 26/18/1 |
| Charlson comorbidity index | 0/1/2/3/4/5/6 | 1/1/23/8/3/8/1 |
| Lesion site | Ce/Ut/Mt/Lt/Ae | 3/12/39/26/1 |
| Macroscopic type | Flat and depressed/elevated | 80/1 |
| Tumor diameter | <10 mm | 33 |
| 10–20 mm | 25 | |
| ≥20 mm | 23 | |
| Preoperative estimated depth of tumor invasion | EP/LPM | 75 |
| MM/SM1 | 6 | |
| Presence or absence of underlying diseases | Present/Absent | 37/8 |
| Presence or absence of antithrombotic therapy | Present/Absent | 10/35 |
PS: performance status; Ce: cervical esophagus; Ut: upper thoracic esophagus; Mt: middle thoracic esophagus; Lt: lower thoracic esophagus; Ae: abdominal esophagus; EP: epithelium; LPM: lamina propria mucosae; MM: muscularis mucosae; SM: submucosa.
Underlying diseases were found in 37 patients (82.2%): liver cirrhosis in 11, heart disease in 6, chronic obstructive pulmonary disease (COPD) in 2, cerebrovascular disease in 2, chronic renal failure in 1, esophageal cancer in 7, hypopharyngeal cancer in 2, gastric cancer in 2, tongue cancer in 1, rectal cancer in 1, lung cancer in 1, and hepatocellular carcinoma in 1. Antithrombotic therapy was being given to 10 patients (28.6%).
The reasons for difficulty in performing endoscopic resection were technical factors in 49 lesions (60.4%), underlying diseases in 26 lesions (32.0%), and other factors in 6 lesions (7.6%) (Table 2). The technical factors were the presence of metachronous multiple lesions in the radiation field after chemotherapy in 22 lesions (27.2%), the presence of metachronous multiple lesions located nearby the scar remaining after EMR in 9 lesions (11.1%), ESD in 6 lesions (7.4%), or EVL in 3 lesions (3.7%), and difficulty in inserting a therapeutic endoscope (caused by disturbed mouth opening or previous surgery for tongue cancer) in 9 lesions (11.1%).
Table 2.
Reasons for difficulty in endoscopic resection.
| Number of lesions | |||
|---|---|---|---|
| Reasons associated with technical difficulty | After chemoradiotherapy (CRT) (Multiple metachronous lesions in radiation field) | 22 | 27.1% |
| Near scar after EMR/ESD/EVL | 9/6/3 | 11.1%/7.4%/3.7% | |
| Difficulty in inserting a therapeutic endoscope | 9 | 11.1% | |
| Subtotal | 49 | 60.4% | |
| Reasons associated with underlying disease | Liver cirrhosis | 14 | 17.2% |
| Chronic respiratory failure | 4 | 4.9% | |
| Chronic heart failure | 4 | 4.9% | |
| Elderly or poor PS | 3 | 3.7% | |
| Chronic renal failure (including hemodialysis) | 1 | 1.3% | |
| Subtotal | 26 | 32.0% | |
| Others | Difficulty in sedation | 2 | 2.5% |
| Prolonged sedation caused by time required to treat other lesions | 2 | 2.5% | |
| Difficulty in long-term hospitalization (because providing care for spouse) | 1 | 1.3% | |
| Patient’s request | 1 | 1.3% | |
| Subtotal | 6 | 7.6% | |
| Total | 81 | 100% |
CRT: chemoradiotherapy; EMR: endoscopic mucosal resection; ESD: endoscopic submucosal dissection; EVL: endoscopic variceal ligation; PS: performance status.
The underlying diseases causing difficulty in endoscopic resection were as follows: liver cirrhosis in 14 lesions (17.2%), chronic respiratory failure in 4 lesions (4.9%), chronic heart failure in 4 lesions (4.9%), elderly or poor performance status in 3 lesions (3.7%), and chronic renal failure including treatment by hemodialysis in 1 lesion (1.3%). APC was selected as the preferred method in patients with liver cirrhosis and bleeding tendency as a result of thrombocytopenia or esophageal varices, and APC was performed because long-time sedation was difficult in patients with chronic respiratory failure or chronic heart failure, elderly patients, and patients with poor performance status.
The other reasons that precluded endoscopic resection were difficulty in inducing sedation in 2 lesions (2.5%), prolonged sedation was required to treat other lesions in 2 lesions (2.5%), long-term hospitalization was difficult because the patient was taking care of her spouse in 1 lesion (1.3%), and the patient’s request in 1 lesion (1.3%).
The median argon gas flow was 2.0 L/min (range, 0.6–2.2). The median high-frequency output was 40 W (range, 30–60). The median treatment time was 22 minutes (range, 2–67). The median number of treatment sessions performed per lesion was 1 (range, 1–3). The median duration of hospitalization was 8 days (range, 4–53). The number of lesions treated in outpatients was 11 (13.6%) (Table 3).
Table 3.
Short-term outcomes
| 45 patients, 81 lesions | ||
|---|---|---|
| Conditions of APC | Median gas flow (range) (L/min) | 2.0 (0.6–2.2) |
| Median high-frequency output (range) (W) | 40 (30–60) | |
| Treatment time | Median (range) (min) | 22 (2–67) |
| Number of treatment sessions performed per lesion | Median (range) (times) | 1 (1–3) |
| Duration of hospitalization | Median (range) (days) | 8 (4–53) |
| Number of lesions treated in outpatients | n (%) | 11 (13.6) |
APC: argon plasma coagulation
As for adverse events, 2 patients (4.4%) had grade 2 hypoxemia. Both cases were caused by over-sedation during APC. However, APC was successfully performed in both patients by increasing the oxygen supply. There were no other serious adverse events (Table 4).
Table 4.
Adverse events.
| Esophagus (45 patients, 81 lesions) | ||
|---|---|---|
| Adverse events | Absent | 43 (95.6%) |
| Present | 2 (4.4%) | |
| Hypoxemia due to over-sedation | 2 (4.4%) | |
| Subsequent bleeding | 0 (0%) | |
| Perforation | 0 (0%) | |
| Gastrointestinal emphysema | 0 (0%) | |
| Stenosis | 0 (0%) |
As for clinical outcomes, local recurrence occurred in 2 lesions (2.5%). Both cases could be controlled by additional APC (Table 5). The first patient had Child’s A liver cirrhosis, and APC was performed to treat SESCN arising in the middle thoracic esophagus. The tumor diameter was 10 mm, and the preoperatively evaluated depth of invasion was EP/LPM. Upper gastrointestinal endoscopy performed after 2 months showed local recurrence near the scar, and APC was additionally performed. As of 51 months after the additional session of APC, the patient remains alive with no metastasis or recurrence.
Table 5.
Clinical outcomes
| 45 patients, 81 lesions | |
|---|---|
| Follow-up period (months)median (range) | 40 (1-158) |
| Local recurrence | 2 (2.5%) |
| Lymph-node metastasis | 0 (0%) |
| Distant metastasis | 0 (0%) |
| Died of esophageal cancer | 0 (0%) |
| Died of other disease | 12 (26.6%) |
In the second patient, sedation was difficult to induce because of restlessness. APC was, therefore, performed to treat SESCN with a tumor diameter of 15 mm, arising in the middle thoracic esophagus. The preoperatively evaluated depth of invasion was EP/LPM. Upper gastrointestinal endoscopy performed 2 months after APC showed local recurrence near the scar, and APC was repeated. A total of 4 sessions of APC were performed, and there was no subsequent evidence of metastasis or recurrence. The patient died of cerebral hemorrhage 96 months after the additional sessions of APC.
No patient had lymph-node metastasis or distant metastatic recurrence or died of esophageal cancer. Twelve patients (26.6%) died of other diseases. The 3-year overall survival rate was 87.0% (Figure 3). The 3-year recurrence-free survival rate was 97.2%, indicating good outcomes (Figure 4).
Figure 3.

Overall survival. The 3-year overall survival rate was 87.0%.
Figure 4.
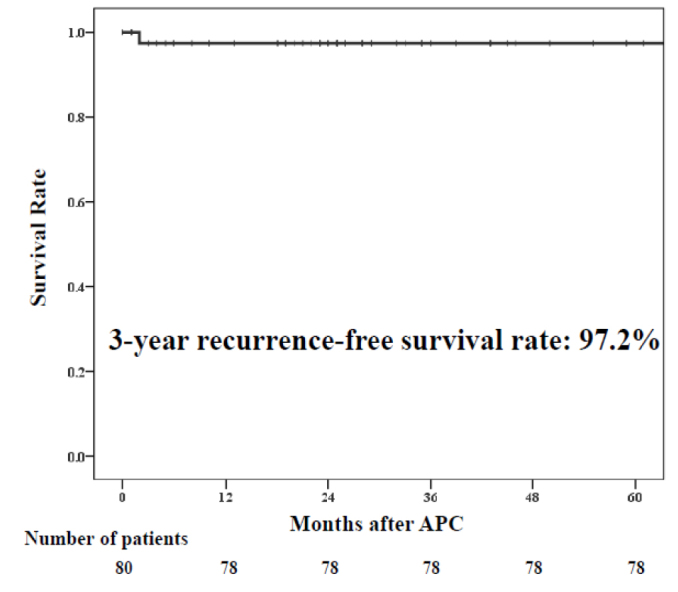
Recurrence-free survival. The 3-year recurrence-free survival rate was 97.2%.
DISCUSSION
In Japan, guidelines for the treatment of SESCN have designated endoscopic resection as the treatment of choice for SESCN with EP/LPM invasion (1). The development of various techniques, such as the clip and line method has increased the rate of en bloc complete resection, shortened the treatment time, and enhanced the safety of treatment (13, 14). However, APC is considered useful for patients in whom endoscopic resection is difficult to perform because of technical factors or patient characteristics (1).
APC is a non-contact technique for thermal cauterization. Tissue cauterized by APC quickly coagulates, increasing the resistance value. Since a high-frequency electric current flows toward tissue with lower resistance values, the depth of tissue degeneration is considered to be about 2–3 mm from the surface. Therefore, as the depth of thermal cauterization induced by APC is confined to the muscularis mucosae, the risk of perforation is considered low (4). However, in contrast to ESD, APC does not permit pathological examination. Thus, careful follow-up examinations are required to confirm the presence or absence of remnant lesions (15).
Nomura et al. (15) reported that APC had a local recurrence rate of 20% (2 of 10 patients), but local control could be achieved by performing additional APC. Min et al. (16) reported a local recurrence rate of 5.3% (1 of 19 patients). The patient with recurrence was treated by additional APC and survived, with no subsequent metastasis or recurrence. Tahara et al. (8) reported that local recurrence occurred in 2 (9.5%) of 21 patients. Both of the patients were treated by additional APC and survived, with no lymphatic metastasis or distant metastasis. In our study, the local recurrence rate after APC was 2.5% (2 of 81 patients) in patients with SESCN. Our 3-year recurrence-free survival rate was higher than the previously reported rate (15, 16). The local recurrence rate after APC was not low, as compared with that after ESD (0% to 1.0%) (17, 18). However, even if local recurrence develops, local control can probably be achieved by follow-up APC for lesions with a diagnosis of shallow invasion. However, since the median follow-up period was 40 months in our study, the possibilities of lymph-node metastasis and distant metastatic recurrence could not be ruled out. Therefore, further follow-up is necessary.
Gastrointestinal perforation associated with APC was reported to have occurred in 6 (1.8%) of 325 patients by Wahab et al. (19) and in 5 (0.31%) of 1,606 patients by Grund et al. (20). As compared with the perforation rate after ESD (0–6.9%), the perforation rate after APC was considered lower (14, 17, 21–25). Since APC is performed with the use of argon gas, emphysema of the gastrointestinal tract has been reported to occur in 0.46% of patients (20).
In our study, grade 2 hypoxemia associated with over-sedation occurred in 4.4% of the patients, but there were no serious adverse events, such as subsequent bleeding, perforation, emphysema of the gastrointestinal tract, or stenosis. Wan et al. (26) reported that the incidence of stenosis was 17% after balloon-based radiofrequency ablation and suggested that prevention of stricture is necessary in patients who have esophageal squamous-cell neoplasms involving a long segment exceeding 9 cm in diameter. In our study, APC was not performed for lesions that occupied the entire circumference of the esophagus or those with a long axis of more than 9 cm. No patient had stenosis and treatment could be performed safely. Thus, it is important to ablate intermittently and to avoid excessive ablation to ensure safe APC treatment.
The time required for ESD is 64–90 minutes, whereas the time required to perform APC in our study was 22 minutes. Thus, treatment could be performed in a relatively short period (14, 22–25). APC was considered safe even in patients in whom long-term sedation was difficult to achieve.
Japan has become an aging society. The majority of patients who are 75 years or older have multiple underlying diseases and functional disorders that influence activities of daily living. In Japan, the proportion of people who regularly visit hospitals for at least one chronic disease is nearly 70% among persons who are 75–84 years of age, which is higher than that in other age groups (27–30).
In our study, we chose to perform APC in patients with underlying diseases, such as liver cirrhosis, chronic heart failure, chronic respiratory failure, and renal failure being treated by hemodialysis. Such patients comprise about 30% of all patients treated in our department. ESD is a technique with a high rate of en bloc complete resection and excellent local control. We, thus, recommend ESD for the treatment of SESCN in patients who can endure surgery and have no technical problems. However, in elderly patients and patients with concurrent disease, the treatment policy of whether to perform ESD or APC should be decided by weighing the risks against the expected benefits of these procedures for individual patients and considering the prognosis of concurrent disease, the quality of daily living, and the desires of patients and their family members. Deciding the treatment strategy is subject to considerable selection bias, and long-term outcomes are likely to reflect the results obtained in clinical practice.
Our study had several important limitations. It was a retrospective study, performed at a single center. Further prospective studies with larger numbers of patients are needed to confirm the usefulness of APC for the management of SESCN.
APC can be considered a promising, highly effective, and safe procedure for the treatment of SESCN in patients whose lesions are difficult to resect endoscopically. Treatment can be accomplished within a short time, and APC can be performed on an outpatient basis in patients in whom hospitalization is difficult. Thus, it is expected to become one of the essential procedures in the aging society. In conclusion, APC can be considered a beneficial, cost-effective procedure for SESCN in patients with a limited life expectancy, poor performance status, or technical difficulty in resecting SESCN.
MAIN POINTS.
Endoscopic resection is the standard treatment for SESCN.
We encounter patients in whom endoscopic resection is difficult to perform the endoscopic resection.
In our study, APC was performed safely and in a short time for SESCN in patients at high risk or with limited endoscopic resectability.
APC can be a useful treatment option for SESCN.
Footnotes
Presented in: This study was presented at the 93rd Congress of the Japan Gastroenterological Endoscopy Society May 12, 2017, Osaka, Japan.
Ethics Committee Approval: Ethics committee approval was received for this study from the Ethics Committee of Kitasato University (Decision date 4.10.2019. Decision number B17-118).
Informed Consent: Written informed consent was obtainedfrom the patients who in this study.
Peer-review: Externally peer-reviewed.
Author Contributions: Concept - K.Y.; Design - T.S.; Supervision-I.K., Y.T.; Materials - K.Y., T.S., I.K., Y.T., W.T., W.A., A.M., K.C., K.W.; Data Collection and/orProcessing - K.Y., T.S., I.K., Y.T.; Analysis and/or Interpretation - K.Y., I.K., Y.T.; Literature Search - K.Y., I.K., Y.T.; Writing Manuscript - K.Y.; Critical Review - T.S., K.W.
Conflict of Interest: The authors have no conflict of interest to declare.
Financial Disclosure: The authors declared that this study has received no financial support.
REFERENCES
- 1.The Japan Esophageal Society. Guidelines for diagnosis and treatment of carcinoma of the esophagus. Tokyo: Kanehara Co Ltd; 2017. [Google Scholar]
- 2.Muto M, Minashi K, Yano T, et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–72. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–95. doi: 10.1016/S0016-5107(03)02532-X. [DOI] [PubMed] [Google Scholar]
- 4.Grund KE, Storek D, Farin G. Endoscopic argon plasma coagulation first clinical experiences in flexible endoscopy. Endosc Surg Allied Technol. 1994;2:42–6. [PubMed] [Google Scholar]
- 5.Grade AJ, Shah IA, Medlin SM, Ramirez FC. The efficacy and safety of argon plasma coagulation therapy in Barrett’s esophagus. Gastrointest Endosc. 1999;50:18–22. doi: 10.1016/S0016-5107(99)70338-X. [DOI] [PubMed] [Google Scholar]
- 6.Sagawa T, Takayama T, Oku T, et al. Argon plasma coagulation for successful treatment of early gastric cancer with intramucosal invasion. Gut. 2003;52:334–9. doi: 10.1136/gut.52.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kitamura T, Tanabe S, Koizumi W, Mitomi H, Saigenji K. Argon plasma coagulation for early gastric cancer: technique and outcome. Gastrointest Endosc. 2006;63:48–54. doi: 10.1016/j.gie.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Tahara K, Tanabe S, Ishido K, et al. Argon plasma coagulation for superficial Esophageal squamous-cell carcinoma in high-risk patients. World J Gastroenterol. 2012;18:5412–7. doi: 10.3748/wjg.v18.i38.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 10.National Cancer Institute. Common terminology criteria for adverse events (CTCAE) ver 4.0. [Accessed 30 January 2018]. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf.
- 11.Oyama T, Inoue H, Arima M, et al. Prediction of the invasion depth of superficial squamous cell carcinoma based on microvessel morphology: magnifying endoscopic classification of the Japan Esophageal Society. Esophagus. 2017;14:105–12. doi: 10.1007/s10388-016-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujimoto K, Fujishiro M, Kato M, et al. Japan Gastroenterological Endoscopy Society Guidelines for gastroenterological endoscopy in patients undergoing antithrombotic treatment. Dig Endosc. 2014;26:1–14. doi: 10.1111/den.12183. [DOI] [PubMed] [Google Scholar]
- 13.Koike Y, Hirasawa D, Fujita N, et al. Usefulness of the thread-traction method in esophageal endoscopic submucosal dissection: randomized controlled trial. Dig Endosc. 2015;3:303–9. doi: 10.1111/den.12396. [DOI] [PubMed] [Google Scholar]
- 14.Higuchi K, Tanabe S, Azuma M, et al. A phase II study of endoscopic submucosal dissection for superficial esophageal neoplasms (KDOG 0901) Gastrointest Endosc. 2013;78:704–10. doi: 10.1016/j.gie.2013.04.182. [DOI] [PubMed] [Google Scholar]
- 15.Nomura T, Miyashita M, Makino H, Okawa K, Katsuta M, Tajiri T. Argon plasma coagulation for the treatment of superficial esophageal carcinoma. J Nippon Med Sch. 2007;74:163–7. doi: 10.1272/jnms.74.163. [DOI] [PubMed] [Google Scholar]
- 16.Min BH, Kim ER, Lee JH, et al. Feasibility and efficacy of argon plasma coagulation for early esophageal squamous cell neoplasia. Endoscopy. 2013;45:575–8. doi: 10.1055/s-0033-1344025. [DOI] [PubMed] [Google Scholar]
- 17.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3:67–70. doi: 10.1016/S1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 18.Ono S, Fujishiro M, Niimi K, Goto O, Yamamichi N, Omata M. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–6. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 19.Wahab PJ, Mulder CJ, den Hartog G, Thies JE. Argon plasma coagulation in flexible gastrointestinal endoscopy: pilot experiences. Endoscopy. 1997;29:176–81. doi: 10.1055/s-2007-1004159. [DOI] [PubMed] [Google Scholar]
- 20.Grund KE, Zindel C, Farin G. Argon plasma coagulation through a flexible endoscope. Evaluation of a new therapeutic method after 1606 uses. Dtsch Med Wochenschr. 1997;122:432–8. doi: 10.1055/s-2008-1047634. [DOI] [PubMed] [Google Scholar]
- 21.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–94. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara R, Ishii H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–72. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous-cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–4. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 24.Repici A, Hassan C, Carlino A, et al. Endoscopic submucosal dissection in patients with early esophageal squamous-cell carcinoma: results from a prospective Western series. Gastrointest Endosc. 2010;71:715–21. doi: 10.1016/j.gie.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 25.Tsujii Y, Nishida T, Nishiyama O, et al. Clinical outcomes of endoscopic submucosal dissection for superficial esophageal neoplasms: a multicenter retrospective cohort study. Endoscopy. 2015;47:775–83. doi: 10.1055/s-0034-1391844. [DOI] [PubMed] [Google Scholar]
- 26.Wang WL, Chang IW, Chen CC, et al. Predictors for postoperative esophageal stricture after balloon-based radiofrequency ablation for early esophageal squamous neoplasia: a multicenter validation study. Therap Adv Gastroenterol. 2016;9:257–64. doi: 10.1177/1756283X16633051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kakushima N, Fujishiro M, Kodashima S, et al. Technical feasibility of endoscopic submucosal dissection for gastric neoplasms in the elderly Japanese population. J Gastroenterol Hepatol. 2007;22:311–4. doi: 10.1111/j.1440-1746.2006.04563.x. [DOI] [PubMed] [Google Scholar]
- 28.Chinda D, Sasaki Y, Tatsuta T, et al. Perioperative complications of endoscopic submucosal dissection for early gastric cancer in elderly Japanese patients 75 years of age or older. Intern Med. 2015;54:267–72. doi: 10.2169/internalmedicine.54.3300. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Public health and aging: trends in aging-United States and worldwide. JAMA. 2003;289:1371–3. doi: 10.1001/jama.289.11.1371. [DOI] [PubMed] [Google Scholar]
- 30.Fried L, Barron J. Older Adults Handbook of Urban Health: Populations, Methods, and Practice. New York, NY: Springer; 2005. [DOI] [Google Scholar]



